Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2/COVID-19) pandemic is a worldwide emergency. An increasing number of diarrhea cases is reported. Here we investigate the epidemiology, clinical presentation, molecular mechanisms, management, and prevention of SARS-CoV-2 associated diarrhea. We searched on PubMed, EMBASE, and Web of Science up to March 2020 to identify studies documenting diarrhea and mechanism of intestinal inflammation in patients with confirmed diagnosis of SARS-CoV-2 infection. Clinical studies show an incidence rate of diarrhea ranging from 2% to 50% of cases. It may precede or trail respiratory symptoms. A pooled analysis revealed an overall percentage of diarrhea onset of 10.4%. SARS-CoV uses the angiotensin-converting enzyme 2 (ACE2) and the serine protease TMPRSS2 for S protein priming. ACE2 and TMPRSS2 are not only expressed in lung, but also in the small intestinal epithelia. ACE2 is expressed furthermore in the upper esophagus, liver, and colon. SARS-CoV-2 binding affinity to ACE2 is significantly higher (10–20 times) compared with SARS-CoV. Several reports indicate viral RNA shedding in stool detectable longer time period than in nasopharyngeal swabs. Current treatment is supportive, but several options appear promising and are the subject of investigation. Diarrhea is a frequent presenting symptom in patients infected with SARS-CoV-2. Increasing evidence indicates possible fecal oral transmission, indicating the need for a rapid and effective modification of the screening and diagnostic algorithms. The optimal methods to prevent, manage, and treat diarrhea in COVID-19 infected patients are subjects of intensive research.
Keywords: COVID-19, SARS-CoV-2, TMPRSS2, Diarrhea, Epidemiology, Angiotensin-Converting Enzyme 2, ACE2
Abbreviations used in this paper: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; MERS, Middle East respiratory syndrome; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
In December 2019, an outbreak of pneumonia of unknown cause in Wuhan, Hubei province, China led to the identification of a new betacoronavirus, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 SARS-CoV-2 is the seventh identified coronavirus that is able to infect humans.2 In addition to animal origin it shares up to 80% of the gene sequence with other members of coronavirus family, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV).2 , 3 The understanding of SARS human-to-human transmission is still evolving, but is currently believed to occur through air droplets, although fecal oral spread and airborne transmission may be other sources of transmission.4 , 5 In a short time, the highly contagious virus has caused a pandemic, destabilizing health systems, economies, and governments around the world.6
SARS-CoV-2 infection can be asymptomatic or be associated with the coronavirus disease 2019 (COVID-19), which has a spectrum of respiratory clinical manifestations ranging from fever, dry cough, and dyspnea to pneumonia, pulmonary edema, acute respiratory distress syndrome, and multiple organ failures, requiring hospitalization in intensive care unit and leading to death in severe cases.7 Less common symptoms include headache, hemoptysis, nausea, vomiting, and diarrhea.8 Although initially found in a small percentage of cases, an increasing number of patients present with diarrhea.9 Diarrhea is a frequent symptom in coronavirus infections; it was detected in up to 30% of patients with MERS-CoV and 10.6% of patients with SARS-CoV.10 , 11 The purpose of this review is to examine the literature on the epidemiology, clinical symptoms, mechanism of action, management, and prevention of COVID-19-associated diarrhea to better characterize this symptom and to identify any preventive measures for patients exposed to virus.
Methods
We searched PubMed, EMBASE, and Web of Science up to March 2020 to identify all studies documenting the presence of diarrhea in patients with confirmed diagnosis of COVID-19. The following search terms alone or matched with the Boolean operators “AND” or “OR” were used: “COVID-19,” “SARS-CoV-2,” “coronavirus,” “pandemic,” “epidemic,” “outbreak,” “diarrhea,” “gastrointestinal symptom,” “stool,” and “feces.” No temporal, study design, or language restrictions were applied. We focused on full-text articles, but abstracts were considered if relevant. Additional studies were identified through the accurate evaluation of the reference list of the included works.
Etiopathogenesis of SARS-CoV-Associated Diarrhea
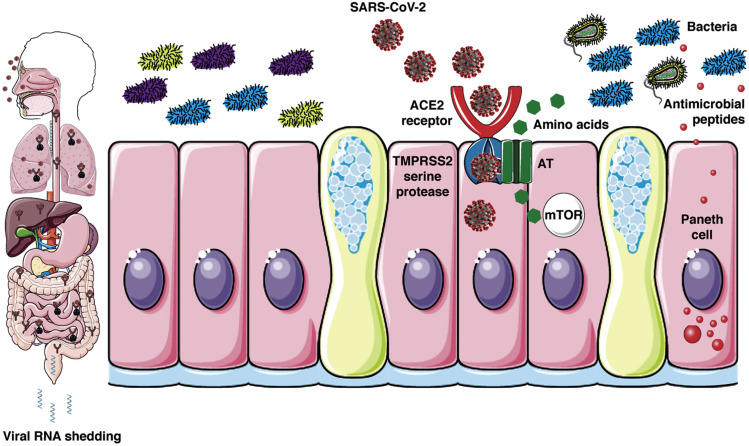
Coronaviruses are a family of single-stranded enveloped RNA viruses and their genome have several open reading frames ranging from 6 to 11.12 The first open reading frame contains most of the viral genome and encodes 16 nonstructural proteins, whereas the other open reading frames encode structural and accessory proteins.13 , 14 The remaining viral genome is responsible for the expression of 4 essential structural proteins: (1) spike glycoprotein, (2) small envelope protein, (3) matrix protein, and (4) nucleocapsid protein.13 , 14 The SARS-CoV entry into a host cell is mediated by the interaction between the envelope-anchored viral spike protein and the host receptor, consisting of angiotensin-converting enzyme 2 (ACE2).13 Genomic characterization of SARS-CoV-2 showed a high homology degree between SARS-CoV-2 and SARS-CoV as regards the receptor-binding domain structure, indicating that the new virus could bind ACE2 and infect humans (Figure 1 ).3 The spike protein is functionally made up of 2 subunits, S1 and S2.13 SARS-2-S uses ACE2 for entry.15 The former mediates virus attachment to the host cell membrane, whereas the latter favors the fusion of the 2 cell membranes.16 This process requires priming by cellular serine proteases (TMPRSS2), which allow spike protein cleavage, regulating the entire mechanism.16 The virus infectivity mainly depends on binding affinity with ACE2 receptor.17 Structural studies17 showed that the new SARS-CoV-2 not only binds ACE2, but its binding affinity for human ACE2 is significantly stronger (10–20 times more)18 than its 2003 SARS-CoV predecessor. A bioinformatics analysis19 based on single-cell transcriptomes revealed that ACE2 was expressed in lung AT2 cells, upper esophagus, and in absorptive enterocytes from ileum and colon. Moreover, other studies provided additional evidence that coronaviruses may infect the gastrointestinal tract, because a high coexpression of ACE2 and TMPRSS2 was detected in enterocytes, and in the esophagus and lungs.4 , 16 , 20 Although the specific mechanisms involved in diarrhea pathogenesis are not entirely known, viral infection is likely to cause an alteration of intestinal permeability, resulting in enterocyte malabsorption.4 In addition, it has been proposed that intestinal ACE2 is involved in the uptake of dietary amino acids, regulating the expression of antimicrobial peptides and promoting the homeostasis of the gut microbiome.21 Mouse models showed that the presence of ACE2 alterations was associated with colitis, suggesting that virus activity may cause enzyme modifications, increasing the susceptibility to intestinal inflammation and diarrhea.21 Further studies are needed to clarify the mechanisms underlying diarrhea in these viral infections and to define the correlation between respiratory and gastrointestinal symptoms.22
Figure 1.
Proposed model for SARS-CoV-2-associated diarrhea. SARS-CoV uses ACE2 and the serine protease TMPRSS2 for entry in lung AT cells. ACE2 and TMPRSS2 are not only expressed in lung, but also the small intestinal epithelia. ACE2 is expressed in the upper esophagus, liver, and colon. ACE2 is also necessary for the surface expression of amino acid transporters of the small intestine. Amino acids, like tryptophan, regulate the secretion of antimicrobial peptides by Paneth cells via mTOR pathway activation. Antimicrobial peptides impact the composition and diversity of the microbiota. Disturbance of this pathway could drive inflammation (enteritis) and ultimately diarrhea. SARS-CoV-2 rendering courtesy of Centers for Disease Control and Prevention. The science cartoon was created with Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. AT, Alveolar Type; mTOR, mammalian target of rapamycin.
Epidemiology of COVID-19-Associated Diarrhea
Epidemiologic data of COVID-19 patients with diarrhea are summarized in Table 1 . The medical records of the National Health Commission of China allowed to evaluate the data of 1099 Chinese patients with an established diagnosis of COVID-19 up to January 31, 2020.23 Forty-two subjects experienced diarrhea (3.8%) and the primary composite outcome (admission to intensive care unit, use of mechanical ventilation, or death) occurred in 4 patients with diarrhea (6%).23 In a retrospective study by Xu et al24 diarrhea occurred in 3 of 62 patients (4.8%). A symptom duration-based analysis showed that diarrhea was experienced only in patients who had symptoms for more than 10 days (3/33; 9%), whereas those who had a shorter duration had no diarrhea.24 In a cross-sectional, Chinese multicenter study9 enrolling 204 patients until early March 2020, diarrhea occurred in 29 cases (29.3%). The time between symptom onset and admission to the hospital was significantly longer in patients with digestive symptoms than in patients without gastrointestinal manifestations (9 vs 7.3 days; P = .02).9 Most patients had nondehydrating loose stools and had an average of 3 evacuations per day.9 No cases of severe diarrhea were detected, although a clinical relationship was reported between diarrhea and worsening of COVID-19 symptoms.9 Wu et al25 investigated a cluster of subjects exposed to the infection at the same time, documenting the presence of diarrhea in 15% of positive patients. In the experience of Huang et al,26 only 1 out of 41 (3%) patients had diarrhea as initial symptom, whereas another Chinese study conducted in the Zhejiang province8 reported a low rate of diarrhea onset in COVID-19 patients (2%). A study by Xiao et al27 analyzed stool samples from 73 COVID-19 patients to assess the clinical significance of measuring SARS-CoV-2 RNA in the feces. Diarrhea was found in 26 patients and the fecal test remained positive until 12 days after the disease onset.27 It is worth mentioning that in 17 patients (23.3%) the stool test was still positive despite negative respiratory tests.27 In addition, in a 78-year-old patient with severe respiratory distress treated with venovenous extracorporeal membrane oxygenation, endoscopic procedures were performed following signs of bleeding (coffee ground material from the nasogastric tube and positive fecal occult blood test).27 No mucosal damage was identified, but multiple esophageal, gastric, duodenal, and rectal biopsies were performed.27 Histologic analysis revealed a high percentage of ACE2 protein in the glandular cells of all examined segments, with the exception of the esophagus (mainly characterized by squamous cells), supporting the theory of a possible effect of the virus on these organs.27 An 81-year-old Japanese woman with COVID-19 had watery diarrhea and the virus was detected in the stool for up to 15 days after disease onset.28 In a descriptive case series29 of the first 18 COVID-19 cases in Singapore, 3 patients had diarrhea (16.6%). None of these 3 patients had complications and none required supplemental oxygen.29 Stool samples were tested by real-time reverse transcriptase polymerase chain reaction and SARS-CoV-2 was found in 4 of 8 patients (50%) from Day 1 to Day 7.29 In a small case series,30 data from 5 patients who tested positive for both SARS-CoV-2 and influenza virus were analyzed, reporting diarrhea in 2 cases (40%). An analysis on the clinical characteristics of 30 medical members (22 doctors and 8 nurses) with COVID-19 showed the presence of diarrhea in 9 of 30 subjects (30%).31 Recently, the first study specifically designed to evaluate patients with gastrointestinal symptoms at disease onset was published.32 One hundred eighty-three patients were included, and diarrhea was detected in 68 cases (37.1%). COVID-19 epidemiologic data in children are lacking; however, in a recent analysis33 of 171 children with a median age of 6.7 years, diarrhea was reported in 8.8% (15) of cases. Furthermore, a recent systematic review and meta-analysis of case studies34 assessed the epidemiologic characteristics of 1995 patients with COVID-19, showing a diarrhea rate of 4.8%. Finally, we performed a pooled analysis of all available studies revealing an overall diarrhea rate of 10.4% in patients with COVID-19.
Table 1.
Epidemiologic Data on Diarrhea in Patients With COVID-19
| First author | Study design | Study country | Hospital | Number of patients | Diarrhea, n (%) | Diarrhea duration | Evacuations per day | Diarrhea pooled analysis,% |
|---|---|---|---|---|---|---|---|---|
| Guan23 | Retrospective cohort study | China | 552 hospitals | 1099 | 42 (3.8) | na | na | |
| Wang7 | Retrospective case series | China | Zhongnan Hospital of Wuhan | 138 | 14 (10.1) | na | na | |
| Huang26 | Retrospective cohort study | China | Jin Yintan Hospital of Wuhan | 31 | 1 (3) | na | na | |
| Xu24 | Retrospective case series | China | 7 hospitals in Zhejiang province | 62 | 3 (4.8) | na | na | |
| Chen8 | Retrospective cohort study | China | Jinyintan Hospital in Wuhan | 99 | 2 (2) | na | na | |
| Xiao27 | Retrospective cohort study | China | Fifth Affiliated Hospital of Zhuhai | 73 | 26 (35.6) | na | na | |
| Luo32 | Retrospective cohort study | China | Zhongnan Hospital of Wuhan University | 183 | 68 (37.1) | na | na | |
| Young29 | Retrospective case series | Singapore | 4 hospitals in Singapore | 18 | 3 (16.6) | na | na | |
| Li34 | Systematic review and meta-analysis | China | na | 1995 | 96 (4.8) | na | na | |
| Pan9 | Cross-sectional study | China | Wuhan Hanan Hospital; Wuhan Union Hospital; Huanggang Central Hospital | 204 | 29 (29.3) | na | 3/d (mean) | |
| Mo38 | Retrospective cohort study | China | Zhongnan Hospital of Wuhan University | 155 | 7 (4.5) | na | na | |
| Lu33 | Retrospective cohort study | China | Wuhan Children’s Hospital | 171 | 15 (8.8) | na | na | |
| Liu31 | Retrospective cohort study | China | Jianghan University Hospital | 30 | 9 (30) | na | na | |
| Wu64 | Retrospective cohort study | China | na | 53 | 8 (15) | na | na | |
| Jin35 | Retrospective cohort study | China | Several hospitals of Zhejiang province | 651 | 53 (8.1) | 4 d | >3 loose stools/d | |
| Spiteri68 | Retrospective cohort study | Europe | na | 38 | 1 (2.6) | na | na | |
| Lescure69 | Retrospective case series | French | na | 5 | 1 (20) | na | na | |
| Chang70 | Retrospective case series | China | Beijing Tsinghua Changgung Hospital; Beijing Anzhen Hospital; Chinese PLA General Hospital |
13 | 1 (7.7) | na | na | |
| Ding30 | Retrospective case series | China | Tongji Hospital | 5 | 2 (40) | na | na | |
| Chan10 | Retrospective case series | China | University of Hong Kong- Shenzhen Hospital | 7 | 2 (28.5) | 3–4 d | 5–8/d | |
| Wang71 | Retrospective case series | China | Shanghai Public Health Clinical Center | 4 | 2 (50) | na | na | |
| Song36 | Case report | China | Weihai Municipal Hospital | 1 | 1 | 4 d | 3–4/d | |
| Hosoda28 | Case report | Japan | Municipal Kawasaki Hospital | 1 | 1 | na | na | |
| Holshue37 | Case report | United States | Snohomish County clinic, Washington |
1 | 1 | 2 d | 3–4/d | |
| 3042a | 292a | 10.4 |
COVID-19, coronavirus disease 2019; na, not available.
The systematic review and meta-analysis of Li was not included in the analysis to prevent some studies from being considered twice.
Characteristics of COVID-19-Associated Diarrhea
Jin et al35 defined diarrhea as the passing of loose stools >3 times per day. In their retrospective study, 53 of 651 patients (8.1%) had diarrhea at onset and the median symptom duration was 4 days.35 The evidence presented by Chan et al10 provided data from a family cluster with COVID-19. Two out of 7 patients experienced 3–4 days of diarrhea with several evacuations ranging from 5 to 8 per day.10 However, in a 22-year-old young man,36 diarrhea resulted in a lower number of evacuations (3–4 per day) and was associated with a low-grade fever. Interestingly, these symptoms disappeared after antiviral therapy (oral lopinavir and ritonavir), supporting the link between symptom and COVID-19 disease.36 The first known case of COVID-19 in the United States also showed diarrheal symptoms for 2 consecutive days.37 A stool sample was collected following loose bowel movements to verify the presence of the virus.37 Importantly, the test was positive 7 days after the presumed onset of the disease, showing a high viral load.37 Unfortunately, in the remaining studies diarrhea was not well characterized and no data were available regarding the total number of evacuations, consistency of the stools (Bristol scale), and duration of symptoms.
Prognostic Implications of Diarrhea
A symptom analysis based on COVID-19 severity (according to the American Thoracic Society guidelines for community-acquired pneumonia) showed a greater diarrhea percentage in patients with severe disease compared with those with nonsevere disease (5.8% vs 3.5%, respectively), suggesting an association between presence of symptom and disease severity.23 Likewise, COVID-19 patients with diarrhea, nausea, and vomiting were more likely to require mechanical ventilation and had acute respiratory distress syndrome compared with patients without gastrointestinal symptoms (6.76% vs 2.08% [P = .034] and 6.76% vs 2.08% [P = .034], respectively).35 However, a case series of 138 patients found that diarrhea was present in 14 patients (10.1%) at disease onset and it was not associated with greater need for intensive care unit care.7 Another study compared the characteristics of COVID-19 patients who underwent regular hospital management with patients who had more severe disease requiring mechanical ventilation or intensive care; the purpose of this analysis was to identify factors associated with poor prognosis in COVID-19.38 Among the 155 included patients, diarrhea occurred in 5 patients with severe disease and in 2 cases in the standard management group.38 Although this finding suggested a worse prognosis in patients with diarrhea, no correlation was found.38 Additional studies are needed to clarify the correlation between diarrhea and the outcomes of COVID-19 patients.
Lessons From Other Coronavirus Infections
Intestinal involvement and diarrhea in association with respiratory symptoms are common features to the other members of the coronavirus family.13 A retrospective study evaluated the gastrointestinal symptoms of the first cohort of patients with SARS in Hong Kong in 2003.39 Watery diarrhea, without blood or mucus, was a frequent symptom, occurring in 28 of 138 patients (20.3%) at disease presentation (Table 2 ).39 In 8 patients (5.8%) diarrhea was combined with fever, whereas 25 additional patients experienced diarrhea in the following 3 weeks, accounting for a total of 53 patients (38.4%).39 Mean duration of diarrhea was 3.7 days and evacuations ranged from few stools to 30 per day.39 Patients with diarrhea had a higher need for ventilatory support (26.4% vs 8.2%; P = .004) and intensive care (49.0% vs 11.8%; P < .001), suggesting a greater disease severity, although no correlation with the mortality rate was found.39 SARS-CoV was also identified in terminal ileal and colonic biopsies and real-time reverse transcriptase polymerase chain reaction fecal samples, where it was detectable up to 10 weeks after the onset of symptoms.39 Data from Toronto SARS patients confirmed the incidence rates of diarrhea, which was reported in 23.6% of the infected patients.40 However, in another study by Peiris et al41 diarrhea was present in 1% of patients at onset, but 73% of infected people developed watery diarrhea after about 1 week. In regards to MERS, a descriptive study showed the presence of diarrhea in 12 cases (26%).42 In a study43 on 186 MERS patients, diarrhea occurred in 26 subjects (14%). However, analyzing the data based on survival, a greater number of subjects who survived had diarrheal symptoms compared with the deceased (76.5% vs 46.4%; P = .05), indicating a less severe disease and a better prognosis in patients with diarrhea.43 Another study44 compared the clinical characteristics of medical staff and patients with MERS, and found a higher percentage of diarrheal symptoms in the former than in the latter (11 cases, 50% vs 5 cases, 23%; P value nonsignificant).
Table 2.
Epidemiologic Data on Diarrhea in Patients With Other Coronaviruses
| First author | Study design | Study country | Virus | Number of patients | Diarrhea at onset disease, n (%) | Late onset of diarrhea, n (%) | Diarrhea duration | Evacuations per day |
|---|---|---|---|---|---|---|---|---|
| Leung39 | Retrospective cohort study | China | SARS-CoV | 138 | 28 (20.3) | 25 (18.1) | 3.7 d (mean) | Up to 30 |
| Booth40 | Retrospective case series | North America | SARS-CoV | 144 | 34 (23.6) | na | na | na |
| Peiris41 | Prospective cohort study | China | SARS-CoV | 75 | 1 (1) | 55 (73) | 3.9 d (mean) | 6.3 (maximum) |
| Assiri42 | Retrospective cohort study | Saudi Arabia | MERS-CoV | 47 | 12 (26) | na | na | na |
| Choi43 | Retrospective cohort study | Republic of Korea | MERS-CoV | 186 | 26a (14) | na | na | na |
| Garbati44 | Retrospective cohort study | Saudi Arabia | MERS-CoV | 48 | 16a (33.3) | na | na | na |
CoV, coronavirus; MERS, Middle East respiratory syndrome; na, not available; SARS, severe acute respiratory syndrome.
This number indicates the total number of diarrhea cases during the entire disease course.
Prevention
So far, no vaccines have been developed to prevent COVID-19, but several potential vaccines are being tested (NCT04299724, NCT04276896, NCT04313127, and NCT04283461). The current precautions adopted to contain the infection are standard measures for respiratory viral pathologies, including the use of masks and gloves, frequent hand hygiene (alcohol-based disinfectants or soap and water), travel restrictions, and avoiding contact with people suspected or confirmed to be infected.45 The presence of virus in the stool and its long fecal persistence time suggest that orofecal transmission is possible, leading to several implications and requiring additional precautions. First, contact with possible sources of contamination (eg, saliva, vomiting, and feces) should be avoided with a greater attention to hygiene.22 Second, outpatient management should be modified. Deferable gastroenterologic consultations and nonurgent endoscopic procedures should be rescheduled and each patient should be stratified according to the symptoms or based on possible contact with infected people or origin from high-risk areas.46 Personal protective equipment including gloves, goggles, gowns, and respiratory protective devices should be adopted by health care professionals of the endoscopy units to avoid spreading the virus (Table 3 ).46 Third, all candidates for fecal microbiota transplantation and healthy donors should be screened for the virus.47 In addition, animal models showed that ACE and angiotensin receptor inhibition were associated with an increase in circulating ACE2 levels.48 Based on diarrhea etiopathogenesis and on the key role of ACE2, the use of ACE or angiotensin receptor blockers should be investigated, particularly in elderly or cardiovascular patients, as it could lead to a higher risk of developing COVID-19 diarrhea.49 Importantly, this hypothesis is not confirmed, and further investigations are needed to demonstrate whether the use of ACE/angiotensin receptor inhibitors is a risk factor for COVID-19.
Table 3.
Recommendations for Health Care Professionals in the Management of Patients With COVID-19 and Diarrhea
| Wear gloves, mask, protective gown, and goggles every time you visit a patient with diarrhea |
| Pay attention to hand hygiene before and after visiting a patient with diarrhea, using alcoholic disinfectants or soap and water |
| Patients with diarrhea should have a personal bathroom and bathroom sanitation should be performed several times per day |
| All endoscopes and reusable accessories should be reprocessed with standard reprocessing procedures |
Treatment
Currently there is no specific treatment for COVID-19 and its management is mainly based on supportive care. No evidence on the efficacy of antidiarrheal drugs is available, but an adequate rehydration and potassium monitoring should be performed as in all patients with diarrhea.50 It is important to underline that antibiotics and antivirals are often used for COVID-19 treatment, involving a likely alteration of the gut microbiota and causing diarrhea.51 , 52 It is therefore plausible that the gut microbiota could be a new therapeutic target and that probiotics could have a role in the management of these patients.22 , 53 Interestingly, China’s National Health Commission recommended the use of probiotics for the treatment of patients with severe COVID-19 to preserve intestinal balance and to prevent secondary bacterial infections.22 Moreover, a rapid improvement in diarrhea was also found after starting antiviral therapy.36 Although no antiviral drug was specifically designed for the treatment of diarrhea, several molecules could have beneficial effects. Some monoclonal antibodies target the receptor-binding domain of the spike protein to inhibit the contact between the virus and ACE2.54 Other attractive target is the TMPRSS2 protease, which plays a crucial role for viral infection.16 Camostat mesylate a known effective inhibitor of TMPRSS2 and is already approved in Japan for management of noninfectious conditions, such as chronic pancreatitis and reflux esophagitis; it is possible that this agent may work for COVID-19.20 A randomized, controlled, open-label trial evaluated the efficacy of lopinavir-ritonavir combination, 2 protease inhibitors, for the treatment of patients with confirmed diagnosis of COVID-19.55 Unfortunately, no significant clinical improvement was found with this therapy.55 Remdesivir is a nucleotide analogue that prevents viral replication and that was effective (in combination with chloroquine) in blocking SARS-CoV-2 infection in vitro.56 Chloroquine and hydroxychloroquine were used for the treatment of SARS and MERS and were effective in reducing coronavirus replication.57 In in vitro studies, both drugs reduced SARS-CoV-2 replication, but hydroxychloroquine had greater inhibitory power than chloroquine.57 A systematic review58 investigated preclinical data and ongoing clinical trials, providing sufficient information on the efficacy of hydroxychloroquine for COVID-19 treatment. Based on this evidence the Food and Drug Administration has recently approved hydroxychloroquine as a therapeutic option for SARS-CoV-2 infection.59 Finally, the use of baricitinib, a JAK kinase inhibitor, was proposed for the treatment of COVID-19.60 It blocks the AP2-associated protein kinase 1 and the cyclin G-associated kinase, which are 2 important regulators of cellular endocytosis, resulting in a theoretical reduction of the viral passage into the host cell.60 The anti-inflammatory function and the antiendocytic activity of baricitinib could be effective in diarrhea and deserve further studies.60
Discussion
In the current SARS-CoV-2 pandemic, most of the attention is still exclusively focused on the respiratory symptoms of the disease. However, it is important to emphasize that the number of COVID-19 patients experiencing diarrhea is significant and cannot be overlooked. We found high variability among published studies in the percentage of patients with diarrhea, ranging from 2% to 50% of cases. Our pooled analysis of the available data revealed an overall diarrhea rate of about 10% in COVID-19 patients. This value is lower than the percentage of diarrhea reported with other coronaviruses,40 , 44 but it is possible that the available data may underestimate the burden of diarrhea associated with COVID-19.61 For example, none of the studies we reviewed provided an explicit definition for diarrhea. The World Health Organization defines diarrhea as 3 or more loose/liquid stools per day or an increase in the number of evacuations compared with the usual.62 Given the subjective nature, it is not surprising that there is marked heterogeneity in the estimates of patients with diarrhea symptoms.61 The collection of reliable epidemiologic information is fundamental, and it should be obtained in the shortest possible time to guarantee adequate preventive measures and to allow the best pandemic management. Data should be recorded with explicit diarrhea definition and characterization of number and duration of evacuations, specifying whether the symptom occurred at the onset or during the course of the disease. The use of the so-called “big data” could be a valid alternative to capture the growing amount of data in a short period as proven in a single Chinese experience.63 In addition, some patients have diarrhea in the absence of respiratory symptoms, and this may lead to underestimation of COVID-19 cases, because further investigations may not be performed in patients with mild symptoms. Another limitation is related to the diagnostic method. The analysis of respiratory tract samples does not allow identification of all infections, resulting in diagnostic delays or undiagnosed cases.64 A recent study used a mathematical model to simulate the dynamics of infection in China.65 It was estimated that around 86% of the infections were not documented and that patients with undocumented cases led to the contagion of most of the identified patients (79%).65 SARS-CoV-2 is similar to SARS-CoV and MERS-CoV, but it is transmitted faster than its predecessors and for this reason a rapid and optimal diagnostic approach is essential to contain the virus dissemination.66 The evidence of SARS-CoV-2 in the stools and in gastrointestinal histologic samples and its prolonged persistence at the stool compared with nasopharyngeal swabs strongly suggests that orofecal transmission is possible, justifying the execution of fecal polymerase chain reaction in suspect patients.27 The homology between SARS-CoV-2 and SARS-CoV and MERS-CoV and the high capacity of these viruses to resist for long periods (even 2 weeks) at low temperatures and for a few days at temperatures between 20°C and 30°C is a further confirmation of possible orofecal transmission and requires an enhancement of preventive hygiene measures.67
In conclusion, the presence of diarrhea should generate suspicion of a possible SARS-CoV-2 infection and should be investigated to reach an early diagnosis of COVID-19. The incidence of diarrhea is currently underestimated and further studies are needed to quantify the exact burden of diarrhea to compare the sensitivity of fecal and nasopharyngeal tests, to evaluate whether diarrhea is a predictive factor for prognosis, and to clarify the effects of COVID-19 in patients with underlying gastrointestinal diseases.
Fasiha Kanwal, Section Editor
Footnotes
Conflicts of interest These authors disclose the following: Daniel C. Baumgart has served as scientific consultant and advisory board member for AbbVie, MSD, Janssen, Takeda, Boehringer-Ingelheim, and Amgen. Silvio Danese has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. Laurent Peyrin-Biroulet has served as a speaker, consultant, and advisory board member for Merck, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC-Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, and Theravance. The other author discloses no conflicts.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. [published online ahead of print, 2020 Mar 3] Gastroenterology. 2020:S0016–S5085. doi: 10.1053/j.gastro.2020.02.054. (20)30281-X. doi:10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doremalen N van, Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anon WHO Director-General’s opening remarks at the media briefing on COVID-19: 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 7.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. [published online ahead of print, 2020 Feb 7] JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L., Mu M., Ren H.G. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. PracticeUpdate. https://www.practiceupdate.com/content/clinical-characteristics-of-covid-19-patients-with-digestive-symptoms-in-hubei-china/98000 Available at: [DOI] [PMC free article] [PubMed]
- 10.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y., Zhao K., Shi Z.-L. Bat coronaviruses in China. Viruses. 2019;11:210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Z., Xu Y., Bao L. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y.-R., Cao Q.-D., Hong Z.-S. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak: an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. [published online ahead of print, 2020 Mar 4] Cell. 2020 doi: 10.1016/j.cell.2020.02.052. S0092-8674(20)30229-4. doi:10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y., Shang J., Graham R. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H., Kang Z., Gong H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020:2020. 01.30.927806. [Google Scholar]
- 20.Kawase M., Shirato K., van der Hoek L. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto T., Perlot T., Rehman A. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X.-W., Wu X.-X., Jiang X.-G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu W.S., Li Y.G., Wei Z.F. [Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:489–493. doi: 10.3760/cma.j.cn112338-20200221-00139. [DOI] [PubMed] [Google Scholar]
- 26.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. [published online ahead of print, 2020 Mar 3] Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. S0016-5085(20)30282-1. doi:10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosoda T., Sakamoto M., Shimizu H. SARS-CoV-2 enterocolitis with persisting to excrete the virus for about two weeks after recovering from diarrhea: a case report. [published online ahead of print, 2020 Mar 19] Infect Control Hosp Epidemiol. 2020:1–4. doi: 10.1017/ice.2020.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. [published online ahead of print, 2020 Mar 3] JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Q., Lu P., Fan Y. The clinical characteristics of pneumonia patients co-infected with 2019 novel coronavirus and influenza virus in Wuhan, China. [published online ahead of print, 2020 Mar 20] J Med Virol. 2020 doi: 10.1002/jmv.25781. 10.1002/jmv.25781. doi:10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M., He P., Liu H.G. [Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia] Zhonghua Jie He Hu Xi Za Zhi. 2020;43:209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clinical Gastroenterology and Hepatology. Available at: 10.1016/j.cgh.2020.03.043. Accessed March 23, 2020. [DOI] [PMC free article] [PubMed]
- 33.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L.-Q., Huang T., Wang Y.-Q. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Mar 12 doi: 10.1002/jmv.25757. doi: 10.1002/jmv.25757. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin X., Lian J.-S., Hu J.-H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. [published online ahead of print, 2020 Mar 24] Gut. 2020 doi: 10.1136/gutjnl-2020-320926. gutjnl-2020-320926. doi:10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Y., Liu P., Shi X.L. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. [published online ahead of print, 2020 Mar 5] Gut. 2020 doi: 10.1136/gutjnl-2020-320891. gutjnl-2020-320891. doi:10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 37.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. [published online ahead of print, 2020 Mar 16] Clin Infect Dis. 2020 ciaa270. doi:10.1093/cid/ciaa270. [Google Scholar]
- 39.Leung W.K., To K.-F., Chan P.K.S. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 41.Peiris J.S.M., Chu C.M., Cheng V.C.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi W.S., Kang C.-I., Kim Y. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect Chemother. 2016;48:118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garbati M.A., Fagbo S.F., Fang V.J. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ungaro R.C., Sullivan T., Colombel J.-F. What should gastroenterologists and patients know about COVID-19? [published online ahead of print, 2020 Mar 17] Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.03.020. S1542-3565(20)30330-X. doi:10.1016/j.cgh.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Repici A., Maselli R., Colombo M. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. [published online ahead of print, 2020 Mar 14] Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.019. S0016-5107(20)30245-5. doi:10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ianiro G., Mullish B.H., Kelly C.R. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 49.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. [published online ahead of print, 2020 Mar 18] J Travel Med. 2020 doi: 10.1093/jtm/taaa041. taaa041. doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avery M.E., Snyder J.D. Oral therapy for acute diarrhea. The underused simple solution. N Engl J Med. 1990;323:891–894. doi: 10.1056/NEJM199009273231307. [DOI] [PubMed] [Google Scholar]
- 51.Bartlett J.G. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 52.Logan C., Beadsworth M.B.J., Beeching N.J. HIV and diarrhoea: what is new? Curr Opin Infect Dis. 2016;29:486–494. doi: 10.1097/QCO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 53.Bradley K.C., Finsterbusch K., Schnepf D. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28:245–256. doi: 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 54.Tian X., Li C., Huang A. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). [published online ahead of print, 2020 Mar 9] Clin Infect Dis. 2020:ciaa237. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapoor K.M., Kapoor A. Role of chloroquine and hydroxychloroquine in the treatment of COVID-19 infection: a systematic literature review. medRxiv. 2020:2020. 03.24.20042366. [Google Scholar]
- 59.Anon Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=040274 Available at:
- 60.Richardson P., Griffin I., Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang W., Feng Z., Rao S. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. [published online ahead of print, 2020 Feb 26] Gut. 2020 doi: 10.1136/gutjnl-2020-320832. gutjnl-2020-320832. doi:10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 62.Anon Diarrhoeal disease. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease Available at:
- 63.Qiu H.J., Yuan L.X., Huang X.K. [Using the big data ofinternet to understand coronavirus disease 2019’s symptom characteristics: a big data study] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:E004. doi: 10.3760/cma.j.cn115330-20200225-00128. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. doi: 10.1001/jama.2020. 2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Li R., Pei S., Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). [published online ahead of print, 2020 Mar 16] Science. 2020:eabb3221. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meo S.A., Alhowikan A.M., Al-Khlaiwi T. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 67.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spiteri G., Fielding J., Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25:2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lescure F.-X., Bouadma L., Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. The Lancet Infectious Diseases 2020;0. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30200-0/abstract Available at: [DOI] [PMC free article] [PubMed]
- 70.Chang D., Lin M., Wei L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Chen X., Lu Y. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]