Abstract
As the coronavirus disease 19 (COVID-19) global pandemic rages across the globe, the race to prevent and treat this deadly disease has led to the “off-label” repurposing of drugs such as hydroxychloroquine and lopinavir/ritonavir, which have the potential for unwanted QT-interval prolongation and a risk of drug-induced sudden cardiac death. With the possibility that a considerable proportion of the world’s population soon could receive COVID-19 pharmacotherapies with torsadogenic potential for therapy or postexposure prophylaxis, this document serves to help health care professionals mitigate the risk of drug-induced ventricular arrhythmias while minimizing risk of COVID-19 exposure to personnel and conserving the limited supply of personal protective equipment.
Abbreviations and Acronyms: COVID-19, coronavirus disease 19; DI-SCD, drug-induced sudden cardiac death; DI-TdP, drug-induced torsades de pointes; ECG, electrocardiogram; FDA, Food and Drug Administration; LQTS, long QT syndrome; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Since its emergence from Wuhan, China, in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), has already claimed the lives of more than 70,000 individuals worldwide.1 , 2 With the number of COVID-19 cases and deaths increasing with each passing day, there is perhaps no more pressing need in medicine than to identify safe and efficacious therapies to prevent SARS-CoV-2 infections as well as to attenuate the severity of the resulting COVID-19 illness.2 Although there are no proven US Food and Drug Administration (FDA)–approved drugs to prevent or treat COVID-19, a number of promising novel (eg, remdesivir) and repurposed (eg, hydroxychloroquine, potentially together with azithromycin) pharmacological agents, reported to inhibit the growth of SARS-CoV-2 in vitro,3 , 4 are being evaluated in randomized clinical trials.
In advance of more definitive evidence, clinicians on the front lines of the pandemic have begun to use these medications under the auspices of “off-label,” “compassionate-use,” or FDA-approved Emergency Use Authorization (for chloroquine and hydroxychloroquine) with anecdotal success.5 , 6 In light of (1) the need for this practice to continue in the absence of viable, evidence-based therapies and (2) the proclivity of many promising COVID-19 pharmacotherapies—specifically antimalarial agents such as hydroxychloroquine—to prolong the QTc, thereby increasing the risk of drug-induced torsades de pointes (DI-TdP) and drug induced-sudden cardiac death (DI-SCD), this document was assembled to help health care professionals safely use these medications and minimize concomitant risks.
Pharmacodynamics and QTc-Prolonging/Torsadogenic Potential of the Antimalarial Medications Chloroquine and Hydroxychloroquine
Chloroquine and its analogue hydroxychloroquine have been used for nearly 80 years as prophylactic pharmacotherapies for malaria. Although still used as antimalarial agents in parts of the world with chloroquine-sensitive Plasmodium falciparum protozoa, hydroxychloroquine has found new life as a disease-modifying antirheumatic drug for the management of conditions such as systemic lupus erythematosus and rheumatoid arthritis.
At the cellular level, these antimalarial drugs accumulate in intracellular vesicles such as endosomes and lysosomes where they are protonated, leading to increased vesicular pH.7 This process in turn inhibits the activity of the pH-dependent proteases involved in the intracellular processing of secretory proteins with a number of immunologic and nonimmunologic effects, including tumor necrosis factor α and interleukin 6.7 Collectively, a reduction in these secretory proteins is believed to result in (1) the accumulation of cytotoxic heme that poisons P falciparum protozoa and (2) modulation of immune cell behavior in a manner that attenuates inflammatory processes.7
In addition, chloroquine and hydroxychloroquine possess antiviral properties in vitro.3 , 4 , 7 , 8 Both chloroquine and hydroxychloroquine are believed to act on the entry and postentry stages of severe acute respiratory syndrome coronavirus and SARS-CoV-2 infection, likely via effects on endosomal pH and the resulting underglycosylation of angiotensin-converting enzyme 2 receptors that are required for viral entry.3 , 4 , 8
Based on this in vitro data, it has been hypothesized that hydroxychloroquine, more so than chloroquine, may have therapeutic efficacy in the COVID-19 pandemic by (1) preventing SARS-CoV-2 infection by inhibiting angiotensin-converting enzyme 2–mediated viral entry (ie, preinfection prophylaxis) and (2) attenuating the postviral cytokine storm observed in severe COVID-19 cases via a multitude of immunomodulatory mechanisms (ie, treatment of active infection/postviral sequelae). Promising in vitro data3 , 4 as well as anecdotal in vivo evidence of therapeutic benefit5 have led many institutions, including Mayo Clinic, to consider the use of hydroxychloroquine as a first-line COVID-19 pharmacotherapy for the time being and spurred an array of clinical trials designed to assess the efficacy of repurposed hydroxychloroquine in both the prevention and treatment of COVID-19.
Although the collective safety profiles of chloroquine and hydroxychloroquine are relatively favorable, both drugs block the KCNH2-encoded HERG/Kv11.1 potassium channel and potentially can prolong the QTc. In at-risk individuals, these so-called HERG blockers can precipitate DI-TdP or, worse, DI-SCD, especially with long-term use (Table 1 ). As a result, the number of DI-SCDs attributable to hydroxychloroquine in particular is not trivial (Table 1). With the theoretical possibility that a substantial proportion of the world population could receive hydroxychloroquine as first-line prophylaxis or treatment, including an estimated 3 million individuals with congenital long QT syndrome (LQTS), the number of hydroxychloroquine-mediated DI-SCDs could increase precipitously unless appropriate QTc monitoring algorithms are instituted. This risk of DI-SCD could be further amplified if multiple medications, each with their own QTc-prolonging/torsadogenic potential (eg, chloroquine/hydroxychloroquine plus azithromycin and/or lopinavir/ritonavir), are used in combination (Table 1).
Table 1.
Torsadogenic Potential and Postmarketing Adverse Events Associated With Possible COVID-19 Repurposed Pharmacotherapiesa
| Possible COVID-19 therapy | In vitro inhibition of SARS-CoV-2 | CredibleMeds classification | VT/VF/TdP/LQTS in FAERSb | Cardiac arrest in FAERSb | References |
|---|---|---|---|---|---|
| Repurposed antimalarial agents | |||||
| Chloroquine | Yes | Known TdP risk | 72 | 54 | 3, 19, 20 |
| Hydroxychloroquine | Yes | Known TdP risk | 222 | 105 | 4, 21 |
| Repurposed antiviral agents | |||||
| Lopinavir/ritonavir | Unknownc | Possible TdP risk | 27 | 48 | 22, 23, 24 |
| Adjunctive agents | |||||
| Azithromycin | Unknown | Known TdP risk | 396 | 251 | 25, 26 |
COVID-19 = coronavirus disease 2019; FAERS = US Food and Drug Administration Adverse Event Reporting System; LQTS = long QT syndrome; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TdP = torsades de pointes; VF = ventricular fibrillation; VT = ventricular tachycardia.
Adverse event reporting from postmarketing surveillance does not account for prescription volume and is often subjected to substantial bias from confounding variables, quality of reported data, duplication, and underreporting of events.
Lopinavir/ritonavir has been found to inhibit other severe acute respiratory syndrome viruses in vitro. However, a recent randomized trial found no benefit in COVID-19.
Mitigating the Potential Risk of DI-TdP and DI-SCD Associated With Widespread Use of Chloroquine/Hydroxychloroquine in the COVID-19 Pandemic
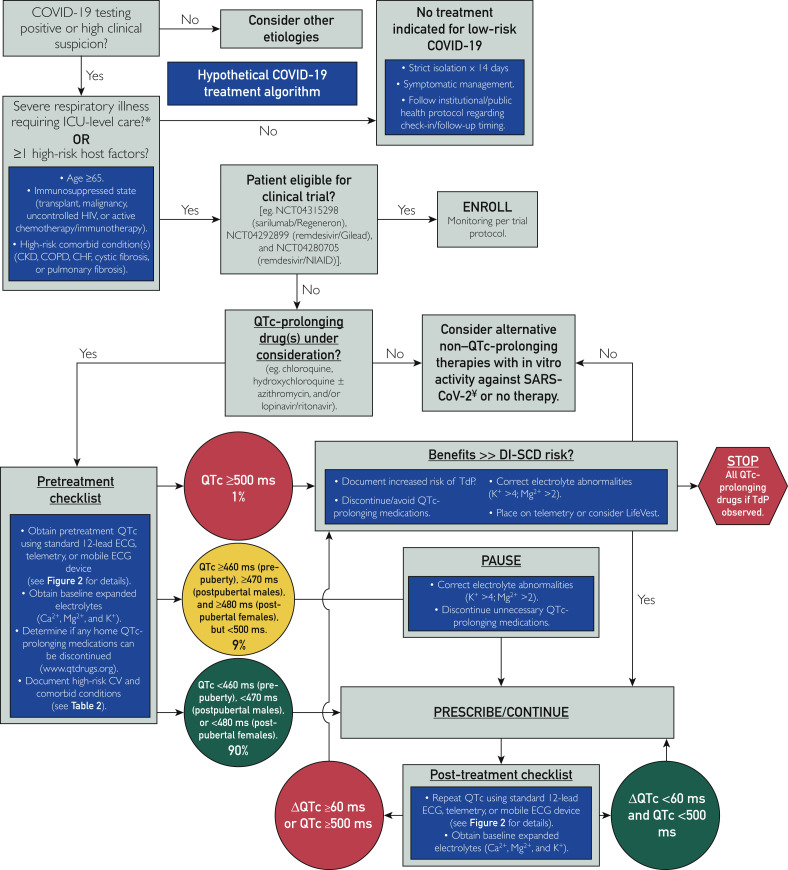
Although some might argue that DI-SCDs in the setting of widespread chloroquine/hydroxychloroquine use represents acceptable “friendly fire” in the war on SARS-CoV-2/COVID-19, we believe that with the institution of a few simple and safe precautions, the risk of DI-TdP and DI-SCD can be mitigated. Ultimately, it comes down to identifying the small subset of individuals who, either secondary to an underlying genetic predisposition (such as congenital LQTS, which is present in 1 in 2000 people) and/or by virtue of the presence of multiple modifiable and nonmodifiable QTc risk factors (Table 2 ),9 have excessive baseline QTc prolongation (QTc ≥500 ms) and/or have an inherent tendency for development of an exaggerated QTc response (ie, ΔQTc ≥60 ms) following exposure to medications with the adverse effect of potential QTc prolongation (Figure 1 ). Although the percentage of individuals at risk is small, given the pandemic nature of COVID-19, in absolute terms the number of individuals potentially at risk for lethal adverse drug effects is large (at least 10,000 individuals of the >1,000,000 COVID-19–positive patients worldwide are expected to be at increased risk for DI-TdP/DI-SCD if treated with these medications). This issue would be especially true if these medications are adopted for postexposure prophylaxis.
Table 2.
| Modifiable risk factors |
| Electrolyte disturbances |
| Hypocalcemia (calcium <4.65 mg/dL) |
| Hypokalemia (potassium <3.4 mmol/L) |
| Hypomagnesemia (magnesium <1.7 mg/dL) |
| QT-prolonging medication polypharmacy |
| Concurrent use of ≥1 medication from www.crediblemeds.com |
| Nonmodifiable risk factors |
| Common diagnoses |
| Acute coronary syndrome |
| Anorexia nervosa or starvation |
| Bradyarrhythmias (heart rate <45 beats/min) |
| Cardiac heart failure (ejection fraction <40%; uncompensated) |
| Congenital long QT syndrome or other genetic susceptibility |
| Chronic renal failure requiring dialysis |
| Diabetes mellitus (types 1 and 2) |
| Hypertrophic cardiomyopathy |
| Hypoglycemia (documented and in the absence of diabetes) |
| Pheochromocytoma |
| Cardiac arrest within preceding 24 h |
| Syncope or seizure within preceding 24 h |
| Stroke, subarachnoid hemorrhage, or other head trauma within preceding 7 d |
| Clinical history |
| Personal or family history of QT-interval prolongation or sudden unexplained death in the absence of a clinical or genetic diagnosis |
| Demographic |
| Elderly (>65 y) |
| Female sex |
A “pro-QTc” score of ≥4 based on risk factors similar to those listed above was an independent predictor of mortality in patients with QT-interval prolongation.9 Unfortunately, the predictive value of these risk factors in patients with normal or borderline QT intervals has not been assessed.
SI conversion factors: To convert calcium values to mmol/L, multiply by 0.25; to convert magnesium values to mmol/L, multiply by 0.411.
Adapted from Neurogastroenterol Motil,10 with permission. © 2018 John Wiley & Sons Ltd.
Figure 1.
Approach to mitigating the risk of drug-induced torsades de pointes (TdP)/drug-induced sudden cardiac death in patients with coronavirus disease 19 (COVID-19) treated following a hypothetical treatment algorithm with “off-label” hydroxychloroquine alone or in combination with azithromycin. Both medications are known HERG blockers with both QTc- prolonging and torsadogenic potential. The estimated 99th percentile QTc values (derived from otherwise healthy individuals), which places a patient in the “green light” category, are less than 460 ms before puberty, less than 470 ms in men, and less than 480 ms in women. We estimate that the baseline QTc assessment will place 90% of patients in green light, 9% in yellow light, and 1% in red light status. ∗Severe COVID-19 cases are defined as a respiratory rate of greater than 30 breaths/min (adults) or 40 breaths/min (children), oxygen saturation of 93% or less, PaO2 to fraction of inspired oxygen ratio less than 300, or lung infiltrates involving more than 50% of the lung field after 24 to 48 hours. ¥Repurposed antiviral alternatives such as lopinavir/ritonavir also have QTc-prolonging effects. CHF = congestive heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CV = cardiovascular; ECG = electrocardiography; ICU = intensive care unit; NIAID = National Institute of Allergy and Infectious Diseases.
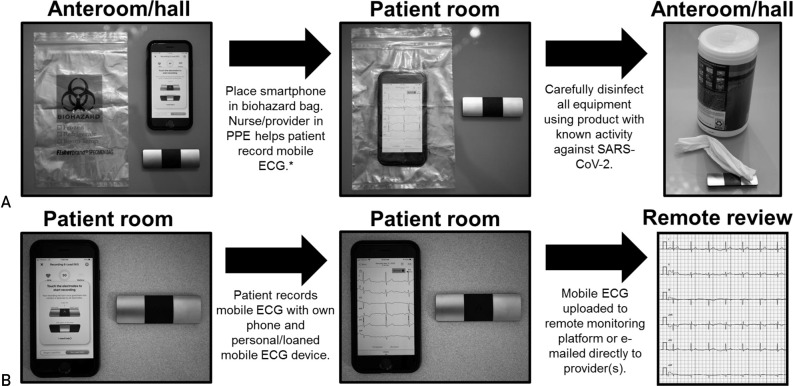
Traditionally, the QTc is calculated from either lead II or V5 of the 12-lead electrocardiogram (ECG) and corrected for heart rate using the Bazett or Fridericia formula before any intraindividual or interindividual QTc comparisons are made. Unfortunately, in the context of the COVID-19 pandemic, acquisition of the patient’s QTc by the 12-lead ECG, which requires additional personnel exposure (ie, ECG technician), and a necessity for serial ECGs, which requires exposure of complex equipment (multiple ECG wires), could further strain the already limited supply of personal protective equipment (PPE) in many countries. Alternatively, some FDA-approved consumer mobile ECG devices are capable of generating accurate QTc measurements.11 To this end, AliveCor, Inc, just received emergency clearance from the FDA for use of the KardiaMobile 6L device (FDA-approved for atrial fibrillation detection) for QTc monitoring of patients with COVID-19 treated with QT-prolonging medications such as chloroquine/hydroxychloroquine (March 20, 2020, 1:15 PM CST). Similarly, many telemetry systems are equipped with real-time QTc monitoring features that could be used for hospitalized patients.
For patients with COVID-19 about to be treated with medications with the increased potential for DI-TdP/DI-SCD (Figure 1), baseline QTc status should be obtained either by a traditional 12-lead ECG or perhaps preferably with the use of a smartphone-enabled mobile QTc meter using the simple infection control measures outlined in Figure 2 to limit personnel exposures and conserve critical PPE. On average, the QTc values for otherwise healthy postpubertal males and females are around 410 ms and 420 ms, respectively. In contrast, a QTc value that exceeds the 99th percentile value for otherwise healthy individuals (ie, 460 ms in both sexes before puberty, 470 ms in postpubertal males, and 480 ms in postpubertal females), in the absence of any exogenous QTc-aggravating factors, may signal an individual at increased risk for QT-related ventricular arrhythmias.12 , 13 In contrast and as a frame of reference, the average QTc value was 470 ms for the more than 1400 patients with congenital LQTS who have been cared for in Mayo Clinic’s Windland Smith Rice Genetic Heart Rhythm Clinic. Furthermore, with very few exceptions (amiodarone being one), patients with a resting QTc of 500 ms or more, whether secondary to congenital LQTS or acquired (QTc-prolonging drugs, QTc-prolonging electrolyte abnormalities such as hypokalemia, or QTc-prolonging disease states as detailed in Table 2), have a considerably greater risk for both DI-TdP and DI-SCD.14, 15, 16
Figure 2.
Protocols for the possible inpatient and outpatient use of a smartphone-enabled mobile electrocardiogram (ECG) to assess and monitor QTc values in patients with coronavirus disease 19. A, Inpatient protocol using dedicated institutional smartphone/tablet and mobile ECG device. Whenever possible, we strongly recommend the use of a dedicated institutional Bluetooth-enabled smartphone or tablet device that is not used for personal use (ie, phone calls or other activities) to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). B, Inpatient or outpatient protocol using personal (or institutionally loaned) smartphone/tablet and mobile ECG device. ∗Currently, the only smartphone-enabled mobile ECG with US Food and Drug Administration approval for QTc monitoring is the AliveCor KardiaMobile 6L device. PPE = personal protective equipment.
Accordingly, the baseline QTc value can be used to roughly approximate the patient’s risk of DI-TdP/DI-SCD following initiation of a medication with QTc-prolonging potential. For patients with QTc values less than the 99th percentile for age/sex (ie, 460 ms in prepubertal males/females, 470 ms in postpubertal males, and 480 ms in postpubertal females [Figure 1 “green light” status]), the risk of DI-TdP/DI-LQTS is low, and chloroquine/hydroxychloroquine (or other QTc-prolonging COVID-19 pharmacotherapies) should be initiated without delay as outlined in the QTc monitoring algorithm. Remember, whether by 12-lead ECG, telemetry, or smartphone-enabled acquisition of the ECG, if the noted QT interval is less than one-half the preceding RR interval, then the calculated QTc will always be less than 460 ms and the patient can be “green light go” for COVID-19 treatments that may have QTc-prolonging potential.
In contrast, those patients with a baseline QTc of 500 ms or greater are at increased risk for DI-TdP/DI-SCD (Figure 1 “red light” status) and every effort should be made to (1) assess and correct for contributing electrolyte abnormalities (eg, replete potassium and magnesium to levels > 4 mmol/L and > 2 mg/dl, respectively), (2) review and discontinue other unnecessary QTc-prolonging medications if present or transition to alternatives with less QTc liability, and/or (3) proceed with closer monitoring (telemetry) or even consideration of more advanced countermeasures such as equipping the patient with a wearable defibrillator (eg, LifeVest [ZOLL Medical Corporation]) if the decision is made to commence therapy.
In the setting of a QTc value of 500 ms or greater, navigating and circumventing this QTc liability depends greatly on the risk-benefit calculus, and the decision rests with the treating clinician and patient. For example, in younger patients with COVID-19 (ie, <40 years) who have only mild symptoms and a QTc of 500 ms or greater, it may be reasonable to avoid treatment altogether because the arrhythmia risk may outweigh the risk of developing COVID-19–related acute respiratory distress syndrome. However, in patients with a QTc of 500 ms or greater presenting with progressively worsening respiratory symptoms or at greater risk (eg, >65 years of age, immunosuppressed, and/or high-risk comorbid conditions) for respiratory complications, the potential benefit of QTc-prolonging COVID-19 pharmacotherapies may exceed the arrhythmia risk. Importantly, if the decision has been made to proceed with such therapies for the patient with a "red light" QTc value (ie, QTc >= 500 ms), it may be reasonable to give magnesium prophylactically, regardless of their magnesium level, as an anti-torsadogenic counter measure. Therefore, the ultimate goal of QTc surveillance in the COVID-19 pandemic should NOT be to identify those who cannot receive these medications but to identify those with compromised or reduced “repolarization reserve” in whom increased QTc countermeasures can and should be taken to mitigate the risk of drug-related death from DI-TdP/DI-SCD.17
Ultimately, much of the risk-benefit calculus awaits determination of the therapeutic efficacy of hydroxychloroquine, with or without concomitant azithromycin. Until such information is available, if the decision has been made to treat a patient with a red light designation (Figure 1) based on their baseline QTc of 500 ms or greater, it seems prudent to start with hydroxychloroquine alone, rather than combination drug therapy with azithromycin. In addition, if combination drug therapy, with hydroxychloroquine and azithromycin, was initiated in a patient with an initial green light/yellow light QTc status and the individual transitions to red light status after self-identification as a “QTc reactor” with a ΔQTc of 60 ms or greater, then consideration should be given to discontinuing azithromycin, optimizing electrolyte status, or intensifying countermeasures further (placing on telemetry for continuous rhythm assessment).
Frequency of QTc Surveillance and Adjustments in the Setting of Wide QRS Complex
Ideally, following a baseline QTc assessment, therapy may be initiated with either QTc reassurance (low risk for the vast majority [90%] of patients) or varying QTc countermeasures in place for those flagged as at increased risk. The timing of on-therapy QTc surveillance will be dictated not only by the pharmacokinetics of the COVID-19 therapies used but also by the practical logistics of an institution’s method of QTc monitoring. For the 12-lead ECG approach, if QTc surveillance is deemed important, then one machine should be designated for acquisition of the data and a limited number of ECG technicians/personnel should be used to minimize PPE utilization and personnel exposure. Also, the number of on-therapy QTc assessments should be constrained to minimize personnel exposure risk and PPE consumption. In this scenario, for those placed in red light status because their baseline QTc is 500 ms or greater, an initial on-therapy QTc should be obtained around 2 to 4 hours after the first dose and then again at 48 hours and 96 hours following treatment initiation. Patients receiving either green light or yellow light status can probably forego the early QTc assessment and wait until 48 hours and 96 hours for their on-drug QTc determination. If the on-therapy QTc is 500 ms or greater or the patient self-identified as a QTc reactor with a ΔQTc of 60 ms or greater, then the QTc countermeasures need to be reexamined or the medications stopped in an effort to neutralize the increased potential for DI-TdP and DI-SCD (Figure 1).
In contrast, for medical centers able to implement the FDA emergency-approved, smartphone-enabled approach (Figure 2) or to determine the QTc from the telemetry strips, ECG technician exposure risk and consumption of PPE by those individuals would be eliminated and the patient’s QTc could be obtained by the health care team already present, for example, with the QTc obtained per shift as another vital sign.18 Such increased QTc surveillance would enable discovery of the QTc reactor and implementation of countermeasures sooner and hopefully would thereby circumvent the potentially preventable tragedy of DI-SCD (Figure 1).
Finally, for patients with a wide QRS complex from either ventricular pacing or right/left bundle branch block, a wide QRS complex QTc adjustment will need to be made. Otherwise, patients will receive a red light signal inappropriately, resulting in therapy delay, discontinuation, or avoidance altogether. In this setting, the simplest approach is to maintain the previously indicated QTc green, yellow, and red light thresholds and apply a simple formula to account for the wide QRS complex (wide QRS complex–adjusted QTc = QTc – [QRS − 120 ms]). For example, if a patient’s left bundle branch block has yielded a QRS complex of 200 ms and a QTc of 520 ms, this scenario would appear to activate the red light pathway (Figure 1). However, the wide QRS complex–adjusted QTc would be 520 ms – (200 − 120 ms) = 520 − 80 = 440 ms, which is not red light status at all but rather “green light go” status with much QTc reassurance that the patient is at low risk for DI-SCD.
Conclusion
As this coronavirus pandemic continues to spread and wreak havoc, economic loss, and more importantly, the tragic deaths of thousands throughout the world, we must all do our part in this war on COVID-19. Washing hands and physical distancing are core components of containment efforts to “flatten the curve.” Development of a coronavirus vaccine is progressing at unprecedented speed but is still at least 12 to 18 months away. In the meantime, there is hope that a long-ago discovered antimalarial drug, hydroxychloroquine, may have lifesaving therapeutic efficacy against COVID-19. And if it does, we hope that this simple QTc surveillance strategy, enabled by innovation and the FDA’s emergency approval, will help prevent altogether or at least substantially reduce the number of drug-induced ventricular arrhythmias and sudden cardiac deaths, particularly if there is widespread adoption and utilization of these medications for COVID-19.
Footnotes
Grant Support: This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.
Potential Competing Interests: Dr Ackerman is a consultant for Abbott, Audentes Therapeutics, BIOTRONIK SE & Co. KG, Boston Scientific Corporation, Invitae Corporation, LQT Therapeutics Inc, Medtronic, and MyoKardia. Drs Noseworthy, Friedman, and Ackerman and Mayo Clinic are involved in an equity/royalty relationship with AliveCor, Inc (not involved in this study). Dr Giudicessi reports no competing interests.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah A., Kashyap R., Tosh P., Sampathkumar P., O'Horo J.C. Guide to understanding the 2019 novel coronavirus [published online ahead of print February 28, 2020] https://doi.org/10.1016/j.mayocp.2020.02.003 Mayo Clin Proc. [DOI] [PMC free article] [PubMed]
- 3.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print March 9, 2020] https://doi.org/10.1093/cid/ciaa237 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 5.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. 2020;03 doi: 10.1101/2020.03.16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19 [published online ahead of print March 4, 2020] https://doi.org/10.1016/j.ijantimicag.2020.105932 Int J Antimicrob Agents. [DOI] [PMC free article] [PubMed]
- 7.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugaa K.H., Bos J.M., Tarrell R.F., Morlan B.W., Caraballo P.J., Ackerman M.J. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315–325. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Giudicessi J.R., Ackerman M.J., Camilleri M. Cardiovascular safety of prokinetic agents: a focus on drug-induced arrhythmias. Neurogastroenterol Motil. 2018;30(6):e13302. doi: 10.1111/nmo.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garabelli P., Stavrakis S., Albert M. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol. 2016;27(7):827–832. doi: 10.1111/jce.12976. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S., Drezner J.A., Baggish A. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39(16):1466–1480. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 13.Vink A.S., Neumann B., Lieve K.V.V. Determination and interpretation of the QT interval. Circulation. 2018;138(21):2345–2358. doi: 10.1161/CIRCULATIONAHA.118.033943. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg I., Moss A.J., Peterson D.R. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;117(17):2184–2191. doi: 10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs J.B., Peterson D.R., Moss A.J. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296(10):1249–1254. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 16.Sauer A.J., Moss A.J., McNitt S. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49(3):329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Roden D.M. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259(1):59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 18.Giudicessi J.R., Noseworthy P.A., Ackerman M.J. The QT interval. Circulation. 2019;139(24):2711–2713. doi: 10.1161/CIRCULATIONAHA.119.039598. [DOI] [PubMed] [Google Scholar]
- 19.Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484(1):41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Stas P., Faes D., Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008;127(2):e80–e82. doi: 10.1016/j.ijcard.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 22.Chen F., Chan K.H., Jiang Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [published online ahead of print March 18, 2020] https://doi.org/10.1056/NEJMoa2001282 N Engl J Med. [DOI] [PMC free article] [PubMed]
- 24.Soliman E.Z., Lundgren J.D., Roediger M.P. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25(3):367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giudicessi J.R., Ackerman M.J. Azithromycin and risk of sudden cardiac death: guilty as charged or falsely accused? Cleve Clin J Med. 2013;80(9):539–544. doi: 10.3949/ccjm.80a.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arellano-Rodrigo E., García A., Mont L., Roqué M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome [in Spanish] Med Clin (Barc) 2001;117(3):118–119. doi: 10.1016/s0025-7753(01)72036-2. [DOI] [PubMed] [Google Scholar]