Abstract
This trial assessed the efficacy of a commercial essential oil (EO) product on the immune response to vaccination against Newcastle disease (ND) and subsequent challenge with virulent ND virus genotype VII (vNDv genotype VII) by using the following experimental groups of broiler chickens (Each group had 21 birds with 3 replicates in each, n = 7): NC (negative control), PC (positive control), VC (vaccinated), and VTC (vaccinated and treated with EOs). Moreover, in a trial to study the effect of EOs on vNDv genotype VII in vivo as a preventive or therapeutic measure, 2 additional ND-vaccinated groups were used (PRV: medicated 1 D before vNDv challenge for 5 D; and TTT: medicated 2 D after vNDv challenge for 5 D). In addition, the immune-modulatory effect of EOs on the avian influenza (AI), infectious bronchitis (IB), and infectious bursal disease (IBD) vaccines was assessed through the serological response. The use of EOs along with administration of ND vaccines (VTC) revealed a lower mortality rate (42.86%), clinical signs, and postmortem lesion score (11) than ND vaccines alone (VC) (52.28% mortality and score 15), in addition to lower hemagglutination inhibition (P < 0.05) (6.5 ± 0.46) and viral shedding (10 log 2.28 ± 0.24) titres 1 wk after challenge in comparison with VC (8.63 ± 0.65 and 10 log 3.29 ± 0.72, respectively). Nevertheless, the EOs mixture (VTC) (1952 ± 28.82) did not significantly (P > 0.05) improve growth performance compared with the nontreated birds (NC and VC) (1970 ± 19.56 and 1904 ± 38.66). EOs showed an antiviral effect on vNDv in vivo (in chickens) as a preventive measure (PRV) as well as some therapeutic effect (TTT) through decreasing the viral shedding titres (loNC0), mortality rate, and severity of clinical signs and postmortem lesions, in addition to serum malondialdhyde level. Regarding the other viruses, the EOs mixture did not improve the immune response to the AI and IB vaccines but significantly (P < 0.05) increased the ELISA antibody titre for IBD virus at the 28th D of age (2,108 ± 341.05). The studied EOs mixture showed an immune-stimulating response to ND and IBD vaccines, antiviral effect against ND virus, especially if administered before the challenge; however, it did not have a growth-promoting effect.
Key words: essential oils, immune response, Newcastle, avian influenza, infectious bronchitis
Introduction
Avian viral diseases caused by avian influenza (AI), infectious bronchitis (IB), infectious bursal disease (IBD), and Newcastle disease (ND) viruses are a major obstacle in the poultry industry worldwide and result in substantial economic losses, especially in broilers, due to respiratory distress, high mortality, impaired growth, or immune suppression. Furthermore, they have the capability of inducing diseases independently or in concurrent infection with each other (Roussan et al., 2008). AI viruses are classified in the genus Influenza virus of Orthomyxoviridae family (Cox et al., 2000), while the etiology of IBD or Gumboro disease is the IBD virus that is a member of genus Avibirnavirus of the Birnaviridae family (Delmas et al., 2019). IB virus is belonging to Coronavirus genus of family Coronaviridae (Enjuanes et al., 2000), while the cause of ND is ND virus, belonging to genus Avulavirus of Paramyxoviridae family (Hines and Miller, 2012). All these viruses have RNA genome enclosed within either enveloped (AI, IB, and ND viruses) or nonenveloped (IBD virus) capsid. Their outbreaks still occur in the field, although several live, inactivated, or recombinant vaccines are commercially available, and different vaccination programs are applied for each disease (Swayne and King, 2003). Many factors are related to this problem, and one of them is the immune suppression or an immature immune system (Skowronski et al., 2008). Moreover, if a virus is endemic in a region, its elimination is difficult, so additional methods are required to combat these viruses along with vaccination. Recently, there has been increased interest in the use of natural antimicrobial agents, and the use of EOs in combination as a strategy to control many pathogens (Gutierrez et al., 2008). Over 3,000 EOs are known, of which approximately 300 are commercially important and used in the flavour and fragrance industries (Van de Braak and Leijten, 1999, Baghban-Kanani et al., 2019). Essential oils are produced by medicinal plants, and their effects vary based on the component, which may have antibacterial (Sokovic et al., 2010, Qorbanpour et al., 2018), antiviral (Koch et al., 2008, Gado et al., 2019), anticoccidial (Mohiti-Asli and Ghanaatparast-Rashti, 2015), antifungal (Pinto et al., 2006), antioxidant (Nunez et al., 2007), or growth-promoting effects (Abd El-Hack et al., 2016a, Abd El-Hack et al., 2016b, Abd El-Hack et al., 2016c, Abd El-Hack et al., 2018). Nevertheless, Reichlinga et al. (2009) concluded in their review that the most tested essential oils exhibited a clear antiviral activity on enveloped DNA and RNA viruses, while they did not affect the nonenveloped viruses. In addition, there are recent studies proving that EOs can improve the cellular and humoral immune responses of chickens (Lee et al., 2011, Roofchaee et al., 2011). We hypothesized that EOs mixture stimulates immune response to different viral vaccines used in broiler flocks, promotes weight gaining, and can be used to the face the viral outbreaks such as ND virus.
The objectives of this study included 1) evaluation of EOs' efficacy on stimulating the immune response to ND vaccines through the challenge with virulent NDv (vNDv) genotype VII; 2) assessment of the antiviral effect of EOs in vivo as a preventive measure and as a therapy for vNDv infection in broiler chickens; 3) evaluation of the effect of commercially prepared EOs on the humoral immune response to the AI, IB, and IBD vaccines in broiler chickens.
Material and methods
Essential Oils
A mixture of EOs produced commercially under the trade name “Broclear” (Nutrex nv-Achterstenhoek Co., Belgium-aBE2165) was supplemented through the drinking water at a dose of 0.5 mL/L. This mixture contained oregano oil (50 g), carvacrol (10 g), thyme oil (33.33 g), eucalyptus oil (50 g), thymol (5 g), eucalyptol (10 g), and acacia (Arabic gum) surfactant (27 g) in water up to 1 L.
The Challenge With Virulent ND Virus (vNDv)
A previously isolated and characterized virulent genotype VII ND virus (Sedeik et al., 2019) was used for challenging experimental groups. The challenge was performed at the 28th D of age (d) with 106.3 EID50/100 μL/bird (Reed and Muench, 1938) via eye drop (50 μL) and the intranasal (50 μL) route (Sedeik et al., 2019). The negative control group was sham challenged with similar volumes and routes using phosphate-buffered saline.
Experimental Chickens and Design
A total of 126 one-day-old unsexed Ross 308 broiler chicks were obtained from a local hatchery and divided, by the ranking method to uniform initial body weight, into 6 groups (n = 21 with 3 replicates in each, n = 7), as follows:
NC: nonvaccinated, untreated, and nonchallenged (negative control)
PC: nonvaccinated, untreated, and vNDv challenged (positive control)
VC: vaccinated, untreated, and vNDv challenged (vaccinated, challenged)
VTC: vaccinated, treated (EOs treatment was started after each vaccine and continued for 3 D), and vNDv challenged (vaccinated, treated, and challenged)
PRV: vaccinated and treated (EOs treatment was started 1 D before vNDv challenge and continued for 5 D) (preventive group)
TTT: vaccinated and treated (EOs treatment was started 2 D after vNDv challenge and continued for 5 D) (therapeutic group)
All birds in the experimental groups except NC (negative control) were challenged with vNDv genotype VII at the 28th D. Vaccinated groups (VC, VTC, PRV, and TTT) received the vaccination program shown in (Table 1) against ND, IB, IBD, and AI. The feeding program consisted of starter feed (crumbles) for the first 12 D with 23% crude protein and metabolizable energy (ME) of 3,008 kcal/kg diet, grower feed (pellets) (21% crude protein and 3,080 kcal/kg diet ME) up to the 26th D, followed by finisher (pellets) feed till the end of the experiment (35 D) with 19% crude protein and 3,190 kcal/kg diet ME. Feed and water were provided to all birds with free access. The chicks were received at 32°C on day 1, and then the temperature was decreased linearly by 2°C per week. All experimental procedures in this study were performed according to the Local Experimental Animal Care Committee and approved by the ethics of the institutional committee of the Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Egypt. All efforts were made to minimize suffering.
Table 1.
Vaccination program of vaccinated experimental groups (VC, VTC, PRV, and TTT).
| Vaccines | Age (days)/route of administration |
|---|---|
| Nobilis MA5 + Clone 30 (infectious bronchitis + Newcastle disease virus strains) (Intervet international B.V. Boxmeer-Holland) | 5th/by the eye drop |
| Dalguban N+ oil vaccine (Newcastle disease virus genotype VII, KBNP-CA152R21 strain) (KBNP, INC. Chungnam, Korea) | 7th/intramuscular (thigh muscle) |
| Avian influenza (H5N1) (MEVAC Co. Egypt) | 9th/subcutaneous (neck) |
| Bursine plus (Zoetis Inc., Kalamazoo, MI, USA) (infectious bursal disease) | 13th/eye drop |
| LaSota (Intervet international B.V. Boxmeer-Holland) (Newcastle disease) | 17th/eye drop |
Abbreviations: PRV, preventive; TTT, therapeutic group; VC, vaccinated and challenged; VTC, vaccinated, treated, and challenged.
Blood Sampling
Ten blood samples without anticoagulant were gathered from additional ten one-day-old chicks by slaughtering for measuring maternally derived antibodies specific for NDv. At weekly interval, blood samples were collected from the wing vein of randomly selected 12 birds per group (four birds/replicate) at the 14th, 21th, and 28th D, while at the 35th D, blood samples were collected from the surviving birds. Sera were collected by centrifugation for 10 min at 1,500 × g and were stored into eppindorff tubes at −20°C until the use for serological tests.
For hematological and phagocytic activity (PA) assays, blood samples were collected at the 28th D using an anticoagulant (sodium citrate 3.8%) from 6 birds in each group (NC, VC, and VTC) (2 birds/replicate) and were sent to the laboratory at the same day.
Immunological and Hematological Assays
Hemagglutination inhibition (HI) and classical ELISA tests were performed to measure antibody titres (IgG) against ND, AI, IBD, and IB. The HI test was performed in U-based microtiter plates using 4 HA units of LaSota antigen (Intervet International B.V. Boxmeer-Holland) and AI H5N1 antigen (clade 2.2.1.1) (MEVAC Co., Egypt) (OIE, 2012). ELISA was performed according to the manufacturer's protocol (Synbiotics Co.). Haematological parameters were determined according to study by Campbell (1991) as follows: total red and white blood cell counts were determined manually using a hemocytometer. The packed cell volume was measured by a capillary tube technique using microhematocrit capillary tubes centrifuged at 2,500 rpm for 5 min. The hemoglobin concentration was measured by the cyanomethemoglobin method. The differential leukocyte count was carried out using blood smears stained with Wrights-Giemsa stain according to their identification characteristics, and the percentage and absolute value for each cell type were calculated.
Phagocytic Assay and Organs Index
Phagocytic assay was determined according to the study by Kawahara et al. (1991): Fifty micrograms of Candida albicans culture was added to 1 mL of citrated blood collected from 6 birds (individually) in each group (NC, VC, and VTC) (2/replicate) and shaken in a water bath at 25°C for 5 h. Blood smears were then stained with Wrights-Giemsa stain. Phagocytosis was estimated by determining the proportion of macrophages that contained intracellular yeast cells in a random count of 300 macrophages and was expressed as the percentage of PA.
The bursa of Fabricius, thymus, spleen, and liver were collected from 6 birds per group for NC, VC, and VTC (2 birds/replicate) at the 28th D and were weighed individually to calculate the relative organ weights and represented as organ index.
Organ index = (organ weight [gram]/body weight [gram]) × 1000 (Hedayati et al., 2005).
Clinical and Postmortem Investigations, Mortality Rate, and Growth Performance
The mortality rate was recorded, and all birds in the experimental groups were daily observed for 7 D after challenge (PC). For clinical signs, including sneezing, rales, swollen head, nasal and ocular discharge, greenish diarrhoea, torticollis, and recumbency, dead birds were examined for postmortem lesions, such as proventricular hemorrhage, intestinal button-like ulcers, ileocecal tonsil hemorrhage, and congested pectoral muscles and tracheal mucosa (OIE, 2012). The birds were individually weighed to determine the body weight at the first, 28th, and 35th D, and weight gain was calculated for each bird at 1- to 28-, 28- to 35-, and 1- to 35-D intervals. The average feed consumption per bird was determined at the same intervals.
The feed conversion ratio (FCR) was calculated as FCR per bird = (total feed consumption in a pen ÷ [weight gain of surviving birds + weight gain of dead birds in the same pen]) (Conway and McKenzie, 2007).
Real-time reverse transcription-polymerase chain reaction (rRT-PCR) for viral shedding titres on oropharyngeal swabs
Oropharyngeal swabs were taken from all surviving birds in each group at the second, fourth, and seventh day PC in 1.5 mL of phosphate-buffered saline–containing gentamicin (200 μg/mL) and penicillin G (1,000 units/mL). The collected swabs were centrifuged at 3,000 rpm for 10 min; the supernatants were collected and stored at −80°C until use. RNA was extracted from 200 μL of the collected oropharyngeal swab fluid using Thermo Scientific Gene JET Viral DNA and RNA Extraction Kits following the manufacturer's recommended procedures. The ND virus fusion protein gene was amplified using the primers and probe according to the study by AlHabeeb et al. (2013) with the Stratagene Real-Time PCR system. The reaction was composed of 5 μL of template RNA, 12.5 μL of rRT-PCR Master Mix, 1.25 μL of RT-enhancer, 0.25 μL of enzyme mix, 1 μL of forward primer (50 pmol), 1 μL of reverse primer (50 pmol), 0.25 μL of probe, and 3.75 μL of RNAse free water. The PCR cycling conditions were 50 cycles at 94°C for 10 s, 58°C for 5 s, and 72°C for 10 s preceded by an initial denaturation for 15 min at 95°C.
Measurement of Antioxidant Enzymes
Malondialdehyde (MDA) and superoxide dismutase (SOD) in serum were estimated according to the studies by Satoh (1978) and Nishikimi et al. (1972), respectively, using commercial kits produced by Biodiagnostic Co. (diagnostic and research reagents) at the 28th and 35th D.
Statistics
The data of growth performance, haematology, serology, antioxidant enzymes, and organ index were analysed using Statistical Analysis System software version 9.2 (SAS, 2004) by one-way analysis of variance followed by post hoc Duncan's test. The data are presented as the average ± standard error. All statements of differences were based on a significance level of P < 0.05. Coefficient of variation percentage of all groups were calculated according to the following equation: (y/x)∗100, where y is standard deviation of the treatment and x is mean value of the same treatment.
Results
Immune Response to NDv and Results of Subsequent vNDv Challenge
Serology
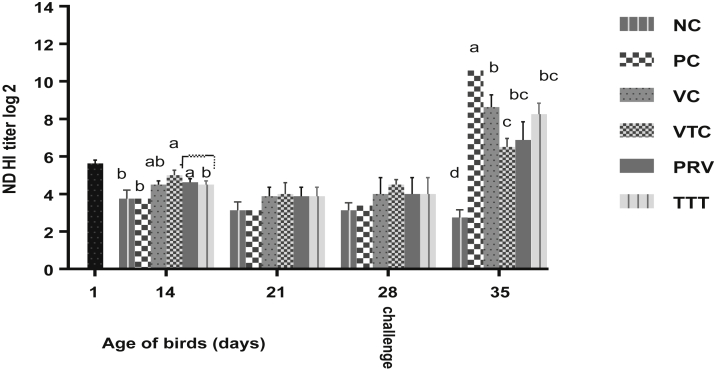
The average level of maternally derived antibodies specific for NDv (HI) was 2 log 5.63 ± 0.18, which decreased over time in the nonvaccinated control groups (NC and PC) to reach values of (2 log 3.13 ± 0.40 and 3.38 ± 0.46, respectively) at the 28th D (challenge day). In addition, ND–vaccinated birds (VC) did not show significantly higher HI titres (P ≥ 0.05) (2 log 4 ± 0.87) at the challenge day than NC or PC birds (nonvaccinated). However, the use of EOs in combination with vaccine application against ND in VTC increased HI antibody titre (2 log 4.5 ± 0.27) (P ≥ 0.05) than VC (vaccinated only).
Positive control birds (PC) showed a significant increase (P < 0.05) of HI titre (2 log 10.57 ± 0.43) for NDv at the 35th D (7 D PC). While, sero-conversion after vNDv challenge was significantly (P < 0.05) lower as a result of using EOs together with ND vaccines in VTC (2 log 6.5 ± 0.46) than the vaccine alone in VC (2 log 8.63 ± 0.65). Also, lower sero-conversion was observed in both groups that received EOs either starting from 1 D before vNDv challenge (PRV) or 2 D after challenge (TTT) and continued for 5 D, but the difference was not significant (P ≥ 0.05) compared to VC or VTC (Figure 1).
Figure 1.
Hemagglutination inhibition (HI) titre (log 2) of Newcastle disease antibodies throughout the experimental period. Geometric mean of 12 serum samples per group (four/replicate) at each observation time. Different letters at each observation time indicate to significant difference (P < 0.05). NC, negative control; PC, positive control; PRV, preventive; TTT, therapeutic group; VC, vaccinated and challenged; VTC, vaccinated, treated, and challenged.
Mortality Percentage and Clinical Protection
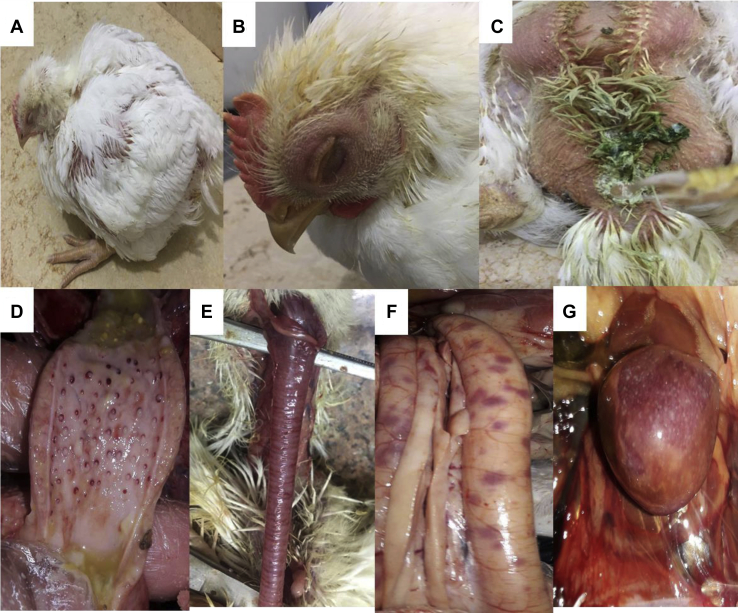
The vNDv challenge in the experimental groups resulted in the death of 17 of 21 birds (80.95%) in PC (nonvaccinated and nontreated), which was reduced by vaccination against ND in VC to 52.28% (11/21). Moreover, the use of EOs with vaccination as an immune modulator in VTC reduced the mortality rate to 42.86% (9/21), whereas preventive and therapeutic groups (PRV and TTT) showed mortality rate of 38.09% (8/21) and 47.62% (10/21), respectively (Table 2). All birds in the negative control group (NC) appeared clinically normal, while vNDv challenge resulted in depression, swollen head, conjunctivitis, rales, sneezing, and nasal discharge beginning at the second day post challenge (PC) in all experimentally challenged groups (PC, VC, VTC, PRV, and TTT). Greenish diarrhoea was also recorded in PC, VTC, and TTT (Figure 2; Table 2).
Table 2.
Clinical signs and postmortem lesion scores throughout 7 D after challenge with vNDv genotype VII according to frequency and severity.1
| Item (affected birds/total) | Experimental groups2 |
|||||
|---|---|---|---|---|---|---|
| NC | PC | VC | VTC | PRV | TTT | |
| Mortality % | 0 | 80.95 (17/21) | 52.28 (11/21) | 42.86 (9/21) | 38.09 (8/21) | 47.62 (10/21) |
| Depression | 0 | 3 (21/21) | 2 (17/21) | 2 (16/21) | 1 (10/21) | 2 (14/21) |
| Conjunctivitis | 0 | 2 (20/21) | 1 (15/21) | 1 (9/21) | 0 (0/21) | 1 (15/21) |
| Sneezing and rales | 0 | 2 (18/21) | 1 (12/21) | 1 (7/21) | 1 (1/21) | 2 (16/21) |
| Nasal discharge | 0 | 2 (16/21) | 1 (9/21) | 1 (5/21) | 1 (3/21) | 1 (13/21) |
| Greenish diarrhoea | 0 | 3 (13/21) | 0 (10/21) | 1 (5/21) | 0 (0/21) | 1 (8/21) |
| Congested pectoral muscles | 0 | 3 (16/17) | 2 (8/11) | 1 (8/9) | 1 (6/8) | 3 (10/10) |
| Tracheal congestion and exudates | 0 | 3 (17/17) | 2 (7/11) | 1 (9/9) | 1 (6/8) | 2 (8/10) |
| Proventricular hemorrhage | 0 | 3 (14/17) | 3 (6/11) | 1 (3/9) | 2 (4/8) | 2 (9/10) |
| Intestinal ulcers | 0 | 2 (12/17) | 2 (4/11) | 1 (1/9) | 1 (2/8) | 1 (6/10) |
| Hemorrhage at ileocecal tonsils | 0 | 1 (11/17) | 0 (5/11) | 0 (0/9) | 1 (1/8) | 1 (6/10) |
| Mottled enlarged spleen | 0 | 3 (17/17) | 1 (9/11) | 1 (4/9) | 1 (3/8) | 2 (10/10) |
| Sum | 0 | 27 | 15 | 11 | 10 | 17 |
Data are an average of clinical signs and the postmortem lesion score, as follows: 0, no; 1, mild; 2, moderate; and 3, severe signs or lesions.
NC: negative control, PC: positive control, VC: vaccinated and challenged, VTC: vaccinated, treated, and challenged, PRV: preventive and TTT: therapeutic group.
Figure 2.
Clinical signs and postmortem lesions in experimental groups challenged with vNDv. A: depression, B: swollen head and closed eye lids, C: greenish diarrhoea, D: proventricular haemorrhage, E: congested trachea, F: button-like ulcers on the intestine, G: enlarged mottled spleen.
Upon examination of dead birds, congestion of the pectoral muscles and trachea, proventricular haemorrhage, button-like ulcers on the small intestine, and a mottled enlarged spleen were observed (Figure 2, Table 2).
Oropharyngeal Shedding of vNDv Using rRT-PCR
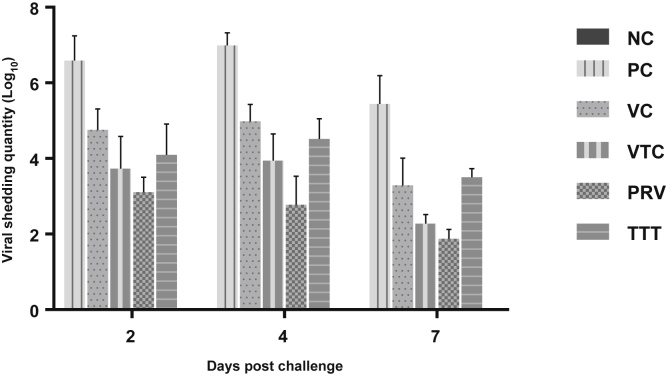
Oropharyngeal swabs were collected from all birds in each group separately at the second, fourth, and seventh day PC and were pooled. No oropharyngeal viral shedding was observed in the negative control birds (NC) throughout the experimental period. The peak of viral shedding (loNC0) was recorded at the fourth day PC in all challenged experimental groups, and the highest titre was produced by the positive control group (PC) at all 3 intervals, whereas the lowest titre (loNC0) was recorded in birds from PRV, followed by VTC and finally TTT as viral shedding titre (loNC0) was 6.59, 4.76, 3.73, 3.11, and 4.1 at second day PC; 6.99, 4.98, 3.94, 2.78, and 4.52 at fourth day PC; and 5.44, 3.29, 2.28, 1.88, and 3.5 at seventh day PC in PC, VC, VTC, PRV and TTT, respectively (Figure 3).
Figure 3.
Viral shedding (loNC0) in the oropharyngeal swabs at 2, 4, and 7 D after challenge in 6 experimental chicken groups. Data are the average of 3 replicates per group (two pooled samples/replicate). NC, negative control; PC, positive control; PRV, preventive; TTT, therapeutic group; VC, vaccinated and challenged; VTC, vaccinated, treated, and challenged.
Body Weight Gain and FCR
There was no significant difference (P ≥ 0.05) in average body weight gain (g) among the experimental groups during the period from 1 D until the day of challenge (first–28th D). However, the average body weight gain during 7 D after challenge was significantly reduced (P < 0.05) in nonvaccinated challenged birds (PC) (387 ± 18.6) compared with negative control ones (NC) (458 ± 25.92), as well as vaccinated challenged groups either treated with EOs (VTC, PRV, and TTT) or nontreated (VC). Moreover, body weight gain of those vaccinated groups (VC-TTT) had no significant difference (P ≥ 0.05) in comparison with negative control birds (NC).
Regarding overall body weight gain (interval first–35th D), the significantly (P < 0.05) high body weight gain was observed in PRV (vaccinated and treated with EOs that started 1 D before challenge and continued for 5 D) (2001 ± 108.79) and NC (negative control) (1970 ± 19.26) when compared with that produced by the challenge with vNDv in PC (1807 ± 28.7) as shown in Table 3. The FCR, at the interval of first to 28th D of age (before challenge), was significantly worse (P < 0.05) in the vaccinated group (VC) (1.89 ± 0.04) when compared with that of negative control birds (NC) (1.66 ± 0.04). Whereas, the use of EOs in VTC along with vaccination resulted in an FCR of 1.77 ± 0.04, with no significant (P ≥ 0.05) difference in comparison with either NC or VC.
Table 3.
Effect of essential oils on the growth performance of broiler chickens ±SE.1
| Parameter | Experimental groups |
|||||
|---|---|---|---|---|---|---|
| NC | PC | VC | VTC | PRV | TTT | |
| First to 28th D (before vNDv challenge) | ||||||
| B. wt gain (g) | 1422 ± 30.59 | 1419 ± 27.59 | 1419 ± 27.14 | 1423 ± 34.50 | 1452 ± 81.83 | 1421 ± 18.14 |
| FCR (g/g) | 1.66 ± 0.04b | 1.68 ± 0.05b | 1.89 ± 0.04a | 1.77 ± 0.03a,b | 1.85 ± 0.13a | 1.91 ± 0.02a |
| 28th to 35th D (period of vNDv challenge) | ||||||
| B. wt gain (g) | 548 ± 25.92a | 381 ± 18.6b | 485 ± 24.79a | 529 ± 23.17a | 540 ± 38.26a | 498 ± 23.87a |
| FCR (g/g) | 1.72 ± 0.09b | 2.83 ± 0.18a | 2.01 ± 0.11b | 1.89 ± 0.09b | 1.80 ± 0.14b | 1.99 ± 0.10b |
| First to 35th D (overall period of the experiment) | ||||||
| B. wt gain (g) | 1970 ± 19.56a | 1800 ± 28.7b | 1904 ± 38.66a,b | 1952 ± 28.82a,b | 1987 ± 108.79a | 1919 ± 20.21a,b |
| FCR (g feed/g gain) | 1.66 ± 0.02b | 2.12 ± 0.03a | 1.91 ± 0.04a | 1.79 ± 0.03a,b | 1.81 ± 0.12a,b | 1.92 ± 0.02a |
Abbreviations: B. wt gain (g), body weight gain (gram); CV%, coefficient of variation percentage; FCR (g/g), feed conversion ratio (gram of feed intake/gram of weight gain); NC, negative control; PC, positive control; PRV, preventive; SE, standard error; TTT, therapeutic group; VC, vaccinated and challenged; vNDv, virulent Newcastle disease virus; VTC, vaccinated, treated, and challenged.
Data are the average of 3 replicates per group, with 7 broilers per replicate. Different superscript letters in the same row indicate a significant difference (P < 0.05). CV% range of experimental groups for both parameters was 0.9:7.8%.
The FCR during 7 D PC was significantly (P < 0.05) higher in birds from PC (positive control) (2.83 ± 0.18) than that of all other experimental groups. The best FCR (1.72 ± 0.09) was recorded by the negative control birds (NC). An improved FCR during the overall period of the experiment (first–35th D) was reported for NC (1.66 ± 0.02), which significantly differed (P < 0.05) from that of all other groups except VTC (1.79 ± 0.03) (P ≥ 0.05) as shown in Table 3.
Antioxidant Status
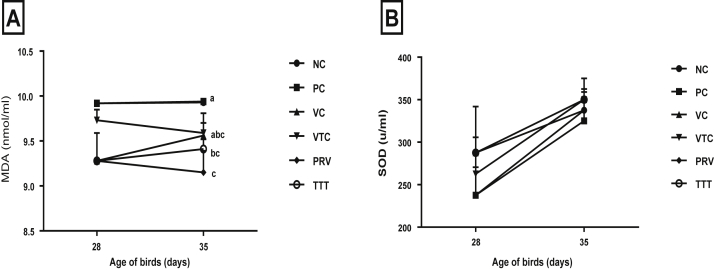
The level of SOD did not significantly differ (P ≥ 0.05) at the 28th D (challenge day) as 237.5, 237.5, 287.5, 262.5, 287.5, and 287.5 U/mL and 35th D (7 D PC) as 337.5, 325, 350, 350, 337.5, and 350 U/mL in NC, PC, VC, VTC, PRV, and TTT, respectively. MDA level did not statistically affect (P > 0.05) at the challenge day (28th D) as 9.92, 9.92, 9.28, 9.73, 9.28, and 9.28 nmol/mL, but it was significantly impacted (P < 0.05) at 7 D after challenge (35th D) as 9.93, 9.94, 9.56, 9.59, 9.15, and 9.41 nmol/mL in NC, PC, VC, VTC, PRV, and TTT, respectively (Figures 4A, 4B).
Figure 4.
(A) Malondialdehyde (MDA) level (nmol/mL) in the serum and (B) superoxide dismutase (SOD) level (U/mL) in the serum of experimental groups (pre- and post-vNDv challenge). Data are means of 6 birds per group (two birds/replicate). Different letters at each observation time indicate to significant difference (P < 0.05). CV% of experimental groups for both parameters was in range of 0.4 to 18.9%. CV%, coefficient of variation percentage; NC, negative control; PC, positive control; PRV, preventive; TTT, therapeutic group; VC, vaccinated and challenged; VTC, vaccinated, treated, and challenged.
Antibody Titres for the AI, IB, and IBD Vaccines
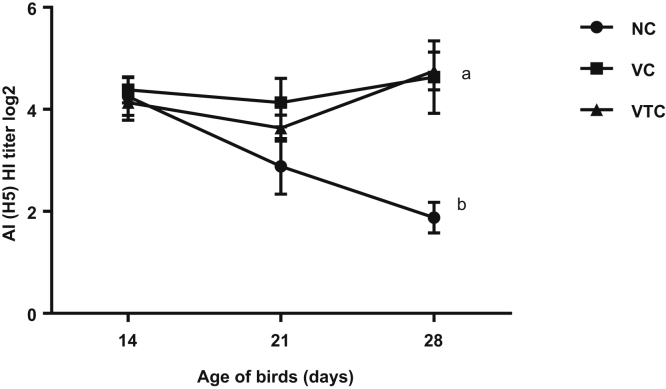
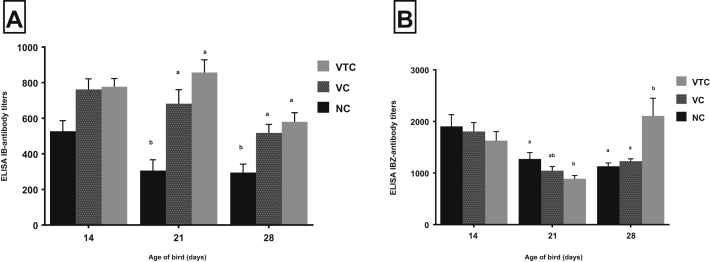
HI titres (loPC) for AI H5, at weekly interval beginning at the 14th D, did not significantly differ (P ≥ 0.05) among NC (negative control), VC (vaccinated), and VTC (vaccinated and EOs treated) with a difference range of 4.13 to 4.38 loPC. At the 28th D, vaccinated groups neither treated (VTC) nor (VC) showed significantly (P < 0.05) higher HI titre than negative control (NC) birds as 1.88, 4.63, and 4.75 loPC in NC, VC, and VTC, respectively (Figure 5). The antibody titre by ELISA for the IB virus did not significantly differ (P ≥ 0.05) among the experimental groups at the 14th D. However, it was increased at the 21th and 28th D on VTC (vaccinated and EOs treated) than VC (P ≥ 0.05) or NC (P < 0.05) as the titres were 527, 762, and 777 at the 14th D, 306, 682, and 857 at 21th D, and 295, 518, and 580 at 28th D in NC, VC, and VTC, respectively (Figure 6A).
Figure 5.
Hemagglutination inhibition (HI) titre (log 2) of avian influenza H5 antibodies. Geometric mean of 12 serum samples per group (four/replicate) at each observation time. Different letters at each observation time indicate to significant difference (P < 0.05). CV% range was 7.2 to 19%. CV%, coefficient of variation percentage; EO, essential oil; NC, negative control; VC, vaccinated and challenge; VTC, vaccinated, EOs treated, and challenged.
Figure 6.
ELISA antibody titres for infectious bronchitis (IB) virus (A) and infectious bursal disease (IBD) virus (B). The ELISA titre was obtained from 12 serum samples per group (four/replicate) at each observation time. Different letters at each observation time indicate to a significant difference (P < 0.05). CV% range among experimental groups for both parameters was 4.6 to 19.7%. NC, negative control; VC, vaccinated and challenged; VTC, vaccinated, EOs treated, and challenged.
Regarding ELISA IBD antibody titres, there was no significant difference among different groups at the 14th D (P ≥ 0.05) as NC, VC, and VTC showed 1904, 1805, and 1826 IBD antibody titres, respectively. However, they decreased at the 21th D in all groups, whereas the significantly (P < 0.05) lower titre was shown by VTC that afterward produced significantly (P < 0.05) higher ELISA IBD antibody titre (at the 28th D) as ELISA IBD antibody titres were 1,273, 1,047, and 890 at the 21th D and 1130, 1230, and 2108 at the 28th D in NC, VC, and VTC, respectively (Figure 6B)
Hematology, Phagocytic Assay, and Organs Index
At the 28th D (challenge day), the readings of red blood cells ( × 106/mm3), PCV%, and hemoglobin (g/dl), that were of difference range of 4.26–4.96, 41.06–44.7, and 14.08–14.96, respectively, did not show significant (P ≥ 0.05) difference among the 6 experimental groups. However, white blood cells count ( × 106/mm3) and heterophils:lymphocytes (H:L) ratio were significantly (P < 0.05) higher in VC (vaccinated) than in NC (negative control) and VTC (vaccinated and EOs treated) as white blood cell count ( × 106/mm3) was 2.24, 2.54, and 2.23 and H:L ratio was 1.06, 1.45, and 1.1 in NC, VC, and VTC, respectively(Table 4).
Table 4.
Effects of essential oils supplementation on hematological parameters, phagocytic assay, and organ indices in broiler chickens at the 28th D ±SE.1
| Items | Groups2 |
||
|---|---|---|---|
| NC | VC | VTC | |
| Hematology and phagocytic assay | |||
| Phagocytic activity (PA) | 48.00 ± 0.58a | 32.67 ± 0.33c | 37.50 ± 0.50b |
| Phagocytic index (PI) | 28.00 ± 0.58a | 10.00 ± 0.58c | 13.50 ± 0.50b |
| H:L ratio | 1.06 ± 0.02b | 1.45 ± 0.01a | 1.10 ± 0.03b |
| WBCs ( × 106/mm3) | 2.24 ± 0.01b | 2.54 ± 0.04a | 2.23 ± 0.11b |
| RBCs ( × 106/mm3) | 4.26 ± 0.02 | 4.61 ± 0.06 | 4.96 ± 0.07 |
| PCV (%) | 44.70 ± 0.05 | 41.06 ± 0.30 | 43.01 ± 0.22 |
| Hb (g/dl) | 14.55 ± 0.02 | 14.08 ± 0.04 | 14.96 ± 0.22 |
| Organ index | |||
| Bursa of Fabricius | 0.54 ± 0.08b | 0.74 ± 0.09a,b | 1.08 ± 0.32a |
| Thymus | 3.67 ± 0.58 | 4.48 ± 0.28 | 3.51 ± 0.37 |
| Spleen | 0.70 ± 0.01 | 0.90 ± 0.10 | 0.95 ± 0.19 |
| Liver | 27.14 ± 2.49 | 23.81 ± 0.75 | 24.57 ± 1.41 |
Abbreviations: Hb, hemoglobin; H:L, heterophils-to-lymphocytes ratio; NC, negative control; PCV, packed cell volume; RBC, red blood cell; SE, standard error; VC, vaccinated and nontreated with EOs; VTC, vaccinated and treated with EOsWBC, white blood cell.
Data are the average of 6 birds per group (two birds/replicate). Different superscript letters in the same row indicate significant differences (P < 0.05). 2Experimental groups.
Experimental groups.
PA and the phagocytic index were significantly higher in NC (negative control) (48 and 28, respectively) than in VC (vaccinated and nontreated with EOs) (32.67 and 10, respectively) and VTC (vaccinated and treated with EOs) (37.5 and 13.5, respectively), with a significant increase (P < 0.05) in VTC in comparison to VC (Table 4).
Spleen, thymus, and liver indices were not statistically different (P ≥ 0.05) between EOs treated group (VTC) and other nontreated ones (NC and VC) at the 28th D with a range of difference of 0.7:0.95, 3.51:4.48, and 23.81:27.14, respectively. However, the bursa of Fabricius index was significantly (P < 0.05) higher in VTC (1.08) than in NC group (0.54) (Table 4).
Discussion
The results of the present study revealed that EOs mixture supplementation in drinking water at 0.5 mL/L beginning at 2 D after each vaccine application for 3 successive days (VTC) did not cause significant increase in ND HI antibody titre (log 2) until the age of challenge (28th D). Consistent with the reports of Sadeghi et al. (2012) and Rahimi et al. (2011). In contrast, Barbour et al. (2014) and Thaje and Ulaiwi (2017) showed that the addition of eucalyptus and mint oils improved the humoral immune responses to ND virus in broilers. In addition, Wati et al. (2015) reported an improvement in the humoral response (HI titre) to ND virus at the 21th D in broilers after using a phytogenic product, but this effect did not last to the 39th D.
Although the obtained result for VTC (vaccinated, EOs treated [after each vaccine] and challenged), an improvement of clinical protection (mortality, clinical signs, PM lesions, and viral shedding titre) was observed. This could be due to stimulating effect on cell-mediated and local immunity (Cserep, 2008) and therefore eliminating some of the viruses from tissues (Awaad et al., 2009). The penetration mechanism of the virus into the cells determines whether the virus is affected by the EOs or not and where ND virus penetrates cells through a fusion protein (Cermelli et al., 2008), which in turn can be resistant to EOs (Barbour et al., 2010), so not all ND viruses are affected by EOs. However, AI virus penetrates the cells through a receptor-mediated endocytosis mechanism (Swayne and King, 2003).
As many studies have discussed the in vitro antiviral activity of EOs against avian viruses after contact between viruses and EOs for certain periods of time at room temperature (Barbour et al., 2010), here we designed PRV and TTT to study the antiviral effect of EOs in vivo (in chickens) at chicken's body temperature (41.5°C) either before (PRV) or after vNDv challenge (TTT). The improvement of the clinical and pathological picture of vNDv was observed in PRV (received the EOs supplement that was started from 1 D before the challenge and was continued for 5 D); in addition, this group had the lowest oropharyngeal shedding titre (log 10), which could be due to eucalyptus, and it has been used in traditional medicine to treat respiratory tract infections (Cermelli et al., 2008) and may exhibit antiviral activity (Barbour et al., 2010) and thus affects the virulence of the challenge virus. Moreover, EOs showed more protective level against vNDv challenge in PRV (preventive group) than TTT (therapeutic group) that may be attributed to the effect of EOs mixture on ND virus adsorption to cell surface and may block cell receptors resulting in interference with virus binding; its accurate mode of action should be studied through tissue culture.
EOs supplementation, during the period of first to 28th D, in VTC did not significantly improve body weight gain compared to the other nontreated groups (NC and VC). Although, VTC showed an improved FCR (1.77 ± 0.03), it did not significantly differ from negative control birds (NC) (1.66 ± 0.04). Vaccination by different live vaccines including ND, IB, and IBD viruses may be the reason for significantly higher FCR in VC (1.89 ± 0.04). The same was observed during 7 D PC for body weight gain and FCR. Nevertheless, the use of ND vaccines, regardless EOs supplementation, has improved (P < 0.05) overall body weight gain in all challenged groups compared with the positive control group. Similar results were obtained by Sedeik et al. (2019). Supplementation of EOs as a preventive drug in PRV (that was started from 1 D before the challenge and continued for 5 D) along with vaccines application has significantly improved the body weight gain compared with positive control birds (PC).
The worst FCR was recorded in PC, followed by VC and TTT. In contrast, El-Ghousein and Al-Beitawi, 2009, Awaad et al., 2016, and Thaje and Ulaiwi (2017) reported that essential oils of eucalyptus, mint, and/or thyme oils resulted in significant increase in the body weight and weight gain, in addition to improving the FCR.The EOs enhance digestibility, balance the gastrointestinal tract microflora, and stimulate the secretion of endogenous digestive enzymes, thus improve growth performance in poultry (Williams and Losa, 2001, Cross et al., 2007). As well, thymol plus carvacrol has reported to significantly improve body weight gain and FCR in broilers (Hashemipour et al., 2013, Saki et al., 2014). The obtained results of body weight gain and FCR in this study were similar to the findings of Rehman et al., 2013, Toghyani et al., 2010 and Lee et al. (2003). The various results of the effect of different types of EOs on bird performance in several studies could be related to variations of dose, active components of the EOs mixture, the period of administration, and the environmental and nutritional factors.
Lipid peroxidation is an autocatalytic process that leads to oxidative damage of cellular membranes (Rhee et al., 1996) and cell death, producing toxic free radicals including MDA which is used as an indicator of lipid peroxidation (Jensen et al., 1997), while the antioxidative enzyme, SOD, protects cells from the damage caused by these free radicals (Yesilbag et al., 2011). In this study, EOs had no significant effect on MDA or SOD in the serum at the 28th D (challenge day), but it caused significant decrease of MDA in PRV and TTT at the 35th D although vNDv challenge did not increase MDA level in PC (positive control). These results make us ask about the role of ND infection on lipid peroxidation and therefore the need for further studies. The decreased level of MDA at the seventh day PC in VTC may be attributed to the antioxidant effect of thyme that is rich in flavonoids (Cook and Samman, 1996). Furthermore, Youdim and Deans, 2000, Botsoglou et al., 2002, and Hashemipour et al. (2013) reported that thymol, carvacrol, thyme oil, and/or oregano have strong antioxidant activity and can inhibit lipid peroxidation.
Regarding the immune-stimulating effect of the EOs mixture for other vaccines, AI and IB antibody titres were not affected by EOs supplementation but that of IBD virus significantly increased only at the 28th D. Similar results were obtained by Saki et al. (2014) for AI and IB but are not consistent for IBD, which could be due to the use of thyme essence in the drinking water at different doses (0, 0.10, 0.15, and 0.20 mL/L) from 8 to 42 D or the inclusion of different components. The hematological and PA assays were conducted only at the 28th D of age to detect the effect of EOs on the immune response to vaccines (regardless of ND challenge). So, the groups were representing by NC, negative control; VC, vaccinated and nontreated with EOs; and VTC, vaccinated and treated with EOs. One of the first lines of defence in the body is leucocytes, which significantly increase in its number when the infection occurs (Ganong, 2003), like what was observed in this study in VC (vaccinated, nontreated). In addition to the lower H:L ratio in VTC (vaccinated and EOs treated), which may be due to the positive influence of this mixture on reducing stress in broilers. It is known that the H:L ratio is considered as an indicator of stress in poultry (Bedanova et al., 2007). Similar results were reported by Hashemipour et al. (2013). In addition, essential oils were able to increase the PA and index in VTC compared to VC. On the other hand, eucalyptus oil stimulates the immune response by enhancing PA of monocytes (El-Ghousein and Al-Beitawi, 2009). Furthermore, Acamovic and Brooker (2005) claimed that the polyphenol compound EO and thymol have a stimulating effect on mononuclear phagocyte system and cellular immunity.
As reported in Table 4, lymphoid organ and liver index, red blood cell counts, hemoglobin percentage, and hematocrit value were not affected by EOs supplementation. Similar findings were reported by Hashemipour et al. (2013). However, Al-Kassie (2009) showed that the supplementation of the oil extract of thyme and cinnamon resulted in increasing red blood cell count, hematocrit, and hemoglobin values compared to those of the control birds.
Conclusions
In conclusion, the EOs mixture used in the present study has a positive preventive effect and some therapeutic effect on ND virus infection in chickens (in vivo) through the decreased viral shedding titre, increased clinical protection, and decreased pathological picture. EOs did not promote the bird's performance or the immune responses to the AI and IB vaccines; nevertheless, it has an immune-stimulating effect for the IBD virus. Further studies are required for determining their antiviral mode of action to help control different field outbreaks and to decrease viral shedding in addition to vaccination programs.
Acknowledgments
This work was funded by Researchers Supporting Project number (RSP 2020/26), King Saud University, Riyadh, Saudi Arabia. The authors extend thanks to their respected universities.
Conflicts of Interest Statement: All authors declare that they do not have any conflict of interests that could inappropriately influence this manuscript.
Contributor Information
Nahed A. El-Shall, Email: nahed.abdelgawad@alexu.edu.eg.
Mohamed E. Abd El-Hack, Email: dr.mohamed.e.abdalhaq@gmail.com.
References
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Inter. J. Pharmacol. 2016;12:232–248. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Saeed M., Arif M., Arain M.A., Bhutto Z.A., Fazlani S.A. Effect of gradual substitution of soybean meal by Nigella sativa meal on growth performance, carcass traits and blood lipid profile of growing Japanese quail. J. Anim. Feed Sci. 2016;25:244–249. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K., Zorriehzahra J., Adel M. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J. Essen. Oil Res. 2016;28:365–382. [Google Scholar]
- Abd El-Hack M.E., Attia A.I., Arif M., Soomro R.N., Arain M.A. The impacts of dietary Nigella sativa meal and Avizyme on growth, nutrient digestibility and blood metabolites of meat-type quail. Anim. Prod. Sci. 2018;58:291–298. [Google Scholar]
- Acamovic T., Brooker J. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 64, 2005:403–412. doi: 10.1079/pns2005449. [DOI] [PubMed] [Google Scholar]
- AlHabeeb M., Mohamed M., Sharaw S. Detection andcharacterization of Newcastle disease virus in clinical samples using real time RT-PCR and melting curve analysis based on matrix and fusion genes amplification. Vet. World. 2013;6:239. [Google Scholar]
- Al-Kassie G. Influence of two plant extracts derived rom thyme and cinnamon on broiler performance. Pakistan Vet. 29 2009:169–173. [Google Scholar]
- Awaad M., Abdel-Alim G., Sayed K., Ahmed K., Nada A. The immunostimulant effects of peppermint and eucalyptus essential oils in chickens. Pak. Vet. J. 36, 2009:61–66. [Google Scholar]
- Awaad M., Afify M., Zoulfekar S., Mohammed F., Elmenawy V., Hafez H. Modulating effect of peppermint and eucalyptus essential oils on vVND infected chickens. Pakistan Vet. J. 2016;36:350–355. [Google Scholar]
- Baghban-Kanani P., Hosseintabar-Ghasemabad B., Azimi-Youvalari S., Seidavi A., Ragni M., Laudadio V., Tufarelli V. Effects of using Artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. J. Poult. Sci. 2019;56:120–127. doi: 10.2141/jpsa.0180050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour E., Yaghi R., Jaber L., Shaib H., Harakeh S. Safety and antiviral activity of essential oil against Avian Influenza and Newcastle disease viruses. Int. J. App. Res. Vet. Med. 2010;8:60–64. [Google Scholar]
- Barbour I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 2014;55:105–114. doi: 10.1080/00071668.2013.873772. [DOI] [PubMed] [Google Scholar]
- Bedanova I., Voslarova E., Chloupek P., Pistekova V., Suchy P., Blahova J., Dobsikova R., Vecere V. Stress in broilers resulting from shackling. Poult. Sci. 2007;86:1065–1069. doi: 10.1093/ps/86.6.1065. [DOI] [PubMed] [Google Scholar]
- Botsoglou N.A., Florou-Paner P., Christaki E., Fletouris D., Spais A. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br. Poult. Sci. 2002;43:223–230. doi: 10.1080/00071660120121436. [DOI] [PubMed] [Google Scholar]
- Campbell T.W. Hematology. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian Medicine: Principles and Application. Wingers Press; Florida: 1994. pp. 176–191. [Google Scholar]
- Cermelli C., Fabio A., Fabio V., Quaglio P. Effect of Eucalyptus essential oil on respiratory Bacteria and viruses. Curr. Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- Conway D., McKenzie M. 3rd ed. Blackwell Publishing Professional; Ames, IA, USA: 2007. Poultry Coccidiosis: Diagnostic and Testing Procedures; p. 57. [Google Scholar]
- Cook N., Samman S. Flavonoids chemistry, metabolism, cardio protective effects and dietary sources. J. Nutr. Biotec. 7, 1996:66–76. [Google Scholar]
- Cox N.J., Fuller F., Kaverin N., Klenk H.D., Lamb R.A., Mahy B.W., McCauley J.W., Nakamura K., Palese P., Webster R.G. Orthomyxoviridae. In: Van Regenmortel M.H., Fauquet C.M., L. Bishop D.H., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., R C., editors. Virus Taxonomy., Seventh Report of the International Committee on Taxonomy of Viruses. Pringl Academic Press; SanDiego: 2000. pp. 585–597. [Google Scholar]
- Cross D., McDevitt R., Hillman K., Acamovic T. The effects of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Cserep T. Poultry Diseases. 6th ed. Butterworth, Heinemann; Edinburgh: 2008. Vaccines and vaccination; pp. 66–81. [Google Scholar]
- Delmas B., Attoui H., Ghosh S., Malik Y.S., Mundt E., Vakharia V.N. ICTV virus taxonomy profile: Birnaviridae. J. Gen. Virol. 2019;100:5–6. doi: 10.1099/jgv.0.001185. [DOI] [PubMed] [Google Scholar]
- El-Ghousein S., Al-Beitawi N. The effect of feeding of rushed thyme (Thymus valgaris L.) on growth, blood constituents, gastrointestinal tract and carcass characteristics of broiler chickens. J. Poult. Sci. 46, 2009:100–104. [Google Scholar]
- Enjuanes L., Brian D., Cavanagh D., Holmes K., Lai M., Laude H., Masters P., Rottier P., Siddell S., Spaan W., Taguchi F., Talbot P. Coronaviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Virus Taxonomy. Classification and Nomemclature of Viruses. Accademic Press; San Diego, Calif: 2000. pp. 835–849. [Google Scholar]
- Gado A.R., Ellakany H.F., Elbestawy A.R., Abd El-Hack M.E., Khafaga A.F., Taha A.E., Arif S.A. M., Mahgoub Herbal medicine additives as powerful agents to control and prevent avian influenza virus in poultry–a review. Ann. Anim. Sci. 2019;19:905–935. [Google Scholar]
- Ganong W.F. 21st ed. MCGraw-Hill Companies Inc.; New York: 2003. Review of Medical Physiology. [Google Scholar]
- Gutierrez J., Barry-Ryan C., Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008;124:91–97. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Hedayati A., Nili A., Bahonar A. Comparisons of pathogenisity and serologic responce of four commercial infectious bursal disease live vaccines. Arch. Razi. Ins. 2005;59:65–73. [Google Scholar]
- Hines N.L., Miller C.L. Avian paramyxovirus serotype-1: a review of disease distribution, clinical symptoms, and laboratory diagnostics. Vet. Med. Int. 2012;2012:17. doi: 10.1155/2012/708216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C., Engberg R., Jakobsen K., Skibsted L., Bertelsen G. Influence of the oxidative quality of dietary oil on broiler meat storage stability. Meat Sci. 1997;47:211–222. doi: 10.1016/s0309-1740(97)00052-1. [DOI] [PubMed] [Google Scholar]
- Kawahara E., Ueda T., Nomura S. Gyobyo Kenkyu; Japan: 1991. In Vitro Phagocytic Activity of White Spotted Shark Cells after Injection with Aeromonas Salmonicida Extracellular Products; pp. 213–214. [Google Scholar]
- Koch C., Reichling J., Schnitzler P. Essential oils inhibit the replication of herpes simplex virus type inhibit the replication of herpes simplex virus type1 (HSV-1) and type 2 (HSV-2. In: Preedy V.R., Watson R.R., editors. Botanical Medicine in Clinical Practice. CABI; Wallingsford: 2008. pp. 192–197. [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Frehner M., Losa R. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Lee S., Lillehoj H., Jang S., Lee K., Bravo D., Lillehoj E. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with eimeria tenella. Vet. Parasitol. 2011;181:97–105. doi: 10.1016/j.vetpar.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prevent. Vet. Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Roa N., Yogi K. The Occurrence of Supeoxide anion in the reaction of reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res. Comm. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Nunez Y., Salabarria I., Collado I., Hernandez-Gala N. Sesquiterpenes from the wood of Juniperus lucayana. Phyto Chem. 2007;68:2409–2414. doi: 10.1016/j.phytochem.2007.05.030. [DOI] [PubMed] [Google Scholar]
- OIE . M. O. D. T. A. V. World Organisation for Animal Health; Paris: 2012. Newcastle disease; pp. 576–589. [Google Scholar]
- Pinto E., Pina-Vaz C., Salgueiro L., Goncalves M., Costa-de-Oliveira S., Cavaleiro C., Palmeira A., Rodrigues A., Martinez-de-Oliveira J. Antifungal activity of the essential oil of Thymus pulegioidesactivity of the essential oil of Thymus pulegioides. J. Med. Microbiol. 2006;55 (Pt 10):1367–1373. doi: 10.1099/jmm.0.46443-0. [DOI] [PubMed] [Google Scholar]
- Qorbanpour M., Fahim T., Javandel F., Nosrati M., Paz E., Seidavi A. Effect of dietary ginger (Zingiber officinale Roscoe) and multi-strain probiotic on growth and carcass traits, blood biochemistry, immune responses and intestinal microflora in broiler chickens. Animals. 2018;8:117. doi: 10.3390/ani8070117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi S., Zadeh T., Torshizi M., Omidbaigi R., Rokni H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agri. Sci. 2011;13:527–539. [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Rehman S., Muhammad K., Yaqub T., Khan M., Hanif K., Yasmeen R. Antimicrobial activity of mentofin and its effect on antibody response of broilers to Newcastle disease virus vaccine. J. Anim. Plant Sci. 2013;23:1008–1011. [Google Scholar]
- Reichlinga J., Schnitzlerb P., Suschkea V., Saller R. Essential oils of Aromatic plants with Antibacterial,Antifungal, antiviral, and Cytotoxic Properties –an Overview. Forsch Komplementmed. 2009;16:79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- Rhee K., Anderson L., Sams A. Lipid peroxidation potential of beef, chicken and pork. J. Food Sci. 1996;61:8–12. [Google Scholar]
- Roofchaee A., XIrani a., Ebrahimzadeh M.A., Akbari M.R. Effect of dietary oregano (Origanumvulgare L.) essential oil on growth performance, cecal microflora and serum antioxidant activity of broiler chickens. Afr. J. Biotechnol. 2011;10:6177–6183. [Google Scholar]
- Roussan D.A., Haddad r., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Sadeghi G., Karimi A., Jahomi S., Aziz T., Daneshmand A. Effect of cinnamon, thyme and turmeric infusions on the performance and immune response in of 1 to 21 day-old male broilers. Braz. J. Poult. Sci. 2012;14:5–20. [Google Scholar]
- Saki A., Kalantar M., Khoramabadi V. Effects of drinking thyme essence (Thymus vulgaris L.) on growth performance, immune ResponseandIntestinal selected bacterial population in broiler chickens. Poult. Sci. J. 2014;2:113–123. [Google Scholar]
- SAS . SAS Int.; Cary, NC, USA: 2004. Statistical User’s Guide. [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chim. Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Sedeik M.E., Elbestawy A.R., El-shall N.A., Abd El-Hack M.E., Saadeldin I.M., Swelum A.A. Comparative efficacy of commercial inactivated Newcastle disease virus vaccines against Newcastle disease virus genotype VII in broiler chickens. Poult. Sci. 2019;98:2000–2007. doi: 10.3382/ps/pey559. [DOI] [PubMed] [Google Scholar]
- Skowronski D., Tweed S., De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is itreal, or is it relevant? J. Infect. Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- Sokovic M., Glamoclija J., Marin P., Brkic D., van Griensven L. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an in Vitro Model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne D., King D. Avian influenza and Newcastle isease. J. Am. Vet. Med. Assoc. 2003;222:1534–1540. doi: 10.2460/javma.2003.222.1534. [DOI] [PubMed] [Google Scholar]
- Thaje F., Ulaiwi A. Effect of AROMAX® on performance, local and humoral immunity against vaccination of Newcastal disease in the low management level in broiler chicken. J. Entomol. Zool. Stud. 2017;5:1986–1990. [Google Scholar]
- Toghyani M., Tohidi M., Gheisari A., Tabeidian S. Performance, immunity, serum biochemical and hematological parameters in broiler chicks fed dietarythyme as alternative for an antibiotic growth promoter. Afr. J. Biotech. 2010;9:6819–6825. [Google Scholar]
- Van de Braak S., Leijten G. Centre for the Promotion of Imports from Developing Countries; Rotterdam, The Netherlands: 1999. Essential Oils and Oleoresins: A Survey in the Netherlands and Other Major Markets in the European Union; CBI; p. 116. [Google Scholar]
- Wati T., Ghosh T., Syed B., Haldar S. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens noculated with enteric pathogens. Anim. Nutr. 2015;1:213–219. doi: 10.1016/j.aninu.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Losa R. The use of essential oils and their compounds in poultry. World's Poult. Sci. J. 17, 2001:14–15. [Google Scholar]
- Yesilbag D., Eren M., Agel H., Kovanlikaya A., Balci F. Effects of dietary rosemary, rosemary volatile oil and vitamin E on broiler performance, meat quality and serum SOD activity. Br. Poult. Sci. 2011;52:472–482. doi: 10.1080/00071668.2011.599026. [DOI] [PubMed] [Google Scholar]
- Youdim K.A., Deans S. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fattyacid composition of the ageing rat brain. Br. J. Nutr. 2000;83:87–93. [PubMed] [Google Scholar]