Abstract
The unexpected discovery of a novel family of antiviral mediators, type III IFNs or IFN-λs, challenged the widely accepted primacy of type I IFNs in antiviral immunity; and it is now well recognized that the IFN-λ-based antiviral system plays a major role in antiviral protection of epithelial barriers. The recent characterization of previously unknown IFN-λ-mediated activities has prompted further reassessment of the role of type I IFNs in innate and adaptive immune and inflammatory responses. Since type I and type III IFNs are co-produced in response to a variety of stimuli, it is likely that many physiological processes are simultaneously and coordinately regulated by these cytokines in pathological conditions, and likely at steady state as baseline expression of both IFN types is maintained by microbiota. In this review, we discuss emerging differences in the production and signaling of type I and type III IFNs, and summarize results of recent studies describing the involvement of type III IFNs in anti-bacterial and anti-fungal, as well as antiviral, defenses.
Keywords: interferon, epithelial barrier, antiviral protection, microbial infection, neutrophils, immune response
1. Introduction
Innate antiviral defenses in mammals, amphibians, reptiles and birds rely on the action of two types of interferons (IFNs), type I and type III IFNs. The first members of the family of proteins which are now known as type I IFNs, were discovered in 1957 [1], and initially appreciated for their ability to protect cells against viral infections. With time, additional activities of these proteins were observed, including their anti-tumor actions and regulation of adaptive host immune responses. Although type III IFNs were discovered decades later, they were also initially characterized as innate antiviral mediators [2, 3] with expression profiles, signaling pathways and gene expression programs resembling those of type I IFNs. Similar to type I IFNs, additional activities of type III IFNs are being discovered, and several recent reviews provide detailed evaluation of the diverse functions of type III IFNs [4–13]. This article is intended to review recent publications about type III IFNs, focusing on their role in resistance to non-viral, as well as viral, infections.
The discovery of type III IFNs in 2003 [2, 3] has led to a major rethinking of innate immune responses. Prior to that time it was appreciated that virus infection or exposure to viral components triggered the synthesis of type I IFNs (IFN-α/β), that play a critical role in antiviral defenses, but the existence of an additional type I IFN-independent antiviral system was unsuspected. The initial discovery of type III IFNs, or IFN-λ, was based on an analysis of sequence data that revealed the existence of a novel receptor subunit, the IFN-λ receptor 1 (IFNLR1). IFNLR1, paired with the IL-10R2 subunit (Fig. 1), was shown to bind and transmit signaling of a novel IFN family, named IFN-λs [2, 3]. Three closely related human cytokines, IFN-λ1, IFN-λ2 and IFN-λ3, were first identified, followed by the discovery of the more distantly related IFN-λ4 [14]. Mice possess only two functional type III IFNs, IFN-λ2 and IFN-λ3 [15]. All four members of the IFN-λ family utilize the same receptor complex for signaling [2, 3, 16], whereas all type I IFNs signal through a receptor complex composed of the IFNAR1 and IFNAR2 subunits (Fig. 1). Initial studies demonstrated that despite the engagement of distinct receptor complexes, activation of either the type I or type III IFN signaling pathway led to the formation of the heterotrimeric transcription factor ISGF3 (IFN-stimulated gene factor 3), and up-regulation of the same set of IFN-stimulated genes (ISGs) which mediate the antiviral response (Fig. 1). The realization that there are two independent antiviral mechanisms has led many groups to ask whether the IFN-α/β and IFN-λ systems have distinct as well as overlapping functions. These characterizations are far from complete, but a picture is emerging whereby the type III IFNs are essential for protecting mucosal surfaces from a variety of insults.
Fig. 1. Division of labor between type I and type III IFNs in antiviral response.
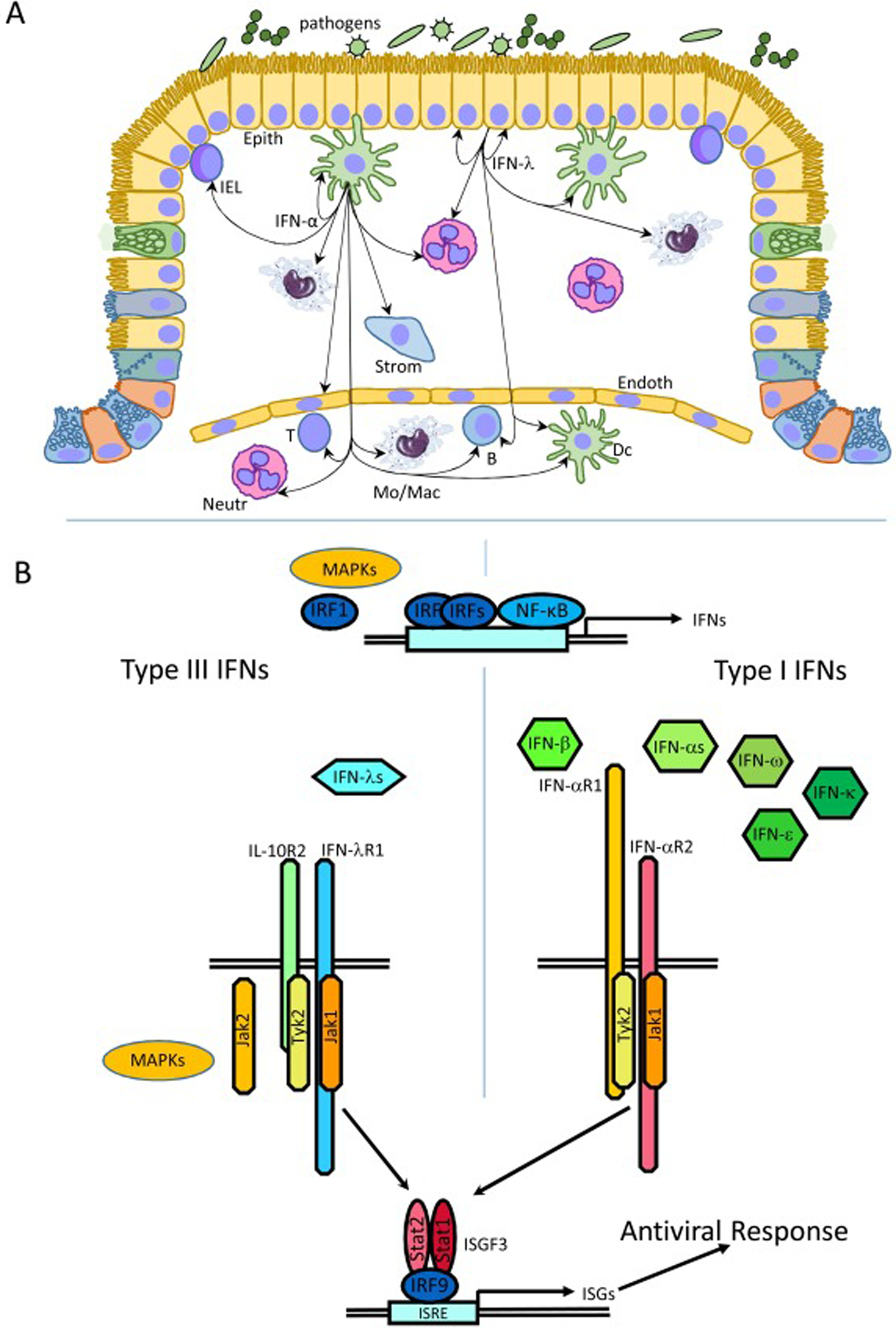
A. Epithelial (Epith) cells are the main source of type III IFNs. Macrophage (Mac), monocytes (Mo) and dendritic cells (DC) can also produce type III IFNs, but primarily produce IFN-αs. IFN-λs act on epithelial cells and tissue-residing neutrophils (Neut), dendritic cells and macrophages, and B cells and plasmacytoid DCs in blood. Most cell types in tissue and in blood including T, B, NK, dendritic, stromal and endothelial cells, Intraepithelial lymphocytes (IEL), macrophages and neutrophils, but not mucosa-lining epithelial cells respond to type I IFNs. B. IRF3, IRF7 and NF-κB are the key transcription factors regulating IFN expression. IRF1 and MAPK signaling has a stronger regulatory effects on type III IFN then on type I IFN production. All type I and all type III IFNs engage distinct IFN-type-specific receptor complexes, but signal through the JAK-STAT pathway resulting in the activation of the same ISGF3 transcription complex and expression of ISGs many of which encode proteins with antiviral functions. Some activities of type III IFNs are also regulated through MAPK signaling and JAK2 involvement.
2. IFN Induction and Signaling
Distinct functions of the two IFN types are well documented in the gastrointestinal (GI) tract, where type III IFNs act almost exclusively on epithelial cells, and immune cells in the lamina propria respond only to type I IFNs [17–22]. Endothelial and stromal cells in the GI tract are also sensitive to type I, but not type III, IFNs. This differential sensitivity of various cell types to IFN-λs is regulated by the levels of IFNLR1 expression. The basis for the nonresponsiveness of intestinal epithelial cells to type I IFNs in vivo is not well understood. Intestinal epithelial cells express lower levels of IFNAR1 and IFNAR2 transcripts than the immune cells within the lamina propria [21], and IFNAR1 expression may be also regulated post-transcriptionally [23]. These and other mechanisms may govern sensitivity of epithelial cells to type I IFNs. Of note, the vast majority of cell lines of epithelial origin, as well as primary cells grown ex vivo, including enteroid and organoid cultures of intestinal cells, are fully responsive to type I IFNs [24–26]. Only within tissues is this differential sensitivity of intestinal epithelial cells to type I IFNs evident, complicating the characterization of the regulatory mechanism(s). Whether IFNs act in compartmentalized manner in other organs remains to be investigated.
The replication strategies and tropism of viruses differ widely, and viruses employ diverse mechanisms to interfere with pathways leading to IFN production by the host cells. Accordingly, the patterns of type I and type III IFN induction differ substantially depending on the type of the virus or even viral strain [22, 27–30]. In general, the same stimuli associated with virus infection, or pathogen-mediated damage to the host, trigger production of type I and type III IFNs through the engagement and activation of pattern recognition receptors (PRRs) and/or pathogen-associated or damage-associated molecular patterns (PAMPs and DAMPs). In this setting epithelial cells predominantly produce type III IFNs [21, 22, 25, 26, 31–37]. Subcellular localization of PRRs, such as TLRs or signaling molecules downstream of these sensors, affects the balance of type I and type III IFN expression levels [38, 39]. Polarized epithelial cells at mucosal surfaces appear to have more peroxisomes [38], as well as cell surface expression of TLRs [40], factors that favor production of type III IFNs over type I IFNs [38, 39]. Epithelial cell-produced IFN-λs then act on virus-infected or neighboring epithelial cells to suppress virus replication and prevent viral spread through the epithelium. The IFN-λ-based antiviral system can therefore be considered an autonomous mucosal defense system, producing ligands which then act on the cells forming the epithelial barrier (Fig. 1). This mechanism ensures the activation of a localized antiviral protection without triggering a more damaging systemic response. Only when the barrier is breached is there a threat of systemic infection that requires a systemic, primarily type I IFN-mediated, antiviral response for containment. Type I IFNs, particularly IFN-αs, are produced primarily by immune cells, such as plasmacytoid dendritic cells, and act on submucosal stromal cells to inhibit viral spread within tissues. Type I IFNs also act on endothelial cells and peripheral blood mononuclear cells to suppress bloodborne spread of the infection.
Within the IFNLR complex, IFNLR1 serves as a high-affinity receptor subunit for IFN-λs [41, 42]. IL-10R2 has a relatively low affinity for ligands but is required to assemble a functional IFNLR complex. There is extensive interaction between the IFNLR subunits, a phenomenon that has not been observed for IFNAR subunits [42, 43]. In addition, binding of IFNLR1 and IL-10R2 to IFN-λ3 leaves a large surface area of IFN-λ3 unoccupied, whereas little surface area of IFN-ω remains exposed in the ternary IFN-ω/IFNAR1/IFNAR2 complex [42, 43].
For either type I or type III IFNs, ligand-guided interaction and conformational changes within the receptor subunits trigger activation of receptor-bound JAK1 and TYK2 kinases. These kinases phosphorylate STAT1 and STAT2, which then heterodimerize, and together with IRF9 form the ISGF3 transcription complex (Fig. 1). Activation of the signaling machinery is thought to employ many of the same interactions, but differences in signal transduction by the IFNAR and the IFNLR complexes have been observed. Although TYK2 was shown to be associated with IL-10R2 [44, 45] and positively regulate IFN-λ-mediated ISG expression [46], TYK2 appears to be dispensable for many IFN-λ-mediated activities [47, 48] despite reduced levels of cell surface IL-10R2 expression on TYK2-deficient cells [47]. This contrasts with the marked reduction of type I IFN-mediated activities in TYK2-deficient cells [47, 48]. Partial suppression of IFN-λ activity by JAK2 inhibitors has been reported [38, 49, 50], but STAT activation in JAK2-deficient cells expressing a chimeric IL-10R1/IFNLR1 chain was not affected by JAK2 deficiency [51].
Distinct features of type I and type III IFN receptor complexes may account for the observed differences in signal transduction by these receptors. It is possible that the extensive IFNLR1 and IL-10R2 interactions required for stable ligand binding make the IFNLR complex less dependent on TYK2, and permit self-activation of IFNLR1-associated JAK1 kinase following ligand binding. IL-10R2 may also have a moderate affinity for JAK2, allowing JAK2 or other JAK kinases to substitute for TYK2 in cells lacking TYK2. The large surface area of IFN-λ that remains unoccupied after IFNLR1 and IL-10R2 binding opens the possibility of an additional interacting partner(s), such as a third receptor subunit, that could introduce another kinase into the signaling complex. This scenario provides a possible mechanism that would allow JAK2 activation by IFN-λ and unaltered activity of this cytokine in TYK2-deficient cells. In addition to JAK-STAT signaling, the mitogen-activated protein kinase (MAPK) pathway is also activated by both IFN types, although antiviral activity of type III IFNs is more sensitive to the pharmacological inhibition of MAPK [25]. Production of type I and type III IFNs is also differentially affected by MAPK inhibitors [38].
Overall, substantial variations in type I and type III IFN production and signaling have been described in both in vitro and in vivo studies. While the many inconsistencies have yet to be explained, recent work in animal models of infection suggests that in vivo compartmentalization of IFN production and receptor binding may play an important role in the innate response to multiple infections. In addition to characterizing the biochemistry of IFN signaling, it seems likely that determining which cells within a given tissue respond to which IFN(s) will be important for understanding the pathogenesis of specific infections. Once the anatomy of IFN responsiveness is well characterized, it may be possible to discover how the differentiation or activation state of a given cell type dictates the availability of signaling components.
3. IFN-λ in antiviral responses
While both type I and type III IFNs have been shown to be important for host protection from respiratory and intestinal virus infection, they have also been shown to have distinct activities. There is clear evidence that, although production of these cytokines is triggered by the same stimuli in vitro, their in vivo production varies with respect to pathogen and source. In both reovirus and rotavirus infections of the GI tract, epithelial cells were the major source of IFN-λ [21, 22], and IFN-λ, rather than IFN-α/β, was the predominant antiviral cytokine induced by rotavirus infection. Like IFN synthesis, IFN responsiveness in vivo was not predicted from in vitro studies. While all cell lines tested are IFN-α/β responsive, mouse models of enterovirus infection have demonstrated that the intestinal epithelium is protected only by IFN-λ after the neonatal period. The basis of this specificity is not understood, but a role for cell polarization has been suggested [52]. Despite this compartmentalization of IFN-α/β and IFN-λ responsiveness, both cytokines are important for host resistance with IFN-λ limiting virus replication in intestinal epithelial cells and IFN-α/β acting on multiple cell types to prevent virus spread beyond the epithelial surface.
IFN-λ is the predominant cytokine induced by influenza A virus infection of the respiratory tract, but the presence of either the IFNAR or IFNLR is sufficient to limit virus production in this model (Jewell 2010, and Mordstein 2010). Virus titers are significantly higher in animals lacking STAT1, STAT2 or both IFNAR1 and IFNLR1 showing that compartmentalized IFN responsiveness is less pronounced in the pulmonary tree. However, the IFN-λ antiviral system appears to reduce virus spread between animals, suggesting that replication of influenza A virus in upper respiratory tract is primarily controlled by IFN-λ [53].
Zika is an emerging flavivirus, transmitted by mosquitos or by sexual contact, which can cause birth defects or fetal demise following infection of pregnant women [54]. There is some evidence that IFN-λ may contribute to preventing vertical and ascending Zika virus infection of the female reproductive tract in mouse models. Initial studies in mice required deletion or blockade of IFNAR1 for virus replication and transmission to the fetus following subcutaneous or intravaginal infection. Fetal and placental virus titers were increased after subcutaneous maternal challenge if female mice and pups were also IFNLR1 deficient [55]. Given the limitations of this model, it has been difficult to determine if there is a significant role for IFN-λ in this infection. Zika virus has been found to antagonize human, but not murine, STAT2 and therefore both type I and type III IFN signaling [56]. Nonetheless, progesterone-treated, anti-IFNAR1 antibody-treated Ifnlr1−/− mice showed enhanced susceptibility to ascending Zika infection from the mucosal surface [57].
The studies described above demonstrate the importance of the type III IFNs for resistance to virus infection, primarily by induction of the antiviral state at the mucosal surface, but there is also evidence that this cytokine is important in innate immune responses to non-viral pathogens. While the effects of IFN-λ on the epithelial compartment is now clearly established, its role in innate immune cell function has yet to be fully characterized. In human PBMCs, only dendritic cells, macrophages and B cells have been shown to respond directly to IFN-λ [13, 58]. However, despite the non-responsiveness of mouse PBMCs to type III IFNs [13], recent publications now demonstrate an essential role for this cytokine in innate responses to bacterial and fungal pathogens which require innate immune cell activation for clearance. Although our understanding of IFN-λ function in non-viral infections is far from complete, these new observations indicate a more nuanced role for type I and type III IFNs than has previously been appreciated.
4. IFN-λ and bacterial infection
Within several years of the discovery of type III IFNs as antiviral cytokines, activation of this pathway by bacteria infection was also observed. IFN-λ induction by Salmonella [59], Listeria monocytogenes [38, 60, 61], Staphylococcus epidermidis [60], S. aureus [62], Enterococcus faecalis [60], Pseudomonas aeruginosa [62], Mycobacterium tuberculosis [60, 63], Borrelia burgdorferi [64], Klebsiella pneumoniae [65] and Cryptosporidium parvum [66] has now been documented. In some cases, the signaling components have been characterized. B. burgdorferi RNA induces type III IFN production by PBMCs via TLR7 and IRF7 [64]. IFN-λ induction by Salmonella and Shigella requires both MyD88 and TRIF [39], as well as p38 MAPK, PI3K and NF-κB pathways in the case of Salmonella [59]. While these studies have been done using cell lines or primary cells studied ex vivo, there is clinical data showing that IFN-λ levels correlate with active, but not latent, M. tuberculosis infection [63].
Recent studies also highlight the impact of type III IFN signaling on bacterial pathogenesis. P. aeruginosa, S. aureus and K. pneumoniae have been examined in models of acute respiratory infection using IFNLR-deficient mice. In all of these infections, type III IFN appears to contribute to pathogenesis; in the absence of the pathway there is improved bacterial clearance and decreased pulmonary pathology [62, 65, 67]. In the case of K. pneumoniae, bacterial clearance was improved 4 days post infection, while dissemination to the spleen was decreased within 24 hours of infection [65]. Ifnlr1−/− mice exhibit improved bacterial clearance and survival against S. aureus [62, 67].
Also of interest is the effect of IFN-λ on the enhanced susceptibility to secondary bacterial pneumonia which occurs following influenza virus infection. This is a major clinical problem associated with significant morbidity and mortality [68], and is of interest here given the robust induction of type III IFNs by this infection [69]. Secondary infection with S. aureus after influenza infection led to significant increases in bacterial burden, which was ameliorated in the absence of type III IFN signaling [70] or further impaired by IFN-λ overexpression [71]. While in vivo co-infection with influenza did not influence P. aeruginosa infection [62], studies in vitro with another virus, RSV, had profound effects. In concert with airway epithelial cells, RSV caused P. aeruginosa to increase its capacity to form biofilms. This was shown to be dependent on IFN-λ using purified protein and thought to be due to dysregulated iron homeostasis [72]. Likewise, bacterial colonization of mouse nares is also influenced by influenza infection. Influenza virus infection was found to enhance nasal colonization with S. aureus in an IFN-λ dependent manner [70].
There are some clues emerging on how IFN-λ may be influencing these phenotypes. One possible mechanism to explain IFN-λ effects on bacterial pathogenesis is its impact on production of IL-1β. In Ifnlr1−/− mice, IL-1β production was significantly reduced in response to S. aureus [62, 67], while IL-1β treatment of these mice impaired clearance [67]. While the mechanism by which this occurs is not yet completely understood, Ifnlr1−/− mice exhibited reductions in capase-1 and neutrophil elastase, two proteases involved in cleaving IL-1β to its active form [67]. In the context of S. aureus-influenza co-infection two observations have been made. In secondary lung infection it was noted that neutrophil recruitment was perturbed as a result of type III IFN signaling, in addition to reduced phagocytosis of S. aureus by neutrophils [71]. In a nasal colonization co-infection model, the nasal microbiome was restructured in a type III IFN dependent manner post-influenza and along with this change there were differences in the proteome associated with cytoskeleton changes, inferring a potential breakdown of barrier function [70]. The theme of barrier dysfunction is also observed in the context of K. pneumoniae infection. IFN-λ was observed to increase airway epithelial cell permeability, which was presumed to contribute to dissemination of the bacterium from the airway to the spleen [65]. These in vivo observations are in contrast to results obtained in vitro where IFN-λ strengthened the barrier function of the epithelial monolayer as assessed by the reduction of Salmonella transmigration [39] and the abrogation of Cryptosporidium-induced loss of paracellular permeability [66]. These discrepancies likely reflect the differences between in vitro and in vivo models. Whereas the polarized monolayer of epithelial cells is relatively static, the epithelial barrier in vivo is a highly dynamic system which undergoes continuous renewal involving processes that may be affected by IFN-λ.
5. Regulation of antifungal immunity by IFN-λ
Neutrophils are essential for defense against infection by extracellular pathogens, especially fungi. Neutropenia renders patients susceptible to invasive fungal infections, a susceptibility that can also be modeled in mice [73–75]. Invasive aspergillosis (IA) is one of the primary causes of lethal fungal infection in susceptible patients, and currently available antifungal drugs are often unable to prevent mortality from IA [76]. Our studies have shown that type III IFNs are crucial regulators of innate antifungal immunity via direct effects on neutrophil function [77]. Upon infection with Aspergillus fumigatus type I IFN is rapidly produced by CCR2+ monocytes. This early production of IFN-α/β promotes optimal induction of the type III IFNs which are required to activate the antifungal activity of pulmonary neutrophils [77]. We found that mice lacking IFNLR1 expression by hematopoietic cells were unable to control fungal infection and succumbed to invasive aspergillosis [77]. Moreover, neutrophil-specific deletion of Ifnlr1 or Stat1 rendered mice unable to contain fungal infection. In this setting, IFN-λ was required as a potent activator of reactive oxygen species (ROS) generation by neutrophils, an essential effector function for the direct elimination of Aspergillus spores [77]. Importantly, we demonstrated that the susceptibility to IA seen in CCR2-depleted mice, was linked to impaired production of type I and III IFNs and that proper antifungal defense could be restored by exogenous administration of these cytokines.
6. IFN-λ and neutrophil function
Although neutrophils are clearly important innate effectors of pathogen elimination, recent studies present a more nuanced view of these cells which can also act as regulators of inflammation. Various studies support the notion that neutrophils facilitate the function of other immune cells, including macrophages, NK cells, B cells and T cells [78–83]. The ability of neutrophils to shape the activity of these diverse cell types is largely due to their ability to rapidly infiltrate tissues and provide cytokines and chemokines that further amplify an inflammatory cascade. As our understanding of the diversity of neutrophil functions has expanded, so has our appreciation of the importance of pathways that can regulate neutrophil function. In this context, IFN-λ has emerged as an unexpected and potent regulator of neutrophil function that can act both as activator and suppressor of neutrophil responses.
In contrast to the robust activation of antifungal neutrophils, studies carried out in other models of infection and inflammation have uncovered a suppressor role for IFN-λ. The suppressive effects of IFN-λ on neutrophil function have been reported to affect recruitment, differential gene expression and inhibition of reactive oxygen species formation. In the context of a murine model of autoimmunity, exogenous treatment with IFN-λ was protective and lessened both recruitment of neutrophils to inflamed joints and IL-1β production [84]. Importantly, neutrophils were found to express the IFNLR1 and respond directly to IFN-λ treatment ex vivo [84]. Therefore, in some contexts, IFN-λs reduce neutrophil responses by limiting neutrophil recruitment.
Additional regulatory effects of type III IFN appear to be mediated via direct modulation of neutrophil transcriptional responses. Among immune cells, neutrophils have emerged as uniquely sensitive to the direct effect of type III IFN stimulation due to constitutive expression of IFNLR1 [50, 77, 84]. In a model of influenza infection, removal of IFNLR1 on neutrophils or epithelial cells both resulted in diminished control of viral infection, suggesting a novel involvement of neutrophils in the control of viral infection [37]. Intriguingly, although neutrophils are equally sensitive to stimulation by type I or type III IFNs, a transcriptional analysis of neutrophils treated ex vivo showed stronger activation of pro-inflammatory cytokines upon treatment with IFN-α as compared to treatment with IFN-λ [37]. In this data set, induction of antiviral genes was comparable. The authors thus suggested a protective role for IFN-λ via activation of antiviral responses in neutrophils while limiting detrimental hyper-inflammation that can lead to neutrophil-mediated tissue damage [37], an effect which may contribute to the post-influenza susceptibility to bacterial infection described above.
Additional suppressive effects of IFN-λ have been reported to occur independent of transcription or translation. As discussed above, activated neutrophils produce ROS as an important effector mechanism that helps eradicate fungi and other extracellular pathogens. ROS can also be the culprits in neutrophil-mediated tissue damage. Ex vivo treatment of TNF or LPS-activated neutrophils with IFN-λ resulted in diminished ROS generation [50]. This anti-ROS effect of IFN-λ on neutrophils was found to mediate important protective effects in a model of DSS-induced intestinal inflammation [50]. A protective in vivo role in limiting neutrophil-mediated tissue damage was shown using a combination of bone marrow chimeric mice and neutrophil-specific IFNLR1-deficient mice [50]. Intriguingly the protective effect of IFN-λ was found to act via JAK2 downstream of the IL-10R2 chain suggesting that the distinct features of the IFN-λ receptor confers unique functions to these cytokines not shared with type I IFNs In aggregate, studies thus far suggest that IFN-λ can affect neutrophil function at multiple levels which include: effects on recruitment, transcription, ROS generation and phagocytosis.
7. Current questions and challenges in IFN-λ biology
Initial in vitro studies of type III IFNs following their discovery in 2003 suggested that, despite their limited homology and distinct receptors, type I and type III IFNs were likely to be functionally redundant. Although distinct roles for these cytokine families have yet to be fully explored, it is clear from the growing number of in vivo studies that IFN-λ has a unique role in protecting and maintaining the mucosal barrier. In the case of viral infection of the GI tract, this IFN is the primary mediator of the epithelial anti-viral response, while it also promotes clearance of fungal pathogens and down regulation of innate immune mediators that may damage mucosal integrity. While different experimental models have sometimes yielded conflicting results, continuing exploration of receptor expression, type III IFN induction by specific pathogens, and direct and indirect effects of IFN-λ on immune effector cells will allow for a more nuanced understanding of innate immune mechanisms at the mucosal surface. In addition, the discovery of type III IFNs in 2003 prompted the reassessment of the functional importance of type I IFNs in homeostasis and disease. It is now evident that numerous studies done with type I IFN receptor-deficient (IFNAR-deficient) mice could not fully evaluate the significance of antiviral cytokines in anti-microbial defenses and other pathologies, since the lack of type I IFN signaling was partially compensated by the presence of an intact type III IFN system in IFNAR-deficient animals. To this point, the availability of mice singly or doubly-deficient in the type I and/or type III IFN receptors [22, 85, 86] as well as mouse strains in which Ifnar1 or Ifnlr1 can be deleted in selected cell types (conditional knockout mice) [77, 87] should help to interrogate specific contributions of each IFN type to the regulation of homeostasis and disease states. Knowledge gained by this approach should provide guidance for the rational design of IFN-based therapeutic strategies for the treatment of infections and other immune and inflammatory diseases.
Funding:
This work was supported in part by the NIH grants: R01HL134870 to D.P., R01AI104669 to S.V.K. and J.E.D., R01AI114647 and a Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease award to A.R.
Abbreviations:
- IFN
interferon
- IL
interleukin
- TNF
tumor necrosis factor
- ISG
IFN-stimulated gene
- IFNAR
IFN-α/β receptor
- IFNLR
IFN-λ receptor
- ISGF3
IFN-stimulated gene factor 3
- PRR
pattern-recognition receptor
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- IRF
IFN regulatory factor
- MAPK
mitogen-activated protein kinase
- ROS
reactive oxygen species
- TLR
Toll-like receptor
- GI
gastrointestinal
- RSV
respiratory syncytial virus
- IA
invasive aspergillosis
- MNV
murine norovirus
- MAVS
mitochondrial antiviral signaling protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: S.V.K. is an inventor on patents and patent applications related to IFN-λs, which have been licensed for commercial development. All other authors declare they have no competing interests.
References
- [1].Isaacs A, Lindenmann J, Virus Interference: 1. The interferon., Proc R Sod Lond B, 1957, pp. 258–267. [Google Scholar]
- [2].Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP, IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex, Nat. Immunol, 4 (2003) 69–77. [DOI] [PubMed] [Google Scholar]
- [3].Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM, IL-28, IL-29 and their class II cytokine receptor IL-28R, Nat. Immunol, 4 (2003) 63–68. [DOI] [PubMed] [Google Scholar]
- [4].Kotenko SV, Durbin JE, Contribution of type III interferons to antiviral immunity: location, location, location, J Biol Chem, 292 (2017) 7295–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Andreakos E, Zanoni I, Galani IE, Lambda interferons come to light: dual function cytokines mediating antiviral immunity and damage control, Curr Opin Immunol, 56 (2019) 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nice TJ, Robinson BA, Van Winkle JA, The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus, Trends Microbiol, 26 (2018) 510–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Syedbasha M, Egli A, Interferon Lambda: Modulating Immunity in Infectious Diseases, Frontiers in immunology, 8 (2017) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zanoni I, Granucci F, Broggi A, Interferon (IFN)-lambda Takes the Helm: Immunomodulatory Roles of Type III IFNs, Frontiers in immunology, 8 (2017) 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lazear HM, Schoggins JW, Diamond MS, Shared and Distinct Functions of Type I and Type III Interferons, Immunity, 50 (2019) 907–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee S, Baldridge MT, Interferon-Lambda: A Potent Regulator of Intestinal Viral Infections, Frontiers in immunology, 8 (2017) 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pott J, Stockinger S, Type I and III Interferon in the Gut: Tight Balance between Host Protection and Immunopathology, Frontiers in immunology, 8 (2017) 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ingle H, Peterson ST, Baldridge MT, Distinct Effects of Type I and III Interferons on Enteric Viruses, Viruses, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ye L, Schnepf D, Staeheli P, Interferon-lambda orchestrates innate and adaptive mucosal immune responses, Nature reviews. Immunology, (2019). [DOI] [PubMed] [Google Scholar]
- [14].Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I, Chen S, Brand N, Tarway M, Liu L, Sheikh F, Astemborski J, Bonkovsky HL, Edlin BR, Howell CD, Morgan TR, Thomas DL, Rehermann B, Donnelly RP, O’Brien TR, A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus, Nat. Genet, 45 (2013) 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV, Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma, Cancer Res, 66 (2006) 4468–4477. [DOI] [PubMed] [Google Scholar]
- [16].Hamming OJ, Terczynska-Dyla E, Vieyres G, Dijkman R, Jorgensen SE, Akhtar H, Siupka P, Pietschmann T, Thiel V, Hartmann R, Interferon lambda 4 signals via the IFNlambda receptor to regulate antiviral activity against HCV and coronaviruses, EMBO J, 32 (2013) 3055–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P, Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections, J Virol, 84 (2010) 5670–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sommereyns C, Paul S, Staeheli P, Michiels T, IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo, PLoS. Pathog, 4 (2008) e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pulverer JE, Rand U, Lienenklaus S, Kugel D, Zietara N, Kochs G, Naumann R, Weiss S, Staeheli P, Hauser H, Koster M, Temporal and spatial resolution of type I and III IFN responses in vivo, J Virol, 84 (2010) 8626–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW, IFN-{lambda} determines the intestinal epithelial antiviral host defense, Proc. Natl. Acad. Sci. U.S.A, 108 (2011) 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mahlakoiv T, Hernandez P, Gronke K, Diefenbach A, Staeheli P, Leukocyte-derived IFN-alpha/beta and epithelial IFN-lambda constitute a compartmentalized mucosal defense system that restricts enteric virus infections, PLoS Pathog, 11 (2015) e1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin JD, Feng N, Sen A, Balan M, Tseng HC, McElrath C, Smirnov SV, Peng J, Yasukawa LL, Durbin RK, Durbin JE, Greenberg HB, Kotenko SV, Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections, PLoS Pathog, 12 (2016) e1005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qian J, Zheng H, Huangfu WC, Liu J, Carbone CJ, Leu NA, Baker DP, Fuchs SY, Pathogen recognition receptor signaling accelerates phosphorylation-dependent degradation of IFNAR1, PLoS Pathog, 7 (2011) e1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Saxena K, Simon LM, Zeng XL, Blutt SE, Crawford SE, Sastri NP, Karandikar UC, Ajami NJ, Zachos NC, Kovbasnjuk O, Donowitz M, Conner ME, Shaw CA, Estes MK, A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection, Proc Natl Acad Sci U S A, 114 (2017) E570–E579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pervolaraki K, Stanifer ML, Munchau S, Renn LA, Albrecht D, Kurzhals S, Senis E, Grimm D, Schroder-Braunstein J, Rabin RL, Boulant S, Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut, Frontiers in immunology, 8 (2017) 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hakim MS, Chen S, Ding S, Yin Y, Ikram A, Ma XX, Wang W, Peppelenbosch MP, Pan Q, Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids, Sci Rep, 8 (2018) 8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, Virgin HW, Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity, Science, 347 (2015) 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW, Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection, Science, 347 (2015) 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB, Persistent LCMV infection is controlled by blockade of type I interferon signaling, Science, 340 (2013) 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG, Blockade of chronic type I interferon signaling to control persistent LCMV infection, Science, 340 (2013) 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A, The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus, Immunity, 42 (2015) 123–132. [DOI] [PubMed] [Google Scholar]
- [32].Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ, HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons, Gastroenterology, 142 (2012) 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ, Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection, J Immunol, 182 (2009) 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marukian S, Andrus L, Sheahan TP, Jones CT, Charles ED, Ploss A, Rice CM, Dustin LB, Hepatitis C virus induces interferon-lambda and interferon-stimulated genes in primary liver cultures, Hepatology, 54 (2011) 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, Himi T, Fujii N, Sawada N, Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells, Virus Res, 160 (2011) 360–366. [DOI] [PubMed] [Google Scholar]
- [36].Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL, Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells, Allergy, 64 (2009) 375–386. [DOI] [PubMed] [Google Scholar]
- [37].Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E, Interferon-lambda Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness, Immunity, 46 (2017) 875–890 e876. [DOI] [PubMed] [Google Scholar]
- [38].Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, Boulant S, Gehrke L, Cossart P, Kagan JC, Diverse intracellular pathogens activate type III interferon expression from peroxisomes, Nat Immunol, 15 (2014) 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Odendall C, Voak AA, Kagan JC, Type III IFNs Are Commonly Induced by Bacteria-Sensing TLRs and Reinforce Epithelial Barriers during Infection, J Immunol, 199 (2017) 3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ioannidis I, Ye F, McNally B, Willette M, Flano E, Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells, J Virol, 87 (2013) 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miknis ZJ, Magracheva E, Li W, Zdanov A, Kotenko SV, Wlodawer A, Crystal structure of human interferon-lambda1 in complex with its high-affinity receptor interferon-lambdaR1, J Mol. Biol, 404 (2010) 650–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mendoza JL, Schneider WM, Hoffmann HH, Vercauteren K, Jude KM, Xiong A, Moraga I, Horton TM, Glenn JS, de Jong YP, Rice CM, Garcia KC, The IFN-lambda-IFN-lambdaR1-IL-10Rbeta Complex Reveals Structural Features Underlying Type III IFN Functional Plasticity, Immunity, 46 (2017) 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, Lee C, Yarden G, Vleck SE, Glenn JS, Nolan GP, Piehler J, Schreiber G, Garcia KC, Structural linkage between ligand discrimination and receptor activation by type I interferons, Cell, 146 (2011) 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kotenko SV, Izotova LS, Pollack BP, Muthukumaran G, Paukku K, Silvennoinen O, Ihle JN, Pestka S, Other kinases can substitute for Jak2 in signal transduction by interferon-gamma, J Biol Chem, 271 (1996) 17174–17182. [DOI] [PubMed] [Google Scholar]
- [45].Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S, Identification and functional characterization of a second chain of the interleukin-10 receptor complex, EMBO J, 16 (1997) 5894–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lumb JH, Li Q, Popov LM, Ding S, Keith MT, Merrill BD, Greenberg HB, Li JB, Carette JE, DDX6 Represses Aberrant Activation of Interferon-Stimulated Genes, Cell Rep, 20 (2017) 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS, El Baghdadi J, Nonoyama S, Mahdaviani SA, Ailal F, Bousfiha A, Mansouri D, Nievas E, Ma CS, Rao G, Bernasconi A, Sun Kuehn H, Niemela J, Stoddard J, Deveau P, Cobat A, El Azbaoui S, Sabri A, Lim CK, Sundin M, Avery DT, Halwani R, Grant AV, Boisson B, Bogunovic D, Itan Y, Moncada-Velez M, Martinez-Barricarte R, Migaud M, Deswarte C, Alsina L, Kotlarz D, Klein C, Muller-Fleckenstein I, Fleckenstein B, Cormier-Daire V, Rose-John S, Picard C, Hammarstrom L, Puel A, Al-Muhsen S, Abel L, Chaussabel D, Rosenzweig SD, Minegishi Y, Tangye SG, Bustamante J, Casanova JL, Boisson-Dupuis S, Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome, J Exp Med, 212 (2015) 1641–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fuchs S, Kaiser-Labusch P, Bank J, Ammann S, Kolb-Kokocinski A, Edelbusch C, Omran H, Ehl S, Tyrosine kinase 2 is not limiting human antiviral type III interferon responses, Eur J Immunol, 46 (2016) 2639–2649. [DOI] [PubMed] [Google Scholar]
- [49].Lee SJ, Kim WJ, Moon SK, Role of the p38 MAPK signaling pathway in mediating interleukin-28A-induced migration of UMUC-3 cells, Int J Mol Med, 30 (2012) 945–952. [DOI] [PubMed] [Google Scholar]
- [50].Broggi A, Tan Y, Granucci F, Zanoni I, IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function, Nat Immunol, 18 (2017) 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dumoutier L, Lejeune D, Hor S, Fickenscher H, Renauld JC, Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription (STAT)1, STAT2 and STAT3, Biochem. J, 370 (2003) 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bhushal S, Wolfsmuller M, Selvakumar TA, Kemper L, Wirth D, Hornef MW, Hauser H, Koster M, Cell Polarization and Epigenetic Status Shape the Heterogeneous Response to Type III Interferons in Intestinal Epithelial Cells, Frontiers in immunology, 8 (2017) 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Klinkhammer J, Schnepf D, Ye L, Schwaderlapp M, Gad HH, Hartmann R, Garcin D, Mahlakoiv T, Staeheli P, IFN-lambda prevents influenza virus spread from the upper airways to the lungs and limits virus transmission, Elife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pierson TC, Diamond MS, The emergence of Zika virus and its new clinical syndromes, Nature, 560 (2018) 573–581. [DOI] [PubMed] [Google Scholar]
- [55].Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, Mysorekar IU, Coyne CB, Diamond MS, Gestational Stage and IFN-lambda Signaling Regulate ZIKV Infection In Utero, Cell Host Microbe, 22 (2017) 366–376 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, Garcia-Sastre A, Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling, Cell Host Microbe, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, Moley KH, Diamond MS, Interferon lambda protects the female reproductive tract against Zika virus infection, Nat Commun, 10 (2019) 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute SB, Garrigues U, Doyle S, Donnelly RP, Kotenko SV, Fitzgerald-Bocarsly P, Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells, J Immunol, 189 (2012) 2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pietila TE, Latvala S, Osterlund P, Julkunen I, Inhibition of dynamin-dependent endocytosis interferes with type III IFN expression in bacteria-infected human monocyte-derived DCs, J Leukoc Biol, 88 (2010) 665–674. [DOI] [PubMed] [Google Scholar]
- [60].Bierne H, Travier L, Mahlakoiv T, Tailleux L, Subtil A, Lebreton A, Paliwal A, Gicquel B, Staeheli P, Lecuit M, Cossart P, Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta, PloS one, 7 (2012) e39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lebreton A, Lakisic G, Job V, Fritsch L, Tham TN, Camejo A, Mattei PJ, Regnault B, Nahori MA, Cabanes D, Gautreau A, Ait-Si-Ali S, Dessen A, Cossart P, Bierne H, A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response, Science, 331 (2011) 1319–1321. [DOI] [PubMed] [Google Scholar]
- [62].Cohen TS, Prince AS, Bacterial pathogens activate a common inflammatory pathway through IFNlambda regulation of PDCD4, PLoS Pathog, 9 (2013) e1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Travar M, Vucic M, Petkovic M, Interferon lambda-2 levels in sputum of patients with pulmonary Mycobacterium tuberculosis infection, Scand J Immunol, 80 (2014) 43–49. [DOI] [PubMed] [Google Scholar]
- [64].Love AC, Schwartz I, Petzke MM, Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-kappaB-dependent cytokines, Infect Immun, 82 (2014) 2405–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ahn D, Wickersham M, Riquelme S, Prince A, The Effects of IFN-lambda on Epithelial Barrier Function Contribute to Klebsiella pneumoniae ST258 Pneumonia, Am J Respir Cell Mol Biol, 60 (2019) 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ferguson SH, Foster DM, Sherry B, Magness ST, Nielsen DM, Gookin JL, Interferon-lambda3 Promotes Epithelial Defense and Barrier Function Against Cryptosporidium parvum Infection, Cell Mol Gastroenterol Hepatol, 8 (2019) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pires S, Parker D, IL-1beta activation in response to Staphylococcus aureus lung infection requires inflammasome-dependent and independent mechanisms, Eur J Immunol, 48 (2018) 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McCullers JA, The co-pathogenesis of influenza viruses with bacteria in the lung, Nat Rev Microbiol, 12 (2014) 252–262. [DOI] [PubMed] [Google Scholar]
- [69].Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE, Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo, J Virol, 84 (2010) 11515–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Planet PJ, Parker D, Cohen TS, Smith H, Leon JD, Ryan C, Hammer TJ, Fierer N, Chen EI, Prince AS, Lambda Interferon Restructures the Nasal Microbiome and Increases Susceptibility to Staphylococcus aureus Superinfection, mBio, 7 (2016) e01939–01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rich HE, McCourt CC, Zheng WQ, McHugh KJ, Robinson KM, Wang J, Alcorn JF, Interferon Lambda Inhibits Bacterial Uptake During Influenza Super-Infection, Infect Immun, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hendricks MR, Lashua LP, Fischer DK, Flitter BA, Eichinger KM, Durbin JE, Sarkar SN, Coyne CB, Empey KM, Bomberger JM, Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity, Proc Natl Acad Sci U S A, 113 (2016) 1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A, Neutrophil function: from mechanisms to disease, Annu Rev Immunol, 30 (2012) 459–489. [DOI] [PubMed] [Google Scholar]
- [74].Borregaard N, Neutrophils, from marrow to microbes, Immunity, 33 (2010) 657–670. [DOI] [PubMed] [Google Scholar]
- [75].Dinauer MC, Primary immune deficiencies with defects in neutrophil function, Hematology Am Soc Hematol Educ Program, 2016 (2016) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC, Hidden killers: human fungal infections, Sci Transl Med, 4 (2012) 165rv113. [DOI] [PubMed] [Google Scholar]
- [77].Espinosa V, Dutta O, McElrath C, Du P, Chang YJ, Cicciarelli B, Pitler A, Whitehead I, Obar JJ, Durbin JE, Kotenko SV, Rivera A, Type III interferon is a critical regulator of innate antifungal immunity, Science immunology, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rivera A, Siracusa MC, Yap GS, Gause WC, Innate cell communication kick-starts pathogen-specific immunity, Nat Immunol, 17 (2016) 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mocsai A, Diverse novel functions of neutrophils in immunity, inflammation, and beyond, J Exp Med, 210 (2013) 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordonez-Rueda D, Barlogis V, Mahlaoui N, Fenis A, Narni-Mancinelli E, Beaupain B, Bellanne-Chantelot C, Bajenoff M, Malissen B, Malissen M, Vivier E, Ugolini S, Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis, J Exp Med, 209 (2012) 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, Boucher JL, Urban JF Jr., Kim CC, Gause WC, Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion, Nat Immunol, 15 (2014) 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A, B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen, Nat Immunol, 13 (2011) 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M, Neutrophil trails guide influenza-specific CD8(+) T cells in the airways, Science, 349 (2015) aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, Doyle S, McCann F, Williams RO, Udalova IA, IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production, J Exp Med, 212 (2015) 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P, Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses, PLoS. Pathog, 4 (2008) e1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U, Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia, Blood, 108 (2006) 3253–3261. [DOI] [PubMed] [Google Scholar]
- [87].Baldridge MT, Lee S, Brown JJ, McAllister N, Urbanek K, Dermody TS, Nice TJ, Virgin HW, Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus, J Virol, 91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


