Abstract
Recent retrospective studies from Wuhan, China suggest Novel Coronavirus Disease 2019 (COVID-19) may be associated with a hypercoagulable state and increased risk for venous thromboembolism. The overlap in the signs and symptoms of COVID-19 associated Acute Respiratory Distress Syndrome (ARDS) and COVID-19 with concurrent pulmonary embolism creates a diagnostic challenge for emergency medicine physicians in patients already at risk for renal impairment. However, identifying features atypical for COVID-19 alone may play a role in the judicious use of Computed Tomography Angiography among these patients. Hemoptysis is seen in roughly 13% of pulmonary embolism cases and infrequently reported among COVID-19 infections. Additionally, the presence of right heart strain on electrocardiography (EKG) is a well described clinical presentations of pulmonary embolism not reported commonly with COVID-19 infections.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Pulmonary embolism, Venous thromboembolism
1. Introduction
Arising in China in the winter of 2019, COVID-19 (caused by the SARS-CoV-2 virus) has caused a global pandemic, and severely stressed medical systems across the world. Although knowledge of this novel coronavirus is emerging, the most common reason for hospitalization of COVID-19 patients is severe respiratory distress [1]. COVID-19 has been well described as causing a proinflammatory and hypercoagulable state with marked elevations seen in Lactate Dehydrogenase, Ferritin, C-reactive protein, D-Dimer, and Interleukin levels [2,3]. While, COVID-19 cardiac complications are well described, an understanding of the role venous thromboembolic disease (VTE) in the pandemic is still developing [4]. Few descriptions of the potential relationship between COVID-19 and VTE exist in the literature [5,6]. If an association between COVID-19 and VTE, particularly PE, exists then clinical features that are atypical for the novel coronavirus like hemoptysis, may aid emergency clinicians with identifying concurrent PE's during the current pandemic. This case illustrates the utility of clinical features that are atypical for COVID-19 infection in identification of concurrent pulmonary embolism with this novel disease.
2. Case report
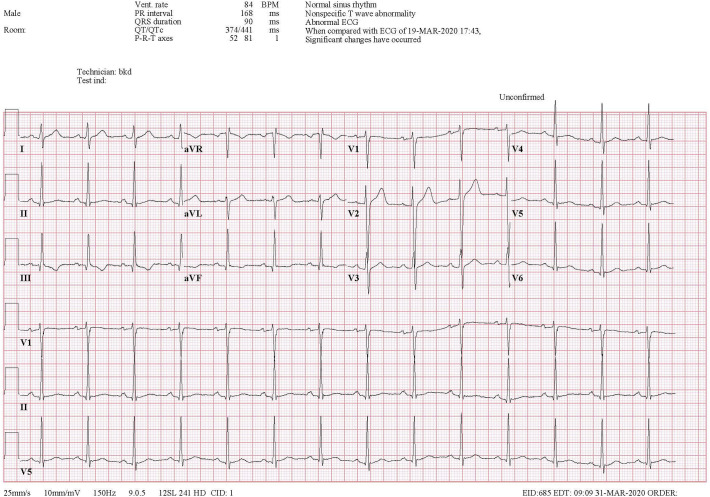
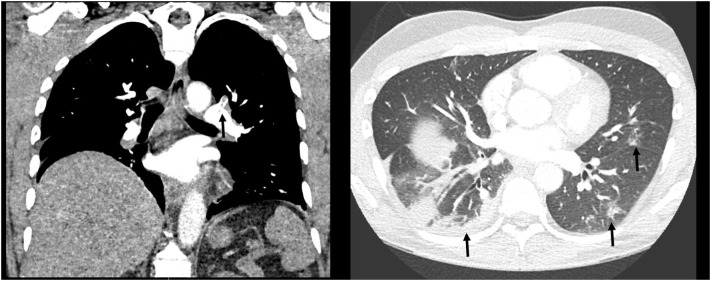
A 42-year-old male without recent travel and no significant personal or familial medical history presented to the emergency department with worsening chest pain, shortness of breath, and hemoptysis. He had been previously diagnosed with mild COVID-19 infection twelve days prior to presentation. He was managed uneventfully at home until the day of presentation when he developed worsening exertional dyspnea, central pleuritic chest pain, and hemoptysis (estimated 10 ml). On presentation he was afebrile and demonstrated a normal heart rate, blood pressure, and oxygen saturation, but he demonstrated a respiratory rate of 30 breaths per minute. His physical exam revealed mild respiratory distress with bibasilar rhonchi but otherwise no other acute findings. Laboratory evaluation was notable for a D-dimer of 4.8 (μg/dl). Electrocardiography (EKG) showed show flattening of the T-waves in the inferior leads as compared to his prior EKGs with right axis deviation and a S1Q3T3 pattern (Fig. 1 ). Chest radiograph was significant for a right lower lobe infiltrate. Given his hemoptysis, evidence of right heart strain on his EKG and elevated D-dimer a Computerized Tomography Angiography (CTA) of the chest was obtained. The test revealed bilateral segmental pulmonary emboli and an additional area of consolidation in the right lower lobe concerning for infarct (Fig. 2 ). Additional findings of peripheral ground glass opacities consistent with COVID-19 pneumonia were also noted. The patient was admitted to a negative pressure room, started on anticoagulation with heparin and eventually discharged to home on a novel oral anticoagulant.
Fig. 1.
EKG with an S1Q3T3 Pattern.
Fig. 2.
(a) CT PA showing segmental clot. (b) CT PA Lung window showing bilateral ground glass opacities.
3. Discussion
The full spectrum of COVID-19 disease is still emerging, but several research studies have highlighted that patients suffering from COVID-19 tend to have high d-dimers, fibrinogen and fibrin degradation products [3,7]. Additional research has suggested that these patients may have a mortality benefit from anticoagulation [7]. During the Severe Acute Respiratory Syndrome (SARS) outbreak in 2002–2003 and H1N1 influenza pandemic in 2009 case reports described concurrent PE with viral lung infections [8,9]. Data from murine models suggests that SARS-CoV may interact with urokinase to produce a hypercoagulable state observed in SARS related acute lung injury [10].
An association between COVID-19 and PE creates a diagnostic challenge for emergency medicine clinicians given the overlap in symptoms between the two clinical entities. Elevated D-Dimer levels (>1.0 mg/dl) have been identified as a potential predictor of increased mortality, but are not specific to the diagnosis of VTE [11,12]. Reliance on D-dimer as a screening tool should be discouraged in this patient population and may lead to over utilization of Computed Tomography Angiography (CTA) in a patient population already at risk for acute kidney injury [1,12] However, hemoptysis has been described as an infrequent COVID clinical symptom (0–5%) by retrospective analysis from Wuhan, China and may be useful in setting pretest probability [11,13]. For comparison, patients in the Prospective Investigation of Pulmonary Embolism (PIOPED) group were noted to have hemoptysis in 13% of PE cases [14]. Hemoptysis was also one of the clinical features with the highest association with PE in the final logistic regression model of the Pulmonary Embolism Rule Out Criteria (PERC) [15]. In addition to clinical features like hemoptysis, signs of right heart strain on adjunctive bedside tests like EKG or point of care ultrasound maybe helpful for clinicians in identifying COVID-19 patients at risk for concurrent pulmonary embolism.
4. Conclusion
This case is one of the first to report segmental PE's in a patient infected with SARS-CoV-2 without an otherwise recognized VTE risk factor. Awareness of the potential association between COVID-19 and PE is important for emergency medicine clinicians. Equally important during this novel coronavirus pandemic is the judicious use of CTA. The use of this imaging modality can by informed by recognition of atypical COVID-19 clinical features like hemoptysis.
Declaration of competing interest
The views expressed in this case report are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. We are military service members. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 28 Feb. 2020 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G., Wu D., Guo W., Cao Y., Huang D. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 Mar 27 doi: 10.1172/JCI137244. (pii: 137244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H., Yang L., Liu R., Liu F., Wu K.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med (CCLM) Mar 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 4.Chen T., Wu D., Chen H., Yan W., Yang D. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 26;368 doi: 10.1136/bmj.m1091. (m1091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 complicated by acute pulmonary embolism. Radiology: Cardiothoracic Imaging. https://pubs.rsna.org/doi/10.1148/ryct.2020200067. Accessed 30 March, 2020. [DOI] [PMC free article] [PubMed]
- 6.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020 Mar 30 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Ning. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 27 Mar. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng K.H.L. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad. Med. J. Jan. 2005;81(956) doi: 10.1136/pgmj.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avnon L.S., Munteanu D., Smoliakov A., Jotkowitz A., Barski L. Thromboembolic events in patients with severe pandemic influenza A/H1N1. Eur. J. Intern. Med. 2015 Oct;26(8):596–598. doi: 10.1016/j.ejim.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Gralinski Lisa E. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. June 2013;4(4) doi: 10.1128/mbio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein P.D., Terrin M.L., Hales C.A., Palevsky H.I., Saltzman H.A. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(3):598. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 15.Kline J.A., Mitchell A.M., Kabrhel C., Richman P.B., Courtney D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J. Thromb. Haemost. 2004 Aug;2(8):1247–1255. doi: 10.1111/j.1538-7836.2004.00790.x. [DOI] [PubMed] [Google Scholar]