A central strategy for healthcare surge control would be to provide remote effective monitoring of patients with Covid-19 and their follow-up. Telemedicine not only allows patients to be carefully screened but also enables a patient-centred system and being conducive to self-quarantine. In addition, patients, clinicians and the community are protected from exposure [1].
Patients with cancer during or after treatment have an increased risk of complication and death related to Covid-19 contagion [2,3].
In this report, physicians in Gustave Roussy Cancer Institute (GRCI), France, a leading European tertiary cancer centre, describe the use of telemedicine for monitoring and optimising referral of Covid-19–positive patients with cancer. The CAPRI telemedicine programme has been set up to monitor patients with cancer undergoing oral therapy [4] (Fig. 1 ). Faced with the Covid-19 crisis, we adapted the system accordingly in a period of two weeks, also drawing inspiration from another programme [5].
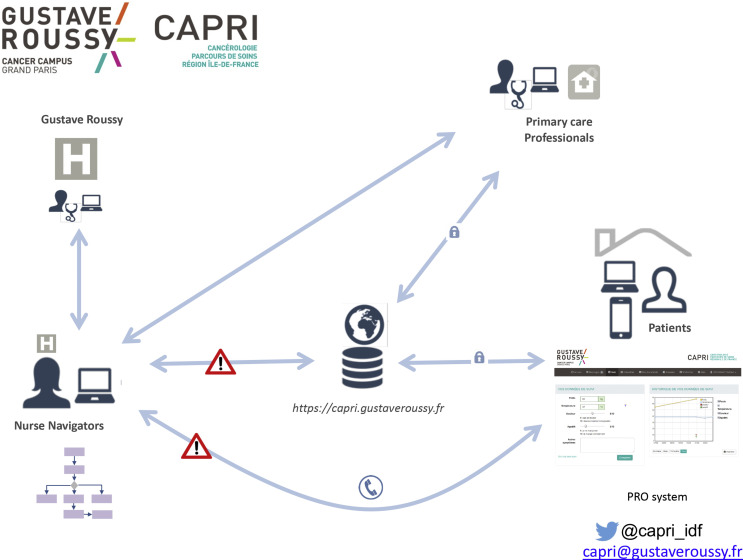
Fig. 1.
CAPRI process for monitoring patients with cancer. PRO, patient reported outcome.
Capri Covid-19 consists of a Web application for patients and a telephone platform with a dedicated call number, the entire procedure being managed by four GR nurse navigators (NNs). It can allow NNs and patients to communicate 24/7 (from 8:30 am to 6 pm; outside the centre, the patient must contact the emergency service) through secure messaging (i.e. the platform complies with the French regulation on data protection). The Web application allows patients to fill in questions specific to Covid-19 based on a patient-reported outcome approach, which has demonstrated its added value in an oncology context [6]. Finally, the application provides NNs with a complete panel to view individual electronic patient medical records.
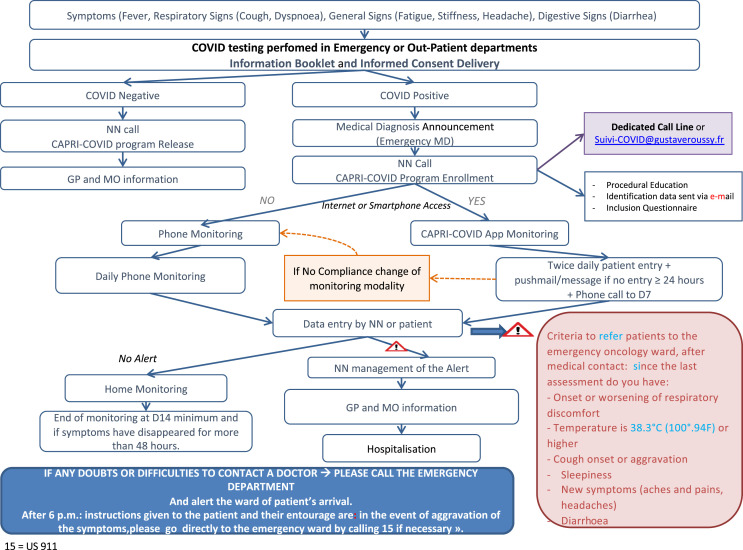
If Covid-19 is suspected, either when the patient comes to the hospital (emergency department or consultation, in which case, the patient is asked to return home while the test results are pending) or at home, the patient is tested. Covid-19–positive patients are included in the CAPRI programme after having provided informed consent and follow a 4-phase remote monitoring strategy (see Fig. 2 ).
Fig. 2.
Algorithm for remote monitoring of Covid-19–positive patients with cancer. NN = nurse navigator; GP = general practitioner; MO = medical oncologist; MD = medical doctor.
1. Initial assessment
The NN calls each patient to assess his/her specific cancer concerns (e.g. medical prescriptions), comorbidities and social conditions (isolation and ability to acquire personal needs during the confinement period). In addition, information on the programme is given when the individual account is created, following the guidelines provided by the French authorities (https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr = 779). Finally, the patient is offered to choose between two monitoring modes: either by phone or by completing an application form (Web or smartphone).
2. Follow-up
This is based on six questions the patients were asked: (i) fever ≥38.3° (100.94° F); (ii) appearance or worsening of respiratory discomfort since the last assessment (LA); (iii) appearance or worsening of a cough since the LA; (iv) drowsiness; (v) any new symptoms since the LA (e.g. muscle aches, headaches) and (vi) appearance or worsening of diarrhoea since the LA. The data are reported following the application twice a day, whereas the data are reported daily in the case of a telephone follow-up.
3. Patient orientation decision
The system generates alerts if one of the six aforementioned questions is answered positively or if the patient has not responded for more than 24 h. The alert is automatically generated via the application or during daily NN phone calls. In this case, the NN contacts the emergency department manager to organise the patient's arrival to the emergency room or the Covid-19 ward directly.
4. Evaluation
A longitudinal assessment is organised, based on five indicators: hospital admissions, emergency visits, access to the intensive care unit after a Covid-19 complication, deaths after a Covid-19 complication and recoveries. These indicators provide a real-time reading of the evolution of the epidemic and will then allow the impact of this intervention to be assessed.
To date, more than hundred patients have been enrolled in the ongoing programme. Our experience shows that Covid-19 crisis is a clinical, epidemiological and organisational issue to overcome. Although telemonitoring cannot solve every problem, it is well suited to the context of Covid-19, and organisations that have already invested in telemedicine are well positioned to expand them and ensure that patients with Covid-19 receive the appropriate care. This decision tree allows not only a collection of data but also a secured organisational process for patient orientation and an optimal physician medical time. Our experience could help other cancer centres, or even healthcare organisations, to implement a rapid effective programme with healthcare professionals monitoring patients at distance while being less exposed. Gustave Roussy Cancer Institute should provide for free the Capri Covid app worldwide to help Covid-infected patients with cancer.
Conflict of interest statement
F.S. reports personal fees from Helsinn, personal fees from MSD, personal fees from Roche, personal fees from Amgen, personal fees from Pierre Fabre Oncology, personal fees from Pfizer, personal fees from Mundipharma, personal fees from Mylan and personal fees from Leo Pharma, outside the submitted work; E.M. has no disclosure to declare. O.M. reports personal fees from Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Eli Lilly, Incyte, Ipsen, Lundbeck, MSD, Novartis, Pfizer, Roche, Servier and Vifor Pharma and other fees from Amplitude Surgical, Ipsen and Transgene, outside the submitted work; F.A. has no disclosure to declare. F.B. reports personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd, Novartis, Merck, MSD, Pierre Fabre, Pfizer and Takeda, outside the submitted work; J.-C.S. reports feesconsultancy fees from AstraZeneca, Astex, Clovis, GSK, GamaMabs, Lilly, MSD, Mission Therapeutics, Merus, Pfizer, PharmaMar, Pierre Fabre, Roche-Genentech, Sanofi, Servier, Symphogen and Takeda, is a full-time employee for AstraZeneca between September 2017 and December 2019, reports other fees from the shareholder Gritstone, during the conduct of the study, and reports personal fees from null, outside the submitted work.
Acknowledgements
The authors are grateful to Richard Medeiros, Medical Editor from Medical Editing International.
References
- 1.Hollander J.E., Carr B. Virtually perfect? telemedicine for covid-19. NEJM. March 11, 2020 doi: 10.1056/NEJMp2003539. Perspective. [DOI] [PubMed] [Google Scholar]
- 2.Guan Wei-jie, Ni Zheng-yi, Hu Yu, Liang Wen-hua, Ou Chun-quan, He Jian-xing. Clinical characteristics of coronavirus disease 2019 in China. NEJM. 2020; February 28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Wenhua, Guan Weijie, Chen Ruchong, Wang Wei, Li Jianfu, Xu Ke. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. March 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gervès-Pinquié, Daumas-Yatim F., Lalloué B., Girault A., Ferrua M., Fourcade A. Impacts of a navigation program based on health information technology for patients receiving oral anticancer therapy: the CAPRI randomized controlled trial. BMC Health Serv Res. 2017 Feb 13;17(1):133. doi: 10.1186/s12913-017-2066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scotté F., Oudard S., Aboudagga H., Elaidi R., Bonan B. A practical approach to improve safety and management in chemotherapy units based on the PROCHE – programme for optimisation of the chemotherapy network monitoring program. Eur J Canc. 2013;49(3):541–544. doi: 10.1016/j.ejca.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Basch E., Deal A.M., Dueck A.C., Scher H.I., Kris M.G., Hudis C. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. J Am Med Assoc. 2017 Jul 11;318(2):197–198. doi: 10.1001/jama.2017.7156. Published online 2017 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]