Abstract
As COVID-19 pandemic continues to explode, cancer centers worldwide are trying to adapt and are struggling with this constantly changing scenario. Intending to ensure patient safety and deliver quality care, we sought consensus on the preferred thoracic radiation regimen in a Canadian province with 4 new R’s of COVID era.
Keywords: Lung cancer, COVID-19
Introduction
The world and health care system is scrambling as COVID-19 pandemic continues to explode [1]. As of 24th March 2020, over 400,000 cases are reported worldwide and the numbers keep rising constantly [1]. Apart from the Artic, it has engulfed every continent and country. With this unimaginable scenario, the healthcare system in the entire world is under immense pressure and struggling to cope.
Available data shows COVID-19 carries high risk or morbidity and mortality for elderly and immuno-compromised individuals [2]. Reports from China show Cancer patients have an aggressive course and carry a 3.5 higher risk of mortality [3]. The study also found patients with cancer deteriorated more rapidly than those without cancer (median time to severe events 13 days vs 43 days) [3].
Cancer centers worldwide are trying to adapt and are struggling with this constantly changing scenario. Hospitals are designing new policy measures and working hard to implement those in place. Radiation Oncology is an integral part of oncology care and the nature of fractionated treatment requires patients to visit cancer centers daily for a significant number of days. The risk of patient contacting/getting exposed to COVID-19 and diffusion of spread is paramount [4]. The risk of decrease in specialized radiation oncology workforce could hamper and even halt operations of radiation oncology centers [5]. Learning from Chinese and Italian experience, aiming to reduce the impact of epidemic operations changes several are recommended [4], [6], [7].
Radiation oncology community must evaluate options of prioritizing radiation treatments (RT), deferring where applicable, omitting where there is no or very minimal benefit and strongly consider reduce numbers of fractions where there is evidence to support [8].
We proposed 4 new R’s in the COVID era [1] ViRtual care (reduce in-person consult/follow up/on treatment visits) [2] Ration radiation (ofer radiation wisely and avoid RT where minimal benefit) [3] defeR radiation (as appropriate) [4] hypofRactionate radiation (where applicable) and came up with provincial preferred thoracic radiation regimen.
Several measures are adopted by Canadian physicians such as virtual care to minimize in-person clinic visits and deferral where needed. Intending to ensure patient safety and deliver quality care, we sought consensus on the preferred thoracic hypofractionated radiation regimen in our provincial cancer center.
Methods
CancerCare Manitoba provides comprehensive oncology services for the province of Manitoba and adjacent areas of Northwestern Ontario and Nunavut. Approximately 3500 new patients are seen/treated annually including approximately 600–650 new lung cancer patients. We are a modern radiation oncology department with 8 modern linear accelerators including True beam and Edge machines and using IGRT, rapid arc and SRS/SBRT techniques in our standard clinical practice.
With provincial emergency declarations, Thoracic Radiation oncology (RO) group was tasked with disease specific COVID-19 emergency preparedness plan. Our Team reviewed the current evidence to assess the role and available options of different radiation regimens in the management of lung cancers.
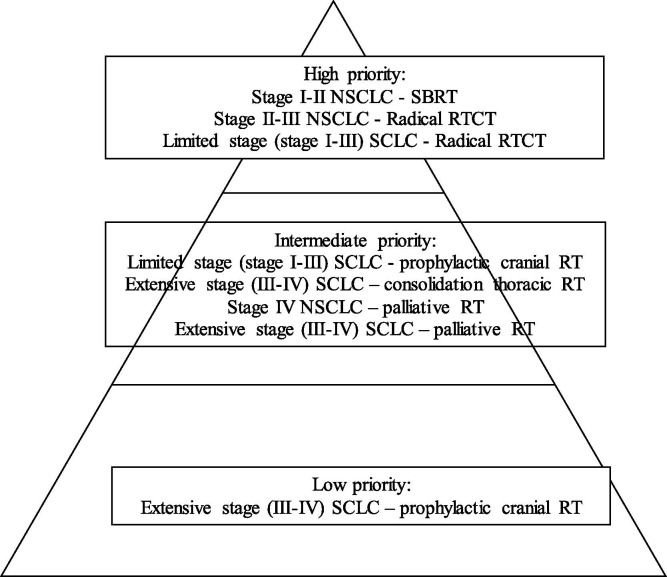
We assessed different common scenarios where radiation would be routinely considered and prioritized the indications. Priority levels were assigned based on prognosis and the expected benefit of radiation treatment. Curative treatments, where delay/deferral would jeopardize the survival outcomes, were considered as a high priority. Treatment indications for symptomatic measures or consolidation (treatment gains in quality of life or progression-free survival) were considered as an intermediate priority. Treatment indications where the role of radiation is debatable (no significant overall survival impact or other options as observation were appropriate) were considered as low priority. Thoracic RO team attained consensus and recommended evidence-based preferred hypofractionated radiation regimen for clinical care at our provincial comprehensive cancer center.
Recommendations
Nonsmall cell Lung Cancers:
Stage I-II NSCLC:
Priority level – high:
RO Consensus: Stage I–II (node negative) NSCLC represents a curative scenario and nonsurgical candidates with good performance status should undergo stereotactic body radiation treatment. Peripheral tumours should be treated with 54 Gy in 3 fractions (FR) and preferred over 48 Gy in 4 Fr option [9], [10]. We do not practice 30–34 Gy in 1 fraction at our center yet, however once available could be preferred for appropriate patients [10]. Central tumours should be treated with 50 Gy in 5 Fr as a preferred option [11]. Group discouraged use of 60 Gy in 8 Fr for central tumours. However, this would be considered a valid option for the treatment of ultra-central tumours. The use of hypofractionated regimen as 60 Gy in 15 Fr is not preferred unless the patient is not a candidate for SBRT [12].
Stage II (node positive) – III NSCLC:
Priority level – high:
RO Consensus: Group agreed inoperable stage II (node positive) – III population with good performance status would be best served with curative concurrent chemo radiation with standard doses of 60 Gy in 30 Fr [13]. Our group did not support concurrent chemo and hypofractionated radiation options. We discouraged the use of a longer regimen such as 66 Gy in 33 Fr at present [13]. We recommended candidates ineligible for concurrent chemo radiation should receive sequential chemoradiation (if suitable) with hypofractionated radiation as 55 Gy in 20 Fr or 40 Gy in 15 Fr.
Stage IV NSCLC:
Priority level – Intermediate:
RO Consensus: Majority stage IV NSCLC patients face significant symptom burden. Palliative radiation offers a significant improvement in symptoms and Quality of life [14]. Group recommended 8–10 Gy in 1 Fr or 16 Gy in 2 Fr (1 week apart) as preferred regimen over other fractionated regimen as 20 Gy in 5 Fr or 30 Gy in 10 Fr especially in patients with poor performance status [15]. There is no strong evidence that any regimen gives greater palliation [16].
Small-cell Lung Cancers:
Limited stage (stage I–III):
Priority level – high:
RO Consensus: Concurrent chemo radiation plays a vital role in the management of this curative group of patients. We adopted 40 Gy in 15 Fr as a preferred consensus recommendation for good performance status candidates [17]. Group discouraged use of 45 Gy in 30 Fr BID or 66 Gy in 33 Fr regimens in the current scenario [18], [19]. The evidence suggests early administration of concurrent RT is superior to delayed administration and RO favoured early administration of RT [20].
Stage I SCLC could account for 10% of screen detected lung cancers. Acknowledging the limitations of available data, SBRT should be considered in stage I SCLC [21], [22]. In such cases group favored SBRT should be offered post chemotherapy [23].
Prophylactic cranial radiation:
Priority level – Intermediate:
RO Consensus: For limited stage SCLC responding to initial therapy, our group supported and recommended standard regimen of PCI (25 Gy in 10 Fr) for eligible patients [24].
Extensive stage (stage III–IV):
Consolidation thoracic radiation:
Priority level – Intermediate:
RO Consensus: For extensive stage SCLC responding to initial therapy, consolidation thoracic radiation improves progression-free survival; reduces intra-thoracic failures, however, does not improve overall survival significantly. For suitable patients group favored 20 Gy in 5 Fr over other regimens [25], [26].
Prophylactic cranial radiation:
Priority level – low:
RO Consensus: For extensive stage SCLC responding to initial therapy, the role of PCI is debatable [27], [28]. For eligible patients group supported and recommended MRI surveillance as a preferred option in the current environment [27], [28].
Palliative radiation in SCLC for symptomatic issues:
Priority level – Intermediate:
RO Consensus: Palliative radiation serves significant improvement in symptoms and Quality of life. The group recommended 8–10 Gy in 1 Fr or 16 Gy in 2 Fr (I week apart) as preferred regimen over other fractionated regimen as 20 Gy in 5 Fr or 30 Gy in 10 Fr.
Summary details of priority and recommended radiation regimen are shown in Fig. 1 and Table 1 respectively.
Fig. 1.
Priority pyramid.
SBRT – sterotactic body radiation treatment, RT – radiation, CT – chemotherapy, NSCLC – non small cell lung cancer, SCLC– small cell lung cancer.
Table 1.
Preferred hypofractionated RT regimen.
| Preferred fractionated RT regimen | Patient visits and RT fractions saved per patient per RT course (with Preferred fractionated RT regimen over other regimens) | |
|---|---|---|
| Lung NSCLC | ||
| SBRT – Peripheral | 54 Gy/3 Fr | 1 Fr |
| SBRT – Central | 50 Gy/5 Fr | 3 Fr |
| SBRT | Continue as usual. You may also wish to assess option of delay for minimally growing tumors | |
| Concurrent CTRT | 60 Gy/30 Fr | 3 Fr |
| Sequential CTRT | 40 Gy/15 Fr or 50 Gy/20 Fr | 7–15 Fr |
| Pall RT lung | 8–10 Gy/1 Fr | 4 Fr |
| Lung SCLC | ||
| Limited stage: Radical | 40 Gy/15 Fr | 15 Fr |
| Limited stage: PCI | 25 Gy/10 Fr | No change |
| Extensive stage: consolidation RT (if needed) | 20 Gy/5 Fr | 5–10 Fr |
| Extensive stage: PCI (if needed) | 25 Gy/10 Fr (Strongly consider the option of no PCI and MRI regularly) | 10 Fr (if RT deferred) |
| Pall RT lung | 8 Gy in 1 Fr | 4 Fr |
Legends: Gy – Gray, Fr – fraction, SBRT – sterotactic body radiation treatment, RT – radiation, CT – chemotherapy, NSCLC – non small cell lung cancer, SCLC – small cell lung cancer.
Discussion
With the current pandemic and associated restrictions, we believe this preferred RT regimen will serve as a policy document for the thoracic radiation community (Table 1 and Fig. 1). The model of viRtual care (reduce in-person consult/follow up/on treatment visits), Ration radiation (ofer radiation wisely and avoid RT where minimal benefit), defeR radiation (as appropriate), hypofRactionate radiation (where applicable) are emerging as new 4R’s of radiation therapy.
These measures will be helpful to minimize patient visits to radiation centers and reduce the risk of patient exposure to infection. The preferred hypofractionated regimen may help minimize the impact of COVID19 on in these unprecedented and unfortunate times. We are hopeful these measures will help mitigate the impact of COVID-19 pandemic on our patients and cancer centers.
Conclusion
In the current times, 4 new R’s of Radiation treatment will help mitigate the impact of COVID-19 pandemic on our patients and cancer centers.
Conflict of interest
None to disclose.
Footnotes
The Editors of the Journal, the Publisher and the European Society for Radiotherapy and Oncology (ESTRO) cannot take responsibility for the statements or opinions expressed by the authors of these articles. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds or experiments described herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made. For more information see the editorial “Radiotherapy & Oncology during the COVID-19 pandemic”, Vol. 146, 2020.
References
- 1.https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi A.R., Russi E., Magrini S.M. Covid-19 outbreak in Northern Italy: first practical indications for radiotherapy departments. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera A.O.N., Thomas E., Miller R., Knoll M.A. he Impact of COVID-19 on radiation oncology clinics and cancer patients in the U.S. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krengli M.F.E., Mastroleo F., Brambilla M., Ricardi U. Running a Radiation Oncology Department at the time of coronavirus: an Italian experience. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simcock R., Mercy C.E., Filippi A.R., Katz M.A., Pereira I.J., Saeed H. COVID-19: Global Radiation Oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020 doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achard V., Tsoutsou P., Zilli T. Radiotherapy in the time of the Coronavirus pandemic: when less is better. Int J Radiat Oncol Biol Phys. 2020 [Google Scholar]
- 9.Timmerman R.D., Paulus R., Pass H.I. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG oncology RTOG 0618 trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Videtic G.M., Paulus R., Singh A.K. Long-term follow-up on NRG oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103:1077–1084. doi: 10.1016/j.ijrobp.2018.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezjak A., Paulus R., Gaspar L.E. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 2019;37:1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swaminath A., Wierzbicki M., Parpia S. Canadian phase III randomized trial of stereotactic body radiotherapy versus conventionally hypofractionated radiotherapy for stage I, medically inoperable non-small-cell lung cancer - rationale and protocol design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE trial. Clin Lung Cancer. 2017;18:250–254. doi: 10.1016/j.cllc.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Bradley J.D., Hu C., Komaki R.R. Long-term results of NRG oncology RTOG 0617: standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathod S., Jeremic B., Fidarova E. Quality of life outcomes in a phase 3 randomized trial of optimization of treatment of advanced non-small cell lung cancer using radiation therapy and chemotherapy: IAEA multicentric randomized phase 3 study ( NCT00864331) Int J Radiat Oncol Biol Phys. 2017;99:S103. [Google Scholar]
- 15.Rodrigues G., Videtic G.M., Sur R. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens R., Macbeth F., Toy E. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD002143.pub4. CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray N., Coy P., Pater J.L. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 18.Faivre-Finn C., Snee M., Ashcroft L. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turrisi A.T., 3rd, Kim K., Blum R. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 20.De Ruysscher D., Lueza B., Le Pechoux C. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27:1818–1828. doi: 10.1093/annonc/mdw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rathod S., Koul R., Bashir B. Role of stereotactic body radiation therapy in early stage small cell lung cancer in the era of lung cancer screening: a systematic review. Am J Clin Oncol. 2019;42:123–130. doi: 10.1097/COC.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 22.NCCN Guidelines – small cell lung cancer, https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf (assessed on 29th March 2020).
- 23.Stahl J.M., Corso C.D., Verma V. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. doi: 10.1016/j.lungcan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Le Pechoux C., Dunant A., Senan S. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): a randomised clinical trial. Lancet Oncol. 2009;10:467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 25.Rathod S., Jeremic B., Dubey A. Role of thoracic consolidation radiation in extensive stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Eur J Cancer. 2019;110:110–119. doi: 10.1016/j.ejca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Slotman B.J., van Tinteren H., Praag J.O. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T., Yamanaka T., Seto T. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663–671. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 28.Slotman B., Faivre-Finn C., Kramer G. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]