Highlights
-
•
The level of SAA and CRP significantly increased in patients with COVID-19.
-
•
As disease progressed from mild to critically severe, SAA and CRP gradually increased.
-
•
Our study indicated that SAA/L, CRP, SAA, and l are valuable in predicting the severity and distinguishing critically ill patients from mild ones.
-
•
Patients with higher initial SAA are more likely to have poor CT imaging.
Keywords: Coronavirus Disease 2019, Serum amyloid A, C-reactive protein, Lymphocyte, Computed tomography imaging, Severity
Summary
Background
To explore the significance of SAA in evaluating the severity and prognosis of COVID-19.
Methods
A total of 132 patients with confirmed COVID-19 who were admitted to a designated COVID-19 hospital in Wuhan, China from January 18, 2020 to February 26, 2020 were collected. The dynamic changes of blood SAA, CRP, PCT, WBC, Lymphocyte (L), PLT, CT imaging, and disease progression were studied. All patients completed at least twice laboratory data collection and clinical condition assessment at three time points indicated for this study; The length of hospital stay was longer than 14 days prior to February 26, 2020.
Results
COVID-19 patients had significantly increased SAA and CRP levels, while L count decreased, and PCT, WBC, and PLT were in the normal range. As disease progressed from mild to critically severe, SAA and CRP gradually increased, while L decreased, and PLT, WBC, and PCT had no significant changes; ROC curve analysis suggests that SAA/L, CRP, SAA, and L count are valuable in evaluating the severity of COVID-19 and distinguishing critically ill patients from mild ones; Patients with SAA consistently trending down during the course of disease have better prognosis, compared with the patients with SAA continuously rising; The initial SAA level is positively correlated with the dynamic changes of the serial CT scans. Patient with higher initial SAA level are more likely to have poor CT imaging.
Conclusions
SAA and L are sensitive indicators in evaluating the severity and prognosis of COVID-19. Monitoring dynamic changes of SAA, combined with CT imaging could be valuable in diagnosis and treatment of COVID-19.
Introduction
COVID-19, recently broke out in Wuhan, China, has spread rapidly throughout China and other countries. This new type of coronavirus could cause severe acute respiratory syndrome and injuries in other systems as well. The disease progresses rapidly, leading to multiple organ failure and death.1 , 2 Quite a few patients have no specific symptoms/signs or radiological abnormalities at the early stage, with only mild symptoms, making the early diagnosis of disease difficult. Therefore, early identification of the infection and creating effective treatment plan are particularly imperative.3 , 4 A series of inflammation factors, such as serum amyloid A (SAA), C-reactive protein (CRP), procalcitonin (PCT), white blood cells (WBC), lymphocyte (L) and platelet (PLT) have been used in clinic as inflammation indicators. In this research, the authors want to explore if these factors can also assist in the diagnosis of COVID-19 infection and estimate of the disease severity. Thus, in this research, the authors systematically studied the dynamic changes of above inflammation indicators in patients infected with COVID-19, in order to evaluate their clinical values in predicting the severity and prognosis of COVID-19.
Methods
Data sources
We conducted a retrospective study focusing on the significance of SAA in evaluating the severity and prognosis of COVID-19. A total of 132 patients with COVID-19 were collected from Tianyou Hospital of Wuhan University of Science and Technology, from January 18, 2020 to February 26, 2020. Tianyou Hospital, Wuhan University of Science and Technology, located in Wuhan, Hubei Province, the endemic areas of COVID-19, is one of the major tertiary teaching hospitals and is responsible for the treatments for COVID-19 assigned by the government. All patients were tested positive with SARS-CoV-2 and hospitalized. Patients completed at least twice laboratory data collection and clinical condition assessment at three time points indicated for this study. The length of hospital stay was longer than 14 days prior to February 26, 2020. The dynamic changes of blood SAA, CRP, PCT, WBC, Lymphocyte (L), PLT, CT imaging, and disease progression were studied. At the same time, clinical conditions were evaluated and CT scans were obtained. Data were collected at three time points: admission, 3–5 days of hospitalization, and at the composite endpoint. Composite endpoint is February 26, 2020.As of February 26, 2020, the number of hospital discharge, inpatients, and the number of dead were counted.
We extracted the medical records of patients and sent these to the data collection center of Wuhan University of Science and Technology. A team of doctors who had been treating patients with COVID-19 collected and reviewed the data. Because of the urgent need to collect data on this emerging pathogen, the requirement for informed consent was waived. If information was not clear, the working group in Wuhan University of Science and Technology contacted the doctor responsible for the treatment of the patient for clarification. This case series was approved by the Medical ethics Review Board of Wuhan University of Science and Technology (No. 202009).
Laboratory confirmation and treatment
Sputum and throat swab specimens collected from all patients at admission were tested by real time polymerase chain reaction for SARS-Cov-2 RNA within three hours. Laboratory confirmation of the virus was performed using real time reverse transcription polymerase chain reaction. Virus detection was repeated twice every 24 h. Laboratory tests were conducted at admission, including a complete blood count, serum biochemistry, and identification of other respiratory pathogens such as influenza A virus (H1N1, H3N2, H7N9), influenza B virus, respiratory syncytial virus, parainfluenza virus, and adenovirus. According to the COVID-19 Diagnosis and Treatment Plan issued by National Health Committee of China, patients received supportive oxygen therapy, antiviral medication, and other supportive treatments.
Clinical condition assessment criteria
(1) Clinical classification
According to COVID-19 Diagnosis and Treatment Plan issued by National Health Committee of China, clinical conditions are classified into four types: mild, moderate, severe, and critically severe.
Mild: mild clinical symptoms, no radiological changes
Moderate: fever, respiratory distress, CT scan indicating pneumonia signs
Severe: Meet any of the following
-
(1)
Shortness of breath, RR>30 times per minute;
-
(2)
At room air, SpO2 lower than 93%;
-
(3)
The partial pressure of Arterial blood oxygen (PaO2)/the fraction of inspired oxygen (FiO2) ≤ 300 mmHg;
-
(4)
CT chest imaging shows that lung damage develops significantly within 24 to 48 h.
Critically severe: Meet any of the following
-
(1)
Respiratory failure requiring mechanical ventilation;
-
(2)
Signs of septic shock;
-
(3)
Multiple organ failure requiring ICU admission.
(2) CT imaging classification: the imaging was classified into four types of normal, mild, progressive, and severe, with scored at 0, 1, 2, and 3, respectively.
-
(1)
Mild: the main manifestations are ground-glass opacities and consolidation. Some cases show very thin, small patchy subpleural ground-glass opacities or ground-glass nodules. Lesions can be single or multiple, and both lung lobes can be involved. Lesions are more common in the middle and lower lobes, and mostly distributed in the outer zones of the lung and subpleural areas.
-
(2)
Progressive: large lesions can be seen and multiple lung lobes in both lungs can be involved. Consolidation and fibrosis of varying sizes are often seen within the lesions. Some cases may be accompanied by bronchial retraction, bronchiectasis, and interlobular pleural thickening. However, pleural effusion and enlarged mediastinal lymph nodes are rare in this type.
-
(3)
Severe: Lesions are diffuse in both lungs and uneven in density. Large areas of consolidation and ground-glass opacities can be seen. The sign of “white lung” can be seen due to large areas of the lung are involved. The interlobular pleura and bilateral pleura are usually thickened, and pleural effusion can be seen.
(3) Outcome of illness: According to clinical progression, cases were divided into four types: fully recovered, improved, exacerbation, and death.
Statistical methods
Statistical analysis was performed using SPSS 25.0 software. Kruskal-Wallis H-test and independent sample chi-square test were used to analyze differences between groups. Due to unequal variance, Tamhane ’s T2 statistical method was a fair measure to perform multiple comparisons among groups of mild, moderate, severe, and critical severe patients for the value of SAA/L. Two-tailed P value less than 0.05 was considered statistically significant. The Receiver Operating Characteristic curve (ROC curve) was used to calculate the area under the curve (AUC) of SAA, CRP, L, and SAA/L in order to evaluate the sensitivity and specificity of these factors. Spearman correlation coefficient was utilized to measure the degree of correlation between the hierarchically ordered variables in this study.
Patient and public involvement
This was a retrospective case series study and no patients were involved in the study design, setting the research questions, or the outcome measures directly. No patients were asked to advice on interpretation or writing up of results.
Results
Demographic characteristics
From January 18, 2020 to February 26, 2020, 693 patients with COVID-19 was treated in Tianyou Hospital, and 132 patients met the requirements of this study. The patients were between 33–89 years old, with an average age of 62 years. And 87 of 132 patients were over 60 years, accounting for 65.9%. Of these patients, 75 were males, accounting for 56.8%, and 57 were females, accounting for 43.2%. (Table 1 )
Table 1.
Demographic characteristics.
| Demographic characteristics | Condition at admission |
Outcome at composite endpoint |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild/moderate | Severe | Critically severe | Total | Discharge | Improved | Exacerbation | Death | Total | |
| Age | |||||||||
| ± s | 57.32 ± 11.52 | 66.55 ± 12.05 | 64.06 ± 13.36 | 62.05 ± 12.68 | 59.50 ± 12.30 | 61.16 ± 12.11 | 63.86 ± 13.70 | 72.25 ± 9.91 | 62.05 ± 12.68 |
| Distribution-n(%) | |||||||||
| <60 y | 29 (22.0%) | 11(8.3%) | 5(3.8%) | 45(34.1%) | 32(24.2%) | 9(6.8%) | 1(0.8%) | 3(2.3%) | 45(34.1%) |
| ≥60 y | 31(23.5%) | 45(34.1%) | 11(8.3%) | 87(65.9%) | 42(31.8%) | 22(16.7%) | 6(4.5%) | 17(12.9.%) | 87(65.9%) |
| Total | 60(45.5%) | 56(42.4%) | 16(12.1%) | 132 | 74(56.1%) | 31(23.5%) | 7(5.3%) | 20(15.2%) | 132(%) |
| Sex | |||||||||
| Male-n(%) | 28(21.2%) | 37(28.0%) | 10(7.5%) | 75(56.8%) | 42(31.8%) | 16(12.1%) | 5(3.7%) | 12(9.1%) | 75(56.8%) |
| Female-n(%) | 32(24.2%) | 19(14.4%) | 6(4.5%) | 57(43.2%) | 32(24.2%) | 15(11.4%) | 2(1.5%) | 8(6.1%) | 57(43.2%) |
| Total | 60(45.5%) | 56(42.4%) | 16(12.1%) | 132(%) | 74(56.1%) | 31(23.5%) | 7(5.3%) | 20(15.2%) | 132(%) |
At the time of admission, 60 patients had mild or moderate symptoms, accounting for 45.5%; and 56 patients had severe symptoms, accounting for 42.4%. 16 of 132 patients were critically severe, accounting for 12.1%. In this study, more patients were male and more patients were more than 60 years, consistently with previous literature reports [3]. (Table 1)
The first test results of SAA, CRP, PCT, WBC, L, PLT and clinical classification
(1) The relationship between the levels of SAA, CRP, WBC, L, PCT and clinical classification at admission.
According to the results showed in Table 2 , with disease progressing from mild to critically severe, SAA and CRP gradually increased, while L gradually decreased (p < 0.05). However, PLT, WBC, and PCT were all within the normal ranges, suggesting that SAA, CRP, and L are closely related to disease classification, while WBC, PCT, and PLT are of little significance.
Table 2.
The 1st test of SAA, CRP, PCT, WBC, L, PLT, and clinical classification.
| Clinical classification | SAA (mg/L) |
CRP (mg/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | ± s | <10 (n) | 10–200 (n) | >200 (n) | n | ± s | <3 (n) | 3–100 (n) | >100 (n) | |
| Moderate | 60 | 123.57 ± 75.81 | 7 | 33 | 20 | 60 | 33.22 ± 32.21 | 6 | 49 | 5 |
| Severe | 56 | 171.91 ± 56.89 | 2 | 11 | 43 | 56 | 66.04 ± 44.89 | 2 | 39 | 15 |
| Critically Severe | 16 | 181.00 ± 40.66 | 0 | 4 | 12 | 16 | 97.44 ± 58.60 | 0 | 7 | 9 |
| Total | 132 | 151.04 ± 69.12 | 9 | 48 | 75 | 132 | 54.93 ± 46.86 | 8 | 95 | 29 |
| H/p | H = 22.80 p = 0.000 | H = 26.19 p = 0.000 | ||||||||
| χ2/p | χ2 = 25.32 p = 0.000 | χ2 = 19.90 p = 0.001 | ||||||||
| Clinical classification | WBC (109/L) |
L (109/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | ± s | <3.5 (n) | 3.5–9.5 (n) | >9.5 (n) | n | ± s | <1.1 (n) | 1.1–3.2 (n) | >3.2 (n) | |
| Moderate | 60 | 4.93 ± 1.91 | 14 | 44 | 2 | 60 | 1.04 ± 0.44 | 37 | 23 | 0 |
| Severe | 56 | 5.81 ± 2.78 | 14 | 33 | 9 | 56 | 0.86 ± 0.54 | 44 | 11 | 1 |
| Critically severe | 16 | 8.34 ± 4.74 | 3 | 7 | 6 | 16 | 0.53 ± 0.29 | 15 | 1 | 0 |
| Total | 132 | 5.72 ± 2.93 | 31 | 84 | 17 | 132 | 0.90 ± 0.50 | 96 | 35 | 1 |
| H/p | H = 8.122 p = 0.017 | H = 22.51 p = 0.000 | ||||||||
| χ2/p | χ2 = 14.50 p = 0.006 | χ2 = 10.24 p = 0.037 | ||||||||
| Clinical classification | PLT (ug/L) |
PLT (109/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | ± s | ≤0.05 (n) | 0.05–0.12 (n) | >0.12 (n) | n | ± s | <125 (n) | 125–350 (n) | >350 (n) | |
| Moderate | 60 | 0.08 ± 0.279 | 55 | 2 | 3 | 60 | 214.05 ± 91.34 | 7 | 46 | 7 |
| Severe | 56 | 0.14 ± 0.353 | 48 | 3 | 5 | 56 | 199.04 ± 73.11 | 8 | 46 | 2 |
| Critically severe | 16 | 0.44 ± 0.512 | 9 | 4 | 3 | 16 | 202.50 ± 85.19 | 4 | 12 | 0 |
| Total | 132 | 0.15 ± 0.360 | 112 | 9 | 11 | 132 | 206.28 ± 82.98 | 19 | 104 | 9 |
| H/p | H = 8.073 p = 0.018 | H = 0.513 p = 0.774 | ||||||||
| χ2/p | χ2 = 13.79 p = 0.008 | χ2 = 5.73 p = 0.220 | ||||||||
Normal reference values: SAA (<10 mg/L); CRP (<3 mg/L); WBC (3.5–9.5 × 109/L); L (1.1–3.2 × 109/L); PCT (<0.05 ug /L); PLT (125–350 × 109/L).
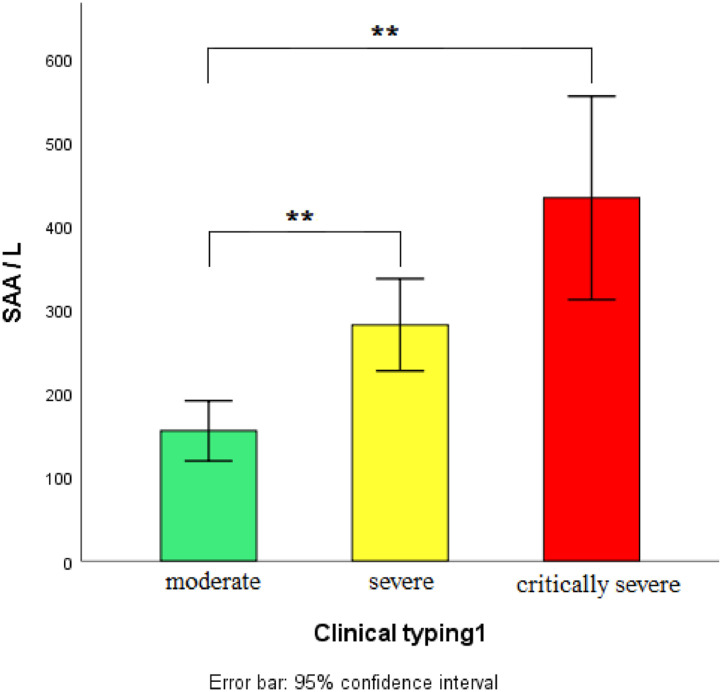
Due to unequal variance, Tamhane ’s T2 statistical method was a fair measure to perform multiple comparisons among groups of mild, moderate, severe, and critical severe patients for the value of SAA/L. The SAA/L of severe/critically severe patients was significantly higher than that of mild/moderate ones, and p < 0.01 indicates significant difference. (Fig. 1 ).
Fig 1.
SAA/L and clinical classification.
(3) SAA/L,CRP,SAA,L and clinical classification
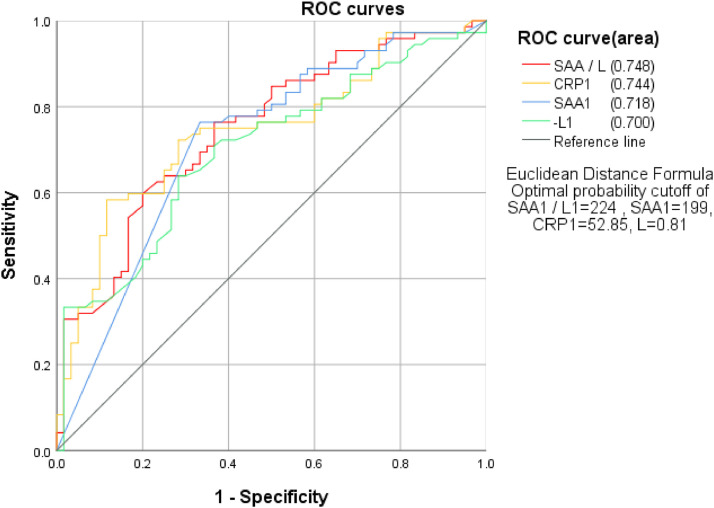
To detect if SAA/L is more sensitive in predicting the severity of disease, the authors used ROC curve analysis to calculate the area under the curve (AUC), regarding mild/moderate type as negative whereas severe/critical severe type as positive. The results showed that AUC from high to low was SAA1/L1 > CRP1 > SAA1 > L1, with the specific value at 0.748, 0.744, 0.718, and 0.700, respectively. Next, the authors used the method of Jordan Index to calculate the critical values of SAA/L that could be utilized as the reference for patient clinical classification (Fig. 2 ).
Fig 2.
SAA/L,CRP,SAA,L and clinical classification.
Relationship between SAA, CRP, L, SAA/L levels and disease progression
The dynamic changes of SAA, CRP, L, and SAA/L reflected the change of patient condition at 3–5 day-hospitalization. Patients with decreased SAA2, CRP2, SAA2/L2 and elevated L2 were more likely to have improved conditions (Table 3 ).
Table 3.
Relationship between SAA, CRP, L, SAA/L levels and disease progression at the time point of 3–5 day-hospitalization (M±SD).
| Clinical changes | N | SAA2 | CRP2 | L2 | SAA2/L2 | |
|---|---|---|---|---|---|---|
| Improved | 26 | 72.32±66.46 | 18.01±24.88 | 1.25±0.48 | 101.97±191.98 | |
| Stable | 78 | 88.30±83.05 | 22.81±35.10 | 1.29±0.78 | 126.85±202.89 | |
| Exacerbation | 18 | 167.22±55.30 | 66.09±41.40 | 0.49±0.26 | 437.57±359.29 | |
| Total | 122 | 96.54±81.51 | 28.17±37.52 | 1.16±0.72 | 167.39±254.53 | |
| H | 15.655 | 20.119 | 27.313 | 29.787 | ||
| p | 0.000 | 0.000 | 0.000 | 0.000 |
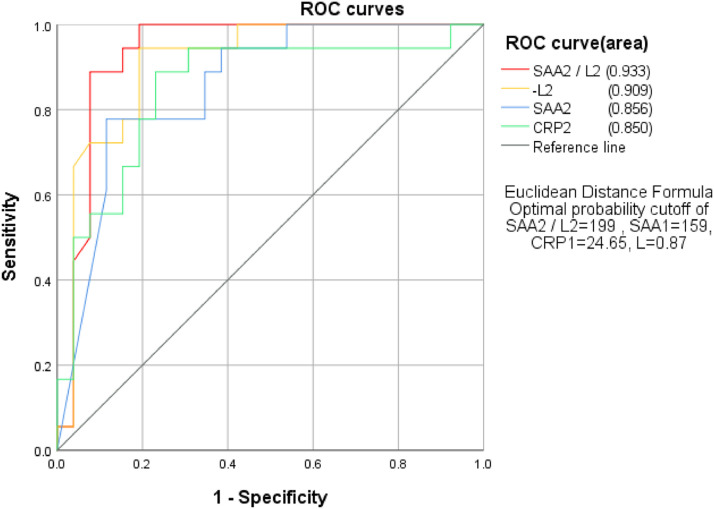
To detect if SAA2/L2 is more sensitive in predicting the progression of disease, the authors used ROC curve analysis to calculate the AUC of SAA2, CRP2, L2, and SAA2/L2, with the criteria of recovering as negative whereas exacerbation as positive. The results showed that AUC from high to low was SAA2/L2 > L2 > SAA2 > CRP2, with the specific value of 0.933, 0.909, 0.856, and 0.850, respectively. Next, the authors used the method of Jordan index to calculate the optimal critical values of them and obtained 199, 159, 24.65, and 0.87 for SAA2/L2, SAA2, CRP2, and L2, respectively (Fig. 3 ).
Fig 3.
Relationship between SAA2/L2, SAA2, CRP2, L2 and the progression of disease.
Relationship between SAA, CRP, L, SAA/L dynamics and the progression of disease
The changes of SAA, CRP, L and SAA/L between the first and second time point. Patients with increased SAA2, CRP2, SAA2/L2 and decreased L2 were more likely to have a worsening condition.Patients with decreased SAA2, CRP2, SAA2/L2 and elevated L2 were more likely to have improved conditions (Table 4 ).
Table 4.
Relationship between SAA, CRP, L, SAA/L dynamics and the progression of disease (M±SD).
| Clinical changes | N | SAA2-SAA1 | CRP2-CRP1 | L2-L1 | SAA2/L2-1 | |
|---|---|---|---|---|---|---|
| Improved | 26 | −96.00±67.76 | −40.09±38.24 | 0.46±0.52 | −196.01±199.40 | |
| Stable | 78 | −53.03±81.19 | −23.97±40.85 | 0.29±0.49 | −80.02±191.84 | |
| Exacerbation | 18 | 1.00±80.92 | −12.89±61.76 | −0.20±0.26 | 125.85±351.44 | |
| Total | 122 | −54.21±83.02 | −25.77±44.39 | 0.25±0.51 | −74.37±241.15 | |
| H | 14.186 | 2.934 | 23.849 | 23.615 | ||
| p | 0.001 | 0.231 | 0.000 | 0.000 |
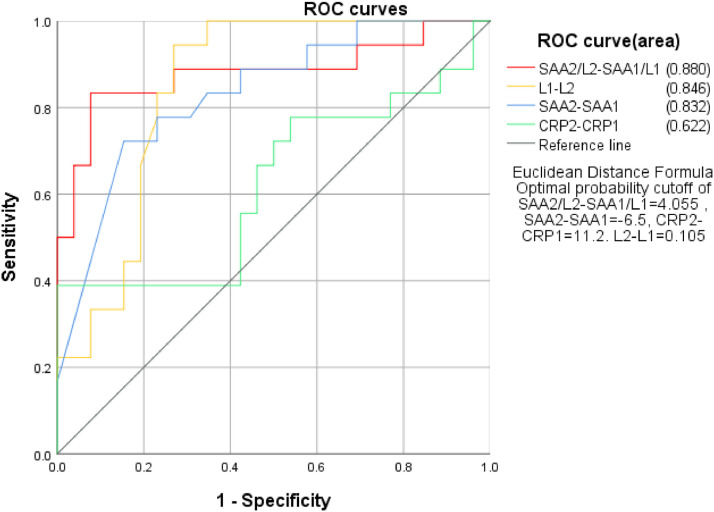
To detect if the dynamic changes of studied inflammation factors were valuable in predicting the progression of disease, the authors used ROC curve analysis to calculate the AUC of the changes of SAA, CRP, L and SAA/L between the first and second time point, regarding recovering as negative whereas exacerbation as positive. The results showed that AUC from high to low were SAA2/L2-SAA1/L1>L1-L2>SAA2-SAA1>CRP2-CRP1, with the specific value at 0.880, 0.846, 0.832, 0.622, respectively. Next, the authors used the method of Jordan index to calculate the optimal critical values according to sensitivity and specificity.
ROC curve analysis results at three time points indicated SAA/L was more sensitive in predicting the progression of disease, especially SAA2/L2 at the second time point, with the sensitivity high at 0.933 (Fig. 4 ).
Fig 4.
Relationship between SAA, CRP, L, SAA/L dynamics and the progression of disease.
Relationship between SAA3, CRP3, L3, SAA3/L3 levels and patient outcome at the composite endpoint
The authors performed a multivariate analysis of variance for SAA levels that were measured at three time points. The results showed a significant correlation of SAA dynamics and patient outcome. Specifically, SAA3, CRP3, and SAA3/L3 levels continued to increase whereas L consistently decreased in exacerbating and deceased patients. While, the levels of SAA3, CRP3, and L3 were within normal ranges in recovered patients (Table 5 )
Table 5.
Relationship between SAA3, CRP3, L3, SAA3/L3 levels and patient outcome at composite endpoint (M±SD).
| Outcome | N | SAA3 | CRP3 | L3 | SAA3/L3 | |
|---|---|---|---|---|---|---|
| Discharge | 51 | 11.02±26.83 | 2.52±3.61 | 1.43±0.41 | 8.60±21.79 | |
| Improved | 27 | 27.67±51.41 | 10.46±18.68 | 1.39±1.05 | 35.45±87.47 | |
| Exacerbation | 5 | 121.40±107.63 | 92.06±85.11 | 0.83±0.61 | 195.60±201.66 | |
| Death | 11 | 169.82±52.85 | 79.92±58.15 | 0.44±0.29 | 540.24±351.73 | |
| Total | 94 | 40.26±69.13 | 18.62±40.94 | 1.27±0.73 | 88.47±215.68 | |
| H | 37.412 | 38.405 | 29.919 | 38.280 | ||
| p | 0.000 | 0.000 | 0.000 | 0.000 |
The relationship between the dynamic changes of SAA, CRP, l, SAA/L/ and clinical outcome at composite endpoint
In patients who were discharged/well recovered, from the first time point at admission to the third time point at composite endpoint, the levels of SAA, CRP, and SAA/L consistently decreased, but L consistently increased. While, in exacerbating and deceased patients, the levels of SAA, CRP, and SAA/L consistently increased, whereas L decreased (Table 6 ).
Table 6.
The relationship between the dynamic changes of SAA, CRP, l, SAA/L and the patient outcome at composite endpoint (M±SD).
| outcome | N | SAA3-SAA1 | CRP3-CRP1 | L3-L1 | SAA3/L3-SAA1/L1 |
|---|---|---|---|---|---|
| Discharge | 51 | −133.55±79.49 | −39.35±36.39 | 0.43±0.41 | −191.97±164.67 |
| Improved | 27 | −128.89±89.70 | −39.73±38.84 | 0.44±0.68 | −196.53±208.92 |
| Exacerbation | 5 | −45.00±154.37 | 4.46±119.26 | 0.08±0.40 | −125.26±248.16 |
| Death | 11 | −3.55±90.00 | −5.24±75.23 | −0.27±0.30 | 198.19±399.65 |
| Total | 94 | −112.29±97.53 | −33.14±50.56 | 0.33±0.54 | −144.07±250.07 |
| H | 17.218 | 4.012 | 20.406 | 14.598 | |
| p | 0.001 | 0.260 | 0.000 | 0.002 | |
| Outcome | N | SAA3-SAA2 | CRP3-CRP2 | L3-L2 | SAA3/L2-SAA1/L2 |
|---|---|---|---|---|---|
| Discharge | 51 | −59.84±76.34 | −13.64±21.55 | 0.12±0.37 | −76.09±139.69 |
| Improved | 27 | −76.96±87.36 | −16.20±32.41 | 0.17±0.45 | −88.40±116.62 |
| Exacerbation | 5 | −7.60±123.01 | 49.58±101.22 | −0.04±0.41 | 3.44±291.33 |
| Death | 11 | 0.82±82.50 | 20.03±64.78 | −0.03±0.27 | 7.44±355.42 |
| Total | 94 | −54.88±85.39 | −7.07±41.74 | 0.11±0.39 | −65.62±180.63 |
| H | 10.812 | 8.721 | 3.178 | 4.987 | |
| p | 0.013 | 0.033 | 0.365 | 0.173 |
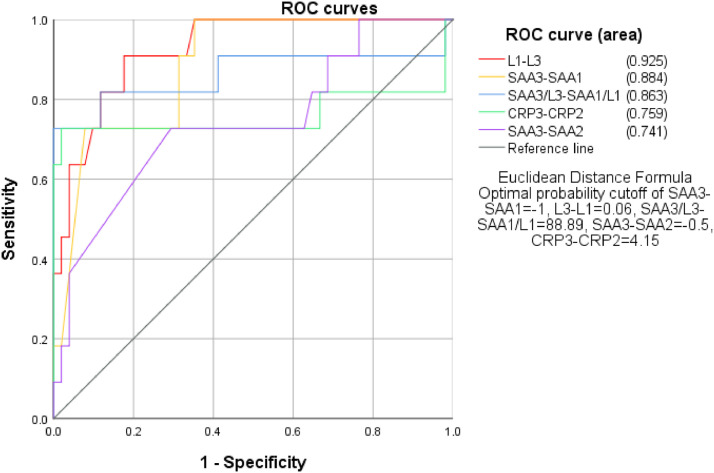
To detect if SAA and L dynamic changes are valuable in predicting the patient outcome, the authors used ROC curve analysis to calculate the AUC of the difference of SAA, L, and SAA/L between the first and third time point, with the criteria of patient discharge as negative whereas patient death as positive. The results showed that AUC from high to low were L1-L3>SAA3-SAA1>SAA3/L3-SAA1/L1, with the specific value of 0.925, 0.884, and 0.863, respectively (Fig. 5 ).
Fig 5.
The correlation of SAA, CRP, L and SAA/L dynamics and patient outcome.
The relationship between CT imaging and clinical outcome
The CT imaging features were evaluated and scored as follows: normal (0 points), mild (1 point), progressive (2 points), and severe (3 points). Of the CT scans performed at admission, 98.5% (130/132) showed abnormalities, and only two cases were normal.
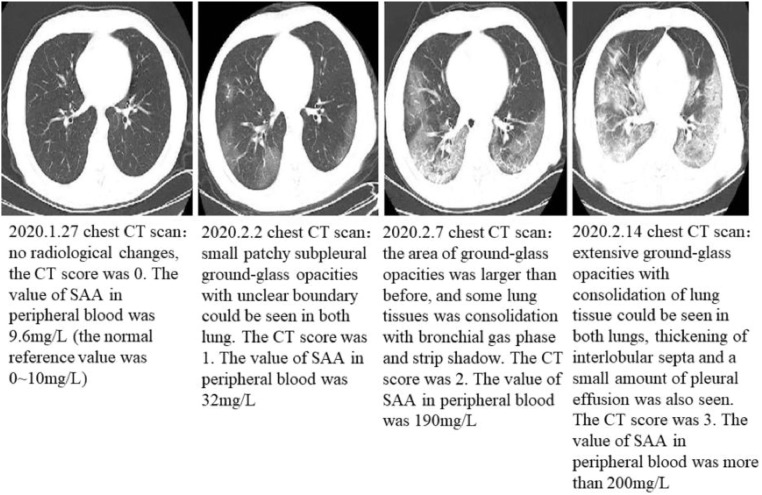
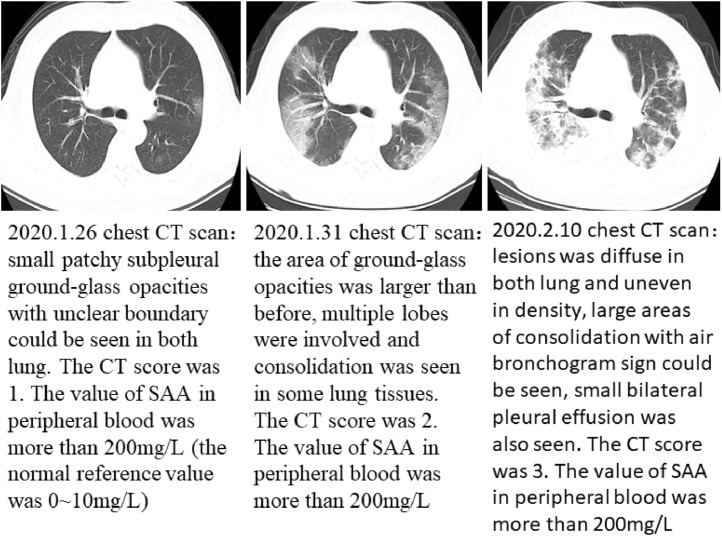
Fig 6, Fig 7 showed the CT imaging changes and SAA dynamic changes of the two patients in different periods. SAA detection is consistent with CT in judging the change of the disease, even more sensitive.
Fig 6.
(64 year-old male patient, febrile and cough for 2 days): serial CT scans and SAA dynamic changes.
Typical serial CT scans and SAA dynamic changes.
Fig 7.
(84 year-old male patient, febrile, hacking cough, short of breath for three days): serial CT scans and SAA dynamics, the first CT scan was mild, but SAA was more than 200 mg/L, and maintained at a high level, which suggest poor prognosis, the later two CT images showed severe (the patient died 21 days later after admission).
Typical serial CT scans and SAA dynamic changes.
Of ten exacerbating patients, seven of them had the second CT scan showing the worsening of disease; Of six deceased patients, five of them had the second CT imaging showing the worsening of disease; Of six exacerbating or deceased patients, five of six had the third CT scan showing the worsening of disease. Additionally, all of deceased patients had CT scans showing the worsening of disease (Table 7 ).
Table 7.
The relationship between CT imaging and clinical outcome.
| Outcome | CT (2nd scan) |
CT (3rd scan) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + (n) | — (n) | 0 (n) | n | χ2/p |
+ (n) | — (n) | 0 (n) | n | χ2/p |
|
| r/p | r/p | |||||||||
| Discharge | 1 | 15 | 35 | 51 | χ2 = 35.91 | 1 | 25 | 14 | 40 | χ2 = 40.66 |
| Improved | 6 | 2 | 19 | 27 |
p = 0.000 |
2 | 13 | 7 | 22 |
p = 0.000 |
| Exacerbation | 2 | 1 | 1 | 4 | r = 0.486 | 2 | 1 | 0 | 3 | r = 0.302 |
| Death | 5 | 0 | 1 | 6 | p = 0.000 | 3 | 0 | 0 | 3 | p = 0.013 |
| Total | 14 | 18 | 56 | 88 | 8 | 39 | 21 | 68 | ||
The relationship between SAA level and serial CT scan
According to the correlation analysis showed in Table 8 , the first SAA level at admission was correlated with the dynamic changes of the first, second, and third CT scans. Specifically, for the patients with higher level of SAA1 at admission, the CT classification tended towards severe. The correlation of the first SAA with the second CT result was higher than that with the first CT result, suggesting the significance of SAA in estimating the progression of disease was higher than CT scan alone (Table 8).
Table 8.
The relationship between SAA level and serial CT scan.
| CT classification | SAA1 | ||||
|---|---|---|---|---|---|
| <10 | 10–200 | ≥200 | 合计 | r/p | |
| CT1 | r = 0.382 | ||||
| Normal | 1 | 1 | 0 | 2 | p = 0.000 |
| Mild | 4 | 10 | 8 | 22 | |
| Progressive | 2 | 17 | 29 | 48 | |
| Severe | 0 | 4 | 18 | 22 | |
| Total | 7 | 32 | 55 | 94 | |
| CT2 | |||||
| Normal | 1 | 0 | 0 | 1 | r = 0.413 |
| Mild | 6 | 13 | 8 | 27 | p = 0.000 |
| Progressive | 0 | 15 | 30 | 45 | |
| Severe | 0 | 4 | 11 | 15 | |
| Total | 7 | 32 | 49 | 88 | |
| CT3 | |||||
| Normal | 0 | 0 | 0 | 0 | r = 0.298 |
| Mild | 5 | 17 | 22 | 44 | p = 0.014 |
| Progressive | 0 | 4 | 15 | 19 | |
| Severe | 0 | 1 | 4 | 5 | |
| Total | 5 | 22 | 41 | 68 | |
Discussion
COVID-19 is an acute infectious disease caused by a new coronavirus (SARS-CoV-2). The main initial symptom is mild to moderate fever.3 , 5 Some patients may have multiple systematic symptoms such as chills, malaise, respiratory distress, or gastroenterological disorders like nausea/vomiting. In some cases, the disease progresses rapidly and patients develop acute respiratory distress syndrome, septic shock, uncompensated acidosis, and coagulation dysfunction within a few days.5, 6, 7, 8 The severity and prognosis of COVID-19 are complicated by the diversity of symptoms, imaging manifestations, and the degree of disease progression.8 , 9 Therefore, early diagnosis and appropriate treatment are essential in reducing the morbidity and mortality of COVID-19-infected patients.
Inflammatory factors, such as SAA, CRP, L, PCT, WBC, and PLT are frequently used to predict, diagnose, and evaluate many inflammatory diseases. SAA is a non-specific acute phase protein mainly produced by cytokines IL-1β, IL-6 and TNF-α in liver cells. As a marker of inflammation, its clinical value is obtaining more attention recently.10, 11, 12 Studies report that patients with severe acute respiratory syndrome had significantly increased level of SAA, suggesting SAA could be used as a biomarker to monitor the progression of respiratory diseases.13 SAA is able to promote inflammatory response through activating chemokine and inducing chemotaxis even at a very low concentration.14 , 15 Studies have suggested that patients infected with COVID-19 had a large amount of IL-1β, IFN-γ, IP-10, MCP-1 and other cytokines present in system, leading to the activation of Th1 cell. Compared with mild patients, critically ill patients may have more IL-1β, IL-6, MCP-1, MIP-1, TNF-α and other cytokines expressed, which boost liver cells to produce SAA.16 , 17
Patients with respiratory virus infection usually have clinical symptoms after 36 to 48 h of infection, and SAA gradually increases and reaches to peak at 3–4 day post infection. During the phase of recovery, it is reported that SAA level continuously decreased and the decrease rate was faster than that of CRP.18 CRP is also an acute phase protein produced when infection occurs. CRP level rises rapidly and the rate of increase is positively correlated with the severity of infection. Lymphocytes, as the key element in immune response, have three types of cells including T cells, B cells, and NK cells. Huang et al.16 suggests that patients with COVID-19 have a large amount of IL-1β, IFN-γ, IP-10, and MCP released, causing the activation of Th1 cells, SAA, CRP, L, PCT, WBC, and PLT. These inflammatory factors can be used as indicators to reflect the body's response to infection.
The authors of this study want to detect if these indicators have clinical value in COVID-19 infection. The results showed that in COVID-19 infection, SAA and CRP levels increased significantly, while L decreased, and PCT, WBC, and PLT were in the normal range, it was consistent with Wang D's results.19 As the disease progressed from mild to critically severe, SAA and CRP gradually increased, while L gradually decreased, but PLT, WBC, and PCT were all within the normal range. In this study, SAA<10 mg/L was used as normal clinical reference value. Of 132 patients, 123 patients had SAA level above 10 mg/L. Almost all severe and critically severe patients had SAA level greater than 10 mg/L, 12 of which were above 200 mg/L, suggesting that the SAA is a sensitive indicator for the severity of COVID-19. ROC curve analysis shows that AUC that from high to low is: SAA/L > CRP > SAA > L, suggesting that SAA/L is a reliable indicator in distinguishing severe COVID-19 infection cases from mild ones.
Compared with the initial levels at the first time point, dynamic changes in SAA, CRP, L, and SAA/L levels at 3–5 day-hospitalization (second time point) and composite endpoint (the last time point) reflected the change of patient's condition. Patients with decreased SAA, CRP, SAA/L and elevated L had improved clinical conditions. In addition, the results showed that SAA levels continued to increase in exacerbating and deceased patients, whereas in patients who were discharged and well recovered, SAA fell below 3 mg/L. The trend could be described as those patients with progressively decreased SAA are more likely to have a better prognosis than the patients who have continuously high level of SAA, suggesting a significant correlation between SAA dynamic change and prognosis. This phenomenon may be related to the activation of body's inflammation response, which stimulates liver cells to produce a large amount of SAA.20 , 21
CT imaging has been proved to be one of the most significant clinical diagnostic methods for COVID-19. According to the correlation analysis in this study, the first SAA level at admission was correlated with the dynamic changes of the first, second, and third CT scan. Specifically, for the patients with higher level of SAA, the CT classification tended towards severe. The correlation of the first SAA with the second CT result was higher than that with the first CT result, suggesting the value of SAA in estimating the progression of disease, especially when combined with serial CT scans during the course of disease.
This study also found that the ratio of SAA to L was more sensitive than SAA or L used alone, as SAA/L had the highest AUC in ROC analysis, compared with SAA and L. The authors found there was a significant statistical correlation of SAA/L with clinical classification and outcomes when using these critical values as a reference for patient categorization.
Limitations of this study
One limitation of this study lies in it was performed in a single medical facility, lacking the control group design due to the emergent situation of COVID-19 breakout. In the future, the researchers will collaborate with a few medical facilities in the area and design the control group to improve the reliability of the study.
Conclusion
Based on the study results, SAA alone or combined with L (SAA/L) could be used as a significant marker to indicate and track inflammation conditions in COVID-19 infected patients. SAA and L are sensitive indicators in evaluating the severity and prognosis of COVID-19. Monitoring dynamic changes of SAA, combined with CT imaging could be a valuable strategy in the diagnosis and treatment of COVID-19.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgments
On behalf of the authors, We sincerely thank clinicians in Tianyou Hospital Affiliated to Wuhan University of Science and Technology for their dedication.
Ethical approval
This study was approved by the Medical Ethics Review Board of Wuhan University of Science and Technology (No. 202009).
Contributor Information
Hui Long, Email: longhui@wust.edu.cn.
Qiang Wang, Email: wangqiang@wust.edu.cn.
Qingming Wu, Email: wuhe9224@sina.com.
References
- 1.Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Based Med. 2020 doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D. A novel coronavirus from patients with pneumonia in China.2019. New Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikari S.P., Meng S., Wu Y.J., Mao Y.P., Ye R.X., Wang Q.Z. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han W.Z., Quan B., Guo Y., Zhang J., Lu Y., Feng G. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92(5):461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H.L. Genomic characterization and epidemiology of 2019 novel coronavirus: implications of virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen T.M., Zhang Y., Pandolfi P.P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020;30(3):189–190. doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19) — China, 2020. China CDC Weekly. 2020;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, china: retrospective case series. BMJ. 2020;368:m792. doi: 10.1136/bmj.m792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baranova I.N., Souza A.CP, Bocharov A.V, Vishnyakova T.G., Hu X.Z., Vaisman B.L. Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye R.D., Sun L. Emerging functions of serum amyloid a in inflammation. J Leukoc Biol. 2015;98(6):923–929. doi: 10.1189/jlb.3VMR0315-080R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang R.Z., Lee M.J., Hu H., Pollin T.I., Ryan A.S., Nicklas B.J. Acute-phase serum amyloid A:an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3(6):e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip T.T.C., Chan J.W.M., Cho W.C.S., Yip T.T., Wang Z., Kwan T.L. Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid a protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin Chem. 2005;51(1):47–55. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly M., Rooney P.R., Mcgarry T., Maratha A.X., Mccormick J., Miggin S.M.. Acute serumamyloid a is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann Rheum Dis. 2016;75(7):1392–1398. doi: 10.1136/annrheumdis-2015-207655. [DOI] [PubMed] [Google Scholar]
- 15.Sack Jr G.H. Serum amyloid A-a review. Mol Med. 2018;24(1):46.. doi: 10.1186/s10020-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.L., Wang Y.M., Li X.W., Ren L.L., Zhao J.P., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, china. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrens K., Alexander W.S. Cytokine control of megakaryopoiesis. Growth Factors. 2018;36(3–4):89–103. doi: 10.1080/08977194.2018.1498487. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y., Chen J., Cai B., Zhang J.L., Li L.X., Liu C. The use of PCT, CRP, IL⁃6 and SAA in critically ill patients for an early distinction between candidemia and Gram positive/negative bacteremia. J Infect. 2012;64(4):438–440. doi: 10.1016/j.jinf.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Wang D.W., Hu B., Hu C., Zhu F.F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, china. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramana C.V., DeBerge M.P., Kumar A., Alia C.S., Durbin J.E., Enelow R.I. Inflammatory impact of IFN-γ in CD8+T cellmediated lung injury is mediated by both Stat1-dependent and-independent pathways. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L650–L657. doi: 10.1152/ajplung.00360.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Me. 2020 doi: 10.1093/jtm/taaa021. pii:taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]