Abstract
Objective:
Using three-dimensional speckle-tracking echocardiography (3D-STE), we aimed to evaluate left ventricular (LV) function in type 2 diabetes mellitus (T2DM) patients with non-alcoholic fatty liver disease (NAFLD).
Methods:
In total, 97 T2: DM patients were categorized into three groups based on hepatic ultrasonography group A (those without NAFLD, n=30), group B (those with mild NAFLD, n=32), and group C (those with moderate-to-severe NAFLD, n=35). Our conventional echocardiographic parameters included transmitral peak early and late diastolic velocity (E and A), septal and lateral early (e’) mitral annular diastolic tissue velocities, and left atrial maximum volume index (LAVImax). LV end-diastolic and -systolic volume, LV mass index (LVMI), and LV ejection fraction were measured using real-time three-dimensional echocardiography. The 3D-STE parameters included LV global radial strain (GRS), global longitudinal strain (GLS), global area strain (GAS), and global circumferential strain (GCS).
Results:
Our results showed that in group C, GCS, GRS, GLS, GAS, and septal and lateral e’ velocity decreased, whereas average E/e’ and LAVImax increased compared to groups B and A (p<0.05). Multiple linear regression analysis showed that NAFLD is independently associated with 3D-STE parameters, and glycosylated hemoglobin also has negative impacts on all LV 3D strains.
Conclusion:
When combined with conventional echocardiography, 3D-STE can assess LV function effectively in T2DM patients with NAFLD. Additionally, the severity of LV dysfunction in the moderate-to-severe NAFLD group (group C) was worse than the mild and absent NAFLD groups (groups A and B).
Keywords: three-dimensional speckle-tracking echocardiography, non-alcoholic fatty liver disease, type 2 diabetes mellitus, left ventricular function
Introduction
Non-alcoholic fatty liver disease (NAFLD) is diagnosed when steatosis is seen in more than 5% of hepatocytes. It is characterized by excessive accumulation of liver fat closely linked to insulin resistance associated with no excessive alcohol consumption or other known liver diseases (1,2). NAFLD has become the most common chronic liver disease in patients with type 2 diabetes mellitus (T2DM) (3), and some studies have indicated that the morbidity of NAFLD is increasing parallelly with that of T2DM (4,5). NAFLD is closely linked to the risk factors that lead to cardiovascular disease, including obesity, hypertension, dyslipidemia, and coronary artery disease (6). However, although the adverse impact of NAFLD has been confirmed on the cardiac function in T2DM patients (7-9), there have been very few studies or reports to date, which assess left ventricular (LV) dysfunction, using three-dimensional speckle-tracking echocardiography (3D-STE) (8,10). A better understanding of the interrelationship between NAFLD, diabetes, and LV function in the ordinary population has important physiological and clinical long-term implications.
As a newly angle-independent echocardiographic technique, 3D-STE is not affected by out-of-plane speckle loss (11). It has also been proven to be a credible method for quantifying LV function (12). At the same time, accuracy and reliability of 3D-STE have been confirmed in studies related to magnetic resonance imaging (MRI) (13).
Methods
Study population and grouping principles
Our study enrolled patients, who had been diagnosed with T2DM from hospital admissions made between October 2016 and August 2018 at the department of endocrinology in the Second Affiliated Hospital of Dalian Medical University, Dalian, China. We excluded patients with (1) a prior history of hypertension and known causes of heart disease (i.e., congenital heart disease, myocardiopathy, or valvular diseases); (2) a prior history of cirrhosis or other known chronic liver diseases; (3) excessive alcohol intake (>30 g/d for males and >20 g/d for females); (4) T2DM-related complications; (5) a prior history of smoking and carotid stenosis; (6) use of any hypoglycemic drugs (oral and insulin). A total of 97 T2DM patients who had normal LV ejection fraction (LVEF >50%) were categorized into three groups according to the results of liver ultrasonography (age range, 26–58 years; mean age, 46.9±8.9 years; male to female ratio, 69:28). Thirty of the T2DM patients were without NAFLD (age range, 26–56 years; mean age, 48.5±10.0 years; male to female ratio, 11:4) and were selected to comprise group A (the control group). The other sixty-seven patients with NAFLD were classified into two groups (mild and moderate-to-severe) according to established criteria: (14) group B (32 mild NAFLD patients; age range, 34–58 years; mean age, 45.3±5.4 years; male to female ratio, 5:3) and group C (35 moderate-to-severe NAFLD patients; age range, 30–58 years; mean age, 47.2±9.7 years; male to female ratio, 27:8).
Our study was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University on human research, and written informed consent was obtained from every participant.
Diagnostic standard of NAFLD and hepatic ultrasonography
The diagnostic criteria for NAFLD were based on the Clinical Practice Guidelines for the management of NAFLD, as published in 2016 (1), combined with characteristic ultrasonographic features (15). All participants underwent hepatic ultrasonography using a GE Vivid E9 device (GE Health Care, American, and Vingmed Ultrasound AS) with a 4C-D probe (1.6–4.0 MHz).
Clinical and biochemical data
The gender, age, height, weight, and heart rate (HR) were recorded for all participants, and each participant’s body surface area (BSA, m2) and body mass index (BMI, kg/m2) were calculated. We measured the blood pressure (BP) after the patients were seated quietly for 10–15 minutes. Blood samples were collected in the morning after overnight fasting. Aspartate aminotransferases (AST) and alanine aminotransferases (ALT) were the liver enzymes measured, whereas triglyceride (TG) and total cholesterol (TC) were the main serum lipid levels measured. Fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c) were also measured.
Conventional echocardiography measurements
The patients were placed in the left lateral decubitus position during a continuous recording using a three-lead electrocardiography. Conventional echocardiography was performed using a GE Vivid E9 scanner equipped with an M5S-D probe (1.5–4.5 MHz), which provided the echocardiographic parameters of the three groups. We recorded standard 2-D images of three consecutive cardiac cycles from apical 4-chamber and long-axis views, while the patients were performing gentle respiration. We measured transmitral peak early diastolic velocity (E) and peak late diastolic velocity (A) using a pulsed-wave Doppler. We used tissue Doppler imaging (TDI) to obtain septal and lateral early (e’) mitral annulus diastolic tissue velocities; thereafter, we calculated E/A and average E/e’. We measured the left atrial maximal volume at the end of LV systole from apical two-chamber and four-chamber views (using modified Simpson rule) and then calculated the left atrial maximum volume index LAVImax (LAVImax=LAVmax/BSA). Interventricular septum thickness diastolic (IVSTd), posterior wall thickness diastolic (PWTd), LV end-diastolic diameter (LVDd), and LV end-systolic diameter (LVDs) were recorded using conventional echocardiography.
Real-time 3D echocardiography and 3D-STE measurements
We acquired 3D echocardiographic LV full-volume images using a matrix-array 4V-D probe (1.7–3.3 MHz). Full-volume images acquisition occurred during four consecutive cardiac cycles; the transducer was kept in a stable position at a frame rate of 25–50 frames/second to ensure the view was maintained in an optimal temporal and spatial resolution. We could manually adjust the frame rate from the machine settings. We took multibeat images while the patient’s breath was held for eliminating any breathing-related motion artifacts. These full-volume images were stitched together from six subvolumes in four consecutive cardiac cycles, including three apical views (four-chamber, three-chamber, and two-chamber) and three short-axis views (basal, middle, and apical). Special attention was paid to the adjustments of the sector width and depth to clearly display the entire LV myocardium, endocardium, and epicardium as well as the entire LV cavity.
We used 4D Auto LVQ software to analyze all the collected full-volume images. The tracks of the endocardial and epicardial borders within a single cycle were detected automatically by the software after the points of mitral valve closing and the LV apex were manually determined, making manual modifications if necessary. Finally, the system provided the LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LVEF, LV mass and regional and global strain values in multiple directions. The LV mass index (LVMI) was then calculated by dividing the LV mass by BSA. The 3D-STE parameters included global longitudinal strain (GLS), global radial strain (GRS), global circumferential strain (GCS), and global area strain (GAS). These were calculated from the weighted average of seventeen myocardial segmental peak strain values in all directions, shown as bull’s eye figures. Two independent and experienced specialists performed all the examinations, and both were unaware of the purpose of our study (to rule out any bias).
Statistical analysis
We carried out the statistical analysis using the Statistical Package for Social Sciences (SPSS™) 19.0 software (SPSS 19.0, Inc., Chicago, IL, USA). Before analyzing the continuous variables, the normal distribution of the continuous variables with the normality test was determined using the Shapiro–Wilk test. Normally distributed continuous data were expressed as mean±standard deviation. Categorical variables were expressed as frequency (percentage, %) and analyzed using Pearson χ2-text. For analyzing normally distributed data of three groups, one-way ANOVA was applied, and Bonferroni test was used for simultaneous multiple comparisons. We used multiple regression analysis to determine the independent predictors of 3D strain parameters in T2DM patients. P values <0.05 were considered statistically significant. We measured intra- and interobserver variabilities in 25 randomly selected subjects, and they were described using the intraclass correlation coefficient.
Ethical approval
All the procedures performed in our studies involving human participants were in accordance with the ethical standards of the Institutional and National Research Committee and 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
No differences were found in gender, age, TG, TC, HR, FPG, and BP among the three groups (p>0.05). NAFLD patients in group C had higher HbA1c, BMI, and ALT and AST levels compared to groups A and B, and ALT, AST levels for group B were higher than for group A (p<0.05; Table 1).
Table 1.
Clinical and biochemical characteristics of the study population (x±s)
| Clinical and biochemical characteristics | Group A (n=30) | Group B (n=32) | Group C (n=35) | P value |
|---|---|---|---|---|
| Male, sex n (%) | 22 (73.3) | 20 (62.5) | 27 (77.1) | 0.290 |
| Age (years) | 48.5±10.0 | 45.3±5.4 | 47.2±9.7 | 0.464 |
| HR (beats/min) | 71±11 | 75±7 | 76±12 | 0.068 |
| BMI (kg/m2) | 24.4±2.6 | 25.5±3.2 | 27.3±3.8*,# | 0.019 |
| Systolic arterial pressure (mm Hg) | 127±9 | 126±6 | 129±8 | 0.438 |
| Diastolic arterial pressure (mm Hg) | 74±6 | 79±8 | 82±8 | 0.187 |
| FPG (mmol/L) | 8.4±2.1 | 8.7±2.2 | 8.8±1.9 | 0.279 |
| HbA1c (%) | 6.9±2.3 | 7.1±3.3 | 9.0±2.1*,# | 0.003 |
| Total cholesterol (mmol/L) | 7.2±3.4 | 5.6±1.4 | 5.3±1.4 | 0.342 |
| TG (mmol/L) | 2.5±1.1 | 2.5±1.3 | 3.0±3.4 | 0.710 |
| AST (U/I) | 11.8±7.9 | 41.9±27.4* | 57.3±36.7*,# | <0.001 |
| AST (U/I) | 13.3±6.8 | 31.0±23.7* | 34.8±19.8*,# | 0.003 |
Data are expressed as mean±SD except for the male to female ratio
P<0.05 versus group A.
P<0.05 versus group B
ALT - alanine aminotransferases; AST - aspartate aminotransferases; FPG - fasting blood glucose; TG - triglyceride HbA1c - glycosylated hemoglobin; BMI - body mass index;
HR - heart rate; SD - standard deviation
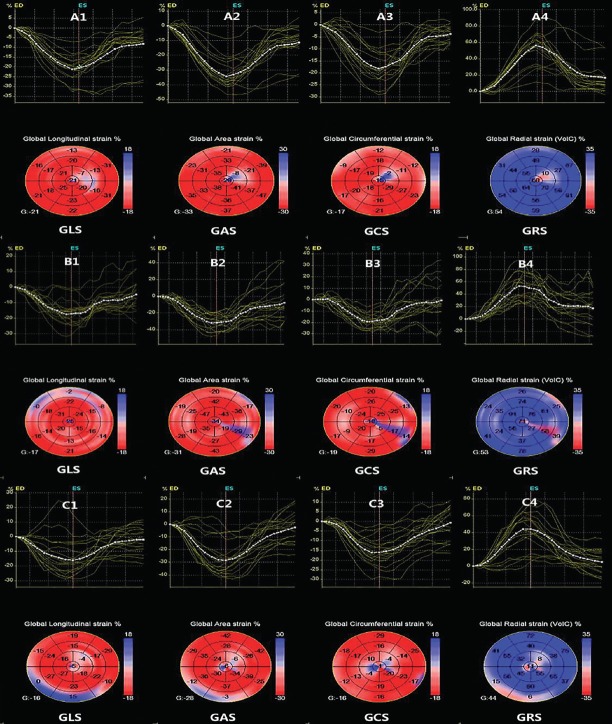
In group C, average E/e’ and LAVImax increased, while septal and lateral e’ velocity decreased compared to groups B and A (p<0.05). There were no differences among the three groups with regard to E/A, IVSTd, PWTd, LVMI, LVDd, LVDs, LVEF, and LVEDV or LVESV (p>0.05). The 3D-STE-derived GCS, GRS, GLS, and GAS values were lower in group C than in groups A and B (p<0.05; Table 2). The 3D-STE parameters of all three groups are shown in Figure 1.
Table 2.
Conventional and 3D echocardiographic parameters and 3D-STE parameters of the study population (x±s)
| Variables | Group A (n=30) | Group B (n=32) | Group C (n=35) | P value |
|---|---|---|---|---|
| 2D | ||||
| E/A | 1.15±0.36 | 1.12±0.31 | 1.10±0.32 | 0.671 |
| Lateral e’ (cm/s) | 14.26±2.43 | 13.81±2.15 | 12.82±2.96*,# | 0.046 |
| Septal e’ (cm/s) | 12.84±2.34 | 11.49±1.67 | 8.83±1.92*,# | 0.025 |
| Average E/e’ | 7.6±2.0 | 9.1±2.3 | 13.2±2.7*,# | 0.017 |
| LVDd (mm) | 47.5±4.5 | 44.0±2.3 | 47.7±5.3 | 0.473 |
| LVDs (mm) | 30.7±3.9 | 27.8±2.3 | 31.3±4.6 | 0.598 |
| IVSTd (mm) | 8.7±1.3 | 8.7±0.9 | 9.6±1.1 | 0.132 |
| PWTd (mm) | 8.5±1.2 | 8.3±1.0 | 9.2±1.2 | 0.116 |
| LAVImax (mL/m2) | 27.21±2.42 | 29.63±2.82 | 33.41±2.2*,# | 0.013 |
| 3D | ||||
| LVMI (g/m2) | 68.9±11.4 | 70.4±8.6 | 71.5±7.3 | 0.102 |
| LVEF (%) | 60.3±5.0 | 59.3±3.7 | 58.9±4.5 | 0.169 |
| LVEDV (mL) | 70.5±10.4 | 71.3±12.6 | 71.9±11.2 | 0.207 |
| LVESV (mL) | 30.2±7.4 | 31.5±11.9 | 32.8±9.8 | 0.115 |
| 3D-STE | ||||
| GLS (%) | -19.0±2.6 | -17.9±3.1 | -14.1.±4.1*,# | <0.001 |
| GRS (%) | 45.7±6.2 | 45.1±9.0 | 40.8±6.8*,# | 0.001 |
| GCS (%) | -19.5±3.3 | -19.4±2.6 | -17.2±3.5*,# | <0.001 |
| GAS (%) | -29.0±3.4 | -27.2±4.3 | -24.3±4.7*,# | <0.001 |
Data presented are mean±SD.
P<0.05 versus group A.
P<0.05 versus group B
Septal and lateral e’- early mitral annular diastolic tissue velocities; GAS - global area strain; SD - standard deviation; 2D - two dimensional; 3D - three-dimensional;
3D-STE - three-dimensional speckle-tracking echocardiography; E/A - transmitral peak early diastolic velocity/ peak late diastolic velocity
Figure 1.
Longitudinal and circumferential area and radial strains of the three groups are generated and presented in both (regional and average) strain curves and color-coded, 17-segment bullseye plots by 3D-STE. The colored lines refer to the regional strain and the white dotted line refers to the global strain. A, B, and C (groups A, B, and C), 1–4 GLS - global longitudinal strain; GRS - global radial strain; GCS - global circumferential strain; GAS - global area strain
In the multiple linear regression analysis, we replaced the negative 3D strain parameters with absolute values. Our study prespecified independent variables based on their statistical significance and their biological plausibility. NAFLD was independently associated with GLS (β=−0.579; p<0.001), GRS (β=−0.568; p<0.001), GCS (β=−0.561; p<0.001), and GAS (β=−0.602; p<0.001). The HbA1c level was independently associated with GLS (β=−0.434; p<0.001), GRS (β=−0.257; p=0.012), GCS (β=−0.387; p<0.001), and GAS (β=−0.464; p<0.001). E/E’ also showed negative effects upon GLS (β=−0.264; p=0.009) and GCS (β=−0.197; p=0.010). In addition, BMI was only associated with GAS (β=0.179; p=0.008; Table 3).
Table 3.
Predictive factors for 3D strain in T2DM patients
| GLS | GRS | GCS | GAS | |||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| Age | 0.063 | 0.432 | 0.052 | 0.543 | 0.032 | 0.712 | 0.046 | 0.528 |
| BMI | -0.094 | 0.286 | -0.084 | 0.353 | -0.187 | 0.053 | 0.179 | 0.008 |
| HbA1c | -0.434 | <0.001 | -0.257 | 0.012 | -0.387 | <0.001 | -0.464 | <0.001 |
| NAFLD | -0.579 | <0.001 | -0.568 | <0.001 | -0.561 | <0.001 | -0.602 | <0.001 |
| E/e’ | -0.264 | 0.009 | -0.096 | 0.087 | -0.197 | 0.010 | -0.104 | 0.169 |
3D - three dimensional; BMI - body mass index; GLS - global longitudinal strain; GRS - global radial strain; GCS - global circumferential strain; GAS - global area strain;
HbA1c - glycosylated hemoglobin; NAFLD - non-alcoholic fatty liver disease; T2DM - type 2 diabetes mellitus; E/e’ - transmitral peak early diastolic velocity/septal and lateral early
Table 4 shows the inter- and intraobserver results of the 3D LV strain.
Table 4.
Inter- and intraobserver analyses for global strain and twist values
| Variables | Intraobserver variability | Interobserver variability, Rho (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| R | Bias (%) | LOA (%) | ICC | R | Bias (%) | LOA (%) | ICC | |
| GAS (%) | 0.84 | 1.24 | -4.34~2.68 | 0.902 | 0.82 | 1.19 | -5.20~4.80 | 0.890 |
| GCS (%) | 0.81 | 2.25 | -6.35~5.27 | 0.857 | 0.79 | 2.85 | -8.04~3.20 | 0.815 |
| GLS (%) | 0.90 | 0.65 | -1.17~2.02 | 0.970 | 0.94 | 0.57 | -0.65~1.17 | 0.989 |
| GRS (%) | 0.82 | 1.93 | -6.05~4.20 | 0.868 | 0.78 | 2.09 | -6.48~4.90 | 0.841 |
CI - confidence interval; GLS - global longitudinal strain; GRS - global radial strain; GCS - global circumferential strain; GAS - global area strain; LOA - limit of agreement; ICC - intraclass correlation coefficient
Discussion
Recently, there has been increased evidence that NAFLD is a common cardiovascular risk factor among T2DM patients (8,16). Moreover, some studies have also reported that NAFLD can lead to subclinical myocardial remodeling and dysfunction in T2DM patients (8, 17, 18). Our study confirms these studies and extends their findings based on 3D-STE, which is more reliable and sensitive in evaluating LV function.
Myocardial strain can reflect the degree of myocardial deformation relative to its original shape, and it has become a common index for evaluating local myocardial systolic function (19,20). 3D-STE is able to track the movement of myocardial speckle, and it can detect myocardial strain in all directions, thus offering more accurate information about the myocardial strain (21,22).
Only a small number of studies have documented the impaired LV systolic function using TDI (23-25). Trovato et al. (26) reported a slight decrease in LVEF but only in male NAFLD subjects. Additionally, most researchers have failed to discover any alterations in systolic function. In our study, all the patients had normal LVEF, and no difference in 3D strain was found between groups A and B. A probable explanation is that in patients with mild fatty liver, only a slight impact on the cardiac function was observed. However, in group C, the GRS, GCS, GLS, and GAS values were lower than those in groups A and B (p<0.05), indicating that moderate-to-severe NAFLD patients have subclinical impairment in LV systolic function. LVEF is generally within a normal range in the early stages of myocardial damage because of the myocardial compensation. This suggests that using this conventional parameter could result overlooking the early stages of LV systolic dysfunction.
The LV myocardium is mainly comprised of longitudinal (70%) and circular fibers (30%) (27). Because of the different directions of motion and arrangement as well as the extent and timing of the shortening and thickening of myocardial fibers, longitudinal fibers play an essential role in LV systolic function (28). In our study, the decrease of GLS precedes the change of LVEF; the most probable interpretation for this was that the longitudinal fibers located in the endocardium were more sensitive to pernicious elements, such as ischemia, hypoxia, and pressure loading (29). Many studies have already described GAS, which is a new parameter (30,31), i.e., the integration of GLS and GCS. It is related to the longitudinal and circumferential motions and represents a deformation of the endocardial area (28). For this reason, a reduction in GAS is a better indication of myocardial systolic dysfunction.
In group C, the patients were characterized by their lower septal and lateral e’ velocity as well as higher than average E/e’ and LAVImax compared to patients in groups A and B (p<0.05), suggesting that NAFLD has an adverse effect on diastolic function. This effect has also been reported in many other published studies (32-34). However, the mechanisms of cardiac dysfunction remain unclear in NAFLD patients. Researchers have suggested various contributing factors, including insulin resistance, lipotoxicity linked to cardiac steatosis, alterations in fatty acid metabolism, and release of inflammatory factors (35,36). Moreover, the content of liver fat as a novel independent indicator plays a crucial role in myocardial insulin resistance in T2DM patients when combined with coronary artery disease (37). Our study shows that both T2DM and NAFLD are risk factors for elevated insulin resistance, and the synergy between the two diseases further aggravates LV dysfunction.
Multiple regression analysis revealed that NAFLD and HbA1c exerted independent effects on 3D strain in T2DM patients. At the same time, E/e’ was also identified as a factor for GLS and GCS. Other research has offered convincing data to indicate that NAFLD is independently associated with a decreasing value of GLS (38). Likewise, Karabay et al. (39) showed that the decrease of GLS and GRS was more remarkable in patients with NAFLD. Our study reveals a negative correlation between NAFLD and 3D strain, indicating that LV systolic dysfunction could be exacerbated by the advance of NAFLD.
Recent evidence has suggested that the level of serum HbA1c is related not only to the presence and severity of NAFLD, but also to the cardiac function in NAFLD patients. Studies have reported some differences in patients’ risks of developing cardiovascular events depending on the varying levels of serum HbA1c (40,41). This evidence suggests that different levels of serum HbA1c potentially leads to varying degrees of cardiovascular disease. Serum HbA1c plays an important role in forecasting cardiovascular events, and researchers have used it as an index of glucose metabolism to evaluate the cardiac function in NAFLD patients (42). For example, in a retrospective study, researchers found that increased serum HbA1c levels were associated with a decrease in LVEF (43). The pathological mechanisms involved the hyperactivation of protein kinase C, excessive deposition of advanced glycation end products, and elevated oxidative stress (44). Studies have confirmed that TDI can precisely evaluate LV filling pressure in patients with normal LVEF (45). In our study, E/e’ was negative associated with GLS and GCS, indicating that an increase in the LV filling pressure exerted an adverse effect on the LV systolic function. This could be the result of an elevation in LV filling pressure because of the rising preload, which might be subjected to strong stress in the mid-layer fibers (46).
Study limitations
Our study also had some limitations. Firstly, a frame rate of 3D-STE will exert an impact on the effect of tracking, i.e., the accuracy of the image is adversely affected when the frame rate is reduced. Secondly, the gold standard of diagnosis for NAFLD is liver biopsy, whereas our study used ultrasound technology, which means a diagnosis of hepatic steatosis can be missed when hepatic fat is <30%. In addition, the population sample in our study was not large enough, as we could not completely adjust for any potentially confounding factors.
Conclusion
LV dysfunction may deteriorate in T2DM patients with moderate-to-severe NAFLD without an obvious decrease in the LVEF. We found that 3D-STE can be used as an efficient and noninvasive method to evaluate LV function effectively in T2DM patients with NAFLD. This has the potential to guide clinical treatment and to decrease morbidity and mortality. It could also provide an accurate and early basis for clinical intervention and treatment.
Acknowledgment:
The authors would like to acknowledge Prof. Li for support to this research. In addition, the authors are thankful for the help and support received from the Department of Ultrasound of the Second Affiliated Hospital of Dalian Medical University.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Y.D., G.L., H.C.; Design – Y.D., G.L.; Supervision – D.H., G.L., H.C.; Funding – D.H., L.S., Y.W., Y.L., W.C.; Materials – L.S., Y.W., Y.L., W.C.; Data collection and/or processing – Y.D., L.S., Y.W., Y.L., W.C.; Analysis and/or interpretation – Y.D., D.H., G.L.; Literature search – Y.D., G.L.; Writing – Y.D., G.L., H.C.; Critical review – Y.D., D.H., G.L., H.C.
References
- 1.European Association for the Study of the Liver (EASL);European Association for the Study of Diabetes (EASD);European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus:A meta-analysis. Medicine (Baltimore) 2017;96:e8179. doi: 10.1097/MD.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231–8. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–9. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 6.de Alwis NM, Day CP. Non-alcoholic fatty liver disease:the mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–12. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–95. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Pichiri I, et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS One. 2015;10:e0135329. doi: 10.1371/journal.pone.0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Kim G, Choi YJ, Huh BW, Lee BW, Kang ES, et al. Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus. Diabetes Metab J. 2019 Feb 28; doi: 10.4093/dmj.2019.0001. doi:10.4093/dmj.2019.0001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G, Byrne CD. Clinical Review:Nonalcoholic fatty liver disease:a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98:483–95. doi: 10.1210/jc.2012-3093. [DOI] [PubMed] [Google Scholar]
- 11.Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking:comparison with MRI. Eur Heart J. 2009;30:1565–73. doi: 10.1093/eurheartj/ehp187. [DOI] [PubMed] [Google Scholar]
- 12.Altman M, Bergerot C, Aussoleil A, Davidsen ES, Sibellas F, Ovize M, et al. Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking:a comparison between 2D and 3D echo modalities. Eur Heart J Cardiovasc Imaging. 2014;15:316–23. doi: 10.1093/ehjci/jet103. [DOI] [PubMed] [Google Scholar]
- 13.Kleijn SA, Brouwer WP, Aly MF, Russel IK, de Roest GJ, Beek AM, et al. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging. 2012;13:834–9. doi: 10.1093/ehjci/jes030. [DOI] [PubMed] [Google Scholar]
- 14.Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY. Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108–12. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 15.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver:a meta-analysis. Hepatology. 2011;54:1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–45. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Zoppini G, Targher G, Golia G, Bonora E. Non-alcoholic fatty liver disease is independently associated with left ventricular hypertrophy in hypertensive Type 2 diabetic individuals. J Endocrinol Invest. 2012;35:215–8. doi: 10.1007/BF03345421. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy S, Hallsworth K, Thoma C, MacGowan GA, Hollingsworth KG, Day CP, et al. Cardiac structure and function are altered in type 2 diabetes and Non-alcoholic fatty liver disease and associate with glycemic control. Cardiovasc Diabetol. 2015;14:23. doi: 10.1186/s12933-015-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favot M, Courage C, Ehrman R, Khait L, Levy P. Strain Echocardiography in Acute Cardiovascular Diseases. West J Emerg Med. 2016;17:54–60. doi: 10.5811/westjem.2015.12.28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducas R, Tsang W, Chong AA, Jassal DS, Lang RM, Leong-Poi H, et al. Echocardiography and vascular ultrasound:new developments and future directions. Can J Cardiol. 2013;29:304–16. doi: 10.1016/j.cjca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, et al. Two- and three-dimensional speckle tracking echocardiography:clinical applications and future directions. Echocardiography. 2013;30:88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 22.Muraru D, Cucchini U, Mihaila S, Miglioranza MH, Aruta P, Cavalli G, et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects:reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr. 2014;27:858–71. doi: 10.1016/j.echo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–63. [PubMed] [Google Scholar]
- 24.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, et al. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease:echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–55. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 25.Kim NH, Park J, Kim SH, Kim YH, Kim DH, Cho GY, et al. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100:938–43. doi: 10.1136/heartjnl-2013-305099. [DOI] [PubMed] [Google Scholar]
- 26.Trovato FM, Martines GF, Catalano D, Musumeci G, Pirri C, Trovato GM. Echocardiography and NAFLD (non-alcoholic fatty liver disease) Int J Cardiol. 2016;221:275–9. doi: 10.1016/j.ijcard.2016.06.180. [DOI] [PubMed] [Google Scholar]
- 27.Henein MY, Gibson DG. Normal long axis function. Heart. 1999;81:111–3. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saccheri MC, Cianciulli TF, Lax JA, Gagliardi JA, Caceres GL, Quarin AE, et al. Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography. 2013;30:1069–77. doi: 10.1111/echo.12216. [DOI] [PubMed] [Google Scholar]
- 29.Brecker SJ. The importance of long axis ventricular function. Heart. 2000;84:577–9. doi: 10.1136/heart.84.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reant P, Barbot L, Touche C, Dijos M, Arsac F, Pillois X, et al. Evaluation of global left ventricular systolic function using three-dimensional echocardiography speckle-tracking strain parameters. J Am Soc Echocardiogr. 2012;25:68–79. doi: 10.1016/j.echo.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Gao Y, Tan K, Xia H, Li P. Assessment of left ventricular function by three-dimensional speckle-tracking echocardiography in well-treated type 2 diabetes patients with or without hypertension. J Clin Ultrasound. 2015;43:502–11. doi: 10.1002/jcu.22268. [DOI] [PubMed] [Google Scholar]
- 32.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–53. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;162:1160–8. doi: 10.1016/j.jpeds.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petta S, Argano C, Colomba D, Camma C, Di Marco V, Cabibi D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease:association with the severity of liver disease. J Hepatol. 2015;62:928–33. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease:implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1155–61. doi: 10.1161/ATVBAHA.114.303034. [DOI] [PubMed] [Google Scholar]
- 36.Bugianesi E. Nonalcoholic fatty liver disease (NAFLD) and cardiac lipotoxicity:Another piece of the puzzle. Hepatology. 2008;47:2–4. doi: 10.1002/hep.22105. [DOI] [PubMed] [Google Scholar]
- 37.Lautamäki R, Borra R, Iozzo P, Komu M, Lehtimäki T, Salmi M, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282–90. doi: 10.1152/ajpendo.00604.2005. [DOI] [PubMed] [Google Scholar]
- 38.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction:A population-based study. Hepatology. 2015;62:773–83. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease:a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–31. doi: 10.1097/MEG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 40.Yu C, Wang L, Xue H, Lin H, Li Y, Chan SO. Association of glycated hemoglobin with the risk of advanced fibrosis in non-alcoholic fatty liver disease patients without diabetes. Clin Res Hepatol Gastroenterol. 2019;43:58–66. doi: 10.1016/j.clinre.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Rigolon R, Pichiri I, Bonapace S, Morani G, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS One. 2017;12:e0185459. doi: 10.1371/journal.pone.0185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score:a retrospective cohort study. Gut. 2017;66:323–9. doi: 10.1136/gutjnl-2016-311854. [DOI] [PubMed] [Google Scholar]
- 43.Shi C, Wang LJ, Hu DF, Li JP, Zhu TQ, Shan Y, et al. Prevalence, clinical characteristics and outcome in patients with chronic heart failure and diabetes. Chin Med J (Engl) 2010;123:646–50. [PubMed] [Google Scholar]
- 44.Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 46.MacGowan GA, Shapiro EP, Azhari H, Siu CO, Hees PS, Hutchins GM, et al. Noninvasive measurement of shortening in the fiber and cross-fiber directions in the normal human left ventricle and in idiopathic dilated cardiomyopathy. Circulation. 1997;96:535–41. doi: 10.1161/01.cir.96.2.535. [DOI] [PubMed] [Google Scholar]