To the editor,
Viral pneumonia is thought to be the most common non-obstetric infectious disease during pregnancy, and is associated with maternal and neonatal morbidity and mortality during pregnancy [1]. Atypical pneumonia known as coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is highly infectious and is currently spreading rapidly around the globe [2]. Before leading to the global emergency, SARS-CoV-2 emerged in Wuhan, Hubei Province, China during December 2019 [3,4]. Several studies focusing on infected individuals from the general population have been reported; however, limited information is available on pregnancy outcomes for women with COVID-19. Chen et al. [5] reported the maternal–neonatal outcomes and vertical transmission potential of COVID-19 pneumonia in pregnant women in the third trimester of pregnancy with an infection history of a maximum of 7 days. However, more studies are needed on maternal and neonatal outcomes of pregnant women. This case series study was conducted to assess the impact of SARS-CoV-2 infection on adverse pregnancy outcomes. In this study, the duration from onset of infection to delivery was 1–26 days. Both health-care workers and women from the general population were included in the study.
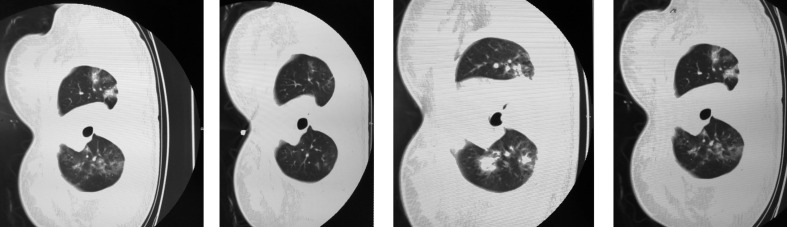
We conducted a case series study on pregnant women (n = 17) infected with SARS-CoV-2 admitted to Hubei general hospital (Renmin Hospital) from 25 January to 15 February 2020. COVID-19 pneumonia was diagnosed according to the New Coronavirus Pneumonia Prevention and Control Program, fifth and sixth editions. All 17 pregnant women were found positive for SARS-CoV-2 using either quantitative RT-PCR (qRT-PCR) or CT scan imaging (Fig. 1), or both. To assess neonatal infection with COVID-19, cord blood and neonatal throat swab samples were collected immediately after delivery in the operating room and were tested using qRT-PCR. All the women underwent caesarean section, and the detailed information collected are presented in Table 1, Table 2 . We conducted a comprehensive literature search for the current outbreak of COVID-19 in pregnant women and a thorough search for the impact of SARS-CoV on pregnancy outcomes.
Fig. 1.
Randomly selected representative Computed Tomography (CT) images.
Table 1.
Clinical details of pregnant women infected with SARS-CoV-2
| Parameter | Average (% and/or range) | Comments |
|---|---|---|
| Occupation | ||
| Health workers | 3/17 (17.6%) | Nurses and nephrologists were included in this study |
| Not health workers | 14/17 (82.4) | Pregnant women who were not related to medical profession |
| General information | ||
| Age (years) | 29.29 (24–34) | |
| Date of hospital admission | Jan 25 to Feb 15 | All women were admitted from 25 January to 15 February |
| Gestational age on admission (weeks+days) | 37.82 (35–41) | Majority of women had gestational age 38–40 weeks |
| Gestational age on delivery (weeks+days) | 38.1 (35+5–41) | Majority of women had gestational age 38–40 weeks |
| Number of days from onset of pneumonia until birth | 4.2 (1–26) | Only two women had ≥10 days of fetus exposure to COVID-19 |
| Source of SARS-CoV-2 infection | ||
| Contact with infected family member | 2/17 (12%) | Only two individuals were infected with SARS-CoV-2 before admission |
| Contact with infected patient | 15/17 (88%) | Majority of individuals, including healthcare workers, contracted infection inside the hospital |
| Mode of delivery and complications | ||
| Caesarean section | 17/17 (100%) | All the women underwent caesarean section; however, some of the women had premature rupture of fetal membrane so underwent emergency caesarean section |
| Complications | 5/17 (29%) | Only five pregnant women had complications that caused preterm delivery |
| Signs and symptoms | ||
| Maximum body temperature | 38.5°C | The minimum body temperature was 36.2°C, most women had a body temperature of c.37°C. Most did not develop fever until the delivery; sputum, shortness of breath and nasal congestion were rare. Comparatively, more individuals had cough |
| Fever | 3/17 (18%) | |
| Cough | 6/17 (35%) | |
| Diarrhoea | 3/17 (18%) | |
| Shortness of breath | 2/17 (12%) | |
| Nasal congestion | 2/17 (12%) | |
| Sputum | 1/17 (6%) | |
| Infected with SARS-CoV-2 | 17/17 (100%) | |
| Treatment/therapy | ||
| Antiviral | 16/17 (94%) | Antiviral drugs oseltamivir or arbidol or both were given orally, while ribavirin was given intravenously according to the severity of symptoms. |
| Antibiotics | 17/17 (100%) | Cefoperazone sulbactam sodium, ceftazidime, azithromycin and moxifloxacin hydrochloride were given to women who needed it |
| Hormones | 8/17 (47%) | |
| Chinese medicine | 15/17 (88%) | All those receiving Chinese medicine received Lianhua Qingwen |
| Laboratory characteristics | ||
| Confirmatory (quantitative RT-PCR) test | 12/17 (70.5%) | Overall 12 women were confirmed only with qPCR testing for nucleic acid form pharynx swab samples |
| Confirmatory (quantitative RT-PCR) test + Chest CT scan | 5/17 (29.5%) | Five out of 17 women were confirmed both with qPCR testing for nucleic acid from pharynx swab samples and chest CT scanning |
| Low or normal leucocyte count (<9·5 × 109 cells/L) | 9/17 (52.9% and5.03-19.97) | The increased leucocyte count was observed in eight women. This increase may or may not be linked to COVID-19 |
| Lymphocyte count ( × 109 cells/L) | 4/17 (23.5% and 0.63-2.37) | Four out of 17 women were found with lymphopenia, which could be linked with COVID-19 |
| Elevated ALT (>45 U/L) | 2/17 (11.7% and 9–46) | Two out of 17 women were found with elevated AST and/or ALT; however, the majority had normal ALT and AST levels |
| Elevated AST (>35 U/L) | 2/17 (11.7% and 12–39) | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, nover coronavirus 2019 infection; SARS-CoV-2, severe acute respiratory syndrome novel coronavirus 2.
Table 2.
Clinical details of neonates born to the COVID-19 infected women
| Parameter | Average (Percent and/or range) | Comments |
|---|---|---|
| Birthweight (g) | 3104.375 (2300–3750) | Birthweight for most neonates was normal; three neonates had weight <2700 g |
| Birth length (cm) | 49.176 (45–52) | Birth length was normal for all neonates |
| Apgar score 9–10 | 16/17 (94.1%) | Only one of the neonates had lower Apgar score 7–9 at 1 min and 5 min |
| Fetal heart rate (normal) | 17/17 (100%) | |
| Ultrasound results (normal) | 17/17 (100%) | All neonates born with a normal heart rate and normal cardiopulmonary function |
| Preterm delivery | 3/17 (18%) | Preterm delivery was not common; risk factors for the three reported preterm cases were not identified |
| Neonatal death | 0/17 (0%) | No neonatal death and stillbirth occurred among the infants delivered. Although no neonate was confirmed with COVID-19, two neonates were suspected of being infected. One of the suspected neonates developed neonatal pneumonia. However, based on lack of evidence we could not confirm if the virus was transferred from mother to neonate. |
| Stillbirth | 0/17 (0%) | |
| Infected with SARS-CoV-2 | 0/17 (0%) | |
| Neonatal pneumonia | 5/17 (29%) |
The age range of the women was 24–34 years, the range of gestational weeks at admission was 35–41 weeks and the range of gestational weeks at delivery was 35+5–41 weeks. In 12 women, the nucleic acid test from the throat swab was positive for SARS-CoV-2 but in only five women did both the CT scan and the nucleic acid test indicate COVID-19 (Fig. 1 ; Table 1). We observed fever in three individuals and complications in five. Other common symptoms were cough (n = 6), diarrhoea (n = 3), nasal congestion (n = 2), shortness of breath (n = 2) and sputum production (n = 1). Women were receiving antibiotics (n = 17), hormones (n = 8), and antivirals together with Chinese medicine (n = 15). A total of 17 neonates, including three preterm neonates, with birthweights from 2300 to 3750 g (mean 3104.375 g) and birth lengths from 45 to 52 cm (mean 49.2 cm) were delivered by caesarean section. There were no fetal or neonatal deaths. The ultrasound results and fetal heart rate were normal for all neonates and Apgar score for 16 neonates was between 9 and 10. Only two neonates (case 6 and case 14) after birth were suspected of having COVID-19 and five neonates were reported with neonatal pneumonia. Three infants were delivered preterm (Table 1, Table 2).
Based on our findings in these 17 women, we suggest that SARS-CoV-2 infection may lead to the occurrence of neonatal pneumonia and preterm delivery. However, we cannot rule out the possibility that these complications can be linked to other biological processes or intrauterine infections [6,7]. We collected all the samples in the operation theatre with care to avoid the chance of contamination. Hence, according to our knowledge, the collected samples were not contaminated. The focus of this study was to investigate the vertical transmission potential of SARS-CoV-2 infection. Two of the neonates (Table 2) had suspected COVID-19, indicating the possibility for vertical transmission, but we could find no convincing evidence to confirm the vertical transmission potential of SARS-CoV-2.
Neonatal pneumonia occurred in five of the 17 neonates. In 15 neonates, SARS-CoV-2 was not detected in the throat swab. The swab samples tested within 24 hours after the delivery were positive in only two neonates (case 6 and case 14). However, intrauterine tissue samples such as placenta, cord blood or amniotic fluid were not tested to confirm if the infection in the neonate was the result of intrauterine transmission. Intrauterine vertical transmission was not reported by either Chen et al. [5] for COVID-19 (n = 9) or Wong et al. [8] for SARS (n = 12).
In summary, we found two neonates suspected for SARS-CoV-2 infection and five neonates with neonatal pneumonia, suggesting the possibility that adverse pregnancy outcomes may be linked to COVID-19 infection.
Ethical statement
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association and informed consent was obtained from the patients and parents of the babies.
Transparency declaration
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors acknowledge a postdoctoral grant from The Second Affiliated Hospital of Zhengzhou University (for SK), and operating grant support from the National Natural Science Foundation of China (grants nos: 81870942, 81471174 and 81520108011), National Key Research and Development Program of China (grant no.: 2018YFC1312200), and Innovation Scientists and Technicians Troop Constructions Projects of Henan Province of China (for MX).
Editor: S. J. Cutler
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.03.034.
Contributor Information
S. Khan, Email: Suliman.khan18@mails.ucas.ac.cn.
G. Han, Email: hg7913@hotmail.com.
M. Xue, Email: xuemengzhou@zzu.edu.cn.
G. Nabi, Email: ghulamnabiqau@gmail.com.
J. Liu, Email: jbliuzz@163.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:1–16. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S., Siddique R., Ali A., Xue M., Nabi G. Novel coronavirus, poor quarantine, and the risk of pandemic. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S., Nabi G., Han G., Siddique R., Lian S., Shi H. Novel coronavirus: how the things are in Wuhan. Clin Microbiol Infect. 2020;26(4):399–400. doi: 10.1016/j.cmi.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;6736:1–7. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atay G.A., Arsan S., Atasay B., Ensari A., Aysev D. The possible role of intrauterine infections in unexplained second trimester abortions and macerated stillbirths: a study from a single center. J Perinatol. 2004;24:679–685. doi: 10.1038/sj.jp.7211167. [DOI] [PubMed] [Google Scholar]
- 7.Kacerovsky M., Pliskova L., Bolehovska R., Gerychova R., Janku P., Matlak P. Lactobacilli-dominated cervical microbiota in women with preterm prelabor rupture of membranes. Pediatr Res. 2019:1–9. doi: 10.1038/s41390-019-0692-1. [DOI] [PubMed] [Google Scholar]
- 8.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.