Abstract
The emerging fields of omics – using large-scale data-rich biological measurements – provide new opportunities to advance and strengthen endocrine disrupting chemicals (EDC) research. While some EDCs have been associated with adverse health effects in humans, our understanding of their impact remains incomplete. Progress in the field has been primarily limited by our inability to adequately estimate and characterize exposure and identify sensitive and measurable outcomes during windows of vulnerability. Evolving omics technologies in genomics, epigenomics, and mitochondriomics have the potential to generate data that enhance exposure assessment to include the exposome – the totality of lifetime exposure burden – and provide biology-based estimates of individual risks. Applying omics technologies to expand our knowledge of individual risk and susceptibility by augmenting biological data to predict variability and response to disease will further advance EDC research. Together, refined exposure characterization and enhanced disease risk prediction help bridge critical EDC research gaps and create opportunities to move the field toward a new vision – precision public health.
Introduction
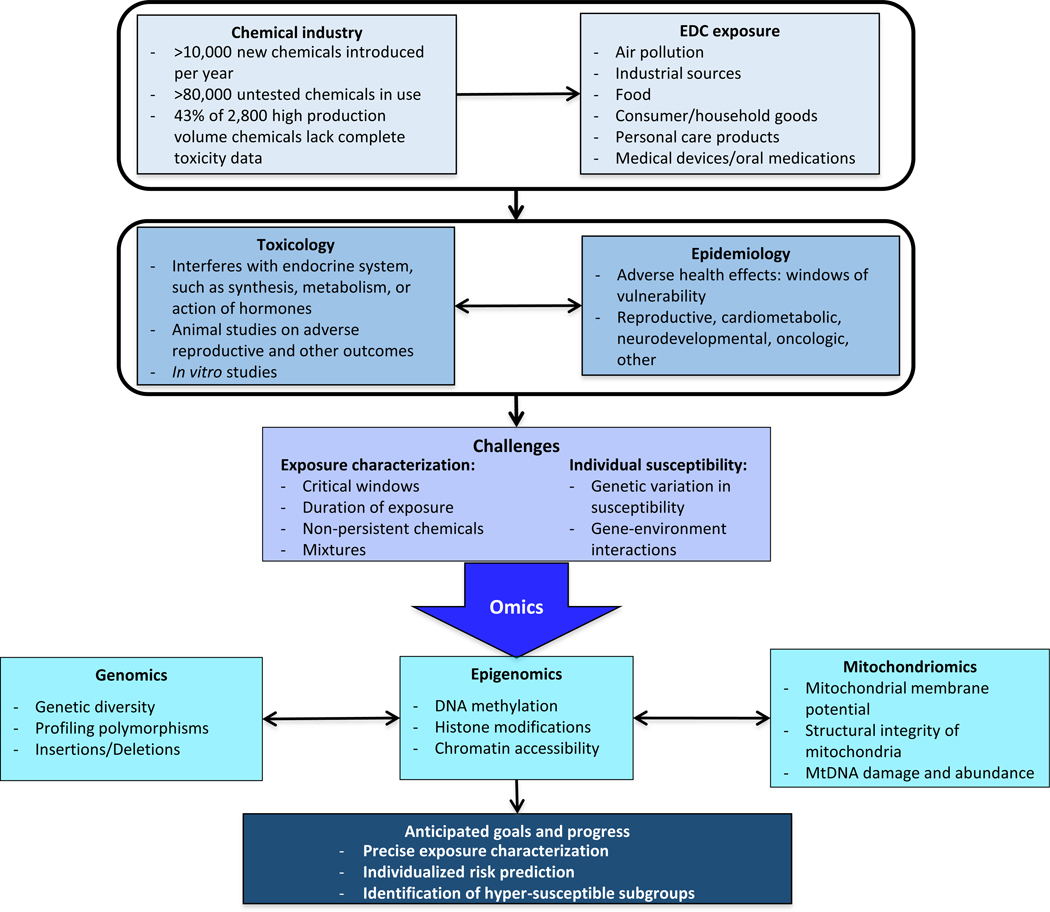
Omics—defined as fields and technologies using large-scale data-rich biology1—offer promising new methods to advance our understanding of the impact of endocrine disrupting chemicals (EDC) on human health2,3._ENREF_13 EDCs are substances found in our environment, food and everyday consumer products that interfere with the endocrine system by altering the synthesis, release, transport, metabolism or action of endogenous hormones_ENREF_24–6. While we lack toxicity data for most of the countless chemicals used in production today, among those we have studied, many have been identified as endocrine disruptors.7 EDCs comprise several classes of compounds including bisphenols, ortho-phthalates, polybrominated diphenyl ethers (PBDEs), per- and polyfluorinated chemicals (PFAs), polychlorinated biphenyls (PCBs) among others5. _ENREF_1Animal and human studies have linked EDCs with a myriad of adverse health effects such as obesity, diabetes and metabolic disease, female and male reproductive alterations including infertility, behavioral and developmental disorders, _ENREF_10and hormone-sensitive cancers8. The estimated economic and health burden associated with exposure to EDCs exceeds $340 and $217 billion US dollars annually in the United States and Europe, respectively9. A recent Endocrine Society Statement called for more mechanistic research and recommended greater consideration of genetic diversity and population differences in order to expand our knowledge of health effects of EDCs8. By adapting a concept underlying the White House Precision Medicine Initiative10, we propose that data-driven omics have the potential to bridge the exposure assessment gap by taking into account individual variability in exposure, dose, biological response, and disease risk. In this perspectives paper, we discuss three relevant omics approaches – genomics, epigenomics, and mitochondriomics – and describe how they can be applied to characterize and estimate exposure and identify individuals at risk of developing EDC-related diseases and disorders (Figure 1).
Figure 1.
Challenges and opportunities of using omics in EDC research.
Omics and EDC research
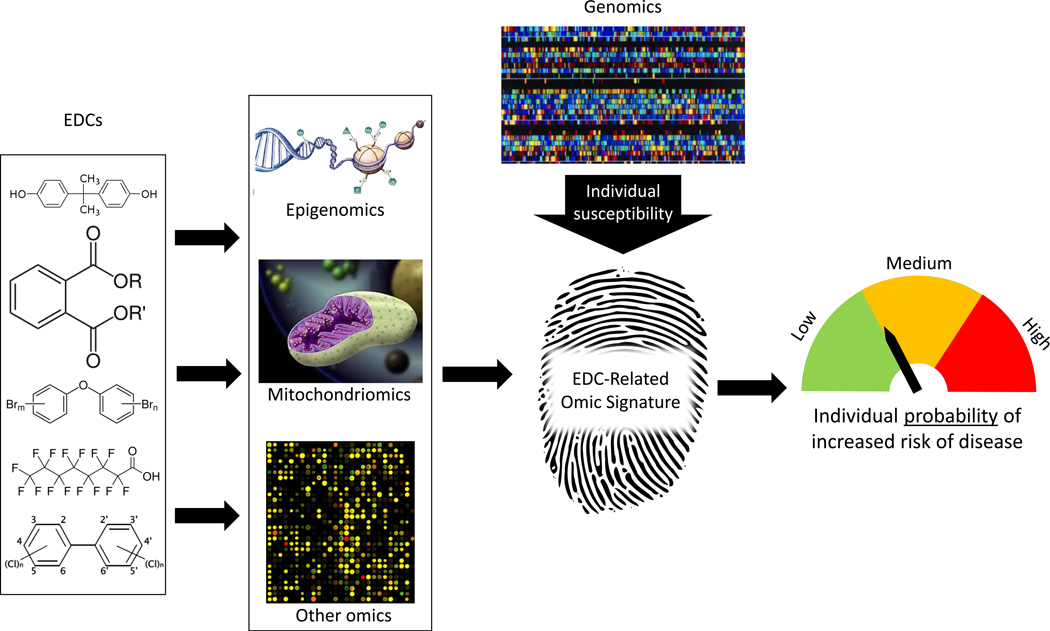
In the last decade, mapping of the human genome has inspired the parallel concept of mapping the ‘exposome’ – the totality of exposure over the life-course11. One of the inherent challenges in EDC research is the difficulty in accurately measuring exposure during critical sensitive windows, or extending exposure assessment to measure the exposome over the life-course11. For many disease endpoints that develop over time and/or have long latency and preclinical phases, exposure estimates are needed months, years, or even decades before the outcome. Moreover, the frequent lack of biological samples during relevant periods along with the need to study EDCs with short half-lives further complicates exposure assessment. Herein we consider genomics, epigenomics, and mitochondriomics, which have overlapping or emerging roles in relation to EDC research. These technologies may ultimately be applied to create unique molecular ‘fingerprints’ that represent personal exposure, dose, biological response, and susceptibility (Figure 2). By incorporating new large-scale data rich approaches with more accurate exposure assessment and improved risk prediction, EDC research has the potential to move toward novel and tailored public health prevention strategies or precision public health12.
Figure 2:
Role of omics in identifying molecular fingerprints in EDC research.
Individual-level variability of biological measures generated by each omics approach is the primary determinant of its potential application in EDC research. While the DNA sequence is static and rarely altered by environmental exposures, including EDCs which are typically non-mutagenic, the DNA sequence can be used to identify individuals whose genetic background make them more or less susceptible to adverse effects of environmental chemicals13–18. Conversely, proteins, metabolites, and RNA expression – used in proteomics, metabolomics, and transcriptomics – are highly sensitive and dynamic and exhibit profound and rapid changes immediately after common, frequent experiences such as eating or physical activity, or following diurnal cycles. While their high temporal variability may be leveraged to identify the impact of current or recent exposure, they are likely less able to characterize long term, prior exposure. In EDC research, we are particularly interested in the long-term exposures and the application of temporally stable omic technologies that accumulate and reflect the influence of these exposures. Finally, other omics have intermediate sensitivity and timing of response to changes and exposures such as DNA methylation, an epigenetic mechanism that has been shown to be modified by environmental factors such as EDCs.3 At least some of these molecular changes can persist over time even if the environmental factor that caused them is removed, thus reflecting a form of biological memory19–25_ENREF_19_ENREF_19_ENREF_19_ENREF_19. The DNA methylome has a wide array of temporal variability, ranging from minutes (e.g., genes related to immune function that need to change expression rapidly to respond to antigen and microbial threats) to years, and some that stay stable over the entire lifetime, including the developmental marks that are established in utero during embryonic development. This demonstrates that DNA methylation has the flexibility to operate over different time frames and can be particularly useful as molecular fingerprinting of past exposures. If exposures to chemicals induce molecular fingerprints that are specific and reflect the dose, duration, and time since cessation of exposure, this information could be vital for assessing past and cumulative exposures and, equally important for predicting risk of future disease. In the following sections, we propose a conceptual model (Figure 2) to describe how genomics, epigenomics, and mitochondriomics can be applied to predict EDC exposure and identify individuals at risk.
Genomics
Genomics is a well-established field that investigates an organism’s genome or complete set of DNA, including all of its genes26. In recent decades, the field has substantially evolved largely as a result of the increased access of technologies that allow for sequencing the human genome in its entirety, or more commonly of the exome, i.e., all the expressed genes in the genome. The genome each of us inherits is virtually unchanged across our lifespan; however, variation does exist between individuals including in single nucleotide polymorphisms (SNPs) and insertions/deletions (INDELs). The genetic variation in SNPs and INDELs among individuals can be measured to generate large-scale data that may serve to signal risk to common chronic diseases27 and be used as a marker for risk prediction. Gene-environment (G X E) interactions – the interplay between the environment and the human genome28 – is the concept that the genetic makeup of an individual determines their susceptibility or resistance to adverse effects in the presence of certain environments29. A well-known clinical example of G X E interaction is that of patients with phenylketonuria (PKU) who have a mutation in the gene coding for phenylalanine hydroxylase, the enzyme that metabolizes phenylalanine, an essential amino acid obtained from dietary sources. This genetic mutation leads to an accumulation of high levels of phenylalanine and consequently to neurotoxicity with concomitant mental retardation30. The G X E concept can be similarly applied to the EDC context where genetic differences may make individuals more susceptible to the effects of environmental chemicals. For example, there is evidence to suggest that genetic polymorphisms modify the anti-androgenic effect of dioxin exposure through differential aryl hydrocarbon-receptor activation resulting in male reproductive disturbances29. Recently, Dunaway et al. explored G x E interactions with polychlorinated biphenyl (PCB) exposure and autism risk. Specifically, they performed genome-wide identification of PBC associated methylation changes to investigate genetic interactions. They identified specific genes involved in autism spectrum disorder (ASD) with altered PCB-related methylation. They concluded that gene-specific epigenetic vulnerability to both genetic and environmental hits are important in identifying different ASD etiologies, and further suggest that such knowledge can be used to develop targeted, individual treatment options 31. While the application of G x E to EDC research is still new, this salient example demonstrates the utility of omics methods to identify at risk groups based on genetic and EDC profiles and proposes using such data toward precision medicine for an outcome with a large public health burden. However, enthusiasm over G x E studies over the last decade has been replaced with caution and skepticism given the inconsistencies in results and null findings.
As technologies continue to evolve, the strengths of both developed and emerging omics methods will need to be considered in light of their inherent limitations. In Table 1, we present relevant omics methods and outline the strengths and limitation to their application. For a more extensive discussion on genomics and the role of genetics in determining susceptibility to toxicants, including EDCs, we refer to prior works.32–35
Table 1.
Strengths and limitations of omics technologies in EDC research
| Type | Biomarkers | Methods | Description | Strengths | Limitations |
|---|---|---|---|---|---|
| Genomics | DNA sequencing | Sanger Sequencing; Next-gen Sequencing | Determines the order of nucleotides within a DNA molecule and allows for full interrogation of the genome, both targeted and global | Not just limited to nuclear DNA - can be extrapolated to mtDNA‡ | Can have high cost, especially for whole-genome sequencing |
| Able to investigate all DNA variants and how they might be related to EDCs^ | Information limited to the DNA sequence | ||||
| GWAS | Genome-wide Association Studies - microarray | Examines specific genetic variants across the genome in different individuals and can be used to establish associations between these variants and disease or quantative traits (4) | Not hypothesis driven - no prior gene information required thus allows for discovery analyses | Hard to use if certain EDC chemicals target variants other than SNPs* or CNV† | |
| Well established for investigating outcomes | |||||
| Allows for association to be examined, especially for environmental exposures | |||||
| Transcriptomics | Gene-expression analyses | RNA-Seq; real-time-quantitative PCR, and microarray | Examines expression patterns of specific genes, an array of genes, or the entire transcriptome and reveals the presence and quantity of RNA in a biological sample (5) | Allows for associations to be examined between EDCs and specific expressed genes or an array of genes | Gene expression varies by tissue type making it more difficult to isolate the biological mechanism |
| Primer design allows for study specific a priori genes to be examined and developed microarrays are readily available | Usually represents data at the point in time the sample was collected; limited in reflecting history of exposures over time | ||||
| Some toxicology or in vitro models in relation to EDCs^ (1)(2) | Relatively few RNA-seq EDC^ studies done in human population | ||||
| Epigenomics | DNA Methylation | Pyrosequencing-Microarray; whole-genome sequencing | Examines the DNA methylome, ranging from gene specific areas to a microarray of about 850K sites to the entire DNA methylome (6) (7), which can impact gene expression | Can examine gene specific methylation and/or epigenome wide DNA methylation (up to 95%) | Tissue specific, so might not be the best representation if target tissue is not obtained |
| Able to examine associations with both EDC^ exposures and outcomes | Only represents data at the point in time the sample was collected, might not reflect the windows of susceptibility | ||||
| Established methods within epidemiology studies that allow for replication of EDC^ findings | |||||
| Methods can also be used to measure methylation in mtDNA‡ | |||||
| Histone Modifications | Chromatin immunoprecipitation - seq | Examines epigenetic marks on histones, including acetylation, phosphorylation, glycosylation, sumolation, methylation and ADP ribosylation, which can impact gene expression by altering chromatin structure (9)(10) | Previous studies have examined the relationship between histone modifications and environmental exposures (Nickel, Arsenic, and few EDCs) (11)(13)(14) | Still a relatively unstudied field in EDC^ (15) | |
| Some studies have linked histone modifications to outcomes, such as obesity(12) | Most studies examining histone modifications are in vitro or in toxicology models (11)(13)(14) | ||||
| Chromatin Remodeling | DNAse-seq; MNase-seq FAIRE-seq; ATAC-seq | Examines the dynamic modification of the chromatin architecture that allows for transcription machinery to adhere to the DNA which can impact gene expression (16) | Provides information on the actual chromatin conformation. Analyzes a cellular state intermediate between the epigenetics marks (e.g. DNA methylation, histone modifications) and gene expression | Most assays require high amounts of cells. | |
| Still not widely applied in EDC studies | |||||
| Mitochondriomics | Mitochondrial Copy Number | Multi-plex real-time-PCR#; Digital-Droplet PCR# | Examines the number of copies of mtDNA‡ compared to nDNAº within a sample (17) | mtDNA‡ copy number can be altered by the presence of environmental chemicals (17)(19) | Measurements are relative to the controls used, so it can be hard to compare between studies |
| This assay has been optimized for toxicologic, in Vitro, and human studies (18) | Still new to the EDC^ field and few studies have examined mtDNA‡ copy number in relation to EDCŝ (18) | ||||
| Mitochondrial Lesions | LongRange quantitative PCR# & Picogreen Fluoroescence | Examines the number of DNA lesions within a fragment of mtDNA‡ | This assay can be used in human studies, allowing for reliable and senstive measures | Measurements are relative to controls used, so it can be hard to compare between studies | |
| Low cost, small amount of DNA needed to start, and PCR# based allows for easy set up and running of the assay | Cannot distinguish the nature or location of DNA damage | ||||
| Not all types of lesions are captured by this method | |||||
| Emerging technology, has not been studied with EDC^ | |||||
| Mitochondrial Sequencing | Next-gen sequencing; MiSeq, MitoExome; sanger sequencing | Like genomic sequencing, allows for gaining the order of nucleotides and allows for full interrogation of the mtDNA‡ genome | Able to investigate all DNA variants and how they might be related to EDCs^ | Information limited to the mtDNA sequence | |
| Measure of mtDNA heteroplasmy vary over time and are in principle influenced by environmental exposures | Emerging technology, has not been studied with EDCs^ | ||||
| mtDNA‡ hyper variable region could be used as a tool for exposure fingerprinting (20) | mtDNA sequence variation is tissue specific, so mechanisms can be missed if measuring in a different tissue type | ||||
| Potential coamplification of nuclear homologs of mtDNA which can lead to inaccurate measures(21) | |||||
SNP Single Polymorphic Nucleotide
CNV Copy Number Variant
mtDNA Mitochondrial DNA
nDNA Nuclear DNA
EDC Endocrine Disrupting Chemical
PCR Polymerase Chain Reaction
Epigenomics
Epigenomics investigates biological mechanisms that change gene expression. If we consider the DNA sequence to be inheritable and fixed, then epigenetic modifications are the markings of this sequence that alter its expression. These marks themselves can be persistent and heritable while they do not change the actual genetic sequence36. In the epigenetics context, ‘persistent’ and ‘heritable’ refer to both the persistence of these marks between parent and daughter cells as well as the inheritance of these marks between parents and offspring. Therefore, one of the underlying properties of epigenetic modifications is that once they are established they do not disappear after the genome is duplicated, but—instead—they can propagate and persist through cell division. DNA methylation and histone modifications are the two epigenetic modifications interrogated in most human studies. DNA methylation is the addition of a methyl group to a cytosine base commonly followed by a guanosine base resulting in a cytosine-phosphate-guanine dinucleotide (CpG)37. Gene-silencing is the best known DNA methylation related mechanism of gene regulation: within the promoter-associated regulatory regions of a gene, the presence of increased methylated CpGs can down-regulate expression of that gene37. However, DNA methylation is not always associated with gene repression. For instance, within the gene body, high levels of methylation are highly correlated with up-regulation of gene expression38.
Today, genome-scale platforms that measure millions of methylation sites are readily available with choices that balance depth of information per sample with sample size and cost (Table 1). DNA methylation has become the more frequently studied epigenetic mark in EDC research because of the availability of robust laboratory methods for analysis3. Large human epidemiologic studies known as epigenome-wide methylation studies often opt for platforms with lower costs per sample, such as the Illumina Infinium Methylation Beadchip, which in its current configuration measures DNA methylation at ~850,000 methylation sites39. Smaller sample sizes in clinical studies have increasingly selected platforms based on deep-sequencing, such as candidate gene pyrosequencing or sequence specific bisulfite sequencing. For instance, Dao et al examined maternal exposure to PBDEs—a class of flame retardants—and promoter methylation in the tumor necrosis factor alpha (TNFa) gene in cord blood40. Higher maternal serum concentrations of PBDE47 were associated with lower cord blood methylation in the TNFa promoter region40. These results suggest that maternal PBDE47 exposure could alter the CpG methylation in the promoter region, which may lead to altered gene expression of TNFa40. Studies such as this could provide a basis from which additional biomarkers could be developed for the purpose of improving EDC exposure characterization and risk assessment. Epigenome-wide methylation methods can measure higher numbers of methylation sites and – at higher cost – can even provide complete coverage of all the 28 million methylation sites in the human genome41. The main advantage of epigenome-wide methylation profiling lies in its ability to determine absolute DNA methylation levels that cover ~95% of the DNA methylome. This method, however, is subject to high costs, is dependent on technical expertise, and has downstream computational requirements38 (see Table 1 for additional examples of epigenomics methods and their strengths and limitations).
While a biomarker reflecting past EDC exposure has yet to be developed and validated, a well-studied example in the tobacco literature provides a potential model. DNA methylation from exposure to tobacco smoke has led to the development of the first known omics biomarker that reflects detailed personal exposure history. Traditionally, assessment of smoking has primarily relied on self-reported data or urinary cotinine measures; however personal recall of smoking is prone to bias and cotinine is largely a reflection of recent tobacco consumption42. A seminal study by Joubert and colleagues identified 26 CpG sites in cord blood that were significantly different among mothers who smoked during pregnancy compared with non-smokers43. Furthermore, a meta-analysis examining 13 cohorts found 6,073 CpG sites differed significantly based on maternal smoking status, and these sites also comprised those identified by Joubert44. Both studies identified cg05575921 – a CpG locus that maps to the aryl hydrocarbon receptor repressor (AhRR) gene and known to be activated by tobacco smoking – as the single most significant site. A combination of studies on adult populations have demonstrated that AhRR methylation is associated with not only smoking status (current, former or never), but also with the average number of cigarettes smoked, years of smoking, pack-years of smoking and—among those individuals who quit—years since quitting43,45,46. While such a biomarker should be evaluated with similar exposures including non-tobacco smoke, DNA methylation of the AhRR receptor may be a potentially useful biomarker to predict past exposure, and has provided motivation for researching DNA methylation biomarkers responsive to EDC exposures. Despite the fact that DNA methylation is a well-developed technology, applying methylation data of specific genes to categorize or predict exposure to EDCs is still new and requires further study and development. Several large human cohorts that assessed EDC exposures have now also generated epigenome-wide methylation data; we expect that a wave of results on the association of EDC exposure and DNA methylation is forthcoming. It is anticipated that results from these new studies will be applied toward gaining understanding on the interrelationship between EDCs and DNA methylation and identifying potential biomarkers.
Developing Omics with Untested Potential in Human Studies
Transgenerational epigenetic inheritance
Transgenerational epigenetic inheritance consists of phenotypic expression transmitted across generations via gametes through epigenetic marks but independently of the genome sequence. Evidence from animal models suggests that environmental stressors, including some EDCs such as pesticides, persistent organic pollutants, and others47,48_ENREF_43, can lead to adverse health outcomes among descendants not directly exposed49–51_ENREF_38. Many examples of transgenerational inheritance occur in rodent models52–54. One study examining di-(2-ethylhexyl) phthalate (DEHP) exposure to F0 pregnant female rats led to the multigenerational inheritance of cryptorchidism (undescended testes). A statistically significant up-regulation of three kinds DNA methyltransferases (Dnmt1, Dnmt3a, and Dnmt3b) was seen in the progeny F1 and F2 male rats’ testes compared to controls52. The main effects were shown in the F1 and F2 generations, however no effect was observed in the F3 and F4 generations. This pattern suggests that the observed phthalate effects may be due to direct exposure of the fetus and its gamete cells in utero rather than to genuine transgenerational inheritance. Indeed, the gametes that will generate F2 are already present in the F1 embryo in utero and may be reprogrammed at that stage to produce demonstrable effects in F2. A rodent study on methoxychlor, an insecticide and pesticide, demonstrated true epigenetic transgenerational inheritance of disease through certain sperm epimutations (differential DNA methylation regions). Only the F0 generation of gestating females was exposed to methoxychlor. Exposure in the F0 gestating females was associated with kidney disease, ovarian dysfunction, and obesity among the unexposed F3 generation descendants. This study also compared epigenetic changes in sperm between the control lineage and methoxychlor lineage F3 rats and found 37 epimutations that were significantly different between the two groups. These 37 sperm epimutations were further compared with exposures, such as other pesticides (DDT and DEET) and plastics (BPA and phthalates) and found that only 4 of 37 overlapped with methoxychlor. This suggests that the transgenerational sperm epimutations found in the F3 generation are exposure-specific and induced by methoxychlor53. However, transgenerational epigenetic inheritance in humans is difficult to determine and requires demonstrating adverse effects of EDCs to the 3rd generation offspring or beyond. Despite evidence in animals, exact mechanisms involved in epigenetic transgenerational inheritance are yet unknown. Designing and conducting human multigenerational studies is particularly challenging, not only because of the time required to follow-up multiple generations but also due to the difficulty of observing exposures and DNA methylation at the appropriate time windows across generations. Therefore, further research is needed to understand the extent to which multigenerational epigenetics inheritance operates in humans.
Mitochondriomics
Investigation of the properties of the mitochondrial DNA (mtDNA) is a relatively new, yet promising field in environmental health and EDC research. Each human cell contains thousands of mitochondria, each carrying 2–10 copies of their own genome, a double-stranded circular mtDNA molecule of approximately 16kb in length. Mitochondria act as the cell’s power plant because they convert energy substrates derived from the breakdown of glucose and fatty acids into adenosine triphosphate (ATP)55. The properties of mtDNA differ from nuclear DNA (nDNA), including the lack of histone-wrapping protection and limited repair mechanisms, making mtDNA more vulnerable to accumulating damage when exposed to environmental chemicals56.
Mitochondrial damage can result from many sources, and damage can impact mitochondrial structure or function, as well as the mtDNA itself. Endogenous reactive oxygen species are a primary agent of mitochondrial damage and these can be amplified in the presence of an external pollutant source, including many environmental chemicals57,58. When reactive oxygen species are produced past the point of homeostatic levels, oxidative stress can lead to alterations to mitochondrial structure as well as to its function, including abnormalities to electron transport chain activity, membrane potential, ion transport, and apoptotic signaling, which can ultimately lead to cell death59. Several studies on sperm function found that abnormal mitochondria and structural alterations to mitochondria60 or its sheath61 were associated with reduced sperm motility. Studies on the effects of EDCs on mitochondrial DNA are scarce. However, studies that showed effects on mitochondrial membrane potential (MMP), which has been extensively used to document mitochondrial dysfunction2, suggest that EDC may affect mitochondria. For instance, one such study found that men with higher phthalate concentrations in semen had lower MMP, and lower MMP was further associated with semen quality58. More recently, the US EPA Tox21 program assessed the potential for some environmental chemicals and pharmaceuticals to affect mitochondria dysfunction by measuring MMP. Among the greater than 8000 different chemicals that were tested in vitro, researchers found that 11% of these decreased MMP including certain classes of EDCs2. While these studies to not provide information on whether EDCs affect the mitochondrial DNA, they do show that the mitochondria are an EDC target.
Damage to mtDNA has also gained attention in the field of mitochondriomics, with biomarkers being used to quantify mtDNA damage and dysfunction. Damaged mtDNA can co-exists with normal mtDNA copies in cells, and the influence of these mtDNA alterations can range between normal, mild and severe according to the proportion of abnormal mtDNA copies62. Biomarkers that measure damage and dysfunction include, among others, mtDNA copy number (a measure of abundance of mtDNA present compared to nDNA) and mtDNA lesions (the amount of damage present on the mtDNA genome)62. There are few studies that have examined mtDNA biomarkers in the context of EDCs, however one in vitro study found that cells treated with TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), the most potent dioxin congener and a known endocrine disruptor, increased mtDNA lesions and reduced mtDNA copy number in the treated cells63. Another study compared mitochondrial function in lymphoblast cells in relation to BPA exposure between children diagnosed with autism and their unaffected siblings. The authors examined several different markers of mitochondrial dysfunction including, MMP, mtDNA copy number in mitochondria genes. The results of this study suggest that among genetically susceptible children, BPA exposure may induce mtDNA dysfunction and act as an important environmental risk factor64.
DNA methylation of the mitochondrial genome is growing in popularity as a potential biomarker in the study of EDCs, though controversies surround the existence and functionality of mtDNA methylation65. Earlier studies—performed more than 30 years ago—reported no cytosine methylation on the mtDNA66. Recently, Shock et al. showed the presence of mtDNA methylation and proposed a possible mechanism of action through DNA methyltrasferases translocating to the mitochondria following a mitochondrial targeting sequence67. However, the unsophisticated structure of the mitochondrial genome has led to the belief that the mtDNA may lack the mechanisms that link DNA methylation with gene expression control in the nuclear genome.
While data in human populations are lacking, a recent animal study examined the epigenetic effects of low doses of PBDE47, a flame retardant chemical, demonstrating that prenatally exposed rats exhibited reduced DNA methylation of a specific mitochondrial gene compared to the control group68. While mtDNA methylation is still largely understudied—partly due to technical limitations of using standard platforms typically used in nDNA methylation as well as to lingering doubts about its functionality62 – other measures of mitochondriomics remain a unique and novel field with potential utility in EDC research. Further development in miochondriomics technology and an expanded awareness of its potential and its limitations will help guide and generate new studies in this area (Table 1).
Transcriptomics, Proteomics and Metabolomics
The central dogma of molecular biology posits that the information contained in genes flows from the DNA sequence to messenger RNA (mRNA) to proteins69. Epigenetic regulation helps control the flow from the DNA into mRNA and, indirectly, into proteins. Proteins have many functions in eukaryotic cells varying from serving as structural components to facilitating transport and storage within the cells. Proteins also serve as enzymes that carry out nearly all the chemical reactions that take place in cells, including all those that transform metabolites. In the past two decades, laboratory technologies, including transcriptomics, proteomics and metabolomics methods, have increasingly allowed for characterizing these interconnected layers of cellular functions. In principle, these technologies—individually or in combination—can be used to determine a biological fingerprinting of EDC exposure70. However, a major challenge of transcriptomics, proteomics and metabolomics is that given mRNAs, proteins, and metabolites are downstream in the central-dogma flow, they are also much more variable over time. Indeed, cells often need to adapt very rapidly in response to environmental conditions and homeostatic signals and consequently the downstream mechanisms that operate cellular responses are also able to change rapidly. Therefore, while these molecular substrates may reflect current exposures, they are less likely to reflect past exposures. In addition, standardized, easily accessible, high-throughput platforms, while existing for transcriptomics, are not readily available for proteomic and metabolomic applications. As a result, these three technologies have not found as much application in EDC research as per our previously outlined methods (see Table 1). For instance, a PubMed search (conducted on March 27, 2017) on “endocrine disrupting chemicals” yielded 56 papers for “transcriptomics OR mRNA microarray OR mRNA sequencing”, 46 for “proteomics”, and 32 papers for “metabolomics”. As a comparison, the same search retrieved 126 papers for “epigenetics and epigenomics” and 747 for “genetics OR genomics”. These search results suggest a limited application of transcriptomics, proteomics, and metabolomics technologies in EDC research thus far, despite the fact that they are extremely informative in understanding mechanisms of action and biological effects of EDCs.
Future Steps – Emerging approaches for obtaining omics-based fingerprints
To date, omics studies have been limited to identifying molecular changes associated with and/or induced by chemical exposures. Developing a chemical fingerprint requires that a biomarker be sufficiently sensitive to modifications by the exposure of concern, however sensitivity is a necessary but insufficient criteria for fingerprint development. Given that individuals are not exposed to a single chemical in isolation, but rather to a multitude of chemicals—as well as to other stressors—simultaneously, biomarkers that can serve as molecular fingerprints of exposure need to inherently also be specific. While a valid and reliable DNA fingerprint in EDC research has yet to be developed, a possible approach may emerge from recent DNA methylation methods developed to predict biological age. For example, Horvath applied DNA methylation arrays to create an algorithm based on elastic-net machine learning technique71 to identify 353 age-related CpG methylation sites and combined them to generate a biological measure highly correlated with chronological age72. The value of this measure of DNA methylation age has been illustrated in a recent meta-analysis of 13 large epidemiology studies of 13,089 participants that showed that individuals who had a positive difference between epigenetic and chronological age at baseline (i.e., those who appeared to be epigenetically older than their actual age) had higher rates of mortality during follow up73. Similarly, machine learning techniques such as those used to construct epigenetic age algorithms may be applied for fingerprinting EDC exposures using not only DNA methylation, but also other omic data, individually or in combination. In principle, machine learning may allow for the identification and discrimination of different EDC exposures and yield omics biomarkers that are both sensitive and specific71. Indeed, machine learning has emerged in biomedical sciences as a method that can use highly dimensional data to maximize biomarker sensitivity and specificity74. Yet, such applications of machine learning have been sparsely used in environmental health and, to the best our knowledge, never applied to EDC research. However, particular challenges inherent in EDC research should not be underestimated. Compared with age, EDC exposures may cause smaller biological differences and therefore be associated with weaker biologic changes. Furthermore, in order to develop a fingerprint that is biologically meaningful, omic data should be combined with reliable measures of EDC exposure over time that can accurately characterize current and past exposure profiles. Such data are rarely available in typical human studies and are especially challenging to collect for EDCs with shorter half-life, which typically require repeated collection of biological samples of exposure quantification over time. Given the various limitations to machine learning technology in the context of EDCs, few studies have applied these methods directly to improving exposure measurement, however these methods are gaining popularity in the field.75,76.
A general challenge in epigenetic studies its tissue specificity. In general, scientists cannot assume—without specific evidence—that the level of an epigenetic mark at a specific locus measured in an easy to access surrogate tissue (e.g., blood) is correlated with that in a more remote, inaccessible tissue (e.g., brain). Furthermore, even in the presence of correlation between two different tissues in a population samples, it cannot be assumed that—when an environmental determinant or a disease changes the levels of the epigenetic mark in one tissue—the second tissues will show the same change. This is a major consideration in epigenetic research to be taken into account in the planning and interpretation of any epigenetic studies. However, for environmental exposures and conditions when simply having a biomarker may be useful, even just working on easy-to-access tissues may be helpful, provided that no inference is claimed on the biological mechanisms affecting the target tissue of concern. Future research is warranted to combine epigenomics, mitochondriomics, and other biomarkers to create novel fingerprinting of the human exposome and to incorporate it with genomic data and other individual-level data to predict individual risk of future disease.31
Conclusion
New and developing technologies in genomics, epigenomics, and mitochondriomics provide new opportunities to bridge exposome and precision public health in EDC research. Although EDCs have been linked with adverse health effects in experimental and human studies, the field requires improved methods to assess human exposure and biological response across different and multiple life stages. In the absence of costly and time-consuming repeated measures of ambient levels and/or biomarkers in biological samples collected over months or years, assessing past exposure remains difficult, if not impossible to reconstruct. Integrating multiple omics technologies will allow for better characterization of past EDC exposures. Applying these technologies will give way to a better understanding of future risk, which may be augmented by the identification of susceptible subgroups. Omics can greatly contribute to developing a comprehensive exposome approach and will help identify individual risk of disease through targeted biomarkers that reconstruct past exposure and predict future risk. Using large-scale data-rich biology in tandem with machine learning will allow for the development of biologically relevant fingerprints of EDC exposures. These tools will bridge the gap between multiple disciplines and help further our understanding of the links between EDC exposure—both past and present—and future risk of disease.
Acknowledgments:
NIEHS Center Grant ES000002; NIEHS Center Grant P30ES009089; NIEHS Grant R01ES021733; NIEHS Grant R01ES021357; NIDDK Grant R01DK100790; NIEHS Grant R21ES024841; NIEHS Grant R21ES027087; NIEHS Grant R01ES009718; CDC/NIOSH Training Grant T42OH008416
Biography
Carmen Messerlian PhD is a post-doctoral research fellow at the Harvard T.H. Chan School of Public Health investigating the effects of phthalates and other emerging chemicals and their mixtures on ovarian reserve, pregnancy loss, preterm birth, birth weight, and child health outcomes. She completed her PhD in epidemiology at McGill University and her MSc in Public Health from the London School of Hygiene and Tropical Medicine.
Rosie M. Martinez MPH is a doctoral student at the Harvard T.H. Chan School of Public Health and conducts her research at the Columbia Mailman School of Public Health. Her research focus is on environmental chemicals that adversely affect male reproductive health and human development. She completed her MPH from Columbia Mailman School of Public Health, receiving a certificate in molecular epidemiology.
Russ Hauser MD, ScD is the Frederick Lee Hisaw Professor of Reproductive Physiology and professor of environmental and occupational epidemiology in the Department of Environmental Health at the Harvard T.H. Chan School of Public Health. He also holds an appointment at the Harvard Medical School, where he is professor of obstetrics, gynecology, and reproductive biology. Dr. Hauser’s research focuses on the health risks posed by exposure to environmental chemicals that adversely affect human development and reproductive health. He received his MD from the Albert Einstein College of Medicine and his MPH and ScD from the Harvard Chan School of Public Health where he also completed a residency in Occupational Medicine.
Andrea A. Baccarelli MD, PhD serves as the Environmental Health Sciences Department Chair and the Director of the Laboratory of Environmental Precision Biosciences. As an epigeneticist and board-certified clinical endocrinologist, Dr. Baccarelli’s research explores epigenetic and molecular mechanisms as potential functional pathways linking exposures to environmental pollutants to human disease. His laboratory research activities are specifically focused on epigenetics, mitochondriomics, and computational epigenomics. Dr. Baccarelli is currently the PI of multiple NIH-funded projects and since 2010, his lab has produced publications at the intersection of epigenetics, molecular epidemiology and environmental health.
Footnotes
Competing Interests: The authors have no competing financial interests to declare.
Reference
- 1.OmicsGateway N. OmicsGateway: About this site, <http://www.nature.com/omics/about/index.html> (2016).
- 2.Attene-Ramos MS et al. Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environmental health perspectives 123, 49–56, doi: 10.1289/ehp.1408642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casati L, Sendra R, Sibilia V. & Celotti F. Endocrine disrupters: the new players able to affect the epigenome. Frontiers in cell and developmental biology 3, 37, doi: 10.3389/fcell.2015.00037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccarelli A, Pesatori AC & Bertazzi PA Occupational and environmental agents as endocrine disruptors: Experimental and human evidence. Journal of Endocrinological Investigation 23, 771–781, doi: 10.1007/bf03345069 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E. et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews 30, 293–342, doi:doi: 10.1210/er.2009-0002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciences, N. I. E. H. Endocrine Disruptors, <https://www.niehs.nih.gov/health/topics/agents/endocrine/> (2010).
- 7.Services, U. S. D. o. H. a. H. National Toxicology Program: About NTP, <http://ntp.niehs.nih.gov/about/index.html> (2016).
- 8.Gore AC et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36, E1–e150, doi: 10.1210/er.2015-1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attina TM et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. The lancet. Diabetes & endocrinology, doi: 10.1016/s2213-8587(16)30275-3 (2016). [DOI] [PubMed] [Google Scholar]
- 10.House TW FACT SHEET: President Obama’s Precision Medicine Initiative, <https://www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative> (2015).
- 11.NIOSH. Exposome and Exposomics, <https://www.cdc.gov/niosh/topics/exposome/> (2014).
- 12.NIH. All of Us Research: Scale and Scope, <https://www.nih.gov/allofus-research-program/scale-scope> (2016).
- 13.Schug TT, Janesick A, Blumberg B. & Heindel JJ Endocrine Disrupting Chemicals and Disease Susceptibility. The Journal of steroid biochemistry and molecular biology 127, 204–215, doi: 10.1016/j.jsbmb.2011.08.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar V. et al. CYP 1A1 polymorphism and organochlorine pesticides levels in the etiology of prostate cancer. Chemosphere 81, 464–468, doi: 10.1016/j.chemosphere.2010.07.067 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Yoshida R. et al. Association of cryptorchidism with a specific haplotype of the estrogen receptor alpha gene: implication for the susceptibility to estrogenic environmental endocrine disruptors. The Journal of clinical endocrinology and metabolism 90, 4716–4721, doi: 10.1210/jc.2005-0211 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Bi Y. et al. Diabetes Genetic Risk Score Modifies Effect of Bisphenol A Exposure on Deterioration in Glucose Metabolism. The Journal of clinical endocrinology and metabolism 101, 143–150, doi: 10.1210/jc.2015-3039 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Nava GA et al. PPARgamma and PPARGC1B polymorphisms modify the association between phthalate metabolites and breast cancer risk. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 18, 493–501, doi: 10.3109/1354750x.2013.816776 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Hung WT, Lambert GH, Huang PW, Patterson DG Jr. & Guo YL Genetic susceptibility to dioxin-like chemicals’ induction of cytochrome P4501A2 in the human adult linked to specific AhRR polymorphism. Chemosphere 90, 2358–2364, doi: 10.1016/j.chemosphere.2012.10.026 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Belinsky SA et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer research 62, 2370–2377 (2002). [PubMed] [Google Scholar]
- 20.Yauk C. et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci U S A 105, 605–610, doi: 10.1073/pnas.0705896105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen BC et al. Aging and Environmental Exposures Alter Tissue-Specific DNA Methylation Dependent upon CpG Island Context. PLOS Genetics 5, e1000602, doi: 10.1371/journal.pgen.1000602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prins GS, Birch L, Tang W-Y & Ho S-M Developmental Estrogen Exposures Predispose to Prostate Carcinogenesis with Aging. Reproductive toxicology (Elmsford, N.Y.) 23, 374–382, doi: 10.1016/j.reprotox.2006.10.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anway MD, Cupp AS, Uzumcu M. & Skinner MK Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards E. Inherited epigenetic variation — revisiting soft inheritance. Nat Rev Genet 7, 395–401 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Dolinoy DC, Weidman JR & Jirtle RL Epigenetic gene regulation: Linking early developmental environment to adult disease. . Reprod Toxicol (2006). [DOI] [PubMed] [Google Scholar]
- 26.Communications, L. H. N. C.f. B . Help Me Understand Genetics: The Human Genome Project, <https://ghr.nlm.nih.gov/primer/hgp/genome> (2016).
- 27.Wu MC et al. Powerful SNP-Set Analysis for Case-Control Genome-wide Association Studies. American Journal of Human Genetics 86, 929–942, doi: 10.1016/j.ajhg.2010.05.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bollati V. & Baccarelli A. Environmental epigenetics. Heredity 105, 105–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brokken LJS & Giwercman YL Gene-environment interactions in male reproductive health: Special reference to the aryl hydrocarbon receptor signaling pathway. Asian Journal of Andrology 16, 89–96, doi: 10.4103/1008-682X.122193 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottman R. Gene–Environment Interaction: Definitions and Study Designs. Preventive medicine 25, 764–770 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunaway KW et al. Cumulative Impact of Polychlorinated Biphenyl and Large Chromosomal Duplications on DNA Methylation, Chromatin, and Expression of Autism Candidate Genes. Cell Reports 17, 3035–3048, doi: 10.1016/j.celrep.2016.11.058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olden K. & Wilson S. Environmental health and genomics: visions and implications. Nat Rev Genet 1, 149–153, doi: 10.1038/35038586 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Alam G. & Jones BC Toxicogenetics: in search of host susceptibility to environmental toxicants. Frontiers in Genetics 5, 327, doi: 10.3389/fgene.2014.00327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundberg Giwercman Y. Androgen Receptor Genotype in Humans and Susceptibility to Endocrine Disruptors. Hormone research in paediatrics 86, 264–270, doi: 10.1159/000443686 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Schwartz DA Environmental genomics and human health. Giornale italiano di medicina del lavoro ed ergonomia 33, 31–34 (2011). [PubMed] [Google Scholar]
- 36.Baccarelli A. Epigenetics Glossary, <https://www.mailman.columbia.edu/research/laboratory-precision-environmental-biosciences/epigenetics-glossary> (2016).
- 37.Rivera Chloe M. & Ren B. Mapping Human Epigenomes. Cell 155, 39–55, doi: 10.1016/j.cell.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stirzaker C, Taberlay PC, Statham AL & Clark SJ Mining cancer methylomes: prospects and challenges. Trends in genetics : TIG 30, 75–84, doi: 10.1016/j.tig.2013.11.004 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Illumina. Introduction to Methylation Array Analysis, <http://www.illumina.com/techniques/microarrays/methylation-arrays.html > (2016).
- 40.Dao T, Hong X, Wang X. & Tang WY Aberrant 5’-CpG Methylation of Cord Blood TNFalpha Associated with Maternal Exposure to Polybrominated Diphenyl Ethers. PloS one 10, e0138815, doi: 10.1371/journal.pone.0138815 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lövkvist C, Dodd IB, Sneppen K. & Haerter JO DNA methylation in human epigenomes depends on local topology of CpG sites. Nucleic Acids Research, doi: 10.1093/nar/gkw124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G. & Tremblay M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine & Tobacco Research 11, 12–24, doi: 10.1093/ntr/ntn010 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Joubert BR et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environmental health perspectives 120, 1425–1431, doi: 10.1289/ehp.1205412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joubert, Bonnie R. et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. American Journal of Human Genetics 98, 680–696, doi: 10.1016/j.ajhg.2016.02.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joehanes R. et al. Epigenetic Signatures of Cigarette Smoking. Circulation. Cardiovascular genetics 9, 436–447, doi: 10.1161/circgenetics.116.001506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philibert RA, Beach SRH & Brody GH Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics 7, 1331–1338, doi: 10.4161/epi.22520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner MK, Bhandari RK, Haque MM & Nilsson EE Environmentally Induced Epigenetic Transgenerational Inheritance of Altered SRY Genomic Binding During Gonadal Sex Determination. Environmental epigenetics 1, dvv004, doi: 10.1093/eep/dvv004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skinner MK Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nature reviews. Endocrinology 12, 68–70, doi: 10.1038/nrendo.2015.206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heard E. & Martienssen RA Transgenerational Epigenetic Inheritance: myths and mechanisms. Cell 157, 95–109, doi: 10.1016/j.cell.2014.02.045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blake GET & Watson ED Unravelling the complex mechanisms of transgenerational epigenetic inheritance. Current Opinion in Chemical Biology 33, 101–107, doi: 10.1016/j.cbpa.2016.06.008 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Guerrero-Bosagna C. in The epigenome and developmental origins of health and disease : 425–437 (Academic Press, 2016). [Google Scholar]
- 52.Chen J. et al. The Mechanism of Environmental Endocrine Disruptors (DEHP) Induces Epigenetic Transgenerational Inheritance of Cryptorchidism. PloS one 10, e0126403, doi: 10.1371/journal.pone.0126403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE & Skinner MK Pesticide Methoxychlor Promotes the Epigenetic Transgenerational Inheritance of Adult-Onset Disease through the Female Germline. PloS one 9, e102091, doi: 10.1371/journal.pone.0102091 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerrero-Bosagna C. et al. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol 34, 694–707, doi: 10.1016/j.reprotox.2012.09.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clare O’Connor JU A. Essentials of Cell Biology. (2014). [Google Scholar]
- 56.Yakes FM & Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A 94, 514–519 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guarnieri M. & Balmes JR Outdoor air pollution and asthma. The Lancet 383, 1581–1592, doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pant N. et al. Correlation of phthalate exposures with semen quality. Toxicology and Applied Pharmacology 231, 112–116, doi: 10.1016/j.taap.2008.04.001 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Meyer JN et al. Mitochondria as a target of environmental toxicants. Toxicological sciences : an official journal of the Society of Toxicology 134, 1–17, doi: 10.1093/toxsci/kft102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gopalkrishnan K, Padwal V, D’Souza S. & Shah R. Severe asthenozoospermia: a structural and functional study. International journal of andrology 18 Suppl 1, 67–74 (1995). [DOI] [PubMed] [Google Scholar]
- 61.Piasecka M. & Kawiak J. Sperm mitochondria of patients with normal sperm motility and with asthenozoospermia: morphological and functional study. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society 41, 125–139 (2003). [PubMed] [Google Scholar]
- 62.Byun HM & Baccarelli AA Environmental exposure and mitochondrial epigenetics: study design and analytical challenges. Human genetics 133, 247–257, doi: 10.1007/s00439-013-1417-x (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen SC, Liao TL, Wei YH, Tzeng CR & Kao SH Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Molecular human reproduction 16, 361–372, doi: 10.1093/molehr/gaq004 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Kaur K, Chauhan V, Gu F. & Chauhan A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radical Biology and Medicine 76, 25–33, doi: 10.1016/j.freeradbiomed.2014.07.030 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Liu B. et al. CpG methylation patterns of human mitochondrial DNA. Scientific reports 6, 23421, doi: 10.1038/srep23421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawid IB 5-methylcytidylic acid: absence from mitochondrial DNA of frogs and HeLa cells. Science 184, 80–81 (1974). [DOI] [PubMed] [Google Scholar]
- 67.Shock LS, Thakkar PV, Peterson EJ, Moran RG & Taylor SM DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proceedings of the National Academy of Sciences 108, 3630–3635, doi: 10.1073/pnas.1012311108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byun HM et al. Epigenetic effects of low perinatal doses of flame retardant BDE-47 on mitochondrial and nuclear genes in rat offspring. Toxicology 328, 152–159, doi: 10.1016/j.tox.2014.12.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crick FH On protein synthesis. Symposia of the Society for Experimental Biology 12, 138–163 (1958). [PubMed] [Google Scholar]
- 70.Sheehan D. The potential of proteomics for providing new insights into environmental impacts on human health. Reviews on environmental health 22, 175–194 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Vidyasagar M. Identifying predictive features in drug response using machine learning: opportunities and challenges. Annual review of pharmacology and toxicology 55, 15–34, doi: 10.1146/annurev-pharmtox-010814-124502 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Horvath S. DNA methylation age of human tissues and cell types. Genome Biology 14, R115–R115, doi: 10.1186/gb-2013-14-10-r115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen BH et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8, 1844–1865, doi: 10.18632/aging.101020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV & Fotiadis DI Machine learning applications in cancer prognosis and prediction. Computational and Structural Biotechnology Journal 13, 8–17, doi: 10.1016/j.csbj.2014.11.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ornostay A, Cowie AM, Hindle M, Baker CJ & Martyniuk CJ Classifying chemical mode of action using gene networks and machine learning: a case study with the herbicide linuron. Comparative biochemistry and physiology. Part D, Genomics & proteomics 8, 263–274, doi: 10.1016/j.cbd.2013.08.001 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Zhang J. et al. In Silico Approach To Identify Potential Thyroid Hormone Disruptors among Currently Known Dust Contaminants and Their Metabolites. Environmental science & technology 49, 10099–10107, doi: 10.1021/acs.est.5b01742 (2015). [DOI] [PubMed] [Google Scholar]