Abstract
Cutaneous metastases are rare and represent a sign of poor prognosis. They are a sign of widespread disease. Breast cancer is the most common neoplasm leading to their appearance. Palliative care is the treatment of choice.
Keywords: breast cancer, chemotherapy, cutaneous, metastases, surgery
Cutaneous metastases are rare and represent a sign of poor prognosis. They are a sign of widespread disease. Breast cancer is the most common neoplasm leading to their appearance. Palliative care is the treatment of choice.
1. INTRODUCTION
Breast cancer is the most common tumor leading to the appearance of cutaneous metastases. Typically, they present as isolated dermal nodules and inflammatory plaques localized on the chest ipsilateral to the primary breast cancer. We report a case of metastatic breast cancer presenting with generalized cutaneous metastases.
Cutaneous metastatic (CM) carcinoma is an unusual clinical finding. It accounts for 0.7%‐9% of all metastases and is considered a grave prognostic sign. CM represent 2% of cutaneous tumors. They are rarely present at diagnosis. Breast cancer is the most common tumor associated with CM in women.1 Diffuse CM all over the body is very rare. The biopsy with immunohistochemistry is the key of diagnosis.
2. CASE REPORT
A 47‐year‐old female without a family history of breast cancer was diagnosed with bilateral breast cancer in May 2017 which was staged T4bN3cM0 in the right‐sided breast (stage IIIC) and T4bN1M0 in the left‐sided breast (stage IIIB) according to TNM classification. Breast biopsy confirmed an invasive ductal carcinoma with cutaneous lymphangitic carcinomatosis, SBR III, HER2‐positive, hormone receptor‐negative with a ki‐67 index of 25%. It was the same immunohistochemical profile on both sides. She underwent neoadjuvant chemotherapy: four courses of FEC regimen (5‐Fluorouracil‐Epirubicin‐Cyclophosphamid) followed by four courses of docetaxel and trastuzumab. Partial clinical response was noted. Bilateral total mastectomy with axillary node dissection was performed and followed by locoregional radiotherapy. Response to treatment was assessed according to Sataloff method: On the left side, there were no viable tumor cells; on the right side, viable tumors cells were <10%. Three months after end of treatment, our patient presented with satellite skin nodules on the right mastectomy scar and complained from cervical bone pain. Biopsy proved cutaneous metastases. CT scan revealed cervical, thoracic, and lumbar spine bone metastases. Radiation therapy was not indicated by our local breast multidisciplinary team as it was metastatic to bone. As first‐line chemotherapy, capecitabin associated with trastuzumab and zoledronic acid was prescribed (dual HER‐2 blockade with pertuzumab and trastuzumab is not available in our country). The clinical examination at each cycle showed a relief in bone pain with stability in chest skin lesions. The patient developed progressively a bilateral lower extremities lymphedema (Figure 1). The Doppler ultrasound imaging did not show any thrombosis. Cardiac ultrasound showed a good left ventricular function. The patient altered her performance status with functional disability of her lower limbs. The skin became progressively thick, erythematous with a “peau d'orange” aspect on the limbs than on the abdomen (Figure 2). No nodule was present initially on the abdomen or limbs. As the patient had stable disease, she was referred to an internist to determine the underlying cause of her edema. No other systemic disease was retained. No other systemic disease was retained.
Figure 1.
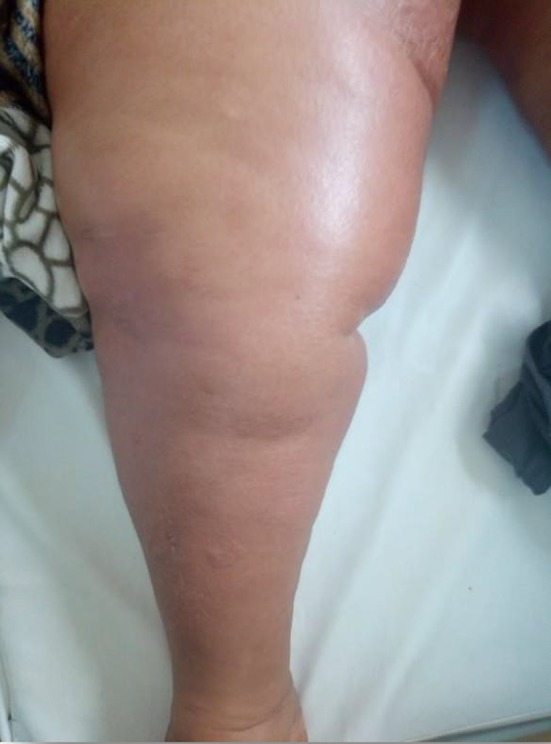
Lymphedema in the lower right limb
Figure 2.
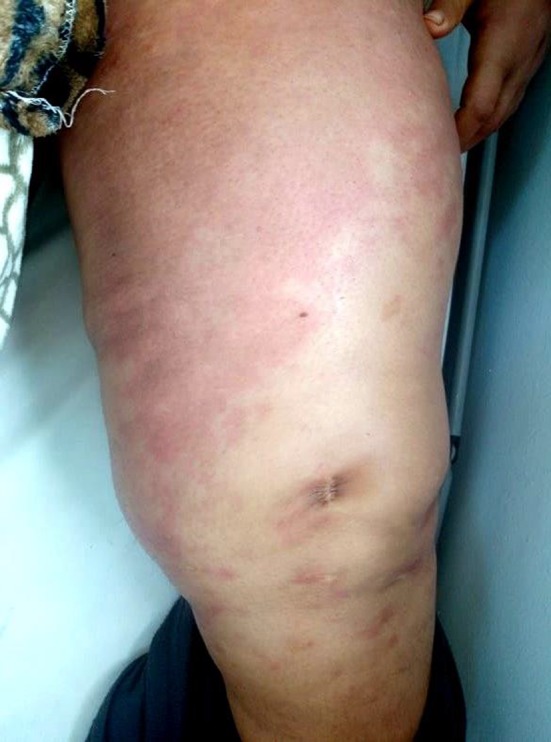
Skin changes with “peau d'orange” appearance in the lower left limb
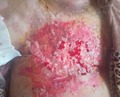
After six cycles of chemotherapy, she developed dyspnea with pleural effusion on the chest X‐ray. Generalized erythematous, hemorrhagic plaques (Figure 3), nodules (Figure 4), and ulcerations have progressively appeared. More than 50% of the patient's total body surface area was invaded. Biopsy specimen from the left inguinal skin lesion (Figure 5) showed proliferation of malignant epithelial cells organized into clusters invading the dermis (Figure 6). Many lymphatic emboli have been found (Figure 7). Immunohistochemical staining was positive for mammaglobin (Figure 8) and for GCDFP15 (Figure 9). HER2 protein overexpression was found, while hormone receptors were negative with high Ki 67 (Figure 10).
Figure 3.
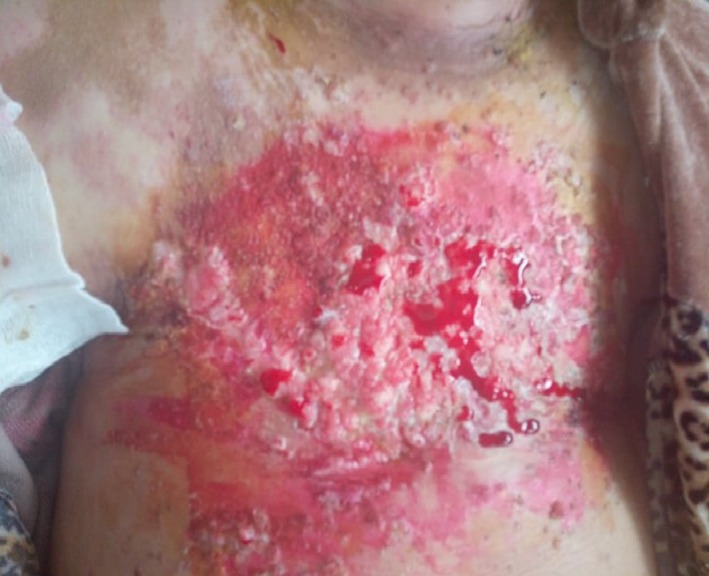
Erythematous with hemorrhagic plaques, nodules, and ulcerations in the chest wall
Figure 4.
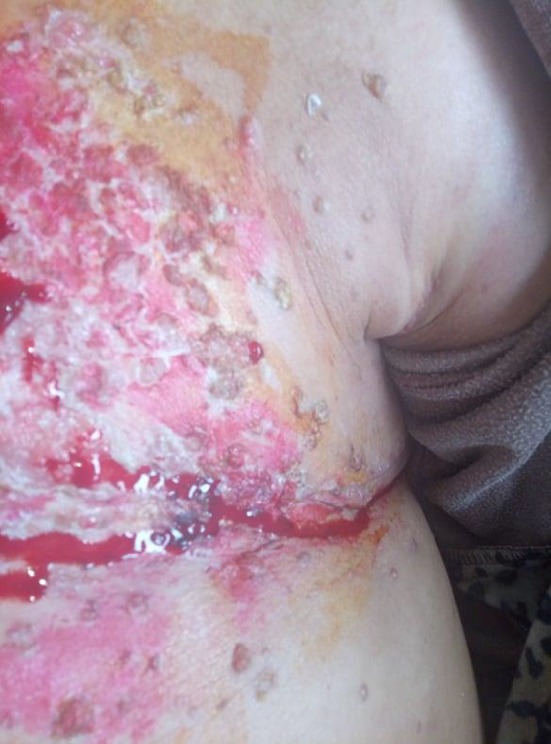
Nodular lesions on the leftt side of the chest
Figure 5.
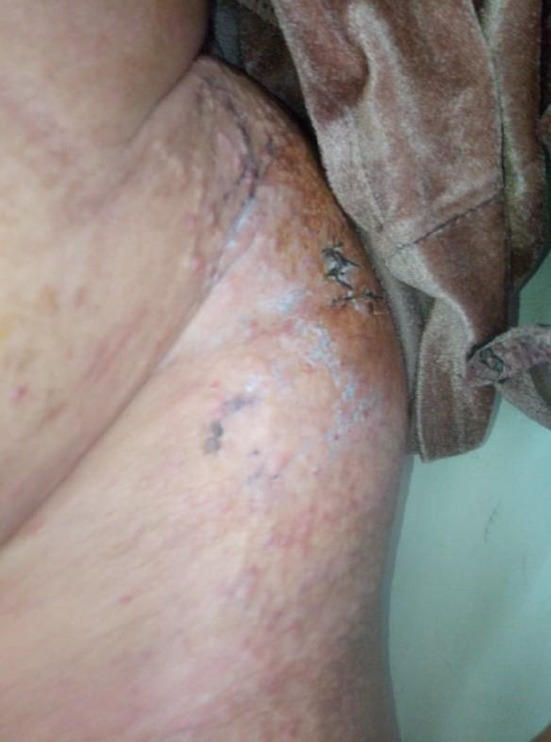
Site of the inguinal biopsy
Figure 6.
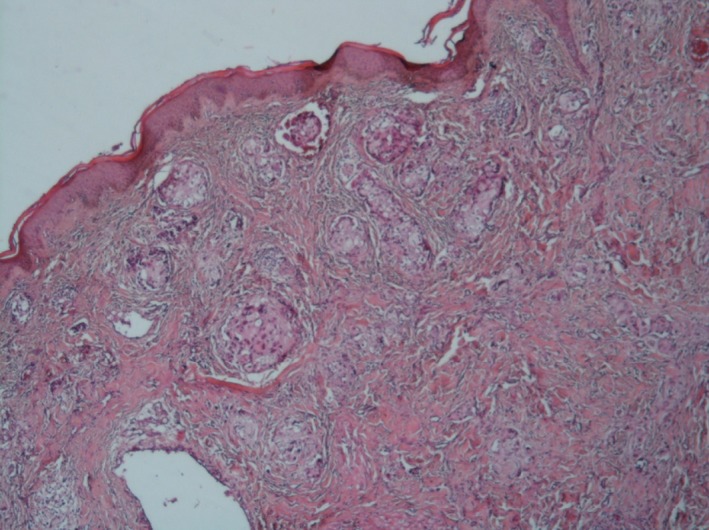
The dermis is invaded by carcinomatous clusters (HE X 40)
Figure 7.
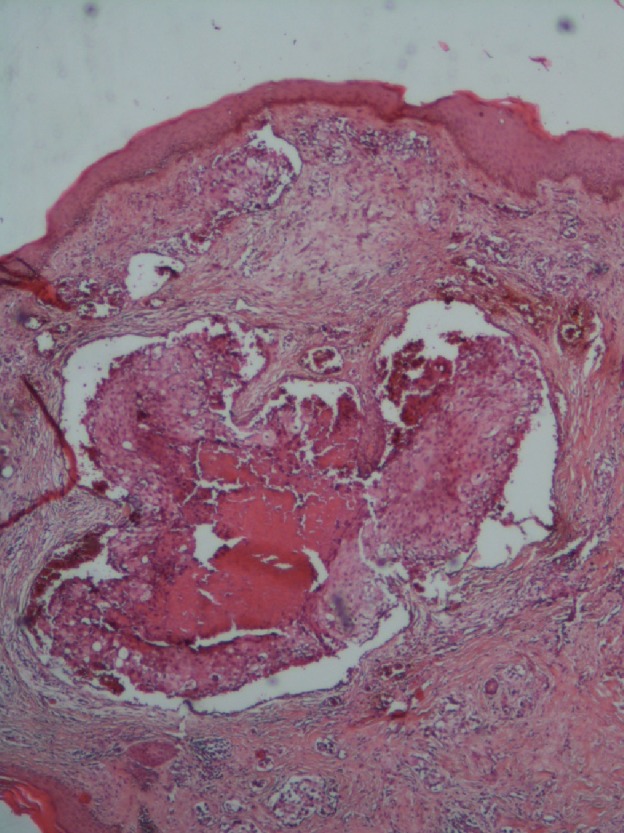
Big lymph embolus surrounded by smaller emboli (HE X 40)
Figure 8.
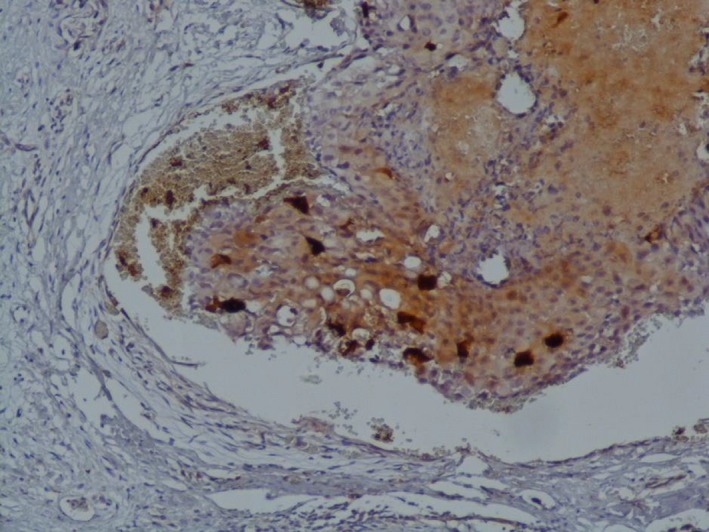
Positivity of mammaglobin (IHCx100)
Figure 9.
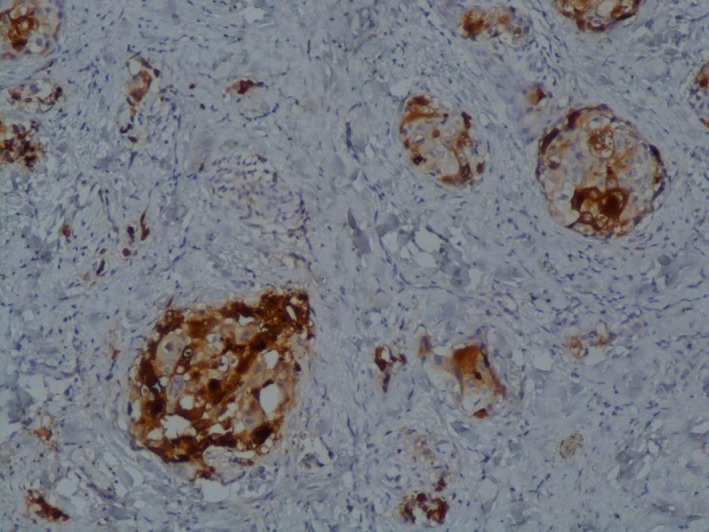
Positivity of GCDFP15 (IHCx100)
Figure 10.
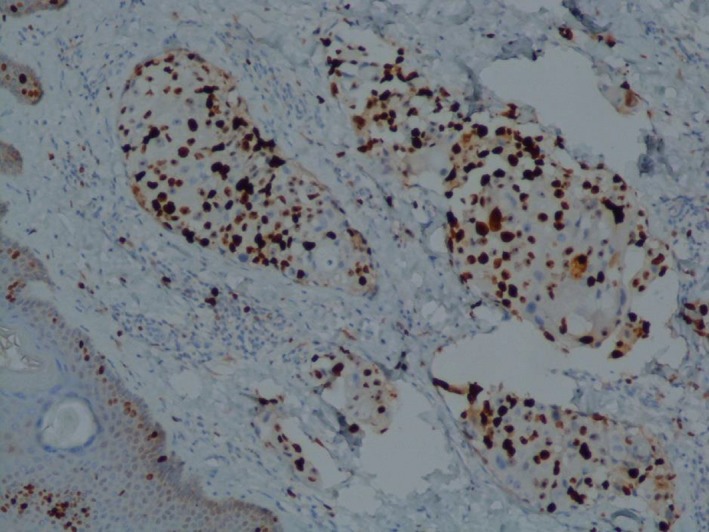
High Ki67 (IHC x100)
She was referred to best supportive care with local vaselin pansement and corticosteroids, as the patient could not come to oncology outpatient (social conditions).
3. DISCUSSION
Cutaneous metastases from carcinoma are uncommon. Breast cancer is the most prevalent type of malignant tumors in women. Breast cancer is the most common tumor (melanoma excluded) associated with cutaneous metastasis.2 CM account for 24% in breast carcinoma.3 They occur few months or years after the tumor diagnosis and commonly at the same time as visceral metastases.4 The most common clinical presentations of CM from breast cancer were described in a retrospective review of 164 cases, by Mordenti et al Clinical features included the following: papules or/and nodules in most cases (80%), telangiectatic carcinoma, erysipeloid carcinoma, en cuirasse carcinoma, alopecia neoplastica, and zosteriform pattern.5 Typically, CM manifest on the chest ipsilateral to the primary tumor. They are usually few in number.6 In our case, the patient had generalized CM affecting more than half of the body surface. Most of the metastases occur due to lymphatic spread of tumor cells. The diagnosis of CM secondary to breast carcinoma is confirmed by pathological examination of an incisional biopsy specimen.
Ductal carcinomas are more frequently associated with CM than lobular carcinomas.6 Immunohistochemical staining is of diagnostic value.1 CM are usually a sign of widespread disease, and it may be uncurable in most cases. If lesions bleed, debridement can be advised. Other options are possible and can be helpful such as imiquimod with good results in localized lesions, trastuzumab in tumors HER2‐positive, systemic chemotherapy, radiation therapy, and immunotherapy.7, 8 Surgical excision may improve patient's quality of life. In our case, because of her impaired performance status, the patient had palliative treatment. The prognosis depends on the type and behavior of the primary cancer. The expected survival is usually <1 year at the time of diagnosis.9 Widespread CM in breast cancer is a particular entity. A few reports of widespread skin metastasis from breast cancer have appeared in the literature.10, 11
4. CONCLUSION
Cutaneous metastatic causes discomfort in patients. They are rare and represent a sign of malignancy and poor prognosis. Generalized CM is a particular presentation. Palliative care is the treatment of choice for patients with widespread disease with impaired performance status.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
NC: contributed to concepts. NC, YZ, and IS: contributed to design. NC, YZ, HB, and SK: contributed to definition of intellectual content. NC, YZ, HB, AG, SK, KR, AM, and IS: contributed to manuscript preparation. NC and YZ: contributed to manuscript editing. NC: contributed to manuscript review. NC: contributed to guarantor.
Chraiet N, Zenzri Y, Bouaziz H, et al. Generalized cutaneous metastases of breast cancer: An uncommon presentation. Clin Case Rep. 2020;8:667–671. 10.1002/ccr3.2693
Contributor Information
Nesrine Chraiet, Email: nesrinechraiet@gmail.com.
Yosr Zenzri, Email: yosr-zenzri@live.fr.
REFERENCES
- 1. Prabhu S, Pai SB, Handattu S, Kudur MH, Vasanth V. Cutaneous metastases from carcinoma breast: the common and the rare. Indian J Dermatol Venereol Leprol. 2009;75:499‐502. [DOI] [PubMed] [Google Scholar]
- 2. Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347‐393. [DOI] [PubMed] [Google Scholar]
- 3. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta‐analysis of data. South Med J. 2003;96:164‐167. [DOI] [PubMed] [Google Scholar]
- 4. Hu S‐S, Chen G‐S, Lu Y‐W, Wu C‐S, Lan C‐C. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735‐740. [DOI] [PubMed] [Google Scholar]
- 5. Mordenti C, Peris K, Concetta Fargnoli M, Cerroni L, Chimenti S. Cutaneous metastatic breast carcinoma. Acta dermatovenerologica. 2000;9:143-8. [Google Scholar]
- 6. Mayer JE, Maurer MA, Nguyen HT. Diffuse cutaneous breast cancer metastases resembling subcutaneous nodules with no surface changes. Cutis. 2018;101:219‐223. [PubMed] [Google Scholar]
- 7. Singh D, Kapoor A, Singhal MK, Singh P, Kumar V, Kumar HS. Cutaneous metastasis involving face in breast cancer: a series of three patients. Clin Cancer Investig J. 2014;3:545‐547. [Google Scholar]
- 8. Ferreira VA, Spelta K, Diniz LM, Lucas EA. Exuberant case of cutaneous metastasis of breast cancer. An Bras Dermatol. 2018;93:429‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox SE, Cruz PD Jr. A spectrum of inflammatory metastasis to skin via lymphatics: three cases of carcinoma erysipeloides. J Am Acad Dermatol. 1994;30:304‐307. [DOI] [PubMed] [Google Scholar]
- 10. Utkan G, Büyükçelik A, Okçu AH, Avci U, Yalçin B, Içli F. Widespread erythematous skin metastasis from breast cancer mimicking generalized drug eruption. Turk J Cancer. 2009;39:66‐68. [Google Scholar]
- 11. Ferreira VA, Spelta K, Diniz LM, Almeida LE. Exuberant case of cutaneous metastasis of breast cancer. An Bras Dermatol. 2018;93:429‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]


