Abstract
Objective:
Diabetes mellitus (DM) is a risk factor for developing in-stent restenosis (ISR) following percutaneous coronary intervention (PCI). This study aimed to examine the presentation and outcomes of drug-eluting stent (DES) ISR in diabetics.
Methods:
This retrospective study included consecutive patients with clinical DES-ISR, who were hospitalized between January 2013 and December 2017 and who were grouped based on the presence or absence of DM. Clinical, angiographic features and 1-year outcomes [composite of death, myocardial infarction (MI), and repeat-target lesion revascularization] were compared.
Results:
Baseline characteristics of the DM group (n=109) were comparable to the non-DM group (n=82), except for the higher prevalence of hypertension and dyslipidemia in the former (60.6% vs. 46.3%, p=0.050; 74.4% vs. 57.8%, p=0.034, respectively). Clinical presentation was similar in both groups [acute coronary syndrome (ACS): 62.4% vs. 61%, p=0.843; MI: 34.9% vs. 34.1%, p=0.918). Diabetics had a higher prevalence of stent-edge restenosis (20.3% vs. 9.2%, p=0.019). The treatment strategy was similar in both groups with 52.3% in the DM group and 57.3% in the non-DM group undergoing PCI (p=0.513). One-year outcomes of the DM group were not different from those of the non-DM group (14.7% vs. 17.1%, p=0.683). Age [hazard ratio (HR), 1.05; 95% confidence interval (CI), 1.01–1.10; p=0.017], MI presentation (HR, 2.34; 95% CI, 1.14–4.80; p=0.020), and chronic kidney disease (CKD: HR, 2.82; 95% CI, 1.21–6.58; p=0.016) were predictors of poor outcomes.
Conclusion:
Stent-edge restenosis is more common in diabetics. Clinical presentation and 1-year outcomes following DES-ISR are similar in diabetics and non-diabetics. Age, MI presentation, CKD, and not DM were predictors of poor outcomes following DES-ISR.
Keywords: coronary restenosis, drug-eluting stent, diabetes mellitus, in-stent restenosis, percutaneous coronary intervention, percutaneous transluminal coronary angioplasty
Introduction
In-stent restenosis (ISR) is the bane of percutaneous coronary intervention (PCI) (1). Although drug-eluting stents (DES) have reduced the incidence of ISR, it continues to be a significant problem affecting 5%–10% of the patients undergoing PCI (2).
Patients with diabetes mellitus (DM) are at higher risk of developing ISR due to excess neointimal hyperplasia, hypercoagulability, increased inflammatory response, endothelial dysfunction, and presence of comorbidities (3). In the bare-metal stent (BMS) era, diabetes was an independent risk factor for both ISR and major adverse cardiac events (MACE) following PCI (4). It is unclear whether diabetics are at increased risk of developing ISR in the DES era. Few studies suggest that diabetes is no longer associated with ISR following DES implantation, while others found it to be associated with an increased ISR risk (3, 5, 6). However, poorer clinical outcomes have been reported in diabetics following PCI compared to their non-diabetic counterparts, even in the DES era (7). Whether patients with diabetes who develop DES-ISR fare poorly compared to their non-diabetic counterparts is unknown because data are scarce regarding the clinical presentation and outcomes in diabetic patients with DES-ISR (8). Few available studies in this area have compared one PCI modality over the other in a clinical trial setting, which is not reflective of the real-world situation (9-11).
We compared the clinical presentation, angiographic features, and outcomes of DES-ISR among patients with and without DM.
Methods
Study population and design
This study was conducted in a tertiary care hospital in South India. All the patients presenting with clinical ISR of DES between January 2013 and December 2017 were included in this retrospective cohort study and grouped according to the presence or absence of DM. The study protocol was approved by the Institutional Ethics Committee and was registered with the Clinical Trials Registry–India (Reg. No. CTRI/2018/10/016181).
Demographic and clinical characteristics, investigations including biochemical test results, and electrocardiographic and echocardiographic findings were noted from patients’ medical records. Two independent cardiologists reviewed the angiographic images to confirm the presence of ISR and to determine the type of ISR (according to the Mehran classification) (12). Details pertaining to the treatment of culprit ISR lesion, including technical details of interventional procedures, were recorded.
Study definitions
ISR was defined as the presence of >50% diameter stenosis on angiography at the stent site or at its edges (adjacent 5 mm segments) (13). Clinical ISR was defined as the presence of symptoms attributable to the ISR lesion.
Patients were considered to be diabetic if they were previously diagnosed or were under treatment for DM or if glycosylated hemoglobin level was ≥6.5% during the index hospitalization. Fasting blood glucose during index hospitalization was not used for diagnosing diabetes, as stress hyperglycemia can lead to false-positive diagnoses.
Clinical presentation at index hospitalization during which ISR was first diagnosed was classified into acute coronary syndrome (ACS) and non-ACS. The ACS group included patients with myocardial infarction (MI) and unstable angina (UA). Non-ACS group included patients presenting with stable angina or silent ischemia.
MI was defined according to the universal definition and was categorized into STEMI (ST-elevation myocardial infarction), when characterized by the ST-segment elevation or new-onset left bundle branch block, and NSTEMI (non-ST elevation myocardial infarction) when there was a rise or fall in cardiac biomarkers (cardiac troponin T) without ST-segment elevation (14).
Stable angina was defined as typical chest discomfort brought upon by physical exertion and relieved by rest and/or nitrates. UA was defined as a recent onset or worsening of typical chest pain, chest pain occurring at rest, or chest pain lasting >20 minutes, with or without dynamic ST-segment changes on electrocardiography and without elevation of cardiac biomarkers. Abnormality on stress tests (the treadmill test or dobutamine stress echocardiography) without typical symptoms was labeled as silent ischemia (15).
Chronic kidney disease was defined according to the Kidney Disease: Improving Global Outcomes 2012 guidelines (16). Congestive cardiac failure was defined as the evidence of fluid retention from cardiac causes. Stent thrombosis, definite or probable, was defined according to criteria from the Academic Research Consortium (17).
Outcome definitions
The primary outcome studied was a composite of all-cause mortality and major adverse cardiac events (MACE) after index hospitalization. MACE included MI and repeat target-lesion revascularization (TLR). MI that led to index hospitalization and the first TLR carried out for index ISR lesion were not included in the cumulative MACE. Only repeat (or second) TLR that occurred during the follow-up was considered as a MACE for the purpose of this study. All deaths were considered cardiac unless another documented cause was found.
Follow-up
Clinical follow-up data were obtained from patients’ medical records for a period of 1 year after index hospitalization to determine the occurrence of an adverse event(s). In patients for whom such data were not available from hospital records, follow-up was conducted by telephonic contact. All adverse events were adjudicated by interventional cardiologists who were blinded to the study objectives.
Study objectives
The main objective of this study was to compare the clinical and angiographic characteristics and outcomes of DES-ISR among patients with and without DM. The secondary objective was to identify the predictors of poor outcomes in patients with DES-ISR.
Statistical analysis
Categorical variables were summarized using frequencies (%). The mean±standard deviation was used for continuous variables. Normality was assessed for continuous data using the Kolmogorov–Smirnov test. The independent samples t-test was used for continuous variables. Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. The Kaplan–Meier method and the log-rank test were used to compare survival (time-to-event) curves of patients with and without DM. The Cox regression analysis was used to calculate the hazard ratios for predictors of clinical outcomes following DES-ISR. A p-value <0.05 was taken as an indicator of statistical significance. A statistical analysis was carried out using the SPSS Inc., Version 16.0 (Chicago, Illinois, USA).
Results
Clinical and angiographic characteristics
This study included 109 patients with DM and 82 patients without DM who presented with clinical ISR of DES (a total of 191 patients with 210 culprit ISR lesions). Approximately, 18,771 diagnostic angiograms were done during the study period, of which 1,318 angiographies were those of patients with a previous PTCA procedure. Therefore, roughly 14.5% (191 study patients out of 1,318) had clinical ISR where an ISR lesion was found to be the culprit. Although this “restenosis rate” is higher than that reported with DES (5%–10%), it should be noted that the decision for repeat angiogram in a patient with previous PTCA was based on clinical suspicion and as per treating physician’s discretion (2). A practice of doing routine check angiograms after a fixed period to look for ISR is not followed in our center.
Patient characteristics at index hospitalization are presented in Table 1. Patients in both groups were similar with respect to age, gender, tobacco consumption, co-existing illness, left ventricular function, and the New York Heart Association class. Patients with DM had a higher prevalence of hypertension and lipid abnormalities compared to non-diabetics, despite similar rates of statin therapy.
Table 1.
Patient characteristics at first clinical in-stent restenosis presentation
| Parameter | DM group (n=109) | Non-DM group (n=82) | P-value |
|---|---|---|---|
| Demographics | |||
| Age | 60.7±9.3 | 61.8±10.8 | 0.464 |
| Men | 90 (82.6%) | 65 (79.3%) | 0.564 |
| BMI | 23.3±3.7 | 23.6±3.1 | 0.690 |
| Clinical characteristics | |||
| Hypertension# | 66 (60.6%) | 38 (46.3%) | 0.050 |
| Chronic kidney disease | 12 (11.0%) | 9 (11.0%) | 0.994 |
| Acute kidney injury | 20 (18.3%) | 12 (14.6%) | 0.496 |
| Dyslipidemia* | 61 (74.4%) | 37 (57.8%) | 0.034 |
| Current tobacco use | 21 (19.3%) | 18 (22.0%) | 0.649 |
| CCF | 21 (19.3%) | 13 (15.9%) | 0.542 |
| NYHA 3, 4 | 9 (8.3%) | 11 (13.4%) | 0.249 |
| LVEF | 52.0±11.1 | 53.5±10.3 | 0.325 |
| Previous MI | 57 (52.3%) | 43 (52.4%) | 0.984 |
| Previous CABG | 10 (9.2%) | 4 (4.9%) | 0.259 |
| Statin therapy | 96 (88.1%) | 67 (81.7%) | 0.218 |
| Clinical presentation | |||
| Non-ACS | 41 (37.6%) | 32 (39.0%) | 0.843 |
| ACS | 68 (62.4%) | 50 (61.0%) | |
| Unstable angina | 30 (27.5%) | 22 (26.8%) | |
| MI | 38 (34.9%) | 28 (34.1%) | |
| NSTEMI | 30 (27.5%) | 22 (26.8%) | |
| STEMI | 8 (7.3%) | 6 (7.3%) | |
| Silent ischemia | 14 (12.9%) | 8 (9.8%) | 0.398 |
| Lab parameters | |||
| HbA1c | 8.2±1.4 | 5.8±0.4 | <0.001 |
| FBS | 176±70 | 104±20 | <0.001 |
| Lipid profile* (mg/dL) | |||
| Total cholesterol | 143±44 | 148±39 | 0.450 |
| LDL | 78±37 | 83±33 | 0.437 |
| HDL | 38±11 | 42±12 | 0.063 |
| Triglycerides | 139±75 | 119±64 | 0.088 |
Dyslipidemia defined as total cholesterol >250 mg/dL, LDL cholesterol >130 mg/dL, HDL cholesterol <40 mg/dL (<50 mg/dL for women) in the fasting state. Data available for 146 patients.
Blood pressure >140/90 mm Hg or the use of antihypertensive therapy. ACS - acute coronary syndrome; BMI - body mass index; CABG - coronary artery bypass grafting; CCF - congestive cardiac failure; HbA1c - glycosylated hemoglobin; LVEF - left ventricular ejection fraction; LDL - low-density lipoprotein;
HDL - high-density lipoprotein; MI - myocardial infarction; NYHA - New York Heart Association
Clinical presentation of DES-ISR was similar among both diabetics and non-diabetics with ACS being the most common presentation mode in both groups. One-third of the patients in both the groups presented with MI (Table 1).
Angiographic features, treatment characteristics, and details of interventional procedures are presented in Table 2. A focal ISR lesion (Mehran Type 1) was the most common lesion type in both groups. Diabetics had higher prevalence of Type 1B lesions (stent-edge restenosis) and a lower prevalence of Type 1C lesions compared to non-diabetics [25 (20.3%) vs. 8 (9.2%), p=0.019; and 37 (30.1%) vs. 36 (41.4%), p=0.039, respectively].
Table 2.
Angiographic characteristics and treatment characteristics at first clinical in-stent restenosis presentation
| Parameter ISR characteristics | DM group (n=123) | Non-DM group (n=87) | P-value |
|---|---|---|---|
| ISR type | 0.866 | ||
| I. Focal | 78 (63.4%) | 56 (64.4%) | |
| Type 1B | 25 (20.3%) | 8 (9.2%) | 0.019 |
| Type 1C | 37 (30.1%) | 36 (41.4%) | 0.039 |
| Type 1D | 16 (13.0%) | 12 (13.8%) | 0.898 |
| II. Diffuse | 14 (11.4%) | 9 (10.3%) | |
| III. Proliferative | 7 (5.7%) | 3 (3.4%) | |
| IV. Complete | 24 (19.5%) | 19 (21.8%) | |
| ISR vessel | 0.583 | ||
| Left anterior descending | 70 (56.9%) | 42 (48.3%) | |
| Left circumflex artery | 25 (20.3%) | 24 (27.6%) | |
| Right coronary artery | 27 (22.0%) | 20 (23.0%) | |
| Left main | 1 (0.8%) | 1 (1.1%) | |
| Proximal ISR location | 70 (56.9%) | 43 (49.4%) | 0.284 |
| Disease burden | (n=109) | (n=82) | 0.195 |
| Single-vessel disease | 37 (33.9%) | 35 (42.7%) | 0.217 |
| Double-vessel disease | 37 (33.9%) | 30 (36.6%) | 0.705 |
| Triple-vessel disease | 35 (32.1%) | 17 (20.7%) | 0.080 |
| Treatment | (n=109) | (n=82) | 0.513 |
| Medical therapy | 20 (18.3%) | 17 (20.7%) | |
| CABG | 32 (29.4%) | 18 (22.0%) | |
| PCI | 57 (52.3%) | 47 (57.3%) | |
| Details of PCI | |||
| Procedural success | 56 (98.2%) | 45 (95.7%) | 0.588 |
| PCI type | 0.427 | ||
| POBA | 14 (24.6%) | 7 (14.9%) | |
| DCB | 5 (8.8%) | 6 (12.8%) | |
| New DES | 38 (66.7%) | 34 (72.3%) | |
| No. of stents | 1.06±0.24 | 1.23±0.43 | 0.051 |
| Stent length | 29.4±8.5 | 30.0±13.3 | 0.828 |
| Stent diameter | 3.00±0.39 | 3.08±0.41 | 0.412 |
| Adjunct devices | |||
| Rotablation | 1 (1.8%) | 1 (2.1%) | 1.000 |
| Cutting or NC balloon | 14 (24.6%) | 10 (21.3%) | 0.692 |
| IVUS guidance | 14 (24.6%) | 11 (23.4%) | 0.891 |
CABG - coronary artery bypass grafting; DCB - drug-coated balloon; DES - drug-eluting stent; IVUS - intravascular ultrasound; ISR - in-stent restenosis; NC - non-compliant; PCI - percutaneous coronary interventional; POBA - plain old balloon angioplasty
Patients with DM had a trend toward a higher prevalence of triple-vessel disease (DM vs. non-DM: 32.1% vs. 20.7%, p=0.080). Both groups were similar with respect to vessels affected by ISR, the ISR location, and treatment received. PCI was the most common treatment modality in both groups, and more than two-thirds of patients undergoing PCI received a new DES (Table 2).
Data on three more variables (length and diameter of previous stent in which ISR developed and time to ISR) were available only in 136 study patients. The average length of the previous stents was 23.4±11.1 mm and 21.5±10.5 mm, respectively, in diabetic and non-diabetic groups (p=0.854). An average diameter of these stents was 2.81±0.3 and 2.97±0.3 mm, respectively, in diabetics and non-diabetics (p=0.370). The mean time to restenosis in diabetics and non-diabetics was 26.7±8.7 months and 32.2±6.4 months, respectively (p=0.431). Although this suggests that diabetics developed ISR earlier and had received longer stents with smaller diameters during their initial PCI, the differences were not statistically significant.
Effect of diabetes on clinical outcomes
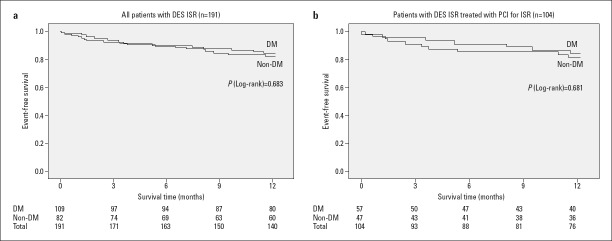
There was no significant difference in the occurrence of the primary composite outcome between the DM group and the non-DM group at the end of the 1-year follow-up period (14.7% vs. 17.1%, respectively, p=0.653). The Kaplan–Meier analysis of 1-year outcomes in DM and non-DM groups is shown in Figure 1. Both groups had comparable rates of all-cause mortality (5.5% vs. 6.1%, p=0.862) and MI (6.4% vs. 4.9%, p=0.650). The DM group had a lower rate of re-TLR compared to the non-DM group (2.8% vs. 6.1%, p=0.253), but the difference was not statistically significant.
Figure 1.
The Kaplan–Meier survival analysis of 1-year outcomes of DES-ISR according to the presence or absence of DM. (a) Entire DES-ISR cohort. (b) Subgroup of patients with DES-ISR treated with PCI for ISR lesion
DES - drug-eluting stent; DM - diabetes mellitus; ISR - in-stent restenosis; PCI - percutaneous coronary intervention
Although the patient number is low for subgroup analysis, we would like to report that there was no difference in outcomes with respect to the type of treatment received (PCI vs. medical therapy vs. CABG; p=0.928) or the type of PCI (DES vs. DEB vs. POBA; p=0.222).
Predictors of outcomes in DES-ISR
On the Cox regression analysis, DM did not appear to be associated with poor outcomes at the 1-year follow-up (Table 3). Among the other clinical and lesion-related parameters, the age, presentation with MI, and chronic kidney disease were associated with poor outcomes following DES-ISR in our study.
Table 3.
Predictors of 1-year clinical outcome following DES-ISR using Cox regression analysis
| Variables | Hazard ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Patient related | ||||
| Age | 1.05 | 1.01 | 1.10 | 0.017 |
| Female gender | 1.39 | 0.60 | 3.24 | 0.446 |
| Presentation with MI | 2.34 | 1.14 | 4.80 | 0.020 |
| Diabetes | 0.86 | 0.42 | 1.77 | 0.684 |
| Hypertension | 1.08 | 0.52 | 2.22 | 0.836 |
| Current tobacco use | 1.52 | 0.68 | 3.42 | 0.309 |
| Dyslipidemia | 1.16 | 0.49 | 2.77 | 0.738 |
| Chronic kidney disease | 2.82 | 1.21 | 6.58 | 0.016 |
| Lesion related | ||||
| Non-focal lesion | 1.55 | 0.75 | 3.19 | 0.236 |
| LAD involvement | 1.26 | 0.61 | 2.62 | 0.531 |
| Proximal ISR location | 1.45 | 0.69 | 3.06 | 0.323 |
CABG - coronary artery bypass grafting; CI - confidence interval; ISR - in-stent restenosis; LAD - left anterior descending; LVEF - left ventricular ejection fraction;
MI - myocardial infarction
Discussion
To the best of our knowledge, our study is the first of its kind to compare the DES-ISR presentation and outcomes among patients with and without DM in a real-world scenario, irrespective of the type of treatment received for DES-ISR. The main findings of the study were the following: (1) Clinical presentation of DES-ISR is similar among patients with and without DM; (2) the ACS is the most common clinical presentation of DES-ISR in both groups; (3) a focal ISR lesion (Mehran Type 1) is the most common lesion type in both groups, but diabetics have a higher prevalence of Type 1B lesions (stent-edge restenosis) and a lower prevalence of Type 1C lesions compared to non-diabetics; (4) DM did not affect 1-year clinical outcomes following DES-ISR; (5) an advanced age, clinical presentation with MI, and the presence of chronic kidney disease were predictors of poor 1-year outcomes following DES-ISR.
Clinical presentation of DES-ISR in patients with DM
Although DES decreased the incidence of ISR, the propensity of ISR to present with ACS remained same in both the BMS and DES eras with up to 70% of patients presenting with ACS and 10%–20% with MI (18-21). Our study shows that patients with DM who develop DES-ISR have a clinical presentation very similar to those without DM. More than 60% of patients in both groups presented with ACS and nearly one-third of them presented with MI in our study. A recent study by Zhao et al. (8) also reported that diabetics and non-diabetics have a similar clinical presentation, but their study suggested that stable angina was the most common clinical presentation, which is at odds with most studies on DES-ISR. Patients with diabetes with de novo coronary artery disease are known to have a higher incidence of atypical symptoms, anginal equivalents, and silent ischemia, which may potentially lead to delayed presentation and higher chances of presenting with an ACS (22,23). However, the presence of DM does not seem to have an effect on the manner of presentation of DES-ISR in our study. The prevalence of silent ischemia was similar in patients with and without DM in our study.
Angiographic characteristics of DES-ISR in patients with DM
In our study, focal restenosis (Mehran Type 1 lesion) was more common than non-focal ISR lesions in both patients with and without DM. This is in agreement with most previous studies, which found that focal restenosis is more common with DES compared to BMS restenosis, which presents more commonly with a diffuse pattern (24). DES, through its antiproliferative effects, seems to effectively reduce intimal hyperplasia and smooth muscle proliferation locally in the stented segment, even among patients with DM who are prone to excessive hyperplasia and restenosis.
A notable finding in our study is the higher incidence of stent-edge restenosis (Mehran Type 1B lesion) among diabetics. Patients with diabetes have a smaller vessel caliber with longer and more diffuse de novo lesions compared to non-diabetics (25). This makes the initial PCI more challenging, especially with respect to choosing an appropriate stent length to cover the entire diseased segment leading to various degrees of geographical miss. This is especially true when visual estimation of lesion length based on angiographic images alone is used to decide the stent length (26). Often, the stent lands in a diseased segment, which then predisposes diabetic patients to the development of edge restenosis. It needs to be determined if intravascular imaging methods like intravascular ultrasound or optical coherence tomography may help overcome this issue in patients with DM.
Effect of DM on clinical outcomes in DES-ISR
Patients with DM in our study had a 1-year outcome (composite of death, MI, and re-TLR), similar to those without DM when both groups were managed with similar treatment strategies. Even in the subgroup managed with PCI, 1-year outcomes were similar in both diabetics and non-diabetics. A recent study comparing 2-year outcomes following the treatment of ISR with second-generation DES among diabetics and non-diabetics also reported that there is no difference between the two groups (8). It appears that DM is not a risk factor for poor outcomes following DES-ISR with currently available therapies. DM has been consistently found to be an independent risk factor for poor outcomes following PCI in several previous studies (4). Although the introduction of DES reduced the restenosis rates, diabetics as a group continued to experience poor outcomes (7). Because outcomes in those who develop restenosis are similar in diabetics and non-diabetics, it suggests that the progression of disease elsewhere in the coronary tree and higher atherosclerotic burden along with comorbidities may be underlying poor outcomes among patients with DM. In this regard, the higher prevalence of triple-vessel disease, hypertension, and dyslipidemia among diabetics in our study supports this hypothesis.
Predictors of clinical outcomes in DES-ISR
We found that older age, chronic kidney disease, and presentation with MI were associated with worse 1-year outcomes following ISR. Lesion-related factors were not the markers of poor outcomes in our study. DES-ISR presenting with MI has been consistently reported to be a predictor of poor outcomes in many of the previous studies, emphasizing the need for a closer follow-up (18,19). Similarly, several studies have shown that patients with chronic kidney disease are at a higher risk of developing restenosis because of a higher incidence of neoatherosclerosis, and have poorer outcomes following PCI (27,28). A subset of patients with chronic kidney disease who develop DES-ISR is known to present with ACS and fare poorly despite treatment (18). Novel therapeutic options need to be explored to improve outcomes of patients with DES-ISR especially when one or more of these poor prognostic factors are present.
Study limitations
Because of the retrospective nature of this study, the results may have been affected by various confounding factors. Therefore, the findings of this study should be considered hypothesis generating.
The possibility of late stent thrombosis masquerading as ISR with MI cannot be excluded, despite the rigorous process of adjudication used. However, recent studies using intravascular imaging modalities have suggested that ISR and stent thrombosis may have a similar underlying pathophysiological basis and therefore may not be entirely distinct clinical entities as once believed.
The impact of the type of DES (first- vs. second-generation DES) on clinical presentation could not be compared because the type of DES received by study patients in their initial procedure (prior to the development of ISR) could not be ascertained in all patients because some of the patients had undergone initial PCI at a different hospital and presented to us for the first time with DES-ISR. However, in our country, a variety of stent types with various combinations of anti-proliferative drugs and polymers are available, which makes it difficult to segregate them into two or three groups for study purposes (29).
Treatment modalities were not compared because patients were treated at physician’s discretion with either PCI, CABG, or medical management. Because re-TLR cannot occur in the latter two groups, re-TLR rates in our study are consequently lower. Further, the type of PCI (New DES, DCB or POBA) may also have influenced outcomes. However, we believe our study is representative of the entire spectrum of clinical ISR in the real-world situation where numerous factors affect treatment decisions and outcomes.
Conclusion
Patients with DM have a similar clinical presentation of DES-ISR compared to patients without DM, with ACS being the most common mode of presentation. Stent-edge restenosis is more common among diabetics. The presence of DM does not affect clinical outcomes at 1 year following contemporary treatment for DES-ISR. Higher age, presentation with MI and chronic kidney disease were predictors of poor outcomes at 1 year following DES-ISR.
Acknowledgment:
Dr. Indu Ramachandra Rao for proofreading the article and her valuable inputs.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – G.P., T.D., R.V.; Design – G.P., T.D.; Supervision – T.D., R.V.; Funding – None; Materials – G.P., T.D., A.J., A.R., M.S.R.; Data collection and/or processing – G.P., T.D., A.J., A.R., M.S.R., R.V., K.N.; Analysis and/or interpretation – G.P., T.D., A.J., A.R., M.S.R., R.V., K.N.; Literature search – G.P.; Writing – G.P., T.D.; Critical review – G.P., T.D., A.J., A.R., M.S.R., R.V., K.N.
References
- 1.Camenzind E. Treatment of in-stent restenosis--back to the future?N Engl J Med. 2006;355:2149–51. doi: 10.1056/NEJMe068215. [DOI] [PubMed] [Google Scholar]
- 2.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, et al. Outcomes associated with drug-eluting and bare-metal stents:a collaborative network meta-analysis. Lancet. 2007;370:937–48. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 3.Iijima R, Ndrepepa G, Mehilli J, Markwardt C, Bruskina O, Pache J, et al. Impact of diabetes mellitus on long-term outcomes in the drug-eluting stent era. Am Heart J. 2007;154:688–93. doi: 10.1016/j.ahj.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, et al. Drug eluting and bare metal stents in people with and without diabetes:collaborative network meta-analysis. BMJ. 2008;337:a1331. doi: 10.1136/bmj.a1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billinger M, Räber L, Hitz S, Stefanini GG, Pilgrim T, Stettler C, et al. Long-term clinical and angiographic outcomes of diabetic patients after revascularization with early generation drug-eluting stents. Am Heart J. 2012;163:876–86. doi: 10.1016/j.ahj.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Tada T, Kimura T, Morimoto T, Ono K, Furukawa Y, Nakagawa Y, et al. Comparison of three-year clinical outcomes after sirolimus-eluting stent implantation among insulin-treated diabetic, non-insulin-treated diabetic, and non-diabetic patients from j-Cypher registry. Am J Cardiol. 2011;107:1155–62. doi: 10.1016/j.amjcard.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Kedhi E, Genereux P, Palmerini T, McAndrew TC, Parise H, Mehran R, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus:analysis from 18 pooled randomized trials. J Am Coll Cardiol. 2014;63:2111–8. doi: 10.1016/j.jacc.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Zhu W, Zhang X, He D, Guo C. Effect of diabetes mellitus on long-term outcomes after repeat drug-eluting stent implantation for in-stent restenosis. BMC Cardiovasc Disord. 2017;17:16. doi: 10.1186/s12872-016-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne RA, Cassese S, Windisch T, King LA, Joner M, Tada T, et al. Differential relative efficacy between drug-eluting stents in patients with bare metal and drug-eluting stent restenosis;evidence in support of drug resistance:insights from the ISAR-DESIRE and ISAR-DESIRE 2 trials. EuroIntervention. 2013;9:797–802. doi: 10.4244/EIJV9I7A132. [DOI] [PubMed] [Google Scholar]
- 10.Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis:results from the PEPCAD China ISR trial. JACC Cardiovasc Interv. 2014;7:204–11. doi: 10.1016/j.jcin.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso F, Perez-Vizcayno MJ, Cardenas A, Garcia del Blanco B, Garcia-Touchard A, Lopez-Minguez JR, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents:The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, et al. Angiographic patterns of in-stent restenosis:classification and implications for long-term outcome. Circulation. 1999;100:1872–8. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 13.Alfonso F, Zueco J, Cequier A, Mantilla R, Bethencourt A, López-Minguez JR. Restenosis Intra-stent:Balloon Angioplasty Versus Elective Stenting (RIBS) Investigators. A randomized comparison of repeat stenting with balloon angioplasty in patients with in-stent restenosis. J Am Coll Cardiol. 2003;42:796–805. doi: 10.1016/s0735-1097(03)00852-0. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–51. [Google Scholar]
- 15.Task Force Members. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease:the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, et al. Kidney disease:Improving global outcomes (KDIGO) CKD work group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA. Academic Research Consortium Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 18.Magalhaes MA, Minha S, Chen F, Torguson R, Omar AF, Loh JP, et al. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ Cardiovasc Interv. 2014;7:768–76. doi: 10.1161/CIRCINTERVENTIONS.114.001341. [DOI] [PubMed] [Google Scholar]
- 19.De Labriolle A, Bonello L, Lemesle G, Steinberg DH, Roy P, Xue Z, et al. Clinical presentation and outcome of patients hospitalized for symptomatic in-stent restenosis treated by percutaneous coronary intervention:comparison between drug-eluting stents and bare-metal stents. Arch Cardiovasc Dis. 2009;102:209–17. doi: 10.1016/j.acvd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Assali AR, Moustapha A, Sdringola S, Denktas AE, Willerson JT, Holmes DR, Jr, et al. Acute Coronary Syndrome May Occur With In-Stent Restenosis and Is Associated With Adverse Outcomes (The PRESTO trial) Am J Cardiol. 2006;98:729–33. doi: 10.1016/j.amjcard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Schwalm T, Carlsson J, Meissner A, Lagerqvist B, James S. Current treatment and outcome of coronary in-stent restenosis in Sweden:a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) EuroIntervention. 2013;9:564–72. doi: 10.4244/EIJV9I5A92. [DOI] [PubMed] [Google Scholar]
- 22.Khafaji HA, Suwaidi JM. Atypical presentation of acute and chronic coronary artery disease in diabetics. World J Cardiol. 2014;6:802–13. doi: 10.4330/wjc.v6.i8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culić V, Eterović D, Mirić D, Silić N. Symptom presentation of acute myocardial infarction:influence of sex, age, and risk factors. Am Heart J. 2002;144:1012–7. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- 24.Corbett SJ, Cosgrave J, Melzi G, Babic R, Biondi-Zoccai GGL, Godino C, et al. Patterns of restenosis after drug-eluting stent implantation:insights from a contemporary and comparative analysis of sirolimus- and paclitaxel-eluting stents. Eur Heart J. 2006;27:2330–7. doi: 10.1093/eurheartj/ehl229. [DOI] [PubMed] [Google Scholar]
- 25.Jensen LO, Thayssen P, Mintz GS, Maeng M, Junker A, Galloe A, et al. Intravascular ultrasound assessment of remodelling and reference segment plaque burden in type-2 diabetic patients. Eur Heart J. 2007;28:1759–64. doi: 10.1093/eurheartj/ehm175. [DOI] [PubMed] [Google Scholar]
- 26.Campbell PT, Mahmud E, Marshall JJ. Interoperator and intraoperator (in)accuracy of stent selection based on visual estimation. Catheter Cardiovasc Interv. 2015;86:1177–83. doi: 10.1002/ccd.25780. [DOI] [PubMed] [Google Scholar]
- 27.Hayano S, Ishii H, Ichimiya S, Kanashiro M, Watanabe J, Suzuki S, et al. Renal dysfunction and atherosclerosis of the neointima following bare metal stent implantation. Am J Nephrol. 2013;38:58–65. doi: 10.1159/000353097. [DOI] [PubMed] [Google Scholar]
- 28.Yonetsu T, Kato K, Kim S-J, Xing L, Jia H, McNulty I, et al. Predictors for Neoatherosclerosis. Circ Cardiovasc Imaging. 2012;5:660–6. doi: 10.1161/CIRCIMAGING.112.976167. [DOI] [PubMed] [Google Scholar]
- 29.Sastry BKS, Nallamalla KR, Kumar N, Kodati D, Menon R. One-year clinical outcomes of different coronary drug eluting stents-Data from a prospective registry. Indian Heart J. 2018;70:580–3. doi: 10.1016/j.ihj.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]