Abstract
Purpose: To characterize the inflammatory response and determine the no-observable-effect level (NOEL) in cynomolgus monkey eyes after intravitreal (ITV) injection of endotoxin.
Methods: The inflammatory response to endotoxin was assessed in a single-dose study in monkeys at doses of 0.01 to 0.51 endotoxin units (EU)/eye. Tolerability was assessed by clinical ophthalmic examinations, intraocular pressure measurements, fundus color photography, optical coherence tomography, and anatomic pathology.
Results: ITV injection of endotoxin at ≥0.04 EU/eye resulted in a dose-related anterior segment inflammatory response. No aqueous flare or cell was noted in the 0.01 EU/eye dose group. A more delayed posterior segment response characterized by vitreous cell was observed beginning on day 5, peaking on day 15, and decreasing in some groups. Microscopic findings of mononuclear cell infiltrates in the vitreous were observed in eyes given ≥0.21 EU/eye.
Conclusion: The NOEL for ITV endotoxin in cynomolgus monkeys was 0.01 EU/eye, suggesting that this species is as sensitive as rabbits to the effects of endotoxin. The vitreous cavity also appears more sensitive to endotoxin than the anterior segment/aqueous chamber. Overall, the magnitude of the inflammatory response at ≥0.04 EU/eye suggests that dose–response curve in monkeys is steeper than in rabbits. These data highlight the importance of assessing endotoxin level in ITV formulations, as levels as low as 0.04 EU/eye may confound the safety evaluations of ITV therapeutics in cynomolgus monkeys.
Keywords: drug development, intravitreal injection, endotoxin, ocular toxicology
Introduction
Given the successful introduction and approval of therapeutics for a variety of ocular conditions over the past decade administered as intravitreal (ITV) injections, the ITV route of administration has now become a common procedure by retina specialists.1 While administering potent therapeutics locally and directly to the back of the eye targeting retina, ITV administration offers a promise to minimize systemic exposure.
Compared to other routes of administration from a toxicology drug development point of view, ITV injection is relatively novel and characterization of key product qualities for ITV formulations presents one of the keys challenges.
Endotoxin, a highly heat-resistant lipopolysaccharide released from a cell wall of gram-negative bacteria is difficult to eliminate from sterile instruments for the use in intraocular surgeries. Clinical cases of acute postoperative sterile uveitis or toxic anterior segment syndrome (TASS) also linked TASS to endotoxin contamination of ophthalmic devices.2–4 In the past few years, ocular inflammation and sensitivity to intracameral effects of endotoxin and endotoxin-contaminated ophthalmic viscosurgical devices (OVDs)5–7 were characterized in rabbits. Endotoxin and ocular inflammation are well described, and ITV endotoxin is utilized to produce experimental animal models of uveitis in mice,8 rabbit9–13 porcine,14 and nonhuman primates (NHPs).15
For the development of ITV therapeutics, endotoxin is perhaps a major potential formulation contaminant, especially if therapeutic candidates are produced in prokaryotic expression systems. Recently, we characterized the sensitivity and time course of the response to endotoxin following ITV administration in rabbits.16 Development of biologics, however, often requires the use of NHPs as the only pharmacologically relevant species. Thus, defining a no-observable-effect level (NOEL) for ITV endotoxin and characterization of the relationship of endotoxin dose to ocular inflammation in the NHP is important and will aid interpretation of ocular nonclinical safety data.
Using the same reference endotoxin material as the recently published ITV NOEL in rabbit,16 the objective of this study was to characterize the acute ocular inflammatory response in a small number of animals and define the NOEL in the NHP to a range of endotoxin doses delivered by ITV injection.
Methods
Test species
Six female naive purpose-bred cynomolgus monkeys of Chinese origin (Covance Research Products, Inc.) were assigned to the study, Groups 1-6 with a single animal per Group (Table 1). The local Institutional Animal Care and Use Committee (Covance Laboratories, Inc. or Genentech, Inc.), in accordance with the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmology and Vision Research, approved the animal protocol. Animals were 3 to 4 years old and ranged in weight from 2.6 to 3.2 kg at study initiation. Animals were pair-housed in stainless steel cages in an environmentally controlled room (individually housed for study-related procedures). Food consisted of Certified Primate Diet #5048 (PMI Nutrition International Certified LabDiet®) and water ad libitum, along with supplemental dietary enrichment.
Table 1.
Study Design
| Group | No. of animals | Treatment (each eye) | Dose level (EU/eye)a |
|---|---|---|---|
| 1 | 1 | Vehicle control (PBS) | 0 |
| 2 | 1 | Endotoxin | 0.01 |
| 3 | 1 | Endotoxin | 0.04 |
| 4 | 1 | Endotoxin | 0.21 |
| 5 | 1 | Endotoxin | 0.35 |
| 6 | 1 | Endotoxin | 0.51 |
Both eyes of each animal received 2 intravitreous injections at a volume of 50 μL/eye/dose. The second injection was administered ∼10 min after the first injection.
EU, endotoxin units; PBS, phosphate-buffered saline.
Test materials
Purified reference endotoxin (i.e., Lipopolysaccharides from Escherichia coli) was obtained from United States Pharmacopeia (reference standard lot H0K354) and reconstituted to a stock concentration of 2000 endotoxin units (EU)/mL using limulus amebocyte lysate (LAL) assay reagent grade water. Endotoxin stock was diluted to appropriate concentrations for dosing using phosphate-buffered saline from Sigma Aldrich. All dose preparations were apportioned on the day of use and stored in a refrigerator set to maintain 2°C to 8°C until released for dose administration. Dosing syringes were filled under a laminar flow hood. Within 6 h before dose administration, vehicle control and test article solutions were drawn through a 1-mL syringe with an 18-gauge needle. The needle was discarded after withdrawal and replaced with a sterile, 30-gauge ½ inch needle for ITV injection. Dose preparation concentrations were analyzed using a kinetic LAL assay with a 4 point standard curve (0.005 to 5.0 EU/mL). Reagent kits were obtained from Charles River Laboratories, and data were analyzed using WinKQCL™ software.
Dose administration
ITV injections were performed by a board-certified veterinary ophthalmologist (E.B.). Before the ITV injections, animals were anesthetized with intramuscular injections of midazolam (0.2 mg/kg), ketamine (5 mg/kg), and dexmedetomidine (0.025 mg/kg). A topical anesthetic (0.5% proparacaine) was instilled in each eye before the dose administration. A wire speculum was used to retract the eyelids. To minimize external ocular irritation, eye preparation was limited to a dose site-specific cleaning with dilute 1% povidone iodine solution (prepared with sterile saline and 5% povidone iodine) and rinsed with sterile saline before the dose administration.
Doses were administered by single ITV injection on day 1, given as 2 × 50 μL at least 10 min apart per eye for a total volume of 100 μL (Table 1). A 1-mL syringe and 30-gauge needle was used for each dose administration. Injections were administered in the inferior temporal region of each eye; injections were alternated such that the first and second injections were administered at approximately the 7 and 8 o'clock positions (right eye) and 4 and 5 o'clock positions (left eye), respectively. A topical antibiotic (Tobrex®) was instilled in each eye following dosing. For anti-inflammatory treatment, topical ocular ointment (neomycin–polymyxin B–dexamethasone; neo-poly-dex; S.A. Alcon-Couvreur N.V., Puurs, Belgium) was used as described in Results.
Medication regimen
Before sedation and at least 30 min before dosing on day 1, animals were administered an oral opioid (Tramadol®; 4 mg/kg). Following recovery from anesthesia, 5–7 h later and at least 16 h later, an intramuscular opioid (buprenorphine; 0.05 mg/kg) was administered; additional doses were administered as appropriate based on individual clinical observations (Table 2).
Table 2.
Palliative Care During the Study
| ITV dose level (EU/eye) | Anti-inflammatory treatment | Days |
|---|---|---|
| 0 | None | n/a |
| 0.01 | None | n/a |
| 0.04 | Neo-poly-dex | 2 thru 6 |
| 0.21 | NSAID | 2 thru 8 |
| Neo-poly-dex, atropine | 2 thru 9 | |
| 0.35 | NSAID | 2 thru 14 |
| Neo-poly-dex, atropine | 2 thru 9 | |
| 0.51 | NSAID | 2 thru 8 |
| Neo-poly-dex, atropine | 2 thru 9 |
Neo-poly-dex: neomycin-polymyxin B-dexamethasone.
NSAID: flunixin megulamine days 2–4; ibuprofen days 5–8 or 14.
On day 1 opioid analgesia was administered before and after dosing to all animals and as needed thereafter.
ITV, intravitreal; n/a, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
Ophthalmic procedures
Ophthalmic examinations (OEs) and intraocular pressure (IOP) measurements were performed predose and on days 1 (post-dose), 5, 8, and 15 (Table 1).
OEs were performed by a board-certified veterinary ophthalmologist (P.E.M.). The anterior segments of both eyes of each animal were examined first via hand-held slit-lamp biomicroscopy (on undilated eyes) followed by a table-mounted slit lamp (on dilated eyes) and indirect ophthalmoscopy. Slit-lamp evaluation included examination of the conjunctiva, cornea, anterior chamber, iris, pupillary reflex, lens, and anterior vitreous. Eyes were scored based on a modified Hackett–McDonald scoring system. The Standardization of Uveitis Nomenclature (SUN) Working Group Grading Scheme17 was used to score anterior chamber flare and cell with a table-mounted slit lamp with the field for evaluation of ∼1 mm diameter spot followed by a second hand-held slit-lamp biomicroscopy examination. Pupils were pharmacologically dilated with tropicamide before indirect ophthalmoscopy. IOP measurements were done in triplicate using a TonoVet rebound tonometer (Icare; Vantaa, Finland). On day 1, IOP was measured just before the first injection, immediately following the first injection, 10 min after the first injection (before the second injection), immediately following the second injection, and 10 min following the second injection. Only values with no or insignificant deviation (steady letter) were used.
Color fundus photography
Color fundus photography digital imaging was done predose, and on days 8 and 15. Animals were anesthetized and eyes were dilated with a mydriatic agent before photography. Photographs were taken of each eye and included stereoscopic photographs of the posterior pole and nonstereoscopic photographs of 2 midperipheral fields (temporal and nasal).
Optical coherence tomography
Spectral domain optical coherence tomography (OCT) was conducted predose, and on days 1, 2, 8, and 15 using a Heidelberg Spectralis® HRA+OCT. Animals were fasted and anesthetized, and eyes were dilated with a mydriatic agent. Eyes were imaged to obtain axial views of the retinal surface in the posterior fundus and the anterior chamber in a subset of animals on day 2. Macular volume scans centered on the fovea, circle scans around the optic nerve, and high-resolution single-line scans in the inferior field of view were acquired. Volume scans and horizontal high-resolution single-line raster scans were collected. Volume scans consisted of 25 horizontal lines, 240 μm apart, which generated a 20 × 20 degree cube of data. High-resolution line scans, which consisted of 40 to 100 averaged B-scans, were collected in the inferior retina, through the fovea and in other locations as needed to document the presence or absence of abnormalities. In addition, high-resolution retinal nerve fiber layer (RNFL) scans consisting of 80 to 100 averaged B-scans in a 12° circle ∼3.4 mm in diameter around the optic nerve were collected. Anterior segment scans were acquired using the Heidelberg Anterior Segment Module. Scans, both 8 and 11 mm in length, were collected using the Cornea and Sclera settings on a subset of animals on day 2. Macular scans and high-resolution single lines were qualitatively examined for the presence of abnormalities, including epiretinal membranes, macular holes or operculum, retinal layer disorganization or discontinuity, hyporeflective areas that would indicate cysts or edema, hyperreflective areas that would indicate increased cell density or changes in metabolic activity, and gross changes in the thickness of retinal layers. Anterior segment OCT scans were collected using the Heidelberg Anterior Segment lens on a subset of animals on day 2.
Image review and interpretation (color fundus photography and OCT) were performed by a vitreo-retinal specialist (T.M.N.).
Histopathology
On day 15 or 16 (Table 1), animals were euthanized, eyes were enucleated, the bulbar conjunctivae was marked for orientation, and the eyes were fixed in modified Davidson's fixative for 48 to 96 h, before transfer to 10% neutral-buffered formalin. The fixed eyes were trimmed into nasal, central, and temporal calottes, processed and embedded in paraffin, and collected sections were stained with hematoxylin and eosin for examination.
Results
Intraocular pressure
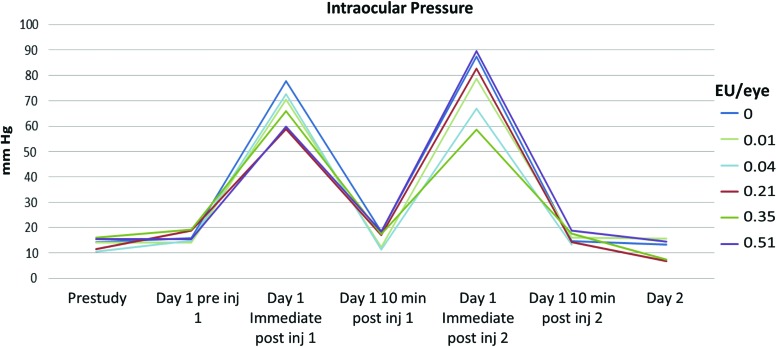
As expected, several fold IOP increase immediately following each ITV 50 μL injection was noted (Fig. 1). Such an increase in IOP was undoubtedly a function of the sudden increase in IOP induced by the injection of a fluid bolus into the vitreous cavity, but returned to baseline preinjection levels within 10 min. The transient pressure increases were not associated with adverse findings and were comparable among treatments. Abnormally low IOP (data not shown) was sporadically observed in animals with marked intraocular inflammation.
FIG. 1.
On day 1, IOP spiked immediately following the first injection, as expected due to bolus administration. Ten minutes after the first injection (before the second injection), IOP returned to pre-ITV injection baseline values. IOP spike was also noted following the second injection, as expected and 10 min afterward it returned to baseline values. IOP, intraocular pressure; ITV, intravitreal. Color images are available online.
Aqueous flare response
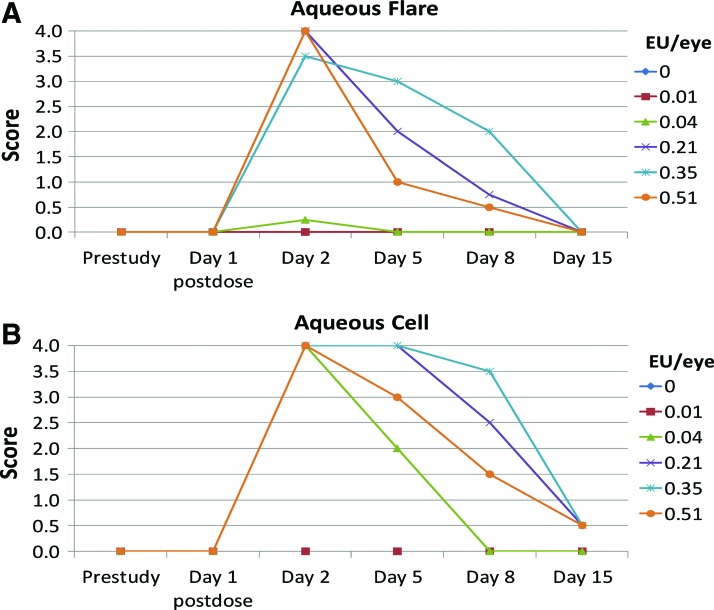
Slit-lamp evaluations revealed a dose-related increase in aqueous flare postdose at ≥0.04 EU/eye (Fig. 2A). This response peaked approximately on day 2 and reached an average of Grade 3–4+ in eyes given ≥0.2 EU/eye. Aqueous flare resolved by day 5 in eyes given 0.04 EU/eye, and by day, 15 in eyes given ≥0.2 EU/eye.
FIG. 2.
Following ITV endotoxin administration, a rapid, transient aqueous inflammatory response was observed. Dose-dependent aqueous flare was observed at ≥0.04 EU/eye (A; results expressed as individual eye measurements). This response peaked approximately on day 2 and reached an average of Grade 3–4+ in eyes given ≥0.2 EU/eye. Aqueous flare resolved by day 5 in a subset of animals given 0.04 EU/eye, and by day 15 in eyes given ≥0.2 EU/eye. Aqueous flare was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye. The aqueous cell response (B) followed a similar dose-related trend. Aqueous flare was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye. EU, endotoxin units. Color images are available online.
Aqueous flare was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye.
Aqueous cell response
Aqueous cell peaked on day 2 in eyes given ≥0.04 EU/eye (Fig. 2B), reaching grade 4+, the highest severity. While aqueous cell had resolved by day 8 in eyes given 0.04 EU/eye, trace levels were observed on day 15 in eyes given ≥0.21 EU/eye.
Aqueous flare was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye.
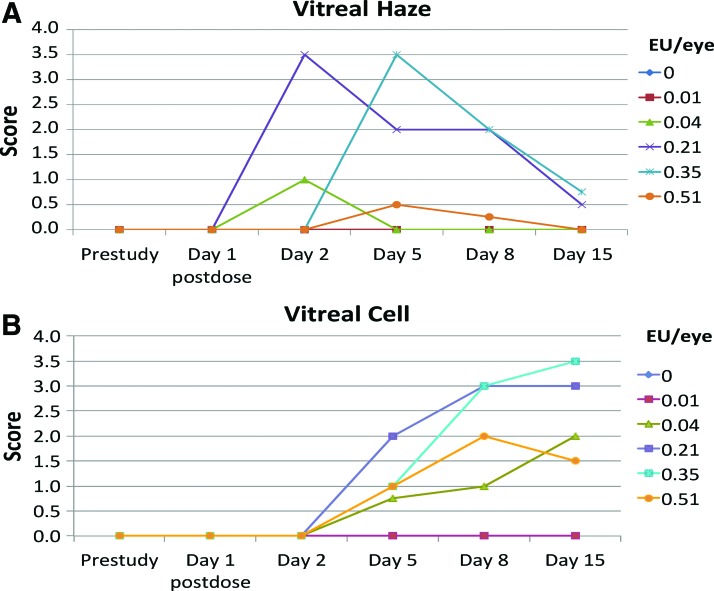
Vitreal haze response
Vitreous haze was observed at doses >0.01 EU/eye (Fig. 3A). In general, vitreous haze scores were dose-related, although impaired visualization of the fundus due to anterior segment inflammation resulted in a diminished ability to score vitreous haze in eyes administered 0.51 EU/eye.
FIG. 3.
The vitreal haze was observed at doses >0.01 EU/eye (A). In general vitreous haze scores were dose-related (A; results expressed as individual eye measurements). Vitreal cell response was first observed on day 2 at low levels and gradually increased in a roughly dose-associated manner through day 8 and then plateauing or increasing slightly after day 8 (B; results expressed as individual eye measurements). Vitreous haze or cell was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye. Color images are available online.
Vitreous haze was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye.
Vitreal cell response
The vitreal cell response was comparable to, but delayed, from that observed for aqueous cell (Fig. 3B). Vitreous cell was first observed on day 2 at low levels and gradually increased in a roughly dose-associated manner through day 8 and then plateauing or increasing slightly after day 8. As for vitreous haze, anterior segment inflammation in some eyes precluded accurately scoring vitreous cell at some intervals, especially in eyes administered 0.51 EU/eye.
Vitreous cell was not noted in vehicle-treated eyes or eyes given 0.01 EU/eye.
Other clinical responses
Endotoxin doses >0.01 EU/eye caused a marked response in 1 or more parameters, and for doses at 0.21 EU/eye and higher, these included conjunctival hyperemia, corneal edema, keratic precipitates (KP), fibrin clots in the anterior chamber, and incomplete dilation of the pupil following application of topical tropicamide. The view of the lens, vitreous, and fundus was substantially impaired when anterior segment scores were high. Other sporadic findings included reflux of blood to Schwalbe's line, white retinal perivascular sheathing, and swelling/hyperemia of the optic disc.
Anti-inflammatory treatment
The animal dosed at 0.05 EU/eye was treated with topical ocular ointment (neomycin–polymyxin B–dexamethasone; neo-poly-dex) from days 2 to 6. In addition to receiving neo-poly-dex and atropine from days 2 to 9 (treatment extended to day 15 for the animal dosed at 0.34 EU/eye), all animals dosed at ≥0.21 EU/eye were administered nonsteroidal anti-inflammatory drugs (NSAIDs) from days 2 to 8 or 14 (Table 2). These treatments were necessary for animal welfare reasons, to prevent pain and discomfort in the treated animals. However, administration of steroids and NSAIDs likely blunted the inflammatory response in these animals, contributing to the lack of a clear dose–response above 0.21 EU/eye.
Color fundus photography
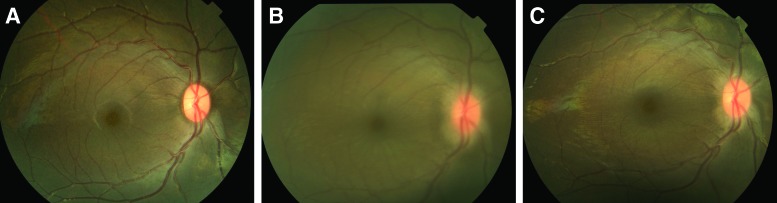
Moderately hazy media and mild optic nerve swelling were noted at doses >0.21 EU/eye on day 8 and resolved by day 15 (Fig. 4).
FIG. 4.
Representative color fundus images of the right eye of an animal that had an ITV injection of 0.35 EU/eye endotoxin are shown here. (A) Baseline; (B) Day 8; and (C) Day 15. Hazy media and optic nerve swelling are seen on day 8 (B), both of which had partially resolved on day 15 (C). Color images are available online.
Optical coherence tomography
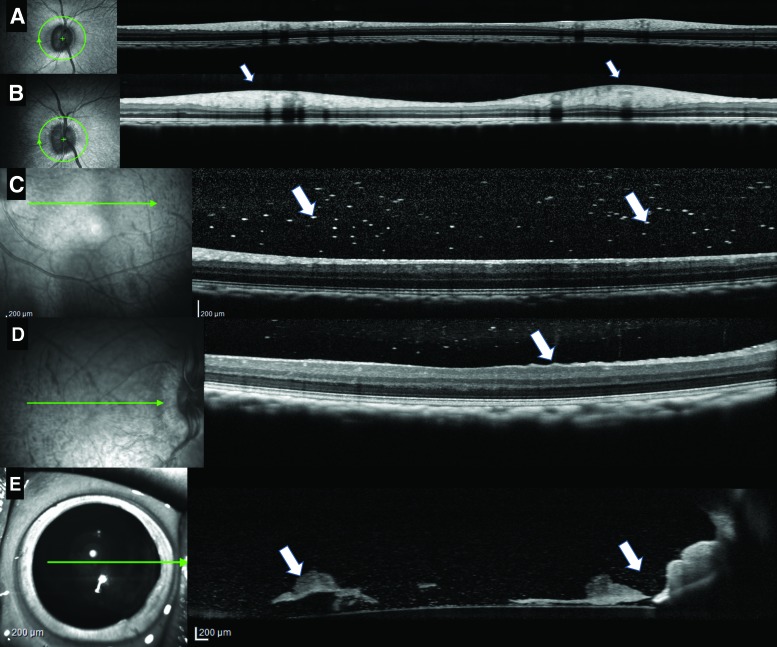
Endotoxin at ≥0.21 EU/eye resulted in RNFL thickening and reflective spots in the vitreous consistent with posterior segment inflammation. At 0.21 and 0.35 EU/eye, RNFL thickening peaked on day 8 (Fig. 5B) and improved by day 15 (data not shown). Reflective spots in the vitreous were noted on day 2 and 8 (Fig. 5C, arrows). Wrinkling of the inner limiting membrane (ILM) was also noted in the 0.21 EU/eye- and 0.35 EU/eye-treated animals on day 8 (Fig. 5D). Anterior segment scans collected on day 2 in the 0.21 EU/eye- and 0.35 EU/eye-treated animals showed an increase in reflective spots in the aqueous (Fig. 5E) consistent with the ophthalmic findings of cells and flare. What appeared to be KP were also noted in the aqueous and on the corneal endothelium (Fig. 5E). Clumps of reflective material that may have been fibrin was noted near the lens in the 0.35 EU/eye-treated animal (Fig. 5E, arrows). Endotoxin at the 0.01 EU/eye dose resulted in mild vitreal haze, inferiorly, at day 8 and 15 (data not shown), while the 0.04 EU/eye dose animal had mild vitreal haze on day 8 only (data not shown). Mild vitreal haze apparent by OCT on day 8 or 15 at a dose of 0.01 EU/eye (compare baseline, Fig. 5A) was not apparent upon clinical ophthalmic examination.
FIG. 5.
OCT images from animals given Endotoxin at 0.21 EU/eye and 0.35 EU/eye showed RNFL thickening (white arrows) compared to baseline (A, B), an increase in white hyper reflective spots in the vitreous (white arrows) (C), ILM wrinkling (white arrows) (D). Anterior segment scans on day 2 showed an increase in reflective spots and clumps (white arrows) that could represent cells and fibrin (E). All of these effects are consistent with an inflammatory response noted on clinical ophthalmic examination. ILM, inner limiting membrane; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer. Color images are available online.
Ocular pathology
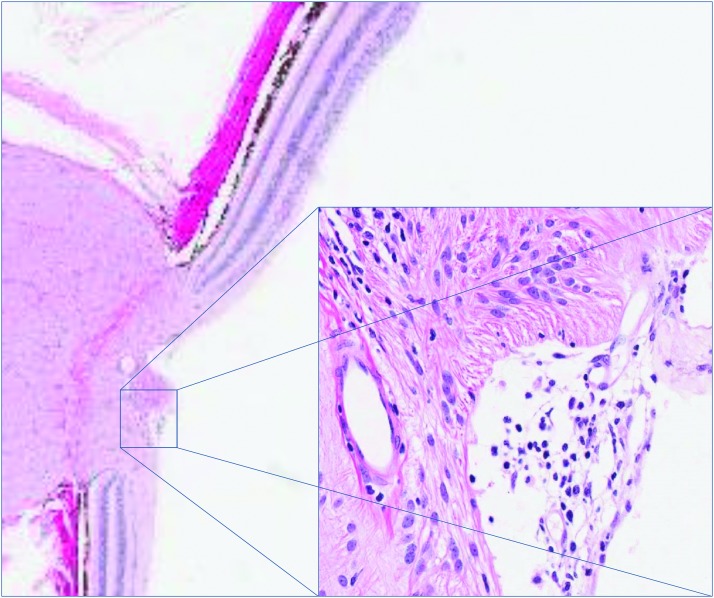
By termination of the study on day 15, consistent with the in-life findings at this time point, limited histopathologic changes were apparent. Microscopic findings of minimal mononuclear cell infiltrates in the vitreous at ≥0.21 EU/eye were noted, which occasionally extended into the optic disc (Fig. 6).
FIG. 6.
Microscopic findings of minimal mononuclear cell infiltrates in the vitreous at ≥0.21 EU/eye were observed on day 15. Infiltrates occasionally extended into the optic disc (inset). Color images are available online.
Discussion
Recently, we defined the response to ITV injection of a reference, purified endotoxin in rabbits, and demonstrated a rapid dose-related anterior chamber reaction, beginning with aqueous flare as early as 4 h postdose and aqueous cell within 48 h postdose.16 In the current study, anterior chamber dose-related response was also noted in monkeys following ITV at ≥0.04 EU/eye. The NOEL for cynomolgus monkeys was the same as in rabbits, 0.01 EU/eye, but the magnitude of response at ≥0.04 EU/eye suggests that dose–response curve in monkey eye is steeper to ITV purified reference endotoxin than the rabbit eye.
Palliative treatment, as indicated in Table 2, was necessary to complete this study. Based on the rabbit study, inflammation in the anterior and posterior segments and presumed ocular pain was anticipated. To alleviate it, a prophylactic protocol was developed specifically for this study. The use of several specific pain medications that in the past showed their effectiveness in preventing anticipated ocular pain was selected. The use of neomycin–polymyxin B–dexamethasone, NSAID (flunixin megulamine initially, followed by ibuprofen through day 8 as needed), was effective in this study to control unacceptable ocular pain and discomfort. It should be noted, however, that the use of these anti-inflammatory treatments likely blunted the inflammatory response in treated animals. This may have contributed to the lack of a clear dose–response at doses above 0.21 EU/eye and suggests that the responses reported in the current study are likely to underestimate the responses observed in untreated NHPs administered >0.21 EU/eye of endotoxin.
OCT effects, including hazy views, RNFL thickening, an increase in white reflective spots in the vitreous, and ILM wrinkling for animals administered ≥0.21 EU/eye were consistent with an inflammatory response seen in clinical OE. Anterior segment OCT scans showed an increase in hyper reflective spots in the aqueous, KP, and clumps of what may have been fibrin in the anterior chamber (Fig. 5). The effects noted on OCT were most pronounced on day 8 and showed some improvement on day 15. It cannot be determined from OCT scans whether RNFL thickening is due to axonal swelling, interstitial edema, or something else, but it is a fairly sensitive marker of inflammation. The default view of 1:1 pixels (as opposed to 1:1 microns) exaggerates the perceived swelling, making small changes in thickness more noticeable. The white reflective spots in the vitreous may have been cells, which would be consistent with an inflammatory response. It is normal to see some reflective spots in the vitreous in monkeys, but they are generally more varied in size than the spots noted in this study and often associated with syneresis. As the inflammation resolved, the effects noted on OCT diminished. The reflective spots, KP, and fibrin noted on the anterior segment scans were also consistent with an inflammatory response. Images taken with the cornea setting, 8 mm, were useful for visualizing KP on the corneal endothelium. They were not useful for estimates of, or grading presence of, cells since it is difficult to capture entire anterior segment images without mirror defects in the monkey eye. Images taken with the sclera setting, with enhanced depth imaging, made it possible to visualize the anterior chamber near the anterior surface of the lens, where fibrin and cells were seen (Fig. 5E). No signs of retinal toxicity were observed on the fundus color photographs of the vehicle control or the animals administered 0.01 and 0.04 EU/eye.
As expected fundus photography was less sensitive at detecting inflammation than slit-lamp biomicroscopy, indirect ophthalmoscopy, and OCT. On review of color fundus photographs, no signs of retinal toxicity were observed in animals administered vehicle control article, 0.01 EU/eye, or 0.04 EU/eye. (Note that animals administered 0.04 EU/eye exhibited an anterior segment inflammatory response that resolved by day 8 on slit-lamp biomicroscopy). Animals administered 0.21 EU/eye or 0.35 EU/eye showed a posterior segment inflammatory reaction on fundus photography (as they did on slit-lamp biomicroscopy and indirect ophthalmoscopy), with hazy media and mildly swollen optic nerves on day 8. These effects resolved by day 15 on fundus photographs, although intraocular inflammation persisted through day 15 on slit-lamp biomicroscopy and indirect ophthalmoscopy. The animal administered 0.51 EU/eye showed no adverse effects on fundus photography on day 8 or 15, although on slit-lamp biomicroscopy and indirect ophthalmoscopy marked inflammation was observed in this animal on day 2 and low levels of aqueous and vitreous cell persisted through day 15.
Overall, based on slit-lamp biomicroscopy, indirect ophthalmoscopy, IOP measurements, color fundus photographs, and OCT evaluations, 0.01 EU/eye is considered the NOEL in this monkey study. This dose level, however, did result in sporadic instances of inferior vitreous haze discernable only on OCT on days 8 or 15.
Microscopic findings correlated to clinical ophthalmic observations, fundus color photography, and OCT and included minimal mononuclear cell infiltrates in the vitreous of animals administered 0.21 or 0.35 EU/eye and mononuclear cell infiltrates observed in the right optic disc of the animal administered 0.21 EU/eye (Fig. 6). It should be noted, however, although histopathology must be included to investigate concurrent toxicities, degenerative changes and the bystander effects of inflammation in the eye, histopathologic examination occurs at a single time point, and even with extended sampling techniques, a very small sample of the globe is examined. The ability to assess the globe in its entirety and potential for longitudinal assessment provided by clinical OE is considered a more sensitive method of identifying and monitoring ocular inflammation.
It should be noted that the translatability of this finding to human is unknown at this time, especially since the exact endotoxin levels are unknown in reported suspected cases of sterile uveitis related to endotoxin contamination.13–15,18 It is also important to note that reference standard endotoxin utilized in this study, while suitable for analytical calibration standards of the bacterial endotoxin test and as a spike in recovery studies, bear minimal resemblance to the real-world endotoxin contamination that might be present during biotechnology/pharmaceutical manufacturing.19 Certainly, when using monkeys as a pharmacologically relevant test species to evaluate local ocular tolerability and distinguish contribution from test article, endotoxin levels of intravitreally administered test article should be kept at ≤0.04 EU/eye for both scientific and animal welfare reasons.
It is important to understand the response to ITV endotoxin from a clinical perspective, that is, endotoxin-induced ocular toxic syndrome20 and know what level of ITV endotoxin is tolerated in the preclinical settings and NHP to better safeguard human safety with adequate endotoxin specifications for ITV products. Characterization of this response would allow for a more informed risk assessment of novel ITV formulations. Importantly, such data would also allow the discrimination of drug-related effects from endotoxin-related effects in nonclinical safety studies for biologic therapies, where nonspecific inflammation to a humanized protein is often observed.
In conclusion, we have defined the dose–response of ITV endotoxin in anterior and posterior segments of cynomolgus monkeys. A NOEL of 0.01 EU/eye was observed, suggesting that the vitreal cavity is at least 2-fold more sensitive to endotoxin administration than the anterior chamber.5–7 Importantly, these levels are well below the levels reported in a survey of endotoxin levels of OVDs,21 and thus may be clinically relevant. As in a rabbit eye,16 the study reported here demonstrates an inflammatory response following ITV injection of significantly lower levels of endotoxin in the monkey eye compared to endotoxin levels of OVDs. As mentioned previously,16 currently there are no regulatory guidances for the acceptable limit level for ITV endotoxin. Overall, when using cynomolgus monkeys as a relevant species to evaluate ocular tolerability following ITV administration and distinguish contribution from therapeutic candidate, ITV endotoxin levels of administered test articles should be kept at ≤0.01 EU/eye for both scientific and animal welfare reasons.
Acknowledgments
Dr. T.M.N. received support from Research to Prevent Blindness, the McPherson Eye Research Institute's Retina Research Foundation Kathryn and Latimer Murfee Chair and the National Institutes of Health (NIH) P30 EY016665.
Author Disclosure Statement
V.B., Genentech, Inc. (E); P.E.M., None; T.M.N., None; C.R., None; A.M., Genentech, Inc. (E); B.J.C., Covance Laboratories, Inc. (E); H.B., Genentech, Inc. (E); E.A.T., Ra Pharmaceuticals, Inc. (E).
References
- 1. Karth P.A., and Blumenkranz M.S. Update on intravitreal injection techniques. Rev. Ophthalmol. 21:81–84, 2014 [Google Scholar]
- 2. Bodnar Z., Clouser S., and Mamalis N. Toxic anterior segment syndrome: update on the most common causes. J. Cataract. Refract Surg. 38:1902–1910, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Kutty P.K., Forster T.S., Wood-Koob C., et al. . Multistate out-break of toxic anterior segment syndrome, 2005. J. Cataract. Refract. Surg. 34:585–590, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Leder H.A., Goodkin M., Buchen S.Y., Calogero D., Hilmantel G., Hitchins V.M., and Eydelman M.B. An investigation of enzymatic detergents as a potential cause of toxic anterior segment syndrome. Ophthalmology. 119:e30–e35, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Sakimoto A., Sawa M., Oshida T., Sugaya S., Hirono T., and Ishimori A. Minimum endotoxin concentration causing inflammation in the anterior segment of rabbit eyes. Jpn. J. Ophthalmol. 53:425–432, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Buchen S.Y., Calogero D., Hilmantel G., and Eydelman M.B. Rabbit ocular reactivity to bacterial endotoxin contained in aqueous solution and ophthalmic viscosurgical devices. Ophthalmology. 119:e4–e10, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Nussenblatt R.B., Calogero D., Buchen S.Y., Leder H.A., Goodkin M., and Eydelman M.B. Rabbit intraocular reactivity to endotoxin measured by slit-lamp biomicroscopy and laser flare photometry. Ophthalmology. 119:e19–e23, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Forrester J.V., Klaska I.P., Yu T., and Kuffova L. Uveitis in mouse and man. Int. Rev. Immunol. 32:76–96, 2013 [DOI] [PubMed] [Google Scholar]
- 9. Csukas S., Paterson C.A., Brown K., and Bhattacherjee P. Time course of rabbit ocular inflammatory response and mediator release after intravitreal endotoxin. Invest. Ophthalmol. Vis. Sci. 31:382–387, 1990 [PubMed] [Google Scholar]
- 10. Fleisher L.N., Ferrell J.B., Olson N.C., and McGanan M.C. Dimethylthiourea inhibits the inflammatory response to intravitreally-injected endotoxin. Exp. Eye Res. 48:561–567, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Fleisher L.N., Ferrell J.B., and McGanan M.C. Ocular inflammatory effects of intravitreally injected tumor necrosis factor-alpha and endotoxin. Inflammation. 14:325–335, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Allen J.B., McGahan M.C., Ferrell J.B., Adler K.B., and Fleisher L.N. Nitric oxide synthase inhibitors exert differential time-dependent effects on LPS-induced uveitis. Exp. Eye Res. 62:21–28, 1996 [DOI] [PubMed] [Google Scholar]
- 13. McGahan M.C., Grimes A.M., and Fleisher L.N. Hemoglobin exacerbates the ocular inflammatory response to endotoxin. Graefe's Arch. Clin. Exp. Ophthalmol. 234:643–647, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Gilger B.C., Abarca E.M., Salmon J.H., and Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest. Ophthalmol. Vis. Sci. 54:2483–2492, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Denlinger J.L., El-Mofty A.A.M., and Balazs E.A. Replacement of the liquid vitreous with sodium hyaluronate in monkeys. II. Long-term evaluation. Exp. Eye Res. 30:101–117, 1980 [DOI] [PubMed] [Google Scholar]
- 16. Bantseev V., Miller P.E., Bentley E., Schuetz C., Streit T.M., Christian B.J., Farman C., Booler H., and Thackaberry E.A. Determination of a no-observable effect level for endotoxin following a single intravitreal administration to dutch belted rabbits. Invest. Ophthalmol. Vis. Sci. 58:1545–1552, 2017 [DOI] [PubMed] [Google Scholar]
- 17. Jabs D.A., Nussenblatt R.B., Rosenbaum J.T; and Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. J. Ophthalmol. 140:509–516, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki T., Ohashi Y., Oshika T., Goto H., Hirakata A., Fukushita K., and Miyata K. Outbreak of late-onset toxic anterior segment syndrome after implantation of one-piece intraocular lenses. Am. J. Ophthalmol. 159:934–939, 2015 [DOI] [PubMed] [Google Scholar]
- 19. Tirumalai R. Naturally occurring endotoxin: a new reference material proposed by the US Pharmacoeia. Am. Pharma Review 2016. (July/August Supplement) www.americanpharmaceuticalreview.com/Featured-Articles/190860-Naturally-Occurring-Endotoxin-A-New-Reference-Material-Proposed-By-the-US-Pharmacopeia/ Accessed October16, 2018
- 20. Wang F., Yu S., Liu K., Chen F., Song Z., Zhang X., Xu X., and Sun X. Acute intraocular inflammation caused by endotoxin after intravitreal injection of counterfeit bevacizumab in Shanghai, China. Ophthalmology. 120:355–361, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Dick H.B., Augustin A.J., Pakula T., and Pfeiffer N. Endotoxins in ophthalmic viscosurgical devices. Eur. J. Ophthalmol. 13:176–184, 2003 [DOI] [PubMed] [Google Scholar]