Abstract
In nonsecretory, oligo‐secretory, and light chain multiple myeloma patients, serial sFLC evaluation could precede biochemical and clinical disease progression, even in extramedullary relapse, thus initiating early treatment with novel anti‐MM agents.
Keywords: disease progression, extramedullary relapse, multiple myeloma, serological markers, serum free light chains
In nonsecretory, oligo‐secretory, and light chain multiple myeloma patients, serial sFLC evaluation could precede biochemical and clinical disease progression, even in extramedullary relapse, thus initiating early treatment with novel anti‐MM agents.
1. INTRODUCTION
Multiple myeloma remains incurable with frequent aggressive relapse. In real life, we cannot always evaluate disease progression with measurable MC, but we can increase the accuracy by using serum free light chain (sFLC) assay. We present four clinical cases where sFLC allowed diagnosis of extramedullary relapse during treatment.
Plasma cell (PC) dyscrasias represent a spectrum of progressively more severe monoclonal gammopathies. Malignant plasma cell clones overproduce and secrete a “monoclonal M‐protein” (MP), an abnormal complete monoclonal antibody or its fragment, called light chain (LC).1, 2
Serum concentrations of free light chains (sFLC) are dependent on the balance between production and renal clearance. Abnormal concentrations of kappa (κ) and lambda (λ) FLC may result from a number of clinical situations including immune suppression, immune stimulation, reduced renal clearance, or monoclonal plasma cell proliferative disorders. The kappa/lambda (κ/λ) FLC ratio (rFLC), however, usually remains normal in these conditions. On the contrary, a significantly abnormal κ/λ rFLC is usually due to plasma cell (or lymphoproliferative) disorders that secrete only one type of FLC in excess and disturb the normal balance between κ and λ secretion.3
The diagnosis and evaluation of PC dyscrasias have been performed, until the early 2000s, using both the protein electrophoresis (PEP) and immunofixation electrophoresis (IFE) of serum and urine, representing the gold standard. These measurements were sufficient for most MGUS and MM patients. However, the tests resulted inadequate for the majority of patients with AL amyloidosis and more than 3% of patients with nonsecretory (NS), oligo‐secretory (OS), and light chain (LC) multiple myeloma. Therefore, FLC assay was introduced and became part of initial screening, together with serum IFE and PEP, for all PC dyscrasias.3
The most commonly used serum FLC assays are the Freelite, the N‐Latex, and the Seralite assay. The most popular is the Freelite assay, that is, based on polyclonal antibodies (pAbs). The vast majority of clinical studies demonstrating the utility of sFLC measurement have been performed using this assay. It is the only method officially mentioned in the International Myeloma Working Group (IMWG) guidelines, considered as a diagnostic tool. Both N‐Latex and Seralite assays are based on monoclonal antibodies (mAbs), but Seralite assay allows the simultaneous measurement of κ and λ FLC, while N‐Latex does not. The three above‐mentioned methods show relevant differences, without a clear superiority of a single method. Hence, the assays cannot be interchangeable, in particular when an assay is used to monitor patient's response to therapy.4 Katzmann and colleagues, referring to Freelite, have defined the normal κ/λ FLC range with ratios superior to 1.65 containing excess of monoclonal κ, and inferior to 0.26 excess of monoclonal λ FLC.5
About a third of patients with MGUS, 70% of patients with smoldering multiple myeloma (sMM), and more than 90% of patients with MM have an altered rFLC at diagnosis. The presence of an abnormal rFLC, and the extent of this abnormality, predict the risk of progression. Dispenzieri and colleagues6 reported that in patients with sMM, an involved to uninvolved (I/U) ratio of 8 or more is associated with about 40% risk of progression within the first 2 years from diagnosis. Larsen et al7 concluded their study on sMM saying that a FLC ratio of at least 100 is a predictor of imminent progression to MM, even in the absence of CRAB criteria, and that such patients should be immediately started on induction therapy. Subsequently, the IMWG included the rFLC of at least 100 as one of myeloma defining biomarkers of malignancy.8
In addition, FLC assay is an integral part of response assessment: stringent complete response (sCR) represents a complete response (CR) plus normal rFLC and absence of clonal cells in bone marrow (BM) biopsy by immunohistochemistry. So far, the routine serial use of sFLC can be indicated only in cases of patients with nonmeasurable (in some cases of NS) or low values of serum (<10 g/L by SPEP) and urine M‐protein (<200 mg/24 h) (OS MM).9
Although the wide use of sFLC assay is already present, none of the clinical trials supporting novel agents’ approval has evaluated treatment response using FLC assay. In main clinical trials evaluating novel anti‐MM therapies from first relapse (MM‐003, ASPIRE, CASTOR, POLLUX), measure of response was calculated using serial monoclonal component (MC) measurements and minimal residual disease (MRD) evaluation, but none of these trials had included patients without measurable monoclonal component, therefore excluding NS MM patients.
In real life, we cannot always evaluate disease progression on the basis of measurable MC, but we can increase the accuracy of disease evaluation by using sFLC assay.
We can, therefore, question whether involved FLC and rFLC measurements could be an independent marker of response in the era of novel anti‐MM novel therapies. Is it time to extend routine FLC evaluation at any time in disease monitoring? Are there any particular subgroups of patients that can benefit from monthly FLC assay evaluation? We are not actually able to answer these questions, but we can reason to this issue and describe our limited experience.
We present four clinical cases where sFLC assay (N‐Latex) anticipated the extramedullary relapse during treatment.
2. CASE REPORTS
2.1. Oligo‐secretory myeloma with EMD following light chain escape
A 57‐year‐old woman was diagnosed with OS MM IgG κ in August 2009 at another center. The disease was characterized by the presence of iliac and lumbar osteolytic lesions, and a lumbar spinal cord plasmocytoma. The mass was surgically removed, and later, the patient underwent an (unknown) induction therapy followed by autologous stem cell transplantation (ASCT) in June 2010, obtaining CR.
In June 2013, the patient was referred to our center for reappearance of MP (0.21 g/dL), together with a single left iliac osteolytic lesion detected by magnetic resonance imaging (MRI), while BM biopsy resulted negative. Given the single disease site, the patient underwent loco‐regional radiotherapy, for a total of 30 Gy, between November and December 2013, resulting in CR with disappearance of MP. The patient was, then, followed with monthly visits and bisphosphonate therapy.
In March 2015, the patient relapsed with multiple osteolytic lesions of the sternum, both femurs and the ribs, revealed on PET/CT scan, and reappearance of MP (0.31 g/dL). The patient was immediately started on 2nd‐line systemic therapy with bortezomib‐cyclophosphamide‐dexamethasone (VCd), administered from April to November 2015, with disappearance of MP and stable bone lesions.
In November 2016, both PET/CT and MRI scan revealed progression of disease with new osteolytic lesions of the skull and right knee, together with progression of the previously known sternum, femurs, and ribs lesions. Neither monoclonal protein nor serum IFE was detected. A 3rd‐line therapy with lenalidomide, 25 mg, and dexamethasone (Rd) was initiated for a total of 7 cycles. Unfortunately, in May 2017 a severe bone pain induced us to perform a low‐dose bone CT scan that revealed further progression bone disease with increase in both the number and volume of the previously known osteolytic lesions. Serum IFE remained negative, while an increase of sFLC κ (175 mg/L; n.v. 6.7‐22.4 mg/L) and rFLC (4.2; n.v. 0.31‐1.56) was recorded.
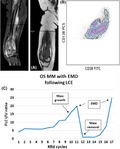
Carfilzomib was then added to lenalidomide and dexamethasone (KRd regimen),10 with a quick improvement of bone pain with the exception of a recurrent pain of the right knee. However, sFLC ratio continued to rise during treatment. Although a CT scan of the right knee in February 2018 did not evidence any new lesions, after completing 11 cycles of KRd, a PET/CT scan in March revealed disappearance of all the previously described lesions, but also the presence of a solid mass in the distal region of the right femur, with the involvement of the surrounding muscular tissue, later confirmed by MRI scan (Figure 1A). Both the BM biopsy and the serum IFE remained negative, while sFLC assay was significantly elevated compared to the initial values (May 2017‐March 2018: sFLC κ 175 vs 340 mg/L, rFLC 4.2 vs 19.9).
Figure 1.
Clinical course of OSMM patient with LCE, showing FLC ratio, diagnostic and immunophenotypic images. A, MRI scan with coronal T1‐W and sagittal STIR sequences of the growing knee mass (1st EMD relapse). B, Dot plot shows the presence of plasma cells CD38 + CD138+, evaluated in cytometry, in extramedullary site. C, FLC curve in OS MM patient with EMD relapse following LCE during KRd therapy (OSMM—oligo‐secretory multiple myeloma; LCE—light chain escape; FLC I/U ratio—free light chain involved/uninvolved ratio (n.v. 0.31‐1.56); KRd—carfilzomib, lenalidomide, dexamethasone; EMD—extramedullary myeloma disease)
Since the knee mass had grown in a matter of weeks, it was surgically removed, followed by a placement of a knee prosthesis. The histology of the mass showed the presence of a monotonous neoplastic plasma cell infiltration (Figure 1B), confirming an extramedullary myeloma disease (EMD). A FISH analysis was performed on plasma cells aspirated from the mass, but resulted negative for standard and high‐risk alterations. Following the mass removal, the sFLC assay returned within normal range, while serum IFE remained negative. The PET/CT scan performed in June 2018, demonstrated only an unspecific uptake at the site of the intervention, that was perceived as postsurgical inflammation.
In June, after completing the rehabilitation period, the KRd regimen was restarted. Patient completed 4 more cycles until November 2018 (15 cycles in total), when the knee mass reappeared, together with an altered sFLC assay (May‐November 2018: sFLC κ 25.8 vs 124 mg/L, rFLC 1.3 vs 25). An echo‐guided mass biopsy confirmed the EMD relapse with the presence of plasma cells and plasmablasts. KRd was discontinued and the patient underwent local radiotherapy for a total of 30 Gy, followed by the 5th line with daratumumab, bortezomib, and dexamethasone (DaraVd). This treatment resulted in a significant reduction of both the diameter of the knee mass, and the sFLC κ (83.4 mg/L) and ratio (18).
At time of this report, the patient is still receiving DaraVd with improvement of general conditions.
Overall, this patient was affected by OS MM IgG κ with history of multiple relapses. At time of extramedullary disease progression, sIFE resulted negative, while sFLC assay was always abnormal in presence of disease. Actually, during the KRd treatment, a monthly monitoring with sFLC assay revealed an important increase compared to initial values (May 2017‐March 2018: sFLC κ 175 vs 340 mg/L, rFLC 4.2 vs 19.9), months before the appearance of the knee mass. After knee mass removal, we observed normalization of sFLC, but when, 4 months later the mass reappeared, once again the assay returned to be abnormal (May‐November 2018: sFLC κ 25.8 vs 124 mg/L, rFLC 1.3 vs 25), anticipating the second EMD relapse and indicating that this assay can be adopted to predict disease progression.
Both the first and the second EMD knee relapse represented the only site of disease, with negative sIFE and BM biopsy, and in both cases the alteration of both the FLC κ and the rFLC has preceded at least by 1 month the aggressive growth of the EMD mass (Figure 1C).
2.2. A rare case of hepatic EMD relapse anticipated by sFLC alteration
A 53‐year‐old man was diagnosed as MM IgG κ in January 2014, stage IIIA (Durie‐Salmon) and stage I (R‐ISS), with FISH positive for t(11;14). The disease presented with numerous osteolytic lesions, and the patient started induction therapy in April 2014 with bortezomib, cyclophosphamide, and dexamethasone (VCd) as part of EMN02/HOVON 95 MM protocol, for a total of 4 cycles, obtaining PR. The patient was then randomized to the transplant arm and underwent 1st (October 2014) and 2nd ASCT (February 2015), obtaining a CR. A maintenance therapy with lenalidomide was started with delay, in August 2015, due to slow hematologic recovery.
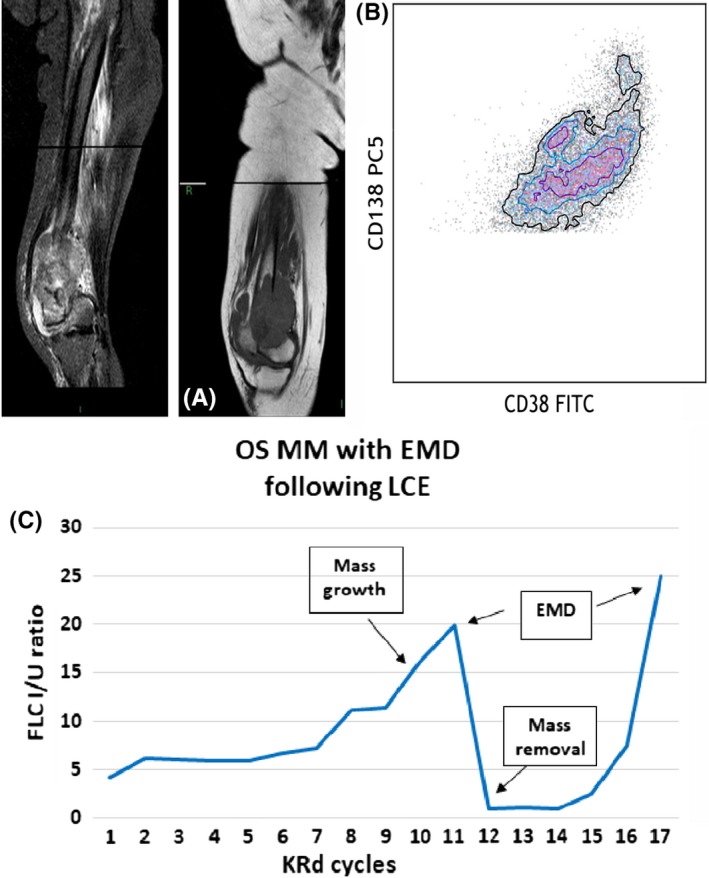
In May 2017, a CT scan demonstrated multiple osteolytic lesions, especially of the vertebral column, with a rise of MP (0.45 g/dL), confirming disease relapse, while the FLC assay was within normal range. The patient received 2nd line of therapy (June 2017), with carfilzomib‐lenalidomide‐dexamethasone (KRd) regimen10 associated with radiation therapy of the vertebral lesions and monthly zoledronic acid. Treatment was well tolerated, and the patient had an improvement of clinical conditions with achievement of PR after only two cycles. The response was maintained until June 2018 (14 completed cycles), when the patient complained diarrhea and abdominal pain, with normal routine examinations. Lenalidomide was temporarily withheld for suspected drug‐related toxicity, while colonoscopy resulted negative. The therapy was restarted, and patient maintained a PR status, but with a slight rise of sFLC (March‐June 2018: rFLC 0.67 vs 1.11). In August, due to the persistence of abdominal pain and a slight increase of MP (0.37 g/dL), patient underwent abdominal CT and MRI scan that revealed multiple hepatic nodularities (Figure 2A). A CT guided biopsy revealed hepatic EMD relapse and the BM biopsy confirmed the progression of disease (Figure 2B) together with an increase of MP (0.99 g/dL) and rFLC (1.67).
Figure 2.
Clinical course of OS MM patient showing FLC ratio, diagnostic and immunophenotypic images. A, Axial sequence TSE T2‐weighted abdominal MRI scan with multiple focal and bilobar lesions, confirmed with echo‐guided mass biopsy as EMD relapse. B, Dot plot shows the presence of plasma cells CD38 + CD138+, evaluated in cytometry, in extramedullary site. C, FLC curve in OS MM patient with hepatic EMD relapse following progressive FLC alteration (OSMM—oligo‐secretory multiple myeloma; FLC I/U ratio—free light chain involved/uninvolved ratio (n.v. 0.31‐1.56); MC—monoclonal component; KRd—carfilzomib, lenalidomide, dexamethasone; EMD—extramedullary myeloma disease)
The KRd regimen was stopped, and 3rd line of therapy was promptly initiated with daratumumab, bortezomib, and dexamethasone (DaraVd) in October 2018. The patient was refractory to the treatment and continued to progress (MP 3.6 g/dL, rFLC 7.26). Unfortunately, due to progressive worsening of the general conditions and blood cell count, the patient passed away before any salvage therapy was started.
In this case, throughout the course of KRd therapy the patient maintained PR with stable MP until the diagnosis of EMD hepatic relapse in September 2018. However, a progressive rise of the rFLC, although within normal range (0.36‐1.52), was noticed since March 2018 (March‐September 2018: rFLC 0.67 vs 1.67), 5 months prior to the first increase of MP (August 2018). In this case, the progressive alteration of the ratio could have been of aid to anticipate the necessary instrumental examinations for the early detection and treatment of hepatic EMD relapse (Figure 2C).
2.3. A case of LCMM with aggressive EMD relapse
A 46‐year‐old woman was diagnosed in September 2014 with LCMM λ, stage IIIA (Durie‐Salmon), stage I (R‐ISS). She presented with numerous osteolytic lesions, and a biopsy of a cranial mass resulted positive for plasmocytoma; FISH analysis showed 13q and 1p32 deletion and 1q21 amplification. The patient was started on induction therapy with bortezomib‐thalidomide‐dexamethasone (VTd) for a total of 4 cycles, obtaining a CR. Given the young age of the patient and 1q21 amplification (considered high risk in our center), according to local policy, the patient underwent double ASCT in July 2015 and March 2016, respectively. The 2nd ASCT was postponed due to grade III gastrointestinal toxicity. After transplant, the patient received a consolidation therapy with 2 further cycles of VTd and maintained CR.
During the first 2 years of follow‐up, the patient was treated with quarterly bisphosphonate therapy. In March 2017, the disease relapsed with numerous osteolytic lesions and severe acute renal insufficiency (creatinine 6.66 mg/dL, urea 218 mg/dL). The laboratory examinations also revealed MP reappearance (0.7 g/dL), FLC λ 2000 mg/L, and rFLC of 171 I/U. FISH was positive for deletion of 17p, along with the previously known alterations. The patient was immediately hospitalized and started on 2nd‐line therapy with carfilzomib, lenalidomide and dexamethasone, KRd scheme,10 with significant pain relief, disappearance of MP and normalization of both rFLC and renal function after only one cycle.
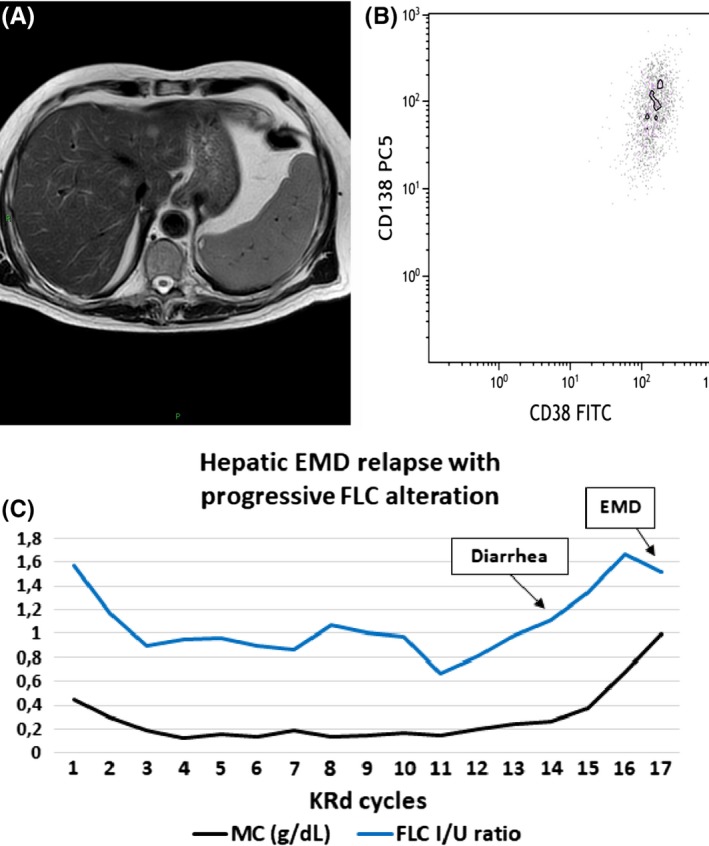
The therapy was well tolerated until July 2018 when, after completing 15 cycles of KRd scheme, both the liver and bilirubin tests showed alterations (AST 112 U/L, ALT 279 U/L, GGT 186 U/L, total bilirubin 1.55 mg/dL), without any clinical sign. The FLC assay has increased since June (sFLC λ 53 mg/dL; rFLC 6), while sIFE was negative. Hepatitis infection and gallstones were excluded with serovirology and abdominal ultrasound, while suspected drug‐related toxicity was treated first with dose reduction and later with lenalidomide discontinuation, along with glutathione antioxidant therapy, without major success.
The patient continued the treatment with carfilzomib and dexamethasone alone, until October 2018, when serum IFE became positive, and in November, the MP reappeared (0.37 g/dL), along with further alteration of sFLC assay (June‐November 2018: sFLC λ 53 vs 207 mg/dL; rFLC 6s 68). The liver and bilirubin tests were getting worse (AST 230 U/L, ALT 474 U/L, GGT 454 U/L, total bilirubin 9.3 mg/dL, direct bilirubin 5.6 mg/dL), so the therapy was stopped after 19 cycles of KRd for disease relapse. Magnetic resonance cholangiopancreatography (MRCP) revealed a massive solid hepatic tissue (Figure 3A), that extended from the hilum, along the biliary tract and the gallbladder, involving the portal vein. The mass resembled a cholangiocarcinoma; however, a tissue biopsy confirmed EMD relapse (Figure 3B). Unfortunately, the patient suffered major abdominal hemorrhage due to the rupture of the vertebral artery, infiltrated by the mass, with fatal outcome, before any salvage therapy could have been started.
Figure 3.
Clinical course of LCMM patient showing FLC ratio, diagnostic and immunophenotypic images. A, Axial T2‐weighted fat saturation sequences MRI scan of cholangio‐EMD with hepato‐hylar growth and portal infiltration. B, Dot plot shows the presence of plasma cells CD38 + CD138+, evaluated in cytometry, in extramedullary site. C, FLC curve in LCMM patient with EMD relapse during KRd therapy (LCMM—light chain multiple myeloma; FLC I/U ratio—free light chain involved/uninvolved ratio (n.v. 0.31‐1.56); KRd—carfilzomib, lenalidomide, dexamethasone; AST—aspartate aminotransferase; ALT—alanine aminotransferase; EMD—extramedullary myeloma disease
In this LCMM λ patient, despite the difficulties of disease monitoring during KRd therapy, monthly sFLC measurements were performed, using the N‐latex assay to detect early relapse.11, 12 The alteration of the sFLC assay was first noticed in June 2018, 1 month prior to first abnormal liver and bilirubin examinations, and 4 months prior to positive serum IFE (June‐November 2018: sFLC λ 53 vs 207 mg/dL; rFLC 6 vs 68 I/U) (Figure 3C).
We can hypothesize that at the onset of the liver tests and FLC increase, a PET/CT scan, although in complete absence of symptoms,13, 14 could have played a role in the early discovery of extramedullary disease, as the abdominal mass was probably present months before the MRCP.
2.4. MM with recurrent EMD relapses
A 67‐year‐old man was diagnosed with MM IgA λ in July 2018, stage IIIA (Durie‐Salmon), stage II (R‐ISS), with FISH positive for 13q deletion, 1q21 amplification and t(14;16) translocation. The disease was diagnosed by soft paravertebral tissues biopsy, along with the presence of associated vertebral osteolytic lesions. Laboratory tests showed MP 2.4 g/dL, FLC λ 128 mg/L, and rFLC 5.68 I/U.
Considering the extension of the paravertebral lesion (bilaterally from D7 to D12 and involving the posterior mediastinum), due to high risk of spinal cord toxicity, loco‐regional radiotherapy was not performed, and the patient was started on 1st‐line induction therapy with bortezomib‐melphalan‐prednisone (VMP).
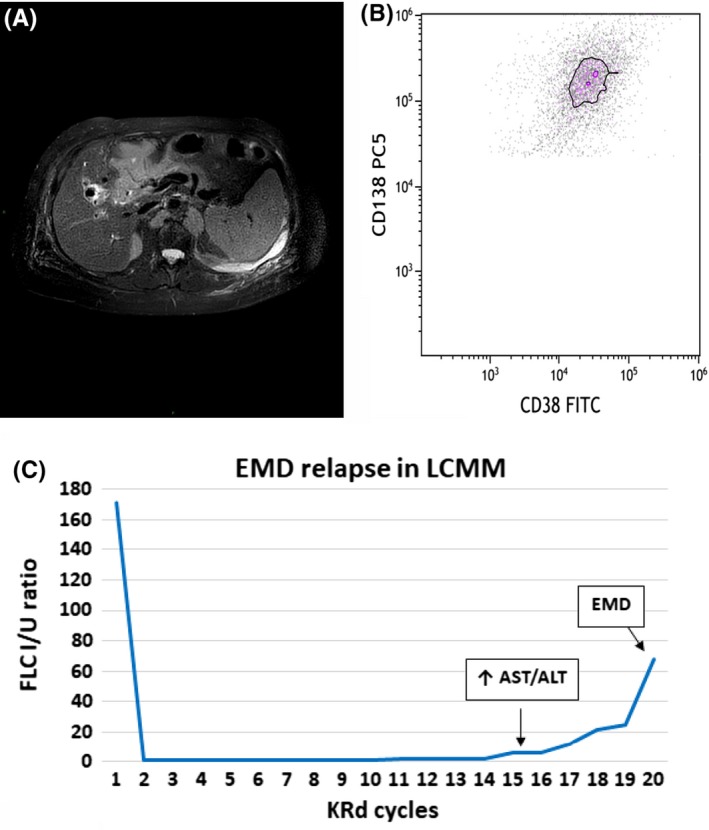
During the 1st cycle, a right ocular lesion appeared, responsible of complete sight loss. The MRI scan (Figure 4A) and the biopsy of the lesion (Figure 4B) confirmed extramedullary localization of disease, characterized by an extremely aggressive growth pattern (Ki67/ Mib1 100%), and accompanied by bone pain, increased levels of FLC λ (263 mg/L) and ratio (29.4 I/U), while the MP was declining (0.22 g/dL).
Figure 4.
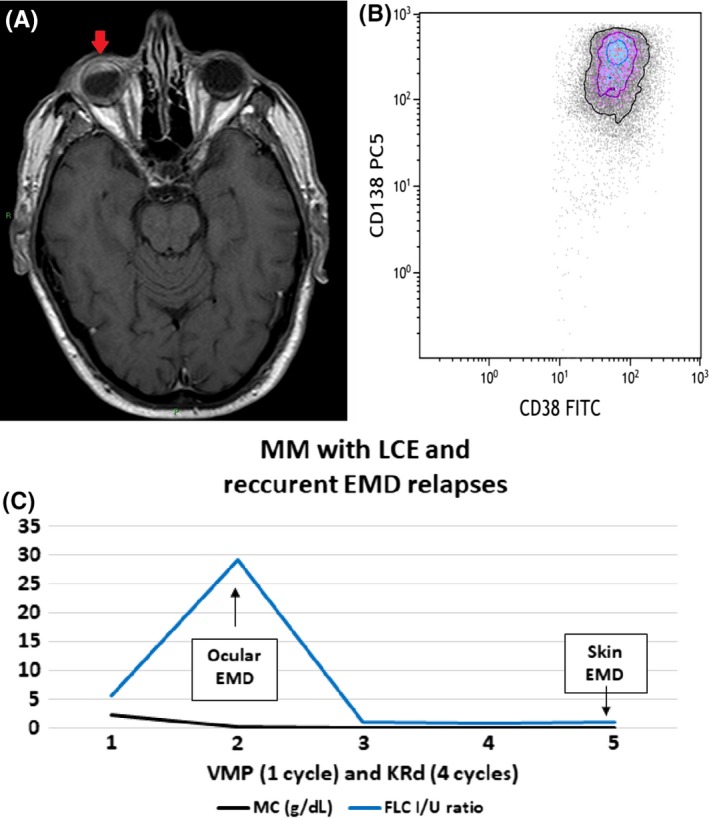
Clinical course of MM patient with LCE and recurrent EMD relapses showing FLC ratio, diagnostic and immunophenotypic images. A, Axial T1‐weighted spin‐echo postgadolinium MRI scan of ocular EMD infiltration. B, Dot plot shows the presence of plasma cells CD38 + CD138+, evaluated in cytometry, in extramedullary site. C, FLC curve in MM patient with LCE and recurrent EMD relapses during therapy (LCE—light chaine scape; FLC I/U ratio—free light chain involved/uninvolved ratio (n.v. 0.31‐1.56); MC—monoclonal component; VMP—bortezomib, melphalan, prednisone; KRd—carfilzomib, lenalidomide, dexamethasone; EMD—extramedullary myeloma disease)
The patient was immediately started on 2nd‐line of therapy with carfilzomib, lenalidomide, and dexamethasone (KRd scheme),10 and after only one cycle he achieved a CR with immediate ocular mass regression, pain relief, disappearance of MP and serum IFE, and progressive reduction of FLC assay (October‐November 2018 FLC λ 263 vs 30 mg/L, rFLC 29.4 vs 1.1 I/U).
In January 2019, during the fourth cycle of KRd regimen, in the absence of any sign or symptom, a cutaneous lesion appeared on his right tibia. Fine‐needle aspiration and cytological examination confirmed cutaneous EMD relapse. PET/CT scan revealed further disease localizations in the subscapular and iliac areas. This relapse occurred in the absence of clear laboratory signs (MP not detectable, serum IFE negative), but with only a slight increase of FLC that was detected the month before (sFLC λ 38.4 mg/L, rFLC 1.0 I/U).
Patient underwent salvage chemotherapy with DCEP scheme (dexamethasone, cyclophosphamide, etoposide, and cisplatin), for a total of 4 cycles, obtaining CR, confirmed by PET/CT scan in May 2019.
In this case, the first relapse occurred during the first cycle of the induction therapy, when ocular EMD involvement was observed, even in the presence of clinical and biochemical response to therapy (regression of paravertebral plasmacytoma and more than 90% reduction of MP); the relapse was associated with a significant increase of the sFLC assay, the light chain escape, (July‐August 2018 sFLC λ 158 vs 263 mg/L, rFLC 5.68 vs 29.4 I/U) (Figure 4C).
Similarly, the second EMD cutaneous relapse occurred during the KRd treatment, effective against underlying disease (negativity of MP and sIFE) and the increase of FLC was the only laboratory abnormality that occurred 1 month prior to relapse (Figure 4C).
3. DISCUSSION
According to the IMWG response criteria, the FLC assay has a restricted role in response evaluation as part of sCR and in a limited population of NS and OS MM, while its use is not recommended for the evaluation in MM with measurable disease (intact immunoglobulin and LC).9 Although the IMWG response criteria are widely used in every line of therapy, there are no clinical data that confirm the validity of this evaluation from the second line of therapy onwards. Moreover, to date there have been several studies that have validated the usefulness of serial FLC measurements with rFLC and differential FLC (dFLC) in MM population, especially in OS, NS, and LC MM with poor prognosis,11, 12, 15, 16, 17, 18 but currently there are no prospective studies performed in the era of novel agents.
Here, we have described four cases where sFLC ratio represented a unique serological marker of aggressive extramedullary progression of disease. Indeed, the described cases represent a particular setting of MM patients since they have a small or light or undetectable monoclonal component. In addition, in all four patients, the disease showed an aggressive behavior, with an extensive extramedullary growth pattern, involving a large portion of tissues and it is well known that extramedullary disease is characterized by a worse long‐term prognosis, resistance to treatment and a greater tendency to relapse.19
According to current international guidelines, only whole‐body low‐dose (WBLD) CT and MRI are recommended in both the initial imaging staging and follow‐up period, in case of increasing bone pain. In addition, PET/CT can be used to detect both medullary and extramedullary myeloma, and SUVmax value has been described to have a prognostic impact on EMD outcome.1, 20 However, radiological evaluation cannot be frequently performed.
Serum FLC assay has proven to be a useful marker of progressive disease and may identify relapse earlier than traditional methods, including urine examinations. Dejoiu and colleagues compared UPEP and sFLC assays as a method of choice in the LCMM population, demonstrating superiority of FLC in response assessment and prognosis (sFLC 100% positive and measurable; UPEP 78% positive; 64% measurable). Another comparison was performed comparing involved FLC (iFLC) and rFLC, showing higher prognostic value of rFLC.11, 12
The impact of FLC tests in response evaluation compared to UPEP was shown not only in LCMM, but also in oligo‐secretory patients with low secretion of intact immunoglobulin (IIMM) at relapse (MP < 1 g/dL). In this specific population, sFLC offers a distinctive advantage over serum IFE to detect disease progression, particularly in IgG MM patients. The conclusion is that oligo‐secretory patients should be controlled routinely with both SPEP and sFLC tests.15 In the meantime, different modified response assessments, including FLC assay, have been proposed, such as inclusion of rFLC and dFLC in response criteria16 and abnormal rFLC and iFLC level with further iFLC increase >50% as a marker of disease progression.18
There has been experience of clonal evolution, with a switch in the monoclonal protein subtype (light chain conversion), described recently also by our group.21 During the course of the disease, a small number of MM patients (3% of cases) can also progress with clones that lose the ability to secrete the original paraprotein (NS escape), or in other cases start secreting only light chains instead of intact monoclonal immunoglobulin (LC escape), first noted by Hobbs in 1969.22 It is also possible that some patients have a significant decrease of original paraprotein secretion levels (oligo‐secretory escape). This subgroup of patents generally have an aggressive clinical course and poor prognosis due to late detection of disease relapse, while almost one‐third experience extramedullary progression.23 Kuhnemund and colleagues described the largest documented series of patients with light chain escape (LCE),24 while Brioli describes the intraclonal heterogeneity of myeloma and different types of disease progression.25 Their conclusion was that, given the evolutive capacity of myeloma clones, routine use of sFLC measurements was recommended in course of therapy and follow‐up, especially in the absence of monoclonal component.
In our analysis, both the first and fourth patient probably experienced LC escape with EMD progression, while both the MP and serum IFE were negative during the course of therapy, until the tumor burden became high enough to become detectable.
The second patient had OS MM relapse (MP < 1g/dL), and during treatment he had stable MP, with rFLC within range, but with a progressive increased until EMD relapse was diagnosed. In this patient, although the FLC values were within normal range, the progressive increase of rFLC was observed 4 months prior the clinical relapse.
The same happened in the third case with LCMM, where disease progression was largely anticipated by monthly FLC measurements.
4. CONCLUSIONS
Due to the development of NS and LC escapes in MM, sFLC assay is necessary for response evaluation in OS, NS, and LCMM. Unlike the IMWG consensus on FLC use, on the basis of our clinical experience in this particular setting of patients, we think that serial sFLC evaluation and, in particular the rFLC, could be of help in monitoring all patients where MP is not clearly evident, thus being able to anticipate both biochemical and clinical relapse. In these patients, an increase of sFLC or rFLC should be a clue for searching an extramedullary relapse, in symptomatic patients not only in OS, NS, or LCMM, but also in standard myeloma patients. Capturing a slight increase of sFLC or rFLC could anticipate treatment and prolong survival of patients.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to all of the following: UM: interpreted the data, drafted the final article, and critically revised it. VL: interpreted the data and participated in the first draft of manuscript. UM, CC, VL, RG, and VDF: selected patients, acquired, analyzed, and interpreted the data. NLP: performed immunophenotypic data acquisition, analysis, and interpretation. MTC: involved in radiographic image acquisition, analysis, and interpretation. CC, AR, DT and FDR: revised the article for important intellectual content and approved the final version for submission.
ACKNOWLEDGMENTS
The work has been supported by PSN 2016 line 6.6 (FDR)
Markovic U, Leotta V, Tibullo D, et al. Serum free light chains and multiple myeloma: Is it time to extend their application?. Clin Case Rep. 2020;8:617–624. 10.1002/ccr3.2636
REFERENCES
- 1. Castillo JJ. Plasma cell disorders. Prim Care. 2016;43(4):677‐691. [DOI] [PubMed] [Google Scholar]
- 2. Willrich MAV, Murray DL, Kyle RA. Laboratory testing for monoclonal gammopathies: focus on monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Clin Biochem. 2018;51:38‐47. [DOI] [PubMed] [Google Scholar]
- 3. Dispenzieri A, Kyle R, Merlini G, et al. International myeloma working group guidelines for serum‐free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215‐224. [DOI] [PubMed] [Google Scholar]
- 4. Graziani MS. Measurement of free light chains ‐ Pros and cons of current methods. Clin Chem Lab Med. 2016;54(6):1015‐1020. [DOI] [PubMed] [Google Scholar]
- 5. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437‐1444. [PubMed] [Google Scholar]
- 6. Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high‐risk smoldering multiple myeloma. Leukemia. 2013;27(4):941‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. [DOI] [PubMed] [Google Scholar]
- 9. Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328‐e346. [DOI] [PubMed] [Google Scholar]
- 10. Conticello C, Romano A, Del Fabro V, et al. Feasibility, tolerability and efficacy of carfilzomib in combination with lenalidomide and dexamethasone in relapsed refractory myeloma patients: a retrospective real‐life survey of the sicilian myeloma network. J Clin Med. 2019;8(6):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dejoie T, Corre J, Caillon H, et al. Serum free light chains, not urine specimens, should be used to evaluate response in light‐chain multiple myeloma. Blood. 2016;128(25):2941‐2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heaney JLJ, Campbell JP, Griffin AE, et al. Diagnosis and monitoring for light chain only and oligosecretory myeloma using serum free light chain tests. Br J Haematol. 2017;178(2):220‐230. [DOI] [PubMed] [Google Scholar]
- 13. Cavo M, Terpos E, Nanni C, et al. Role of 18F‐FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18(4):e206‐e217. [DOI] [PubMed] [Google Scholar]
- 14. Bailly C, Leforestier R, Jamet B, et al. Pet imaging for initial staging and therapy assessment in multiple myeloma patients. Int J Mol Sci. 2017;18(2):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet‐Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dejoie T, Corre J, Caillon H, Moreau P, Attal M, Loiseau HA. Responses in multiple myeloma should be assigned according to serum, not urine, free light chain measurements. Leukemia. 2019;33(2):313‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacchetti P, Pezzi A, Zamagni E, et al. Role of serum free light chain assay in the detection of early relapse and prediction of prognosis after relapse in multiple myeloma patients treated upfront with novel agents. Haematologica. 2017;102(3):e104‐e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zamarin D, Giralt S, Landau H, et al. Patterns of relapse and progression in multiple myeloma patients after auto‐SCT: implications for patients’ monitoring after transplantation. Bone Marrow Transplant. 2013;48(3):419‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over‐represented in high‐risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zamagni E, Nanni C, Mancuso K, et al. PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin Cancer Res. 2015;21(19):4384‐4390. [DOI] [PubMed] [Google Scholar]
- 21. Markovic U, Calafiore V, Martino E, et al. A rare case of multiple myeloma with intracranial extramedullary relapse: one or more myeloma clones? Clin case reports. 2019;7(9):1629‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hobbs JR. Growth rates and responses to treatment in human myelomatosis. Br J Haematol. 1969;16(6):607‐617. [DOI] [PubMed] [Google Scholar]
- 23. Patel UH, Drabick JJ, Malysz J, Talamo G. Nonsecretory and light chain escape in patients with multiple myeloma. Clin Lymphoma, Myeloma Leuk. 2018;18(12):e515‐e519. [DOI] [PubMed] [Google Scholar]
- 24. Kühnemund A, Liebisch P, Bauchmüller K, et al. ’Light‐chain escape‐multiple myeloma’‐an escape phenomenon from plateau phase: report of the largest patient series using LC‐monitoring. J Cancer Res Clin Oncol. 2009;135(3):477‐484. [DOI] [PubMed] [Google Scholar]
- 25. Brioli A, Giles H, Pawlyn C, et al. Serum free immunoglobulin light chain evaluation as a marker of impact from intraclonal heterogeneity on myeloma outcome. Blood. 2014;123(22):3414‐3419. [DOI] [PubMed] [Google Scholar]


