Abstract
Interleukin-12 (IL-12), which is composed of a p35 and a p40 subunit, is a proinflammatory natural-killer (NK) cell-stimulating, Th1-inducing and Th1-maintaining cytokine, which promotes cell-mediated immunity. On activation, heterodimeric IL-12 is found in small amounts, whereas free p40 is produced in excess. Besides IL-12, other p40-dependent molecules exist that orchestrate Th1 responses. Homodimeric p40 can act as an IL-12 antagonist by competing for its receptor. Recent data also reveal potential immunostimulatory functions of p40. In addition, p40 can be covalently linked to a p35-related protein p19. This heterodimer is known as IL-23 and has activities on memory T cells. Finally, IL-27, the latest addition to this family, is a heterodimer composed of the p40-related protein EBI3 (Epstein–Barr virus-induced gene 3) and the p35-related protein p28. IL-27 is involved in early Th1 initiation.
With the discovery of interleukin-12 (IL-12) as a heterodimeric cytokine composed of a p35 and a p40 subunit, free p40 (mostly monomeric and homodimeric) was also found and characterized. About 10 years later a novel p40p19 heterodimeric molecule (IL-23) was described, and this was shortly after followed by a p40-related molecule dimerized with a p28 protein (IL-27). These novel molecules not only establish the IL-12 family but also help to reveal distinct cellular and functional stages of Th1 development.
1. IL-12 – the prototypic Th1-inducing and Th1-maintaining molecule
IL-12 (also termed IL-12p75 or IL-12p70 but commonly designated IL-12) is an immunoregulatory cytokine that promotes cell-mediated immunity 1, 2. IL-12 stimulates production of interferon-γ (IFNγ) from T cells and natural-killer (NK) cells. Studies in IL-12-deficient mice have demonstrated an essential role for IL-12 in the induction of Th1 responses, which are especially required for protection against intracellular microorganisms 3, 4. IL-12 is not only involved in induction but also in maintenance of Th1 responses 5, 6. Among the cytokine family, IL-12 has an atypical heterodimeric structure. It is composed of a p35 and a p40 subunit (Table 1), each of which is expressed by its own gene and on different chromosomes. The p35 gene has homology with other class I cytokines, especially IL-6 and granulocyte-colony stimulating factor (GCSF) [7]. Interestingly, the p40 subunit is homologous to the extracellular domain of the hemopoietic cytokine receptor family, in particular to the extracellular domains of the IL-6 receptor α-chain (IL-6Rα) and the ciliary neurotrophic factor receptor (CNTFR) [8]. Coexpression of both chains of IL-12 in one cell is required to generate bioactive IL-12 [9]. Although p35 transcripts are found in many cell types, free p35 is not secreted [10]. Production of IL-12 by activated macrophages and dendritic cells (DCs) results in secretion of a 10–1000-fold excess of free monomeric and homodimeric p40 relative to heterodimeric IL-12 [10]. The activities of IL-12 are mediated by a high-affinity receptor composed of two subunits, designated β1 and β2 [1] (Table 1). Both β1 and β2 are members of the class I cytokine receptor family and are most closely related to glycoprotein gp130 and the receptors for leukemia-inhibitory factor and GCSF. Both receptor subunits can be expressed on NK and T cells and are required for IL-12 bioactivity 1, 11. The β2 subunit acts as signal transducer by providing a cytoplasmic STAT4 (signal transducer and activator of transcription 4) binding site, which enables STAT4-mediated responses to IL-12 to occur 12, 13. Studies of β1−/− and β2−/− mice confirmed the essential role for both subunits in mediating the biological functions of IL-12 on NK and T cells 14, 15.
Table 1.
Members of the IL-12 family, their receptors and their functiona
| Cytokine | IL-12 | p40 | (p40)2 | IL-23 | IL-27 |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Cytokine receptor | |||||
| Binding chain | IL-12Rβ1 | IL-12Rβ1 | IL-12Rβ1 | IL-12Rβ1 | WSX-1b |
| Signaling chain | IL-12Rβ2 | ? | ? | IL-23R | ? |
| Function | Th1 activation+Th1 maintenance | ? | Th1 inhibition+Th1 activation | Th1 activation | Early Th1 initiation |
| DTH, granuloma formation, macrophage recruitment | T-cell memory | ||||
Abbreviations: DTH, delayed-type hypersensitivity; IL-12, interleukin-12; (p40)2, homodimeric p40.
WSX-1 is identical to TCCR.
2. p40 – a Th1 regulator with two faces: not only an in vitro and in vivo antagonist of IL-12 but also a potential agonist
In vitro, the soluble p40 subunit of IL-12 is able to antagonize IL-12 bioactivity [16]. In vivo, about a third of the free p40 in the serum of endotoxin-challenged mice is present in the homodimeric form, the remainder consists of monomeric p40 [17]. For both murine and human homodimeric p40, (p40)2 is able to act as antagonist of IL-12 by binding to the β1 subunit of the IL-12 receptor 18, 19. Pretreatment of mice with homodimeric p40 was able to reduce serum IFNγ levels and protect mice from lipopolysaccharide (LPS)-induced death 17, 20. The antagonistic potential of (p40)2 was further confirmed by reduced Th1 responses of transgenic mice in which the p40 gene is regulated by a liver-specific promoter [21]. However, because of its low affinity to the human IL-12R, human (p40)2 has only a minor ability to antagonize IL-12 functions compared to murine (p40)2 [22]. The antagonistic potential of (p40)2 might, therefore, represent a phenomenon specific for the mouse but could be different in humans. Murine homodimeric p40 is 25–50-fold more potent as an IL-12 antagonist than monomeric p40, as shown in both binding assays and bioassays [18]. In physiological situations the effect of IL-12 appears to be dominant despite the excess of p40. However, reducing the excess of p40 (without changing the levels of IL-12p75) by generating N-glycosylation mutants of p40 led to enhanced cytotoxic T-lymphocyte (CTL) responses and to better protection against tumor challenge [23]. This suggests that excessive levels of p40 physiologically dampen IL-12-dependent bioactivity as a result of its antagonistic properties.
The first evidence of an agonistic activity of homodimeric p40 was shown in vitro. Induction of CD8+ Th1 differentiation in a mixed lymphocyte culture was enhanced in the presence of (p40)2 [24]. Moreover, the same group subsequently showed in vivo that endogenous p40 produced by allografted IL-12p35−/− mice is able to stimulate alloreactive CD8+ Th1 development [25]. By contrast, IL-12p40−/− mice, which are unable to produce p40, generated less IFNγ than IL-12p35−/− mice [25]. Treatment of allografted IL-12p35−/− mice with a monoclonal anti-IL-12p40 antibody was able to abrogate this IFNγ production [25]. Therefore, IL-12p40 is, in addition to its antagonistic activity, able to act in vitro and in vivo as an agonist similar to IL-12. However, in light of the recently discovered p40-composed heterodimer p40p19 (designated IL-23) it cannot be ruled out that heterodimeric IL-23 was also neutralized by the anti-IL-12p40 monoclonal antibody.
The p40-dependent agonistic function was further confirmed by comparing p40-producing IL-12p35−/− with IL-12p40−/− mice in models of infectious diseases. IL-12p35−/− mice, which are able to produce endogenous p40, cleared Mycobacterium bovis BCG (bacillus Calmette–Guérin) and showed reduced susceptibility to pulmonary Mycobacterium tuberculosis infection 26, 27. By contrast, IL-12p40−/− or IL-12p35/40−/− mice (unable to produce p40) were highly susceptible in both models of infection [26]. Treatment of infected IL-12p35/40−/− mice with homodimeric p40 restored the observed defect in antigen-specific delayed-type hypersensitivity (DTH) responses [26]. Thus, endogenous and exogenous p40 induces protective immunity in mycobacterial infection. The reconstitution experiments could not exclude the possibility that p40 is able to associate with extracellular p19 to form bioactive IL-23. However, because p19 is not secreted on its own (as is the case for p35) 10, 28, it is rather unlikely that extracellular monomeric p40 is able to form bioactive IL-23.
In addition, in a paramyxoviral bronchitis model, p40-dependent (monomeric and homodimeric p40) epithelial macrophage accumulation and increased mortality was observed [29]. Interestingly, the results from this virally induced murine model of airway inflammation could be extended to patients with asthma, in which airway levels of predominantly homodimeric p40 and airway macrophages were elevated relative to normal subjects [29]. This suggests that the agonistic effect of (p40)2 as opposed to its antagonistic property is also significant in humans. Together, excessive levels of p40 associated with macrophage accumulation in Sendai-virus infected IL-12p35−/− mice and in patients with asthma strongly argue for an agonistic potential of homodimeric p40, which can even result in immunopathological responses. Recently, homodimeric p40 was found to be a chemoattractant for macrophages in vitro and in vivo, supporting the discussed effects during bronchitis and asthma [30]. Also, p40-dependent pulmonary fibrosis and macrophage infiltration was observed in a murine model of silica-induced lung fibrosis [31]. Administration of recombinant (p40)2 to silica-treated mice resulted in transient lung fibrosis and macrophage influx to the lung [31]. Taken together, endogenous and exogenous p40 are able to induce lung macrophage accumulation and fibrosis.
In vitro, a direct activating effect of homodimeric p40 on macrophages was further supported by Pahan et al. [32], who showed that inducible nitric oxide (iNOS) expression and NFκB activation could be induced by (p40)2 in mouse primary microglia and peritoneal macrophages but not in mouse primary astrocytes. Interestingly, even the monomeric p40 was found to stimulate NO production provided the monocytic cells were activated by IFNγ [32].
Homodimeric p40 binds to IL-12Rβ1 but not to IL-12Rβ2 [33]. Similar to IL-12, murine (p40)2 binds with both high and low affinity to IL-12Rβ1 on Concanavalin A blasts and B cells [34]. The binding of (p40)2 to IL-12Rβ1 (thereby preventing IL-12 binding) provides a molecular mechanism for the antagonistic effect of (p40)2 on IL-12. Moreover, it is conceivable that IL-12Rβ1 is also responsible for the agonistic effect of (p40)2. IL-12Rβ1 and IL-12Rβ2 associate with different Janus kinases and therefore might contribute to distinct signaling pathways: the cytoplasmic domain of IL-12Rβ1 associates with tyrosine kinase 2, and the cytoplasmic domain of IL-12Rβ2 interacts with Janus kinase 2 [35]. Hence, IL-12Rβ1 might be capable of transducing IL-12p40 signals through tyrosine kinase 2. In addition, a yet to be discovered novel receptor subunit might associate with IL-12Rβ1 for (p40)2-mediated agonistic activities. A hitherto unidentified third component associating with the IL-12Rβ1 subunit was recently reported [36].
3. IL-23 – the late actor in the Th1 program: a p40–p19 heterodimer with activity on memory T cells
Searching sequence databases with a computationally derived profile of IL-6 subfamily structures, a novel protein p19 was identified that is able to build a disulfide-bridged complex with the p40 subunit originally described for IL-12 [28]. This novel heterodimeric molecule was designated IL-23. The p19 protein is, similar to the p35 protein, biologically inactive by itself [28]. IL-23 requires interaction with IL-12Rβ1 [28] and an additional, novel β2-like receptor subunit designated IL-23R with a cytoplasmic STAT4 binding domain [37]. Similar to IL-12, coexpression of p19 and p40 in the same cell appears to be required for secretion of IL-23 [28]. The p40–p19 complex is secreted by activated murine and human DCs [28]. In the mouse, the biological activities of IL-23 are distinct from IL-12. Murine IL-23 was not found to induce significant amounts of IFNγ. Murine IL-23 induces strong proliferation of memory T cells but not of naı̈ve T cells, whereas IL-12 has no effect on memory T cells [28]. In addition, recently it was shown that IL-23 (but not IL-12) can activate murine memory T cells for production of the proinflammatory cytokine IL-17 [38]. In humans, IL-23 has biological activities that are less distinct from IL-12 (proliferation of memory T cells, modest IFN-γ production by naı̈ve and memory T cells compared to IL-12) [28]. Transgenic expression of p19 leads to multi-organ inflammation, runting, infertility and premature death [39]. The p19-transgenic mouse shows infiltrates of lymphocytes and macrophages in organs, elevated serum tumor necrosis factor-α (TNF-α) and IL-1 levels, increased numbers of circulating neutrophils and constitutive expression of acute phase proteins in the liver [39]. In the light of these data it is possible that the p40-dependent but p35-independent defect in granuloma formation and DTH responses observed in several infectious disease models might in fact be due to a lack of IL-23 40, 41. Indeed, reconstitution of Cryptococcus neoformans-infected IL-12p40−/− mice with recombinant monomeric or homodimeric IL-12p40 failed to protect these mice from a fatal course [40]. Mice with keratinocyte-specific transgenic expression of p40 developed an inflammatory skin disease phenotype [42] reminiscent of the p19-transgenic mice [39]. Interestingly, the inflammatory response following transgenic p40 expression could not be mimicked by injection of homodimeric p40 into the skin of littermate mice [42]. This argues for a molecule different from homodimeric p40, for example, IL-23, responsible for the observed eczematous skin disease. Studies in recently generated p19-deficient mice might clarify these questions.
4. IL-27 (EBI3–p28) – the early riser in Th1 responses, which was found latest: a p40 relative involved in Th1 initiation
Recently, a further member of the IL-12 family was described and termed IL-27 [43]. IL-27 is a heterodimeric protein that consists of Epstein–Barr virus (EBV)-induced gene 3 (EBI3), a p40-related protein, and p28, a newly discovered IL-12p35-related polypeptide. IL-27 appears to be produced early by activated antigen-presenting cells. It is able to induce clonal proliferation of naı̈ve but not memory CD4+ T cells and synergizes with IL-12 in IFNγ production by naı̈ve CD4+ T cells [43]. Recently, an orphan receptor was described with 26% homology and 37% similarity to the IL-12Rβ2 subunit and to gp130, designated TCCR [44] or WSX-1 [45]. This receptor is essential for early initiation of Th1 responses but neither binds IL-12 nor associates with the IL-12R subunits 43, 44. Instead, this receptor was identified as one of the receptor subunits for IL-27 and as necessary but not sufficient for IL-27 function [43]. Interestingly, whereas activation of TCCR or WSX1 is required for the early initiation of a Th1 response, it is not necessary for the maintenance of Th1 responses 5, 6, 45. It is possible that IL-27 and IL-12 function sequentially in initiating and maintaining Th1 responses, respectively 43, 45. Such a view is supported by high TCCR mRNA expression on undifferentiated Th cells but low expression on differentiated Th1 and Th2 cells enabling IL-27 to activate Th0 cells [44]. However, on naı̈ve Th cells IL-12Rβ2 is upregulated on antigen activation and during IL-12-driven Th1 development 46, 47. This suggests that IL-27 can act before IL-12.
EBI3 was initially identified in B lymphocytes infected with EBV [48]. EBI3 is related to p40 showing 27% homology at the amino-acid sequence level [48]. Importantly, EBI3 is expressed in monocytes and macrophages, as is IL-12. Thus, pokeweed-mitogen activated peripheral-blood mononuclear cells express EBI3 [48]. Human monocytes activated for IL-12 production also induced EBI3 gene transcription, although with slower and more prolonged kinetics than for IL-12 [2]. Interestingly, EBI3 is expressed throughout human pregnancy by cells of fetal origin [49] and some of the EBI3 expressed in placental trophoblasts was found to be associated with the p35 subunit of IL-12 [50]. Expression of EBI3 in EBV-infected cells as well as during pregnancy led to the suggestion that free EBI3 and/or EBI3–p35 might counter-regulate type 1 responses [48] similar to the way by which (p40)2 was found to antagonize the immunostimulatory action of IL-12. However, the function of EBI3 as a potential downregulator of IL-27 action remains speculative. Recently, it was shown in EBI3−/− mice that EBI3 or an EBI3-dependent homo- or hetero-dimeric factor is essential for growth and differentiation of IL-4 producing invariant NKT cells [51]. EBI3−/− mice exhibited a reduced number of invariant NKT cells, a sustained decrease in IL-4 production and were resistant to Th2-mediated immunopathology associated with oxazolone-induced colitis [51]. Interestingly, IFN-γ production was only transiently decreased in EBI3−/− mice and EBI3-deficient mice were as susceptible as wild-type mice in a Th1-mediated colitis model induced by trinitrobenzene sulfonic acid [51]. These data suggest that an EBI3-dependent factor different from IL-27 is involved in IL-4-mediated Th2 responses (Fig. 1).
Fig. 1.

Regulation of Th-cell differentiation by activating and inhibitory members of the IL-12 family. IL-12, IL-23 and IL-27 are involved in differentiation and activation of Th1 cells at different stages of Th1 development. Homodimeric p40 can act both in Th1 activation as well as in Th1 inhibition. EBI3x is able to directly drive Th2 responses by supporting the growth and activation of invariant NKT cells, which produce IL-4 [51]. This expands the IL-12 family members beyond exclusively Th1 regulating factors. Additonal mechanisms beyond Th1/Th2 regulation (such as IL-23-induced IL-17 production by memory T cells) are operative [38]. Abbreviations: EBI3, Epstein–Barr virus (EBV)-induced gene 3; IL-12, interleukin-12; NK, natural killer; x, as yet uncharacterized binding partner of EBI3.
5. Role of the IL-12 family members in infectious diseases
IL-12 has a key role in protection against intracellular protozoan, fungal and bacterial infections 4, 5, 40, 52, 53 (Table 2). The role of IL-12 in protection and pathology during viral infections depends on the type of virus 54, 55, 56, 57. Interestingly, for several viruses (shown in Table 2) protective type-1 immunity was completely IL-12-independent and also independent of other members of the IL-12 family, pointing to other Th1-inducing factors for immunity against these viruses. This confirms data from patients with mutations in the IL-12p40 or the IL-12Rβ1 gene who responded normally to standard viral immunizations but developed chronic courses of salmonellosis or mycobacteriosis [58]. In some intracellular fungal, bacterial and some viral infections other p40-dependent and p40-related proteins (different from IL-12), that is, (p40)2, IL-23 and IL-27 (and potentially other as yet unknown members of the IL-12 family), are able to contribute to type-1 responses (Table 2). It is of special interest that many of these infections (especially mycobacterial infections) tend to develop a chronic course by a pathogen with low virulence. Moreover, it is noteworthy that for salmonellosis other p40-dependent proteins different from IL-12 only have a detectable role at low infective doses (Table 2) [41]. Thus, the function of members of the IL-12 family appears to depend on the type of pathogen as well as on the dose of the pathogen. Low-dose infections can be controlled independently of IL-12 but depend on other members of the IL-12 family in some experimental murine models. By contrast, high-dose infections require IL-12 for Th1 induction. This hypothesis could be based on a dose- and pathogen-specific regulation of synthesis of p40, p35, p19, EBI3 and p28. In addition, there might be a characteristic time course for synthesis of p40, p35, p19, EBI3 and p28 during infection [43].
Table 2.
Role of endogenous IL-12 versus other p40-dependent proteins in type-1 response induction in different murine infection modelsa
| Infection model |
Type-1 response induction |
Refs | |
|---|---|---|---|
| IL-12-dependentb | Dependent on other p40 cytokinesc | ||
| Leishmania major | + | − | [4] |
| Trypanosoma cruzi | + | − | [63] |
| Trypanosoma brucei brucei | + | − | F. Brombacher et al., unpublished |
| Cryptococcus neoformans | + | + | [40] |
| Salmonella Enteritidis | |||
| 50 cfu | − | + | [41] |
| 500 cfu | − | + | |
| 5000 cfu | − | + | |
| >10 000 cfu | + | − | |
| Francisella tularensis | − | + | [64] |
| Mycobacterium tuberculosis, Mycobacterium bovis BCG | + | + | 26, 27 |
| Listeria monocytogenes | +/−d | + | [65], F. Brombacher et al., unpublished |
| Sendai virus | − | + | [29] |
| Murine cytomegalovirus (MCMV) | − | + | [55] |
| Vesicular stomatitis virus (VSV), lymphochoriomeningitis virus (LCMV) | − | − | [56], H. Pircher et al., unpublished |
| Herpes virus (pseudorabies, HSV-1, bovine herpes virus 1 and 5) and corona virus (murine hepatitis virus) | − | − | [54], M. Suter et al., unpublished |
Abbreviations: BCG, bacillus Calmette–Guérin; cfu, colony forming units; HSV-1, herpes simplex virus-1; IL-12, interleukin-12; NK, natural killer.
Endogenous IL-12 essential.
Endogenous p40-dependent proteins different from IL-12 involved.
Endogenous IL-12 only required at high infective doses for NK-cell activation.
Interestingly, microbial components appear to induce primarily p40 and relatively low amounts of IL-12, whereas subsequent cross-linking of CD40 on antigen-activated DCs by CD40L-expressing T cells results in amplification of IL-12 production by increasing p35 expression 59, 60. More recently, IFNβ was shown to differentially affect CD40L or IFNγ-induced induction of the IL-12 family members (inhibition of p35 and p40, enhancement of p19 and EBI3 transcription) in DCs resulting in inhibition of IL-12 production [61].
Multiple activating and inhibitory members of an emerging cytokine family contribute at different stages to induction and inhibition of type-1 cellular immunity (Fig. 2). According to currently available data the receptor expressed on the target cell has a crucial role. Expression of the receptor for IL-27 on naı̈ve Th cells enables the initiation of Th1 responses 43, 44, subsequent upregulation of IL-12Rβ2 [46] enables IL-12 to become active in induction and maintenance of effector Th1 responses 5, 6. However, activation of Th1 memory cells appears to be restricted to IL-23 [28]. Moreover, the IL-1-related cytokine IL-18 will synergize with IL-12 as soon as its receptor is induced by IL-12 [62]. The orchestration of Th development ultimately results in regulated effector mechanisms, such as IFNγ-dependent macrophage activation, granuloma formation and DTH responses.
Fig. 2.
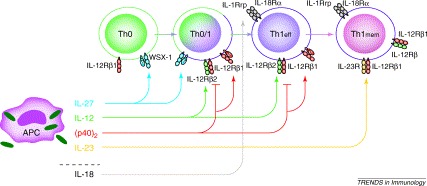
Current working hypothesis for the regulation of Th1 development by IL-12 family members and by IL-18. On infection or pathogen uptake (green symbols in APC), APCs secrete cytokines, which act on Th cells at different stages of their development. IL-27 appears to activate Th0 and Th0/1 cells, which express WSX-1 (identical with TCCR). After induction of IL-12Rβ2 expression IL-12 is able to drive further differentiation and maintenance of Th1eff cells. Homodimeric p40 is able to antagonize IL-12 at its receptor or to contribute to Th1 activation by interacting with IL-12Rβ1 and possibly a putative second receptor component. IL-23 is able to activate Th1mem cells. There is synergy between IL-27 and IL-12, as well as between IL-12 and IL-18. IL-12 induces the receptor for IL-18 on Th cells [66]. The receptors for IL-12 and IL-23 are also expressed on APCs or APC subpopulations (not shown in this figure). Thus, regulation of APC activity might be modulated, as well as T-cell development, by IL-12 and IL-23. Abbreviations: APC, antigen-presenting cell; IL-12, interleukin-12; R, receptor; Th0, naı̈ve T cells; Th0/1, differentiating naı̈ve T cells; Th1eff, effector T; Th1mem, memory Th1.
Acknowledgements
We are grateful to Uwe Müller for helping with preparation of the figures. We thank the members of our laboratories for their contributions to this work. This work was supported by a research contract from Hoffmann–La Roche Ltd, Basel (Switzerland) and grants to G.A. from the Deutsche Forschungsgemeinschaft (AL 371/3–1, AL 371/3–3). F.B. is the holder of a Wellcome Trust Research Senior Fellowship for Medical Science in South Africa (Grant 056708/Z/99) and supported by NRF and MRC South Africa. DNAX is supported by Schering-Plough.
References
- 1.Gately M.K. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 3.Magram J. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 4.Mattner F. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2-cell response. Eur. J. Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 5.Park A.Y. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 2000;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- 6.Yap G. Cutting edge: IL-12 is required for the maintenance of IFNγ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 7.Merberg D.M. Sequence similarity between NKSF and the IL-6/G-CSF family. Immunol. Today. 1992;13:77–78. doi: 10.1016/0167-5699(92)90140-3. [DOI] [PubMed] [Google Scholar]
- 8.Gearing D.P., Cosman D. Homology of the p40 subunit of natural-killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin-6 receptor. Cell. 1991;66:9–10. doi: 10.1016/0092-8674(91)90131-h. [DOI] [PubMed] [Google Scholar]
- 9.Gubler U. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Andrea A. Production of natural-killer cell stimulatory factor (interleukin 12) by peripheral-blood mononuclear cells. J. Exp. Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Presky D.H. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan M.H. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 13.Thierfelder W.E. Requirement for Stat4 in interleukin-12-mediated responses of natural-killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 14.Wu C. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)-deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J. Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- 15.Wu C. IL-12 receptor β2 (IL-12Rβ2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 16.Mattner F. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur. J. Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel F.P. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 18.Gillessen S. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 19.Ling P. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 20.Mattner F. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor α-dependent shock. Infect. Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto T. Reduced T helper 1 responses in IL-12 p40 transgenic mice. J. Immunol. 1998;160:588–594. [PubMed] [Google Scholar]
- 22.Germann T. The IL-12 p40 homodimer as a specific antagonist of the IL-12 heterodimer. Immunol. Today. 1995;16:500–501. doi: 10.1016/0167-5699(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 23.Ha S.J. Engineering N-glycosylation mutations in IL-12 enhances sustained cytotoxic T-lymphocyte responses for DNA immunization. Nat. Biotechnol. 2002;20:381–386. doi: 10.1038/nbt0402-381. [DOI] [PubMed] [Google Scholar]
- 24.Piccotti J.R. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4+ and CD8+ IFNγ-producing cells. J. Immunol. 1997;158:643–648. [PubMed] [Google Scholar]
- 25.Piccotti J.R. Alloantigen-reactive Th1 development in IL-12-deficient mice. J. Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 26.Hölscher C. A protective and agonistic funciton of IL-12p40 in mycobacterial infection. J. Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 27.Cooper A.M. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 28.Oppmann B. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Walter M.J. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J. Exp. Med. 2001;193:339–351. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha S.J. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J. Immunol. 1999;163:2902–2908. [PubMed] [Google Scholar]
- 31.Huaux F. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol. 2002;169:2653–2661. doi: 10.4049/jimmunol.169.5.2653. [DOI] [PubMed] [Google Scholar]
- 32.Pahan K. Induction of nitric-oxide synthase and activation of NFκB by interleukin-12 p40 in microglial cells. J. Biol. Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Presky D.H. Evidence for multiple sites of interaction between IL-12 and its receptor. Ann. New York Acad. Sci. 1996;795:390–393. doi: 10.1111/j.1749-6632.1996.tb52702.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang X. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur. J. Immunol. 1999;29:2007–2013. doi: 10.1002/(SICI)1521-4141(199906)29:06<2007::AID-IMMU2007>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Zou J. Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits β1 and β2 and JAK kinases. J. Biol. Chem. 1997;272:6073–6077. doi: 10.1074/jbc.272.9.6073. [DOI] [PubMed] [Google Scholar]
- 36.Kawashima T. Interleukin-12 induces tyrosine phosphorylation of an 85-kDa protein associated with the interleukin-12 receptor β1 subunit. Cell. Immunol. 1998;186:39–44. doi: 10.1006/cimm.1998.1294. [DOI] [PubMed] [Google Scholar]
- 37.Parham C. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal S. Interleukin-23 promotes a distinct CD4 T-cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 39.Wiekowski M.T. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility and premature death. J. Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 40.Decken K. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann J. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella Enteritidis. J. Immunol. 2001;167:5304–5315. doi: 10.4049/jimmunol.167.9.5304. [DOI] [PubMed] [Google Scholar]
- 42.Kopp T. Inflammatory skin disease in K14/p40 transgenic mice: evidence for interleukin-12-like activities of p40. J. Invest. Dermatol. 2001;117:618–626. doi: 10.1046/j.1523-1747.2001.01441.x. [DOI] [PubMed] [Google Scholar]
- 43.Pflanz S. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naı̈ve CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida H. WSX1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 46.Szabo S.J. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogge L. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devergne O. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J. Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devergne O. Expression of Epstein–Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am. J. Pathol. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devergne O. Epstein–Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieuwenhuis E.E. Disruption of T helper 2-immune responses in Epstein–Barr virus-induced gene 3-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastroeni P. Effect of interleukin 12 neutralization on host resistance and γ interferon production in mouse typhoid. Infect. Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper A.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schijns V.E. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J. Immunol. 1998;160:3958–3964. [PubMed] [Google Scholar]
- 55.Carr J.A. The role of endogenous interleukin-12 in resistance to murine cytomegalovirus (MCMV) infection and a novel action for endogenous IL- 12 p40. J. Interferon Cytokine Res. 1999;19:1145–1152. doi: 10.1089/107999099313082. [DOI] [PubMed] [Google Scholar]
- 56.Oxenius A. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- 57.van Den B.M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFNγ and nitric oxide synthetase 2. J. Immunol. 2000;164:371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 58.Ottenhoff T.H. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today. 1998;19:491–494. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 59.Reis e Sousa C. Conditioning of dendritic cells by pathogen-derived stimuli. Immunobiology. 2001;204:595–597. doi: 10.1078/0171-2985-00097. [DOI] [PubMed] [Google Scholar]
- 60.Schulz O. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 61.van Seventer J.M. Interferon-β differentially regulates expression of the IL-12 family members p35, p40, p19 and EBI3 in activated human dendritic cells. J. Neuroimmunol. 2002;133:60–71. doi: 10.1016/s0165-5728(02)00362-4. [DOI] [PubMed] [Google Scholar]
- 62.Afkarian M. T-bet is a STAT1-induced regulator of IL-12R expression in naı̈ve CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 63.Müller U. IL-12-independent IFNγ production by T cells in experimental Chagas’ disease is mediated by IL-18. J. Immunol. 2001;167:3346–3353. doi: 10.4049/jimmunol.167.6.3346. [DOI] [PubMed] [Google Scholar]
- 64.Elkins K.L. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 2002;70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brombacher F. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 66.Takeda K. Defective NK-cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]


