Abstract
Rationale:
Cocoa and its major flavanol component, epicatechin, have therapeutic properties that may improve limb perfusion and increase calf muscle mitochondrial activity in people with lower extremity peripheral artery disease (PAD).
Objective:
In a phase II randomized clinical trial, to assess whether six months of cocoa improved walking performance in people with PAD, compared to placebo.
Methods and Results:
Six-month double blind randomized clinical trial in which participants with PAD were randomized to either cocoa beverage vs. placebo beverage. The cocoa beverage contained 15 grams of cocoa and 75 mgs of epicatechin daily. The identical appearing placebo contained neither cocoa nor epicatechin. The two primary outcomes were six-month change in six-minute walk distance measured 2.5 hours after a study beverage at 6-month follow-up and 24 hours after a study beverage at 6-month follow-up, respectively. A one-sided P value <0.10 was considered statistically significant. Of 44 PAD participants randomized (mean age: 72.3 years (±7.1), mean ankle brachial index 0.66 (±0.15)), 40 (91%) completed follow-up. Adjusting for smoking, race, and body mass index, cocoa improved six-minute walk distance at 6-month follow-up by 42.6 meters (90% Confidence Interval (CI): +22.2,+∞, P=0.005) at 2.5 hours after a final study beverage and by 18.0 meters (90% CI:−1.7, +∞, P=0.12) at 24 hours after a study beverage, compared to placebo. In calf muscle biopsies, cocoa improved mitochondrial cytochrome c oxidase activity (P=0.013), increased capillary density (P=0.014), improved calf muscle perfusion (P=0.098), and reduced central nuclei (P=0.024), compared to placebo.
Conclusions:
These preliminary results suggest a therapeutic effect of cocoa on walking performance in people with PAD. Further study is needed to definitively determine whether cocoa significantly improves walking performance in people with PAD.
Clinical Trial Registration:
Keywords: Peripheral artery disease, intermittent claudication, 6-minute walk, functional impairment, flavanols, limb perfusion, clinical trial, mitochondria, muscle, functional performance, muscle biopsy
Subject Terms: Peripheral Vascular Disease, Vascular Disease
Graphical Abstract

INTRODUCTION
People with lower extremity peripheral artery disease (PAD) have shorter six-minute walk distance compared to people without PAD (1,2). Without an effective therapeutic intervention, people with PAD typically decline in walking performance over time (3–6). Cocoa flavanols, including (–) epicatechin, that are present in dark chocolate have therapeutic properties that may improve walking performance in people with PAD. Pre-clinical evidence and preliminary evidence from humans without PAD suggest that cocoa may increase limb perfusion and improve skeletal muscle mitochondrial activity and muscle regeneration (7–13). In a single-blind cross-over pilot study of 20 people with PAD, one 40 gram dose of dark chocolate (>85% cacao) increased maximal treadmill walking distance by 11% and increased serum nitrite/nitrate levels two hours after the dark chocolate was administered, while milk chocolate had no acute effect on treadmill walking distance or serum nitrite/nitrate (14). However, effects of chronic consumption of cocoa on walking ability in PAD are unknown.
The COCOA-PAD Study was a double-blind, pilot randomized clinical trial designed to test the hypothesis that daily cocoa consumption for six months improves or prevents decline in six-minute walk distance at six-month follow-up, compared to placebo.
METHODS
Data supporting the findings of this study are available upon reasonable request.
The study was a parallel-design, double blind randomized clinical trial performed at Northwestern University in Chicago. Northwestern University’s Institutional Review Board approved the protocol. Participants gave written informed consent. Participants were randomized to either a flavanol-rich cocoa beverage or a similar appearing and tasting beverage without cocoa or flavanols (Online Table I). The first participant was randomized January 18, 2017. Final follow-up occurred October 30, 2018. Enrollment stopped when the target sample size was reached. Muscle samples were analyzed after follow-up visits and were completed October 3, 2019.
Participant identification.
Participants were recruited using Chicago Transit Authority advertisements and through recruitment postcards mailed to people 60 and older in the Chicago area. People who previously participated in research with the principal investigator (MMM) and expressed interest in future research were contacted.
Inclusion and exclusion criteria.
Inclusion criteria were age ≥ 60 years and presence of PAD, defined as an ankle brachial index (ABI) ≤ 0.90 in either leg or vascular laboratory or angiographic evidence of PAD (2,3,5). Absence of classical symptoms of intermittent claudication was not an exclusion criterion, since most people with PAD do not have intermittent claudication symptoms (1–6,15).
People unwilling to give up major dietary sources of chocolate, allergic to chocolate, or unable to consume products manufactured on equipment processing nuts, egg, wheat, soy, or milk were excluded. People with major leg amputation, critical limb ischemia, wheel-chair confinement, walking aid use, walking impairment for a reason other than PAD, and significant visual or hearing impairment were excluded. Individuals on dialysis, those requiring oxygen, those with a major cardiovascular event, revascularization, or major surgery in the past three months, and those planning revascularization or major surgery in the next six months were excluded. People treated for cancer in the past two years were excluded unless prognosis was excellent. Those with baseline six-minute walk < 500 or > 1600 feet, and those with a Mini-Mental Status Examination score < 23 at baseline were excluded (5,6,15).
Interventions.
Flavanol rich cocoa and matching placebo beverages were manufactured by The Hershey Company and dispensed in packets. The study was double blinded. Dispensed packets looked identical except for labeling with ‘A’ or ‘B’ for each group. The packet contents in each group contained similar looking powder and participants only saw the contents of their own assigned packets and did not have opportunity to compare packet contents. Throughout the trial, investigators, study staff, and the data management team were unaware of whether the cocoa intervention was Group A or B. Participants were instructed to mix packet contents with water or milk and consume three packets daily. Dietary composition of each beverage is shown in Online Table I. To avoid weight gain from the beverage, participants were individually counseled on foods with similar caloric content to study beverages that could be eliminated during the study. Participants brought remaining unopened packets to monthly study visits, where investigators counted them. At the end of the study, the Hershey Company notified the data management team which group (A or B) received cocoa and which received placebo.
Randomization.
Participants were randomized to Group A or B by the data management team, using a randomly permuted block method, stratified by baseline six-minute walk distance. Randomization was performed separately for participants with baseline six-minute walk distance less than 365.75 meters (1,200 feet) vs. 367.65 meters or more.
Ankle brachial index measurement.
A hand-held Doppler probe (Pocket Dop II; Nicolet Biomedical, Inc, Golden, CO) was used to obtain systolic pressures twice in each brachial, dorsalis pedis, and posterior tibial artery using established methods (1,2). The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the four brachial pressures (1–3,16).
Medical history.
Medical history, race, and demographics were obtained by a certified health interviewer using questionnaires administered to the participants as in previous randomized trials (5,6,15).
Leg symptoms.
Leg symptoms were characterized using the San Diego Claudication Questionnaire. Intermittent claudication (IC) was defined as exertional calf pain that does not begin at rest, causes the participant to stop walking, and resolves within ten minutes of rest (1–3). Participants without IC were either asymptomatic (i.e. reported no exertional leg symptoms) or had leg symptoms not meeting criteria for IC (1–3).
Primary and secondary outcomes.
Outcomes were measured before randomization and at six-month follow-up by staff unaware of group assignment. To distinguish between acute vs. chronic intervention effects, there were two primary outcomes: Six-month change in six-minute walk distance, measured at 2.5 hours after a study beverage and at 24 hours after a study beverage was consumed.
Secondary outcomes were six-month change in brachial artery flow-mediated dilation (FMD), measured both 2.5 hours and 24 hours after study beverage at 6-month follow-up, and six-month change in maximal and pain-free treadmill walking distance measured 48 hours after beverage. To obtain measures at 2.5 hours, 24 hours, and 48 hours after a beverage, at the six-month follow-up time point, participants were instructed to discontinue their study beverage temporarily (at specific times to facilitate correct timing of follow-up measures) and resume the beverage for muscle biopsy and perfusion measurements.
Additional secondary outcomes were obtained at 6-month follow-up while participants were taking beverages daily and included calf muscle biopsy measures of peroxisome proliferator activated receptor-γ co-activator 1α (PGC-1α), follistatin, and myostatin levels and citrate synthase, and cytochrome c oxidase (COX) activity. Exploratory outcomes were six-month change in calf muscle perfusion and calf biopsy measures of satellite cell abundance, capillary density, centrally nucleated and embryonic myosin heavy chain-expressing fiber abundance, and oxidative stress measures (nitrotyrosine and 4-hydroxynonenal (4-HNE)) (online Table II).
Six-minute walk test.
The six-minute walk has been well validated in people with PAD and simulates the type of walking typically performed in daily life (i.e. corridor walking) (1–6,17). Small meaningful change for six-minute walk distance has been defined as 12 to 20 meters (17,18). Following a standardized protocol (1–3,17), participants walked up and down a 100-foot hallway for six minutes after instruction to cover as much distance as possible.
Treadmill walking performance
Maximal treadmill walking time and time to ischemic leg symptom onset were measured using the Gardner-Skinner protocol at baseline and six-month follow-up (5,6,19).
Brachial artery flow-mediated dilation
Brachial artery FMD was measured in the proximal brachial artery (B-mode and Doppler) after a 12-hour fast by Registered Diagnostic Cardiac Sonographers using a linear array vascular ultrasound transducer (Siemens Medical Solutions, Sequoia Model #256, frequency 8MHz, range 5–8MHz) (5,6). A cuff proximal to the visualized brachial artery segment was inflated for four minutes at 50 mm Hg above systolic pressure. Brachial artery images were obtained 60 seconds after cuff deflation and interpreted by a single reader, blinded to group assignment, at the University of Wisconsin Atherosclerosis Imaging Research Program Core Laboratory.
Calf muscle perfusion.
Arterial spin labeling with cardiovascular magnetic resonance imaging was used to measure changes in calf perfusion at 3 Tesla between PAD participants receiving cocoa vs. placebo (20). A thigh cuff was inflated to 250 mm Hg in the leg with lowest ABI and rapidly deflated after five minutes. Seven control-tagged image pairs were acquired over 60 seconds using pulsed arterial spin labeling pulse sequence with single-shot echo-planar imaging readouts (field of view 200×200 mm, matrix 64×64, repetition time 4000 ms, echo time 32 ms, slice thickness 10 mm). Perfusion was measured and quantified on a Siemens Healthcare workstation by co-investigator CMK.
Physical activity.
Free-living physical activity was acquired over seven days with the ActiGraph accelerometer. The accelerometer was worn on the right hip and removed only for bathing or sleeping (17,21).
Calf muscle biopsy.
An open muscle biopsy at baseline was performed in the medial head of the gastrocnemius muscle. Anesthesia was achieved with subcutaneous lidocaine (6,22). Subcutaneous tissue was dissected and approximately 250 mgs of muscle was removed and immediately prepared for freezing at −80 degrees Celsius. At six-month follow-up, the biopsy was repeated, adjacent to the original biopsy, identifiable by the scar.
Mitochondrial measures.
For enzyme activity assays (citrate synthase and COX), whole muscle tissue homogenates were prepared and enzyme activity was measured as described (6,22–24). For immunoblotting (PGC-1α, follistatin, myostatin, nitrotyrosine, 4-HNE), whole muscle tissue homogenates were analyzed as described (6,22,24). Detailed methods are in the online supplement.
Immunohistochemistry.
Frozen 7-μm sections of muscle were processed and stained for detection of nuclei using 4’6-diaminidino-2-phenylindole (DAPI), satellite cells using anti-Pax7 and fiber borders with anti-laminin as described (25,26), except that anti-embryonic myosin heavy chain (eMyHC) antibody was used. Validation of central nuclei was carried out by detection of the nuclear lamina protein Lamin A/C. Capillary density was determined using lectins Ulex europaeus and Griffonia Simplicifolia. Whole cross section images were acquired on an AxioImager M1 microscope (Zeiss; Oberkochen, Germany) equipped with ZEN (v2.0) software. DAPI+ central fiber nuclei, Pax7+/DAPI+ satellite cells, eMyHC+ fibers and lectin + capillaries were expressed relative to fiber number (details in online supplement).
Other measures.
Height and weight were measured at baseline and follow-up. Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Flavanol metabolites were measured as the structurally related (–)epicatechin metabolites (SREM) and 5-(3ʹ,4ʹ-dihydroxyphenyl)-γ-valerolactone metabolites (gVLM) as described previously (27–29). Theobromine was measured via UV detection at 274 nm.
Sample size calculations.
The sample size calculation was based on an anticipated drop-out rate of 10% (5,6). Using the standard deviation (SD) of 52 meters of change in six-minute walk distance from prior trials (15,30), 20 participants per group provided 70% power to detect a difference of 0.58 SD, representing approximately 30 meters change in six-minute walk between the cocoa group (n=20, respectively) and the placebo group (n=20), based on a one-sided two-sample t-test with a significance level of 0.10. The level of statistical significance and power were selected because the trial was a pilot intended to collect preliminary data.
Statistical analyses.
Primary analyses were performed using the intention to treat principle. All participants were asked to return for follow-up testing and data were analyzed according to their assigned group even if they discontinued the study beverage. For participants who declined to return for follow-up but remained alive, multiple imputation was performed using 20 imputations to account for missing data. In sensitivity analyses, analyses were repeated excluding participants who underwent lower extremity revascularization during follow-up and adjusting for beverage adherence, respectively. Baseline characteristics were summarized as means and standard deviations, frequencies and percentages, as appropriate. T-tests and Chi-square tests were used to compare continuous and categorical characteristics of participants between the two study groups, respectively. Two sample one-tailed t-tests were used to compare changes in each outcome at 6-month follow-up between cocoa and placebo. A one sided P value < 0.10 was considered statistically significant. Analyses were repeated using analysis of covariance adjusting for baseline differences in characteristics of the intervention and control groups. Statistical analyses were performed using SAS version 9.4.
RESULTS
Of 118 participants who provided written informed consent, 44 were randomized: 23 to cocoa and 21 to placebo (Figure 1). Of 74 who signed a consent but were not randomized, 58 had an ABI > 0.90, 10 did not complete baseline testing or declined participation, three had a baseline six-minute walk distance that met the exclusion criterion for this measure, one was scheduled for coronary revascularization after completing the baseline treadmill test, one had a mini-mental status examination score <23, and one had walking impairment not due to PAD. Forty of 44 randomized (90%) completed one or more six-month follow-up measures reported here. Of these, 36 completed both six-minute walk tests at 6-month follow-up, one completed the six-minute walk at 2.5 hours but not at 24 hours after study beverage, and three completed the six-minute walk at 24 hours but not at 2.5 hours after the beverage. Twenty-two participants underwent baseline muscle biopsy, but muscle tissue was only obtained for 21. Of these, 17 completed a follow-up biopsy. Of the four people who did not undergo follow-up muscle biopsy, one moved out of state, one had a stroke and could not return, one underwent the biopsy procedure, but no muscle was obtained, and one repeatedly cancelled his muscle biopsy appointment.
Figure 1.

Overview of recruitment, randomization, and follow-up rates in the COCOA-PAD Study
Participants randomized to cocoa had lower BMI and higher proportions of current smokers and African-Americans than those randomized to placebo (Table 1). Comparisons between the cocoa vs. placebo groups adjusted for these baseline differences.
Table 1.
Baseline characteristics of participants by group assignment
| Baseline variable | Overall (N=44) | Trial Assignment | |
|---|---|---|---|
| COCOA (N=23) | Placebo (N=21) | ||
| Age (year), mean (SD) | 72 (7) | 71 (7) | 73 (7) |
| Male, n (%) | 29 (66) | 15 (65) | 14 (67) |
| African American, n (%) | 31 (70) | 19 (83) | 12 (57) |
| Ankle brachial index, mean (SD) | 0.66 (0.15) | 0.67 (0.14) | 0.64 (0.15) |
| Body mass index (kg/m2), mean (SD) | 29 (5) | 27 (5) | 31 (5) |
| Current smoker, n (%) | 14 (32) | 11 (48) | 3 (14) |
| Former smoker, n (%) | 24 (55) | 9 (39) | 15 (71) |
| Myocardial infarction, n (%) | 6 (14) | 4 (17) | 2 (10) |
| Heart failure, n (%) | 7 (16) | 3 (13) | 4 (19) |
| Stroke, n (%) | 10 (23) | 6 (26) | 4 (19) |
| Angina, n (%) | 7 (16) | 4 (17) | 3 (14) |
| Pulmonary disease, n (%) | 11 (25) | 4 (17) | 7 (33) |
| Diabetes, n (%) | 24 (55) | 13 (57) | 11 (52) |
| Intermittent claudication, n (%) | 6 (14) | 2 (9) | 4 (19) |
| Leg pain other than claudication, n (%) | 34 (77) | 19 (83) | 15 (71) |
| No exertional leg pain, n (%) | 4 (9) | 2 (9) | 2 (10) |
| Six-minute walk distance (meters), mean (SD) | 334 (81) | 337 (75) | 331 (88) |
| Maximal treadmill time (minutes), mean (SD) | 9 (6) | 9 (5) | 10 (7) |
SD=Standard deviation
Beverage adherence rates were 68% and 80% in the cocoa and placebo groups, respectively, based on packet counts. After excluding seven participants who discontinued beverages but returned for follow-up, adherence was 80% and 87%, respectively. Six-month change in epicatechin metabolites and theobromine were significantly greater in participants randomized to cocoa compared to placebo, consistent with good adherence (Table 2).
Table 2.
Six month change in epicatechins, valerolactones, and theobromine by group assignment
| No | Mean (SD) | Mean (90% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6-M follow-up | Within-group Change | Difference in Changes | P value | ||
| Structurally related epicatechin metabolites (nM) | ||||||
| Cocoa | 17 | 18.6 (22.2) | 148.2 (106.4) | +129.6 (+80.8, +178.3) | +113.1 (+63.7, +162.4) | 0.001 |
| Placebo | 19 | 15.7 (19.1) | 32.3 (23.4) | +16.5 (+7.8, +25.2) | --- | --- |
| γ valerolactone metabolites (nM) | ||||||
| Cocoa | 17 | 23.7 (43.4) | 69.0 (137.0) | +45.3 (−17.3, +107.9) | +36.5 (−30.8, +103.9) | 0.36 |
| Placebo | 19 | 17.7 (41.9) | 26.4 (54.4) | +8.7 (−18.9, +36.4) | --- | --- |
| Theobromine (μM) | ||||||
| Cocoa | 17 | 1.78 (1.49) | 9.23 (8.60) | +7.45 (+3.98, +10.92) | +6.86 (+2.66, +11.07) | 0.010 |
| Placebo | 19 | 2.86 (3.76) | 3.44 (6.99) | +0.59 (−1.99, +3.16) | --- | --- |
Primary outcome.
In intention to treat analyses, adjusting for BMI, race, and smoking, participants randomized to cocoa improved six-minute walk distance at six-month follow-up by +42.6 meters (P=0.005, 90% Confidence Interval (CI): +22.2,+∞) at the 2.5-hour time point and by +18.0 meters (P=0.12, 90% CI:−1.7,+∞) at the 24-hour time point, compared to placebo (Table 3). Results became statistically significant according to our a priori definition of statistical significance at the 24-hour time point when missing data were imputed (Table 3) (+32.0 meters (P=0.040), 90% CI:+8.6, +∞) at the 2.5 hour time point and +21.5 meters ((P=0.084), 90% CI:+1.5, +∞) at the 24 hour time point. Results were became statistically significant at the 24 hour time point when analyses were repeated, excluding the two participants in the cocoa group who underwent lower extremity revascularization (+42.3 meters, P=0.006, 90% CI:+21.4, +∞ at the 2.5 hour time point and +25.2 meters, P=0.058, 90% CI:+4.8, +∞ at the 24-hour time point). Results were not substantially changed when analyses were repeated, adjusting for beverage adherence (+46.5 meters, P=0.002, 90% CI:+26.6, +∞ at the 2.5 hour time point and +18.9 meters, P=0.114, 90% CI:−1.2, +∞ at the 24 hour time point).
Table 3.
Adjusted effects of cocoa on change in six-minute walk distance at 6-month follow-up*
| No. | Mean (SD) | Mean (90% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6-M follow-up | Within-group Change | Difference in Changes | P value | ||
| INTENTION TO TREAT RESULTS | ||||||
| Six month change in six-minute walk distance at 2.5 hours after the study beverage (meters) | ||||||
| Cocoa | 17 | 348.6 (74.2) | 356.6 (64.0) | +18.4 (+0.34, +36.5) | +42.6 (+22.2, +∞) | 0.005 |
| Placebo | 20** | 337.3 (85.2) | 322.0 (96.4) | −24.2 (−40.7, −7.8) | --- | --- |
| Six-month change in six-minute walk distance at 24 hours after the study beverage (meters) | ||||||
| Cocoa | 19 | 347.7 (74.3) | 353.0 (76.9) | +15.1 (−1.9, +32.1) | +18.0 (−1.7, +∞) | 0.12 |
| Placebo | 20** | 329.1 (90.4) | 335.4 (92.5) | −2.9 (−19.4, +13.6) | --- | --- |
| RESULTS IMPUTED FOR MISSING DATA | ||||||
| Six-month change in six-minute walk distance at 2.5 hours after the study beverage (meters) | ||||||
| Cocoa | 22 | 338.9 (74.7) | 337.7 (68.6) | +9.8 (−9.8, +29.4) | +32.0 (+8.6, +∞) | 0.040 |
| Placebo | 21 | 330.7 (86.4) | 320.0 (92.3) | −22.2 (−41.5, −3.0) | --- | --- |
| Six-month change in six-minute walk distance at 24 hours after the study beverage (meters) | ||||||
| Cocoa | 22 | 338.9 (74.7) | 346.6 (74.2) | +17.2 (+0.50, +34.0) | +21.5 (+1.5, +∞) | 0.084 |
| Placebo | 21 | 330.7 (86.4) | 336.4 (88.3) | −4.3 (−20.7, +12.1) | --- | --- |
Data adjust for smoking, body mass index and race. Baseline and six-month follow-up distances do not sum to the within-group change value, due to adjustment for smoking, body mass index, and race.
Note that 1 participant randomized to placebo participated in the 2.5 hour data collection but not the 24 hour data collection and 1 participant randomized to placebo participated in the 24 hour data collection but not the 2.5 hour data collection, resulting in differences in baseline values for the placebo group in the intention to treat analyses.
Secondary outcomes.
Cocoa had no statistically significant effect on change in treadmill walking time measured 48 hours after a study beverage or brachial artery FMD measured 2.5 hours and 24 hours after study beverage, compared to control (Table 4). Cocoa increased calf muscle biopsy COX activity compared to placebo, but had no statistically significant effect on PGC1α, myostatin, or follistatin.
Table 4.
Adjusted effects of cocoa on change in secondary outcomes at 6-month follow-up*
| No. | Mean (SD) | Mean (90% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6-M follow-up | Within-group Change | Difference in Changes | P value | ||
| Six-month change in brachial artery FMD 2.5 hours after study beverage (%) | ||||||
| Cocoa | 15 | 6.47 (3.25) | 5.93 (2.69) | −0.65 (−2.08, +0.78) | −1.28 (−2.94, +∞) | 0.84 |
| Placebo | 17 | 5.76 (3.34) | 6.29 (4.57) | 0.63 (−0.69, +1.96) | --- | --- |
| Six-month change in brachial artery FMD 24 hours after study beverage (%) | ||||||
| Cocoa | 17 | 6.38 (3.34) | 6.10 (3.85) | 0.04 (−1.23, +1.32) | +0.63 (−0.96, +∞) | 0.30 |
| Placebo | 15 | 6.03 (3.41) | 5.81 (4.31) | −0.59 (−1.96, +0.79) | --- | --- |
| Six-month change in pain-free treadmill walking time at 48 hours after study beverage (minutes) | ||||||
| Cocoa | 18 | 6.08 (4.28) | 6.22 (3.81) | −0.07 (−1.13, +1.00) | −0.46 (−1.70, +∞) | 0.68 |
| Placebo | 19 | 5.23 (3.38) | 5.44 (3.20) | +0.39 (−0.64, +1.43) | --- | --- |
| Six-month change in maximal treadmill walking time at 48 hours after study beverage (minutes) | ||||||
| Cocoa | 18 | 8.86 (4.86) | 9.14 (4.43) | −0.03 (−1.16, +1.10) | −0.31 (−1.60, +∞) | 0.62 |
| Placebo | 20 | 9.76 (7.40) | 9.76 (8.57) | +0.28 (−0.79, +1.34) | --- | --- |
| Six-month change in physical activity (activity counts) | ||||||
| Cocoa | 15 | 97,812 (25,410) | 98,551 (43,057) | −1,919 (−17,743, +13,904) | +7,062 (−10515, +∞) | 0.30 |
| Placebo | 19 | 89,527 (54,517) | 78,447 (40,182) | −8,981 (−22,743, +4,780) | --- | --- |
| Six month change in calf muscle biopsy abundance of PGC1α (arbitrary units) | ||||||
| Cocoa | 10 | 0.59 (0.22) | 0.67 (0.29) | +0.09 (−0.09, +0.28) | +0.05 (−0.21, +∞) | 0.40 |
| Placebo | 6 | 0.57 (0.28) | 0.63 (0.53) | +0.04 (−0.21, +0.29) | --- | --- |
| Six month change in calf muscle biopsy abundance of myostatin (arbitrary units) | ||||||
| Cocoa | 10 | 1.27 (0.42) | 1.52 (0.42) | +0.26 (+0.05, +0.47) | +0.11 (−∞, +0.40) | 0.69 |
| Placebo | 6 | 1.25 (0.70) | 1.43 (0.68) | +0.15 (−0.14, +0.43) | --- | --- |
| Six month change in calf muscle biopsy abundance of follistatin (arbitrary units) | ||||||
| Cocoa | 10 | 0.73 (0.46) | 0.71 (0.29) | −0.03 (−0.20, +0.15) | −0.12 (−0.37, +∞) | 0.74 |
| Placebo | 6 | 0.95 (0.63) | 1.03 (0.60) | +0.09 (−0.15, +0.34) | --- | --- |
| Six month change in calf muscle biopsy citrate synthase activity (nmol/min/mg protein) | ||||||
| Cocoa | 10 | 11.43 (4.73) | 11.66 (4.24) | −0.25 (−3.85, +3.36) | +0.86 (−4.12, +∞) | 0.41 |
| Placebo | 6 | 14.13 (4.75) | 12.24 (5.45) | −1.10 (−5.98, +3.77) | --- | --- |
| Six month change in calf muscle biopsy COX activity (nmol/min/mg protein) | ||||||
| Cocoa | 10 | 65.1 (64.3) | 55.8 (32.8) | −4.6 (−37.2, +28.0) | +85.5 (+40.4, +∞) | 0.013 |
| Placebo | 6 | 131.4 (95.4) | 49.2 (26.4) | −90.0 (−134.1, −46.0) | --- | --- |
Data adjust for smoking, body mass index and race. Baseline and six-month follow-up values do not sum to the within-group change value, due to adjustment for smoking, body mass index, and race. COX = cytochrome c oxidase. Six month outcomes obtained on study beverage unless otherwise specified.
Exploratory outcomes.
Relative to placebo, cocoa reduced centrally located myofiber nuclei, increased capillary density, and increased calf muscle perfusion, but did not significantly change measures of oxidative stress, eMyHC myofibers, or abundance of Pax-7+ cells (Table 5 and Figure 2A).
Table 5.
Adjusted effects of cocoa on changes in exploratory outcomes at six-month follow-up*
| No. | Mean (SD) | Mean (90% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6-M follow-up | Within-group Change | Difference in Changes | P value | ||
| MRI-measured calf muscle perfusion (ml/min/100 gram)** | ||||||
| Cocoa | 13 | 2.91 (0.58) | 2.93 (0.46) | +0.10 (−0.25, +0.45) | +0.42 (+0.004, +∞) | 0.098 |
| Placebo | 13 | 3.01 (0.63) | 2.78 (0.59) | −0.32 (−0.67, +0.03) | --- | --- |
| Calf Muscle Biopsy Measures | ||||||
| #Pax7+/100 fibers | ||||||
| Cocoa | 11 | 11.87 (8.28) | 14.85 (10.98) | +3.50 (−1.85, +8.84) | +3.23 (−4.30, +∞) | 0.29 |
| Placebo | 6 | 18.28 (12.81) | 19.50 (10.38) | +0.27 (−7.31, +7.84) | --- | --- |
| Embryonic+ fibers (% of total) | ||||||
| Cocoa | 11 | 0.16 (0.54) | 0.04 (0.09) | −0.11 (−1.17, +0.96) | −1.09 (−∞, +0.42) | 0.17 |
| Placebo | 6 | 0.48 (0.56) | 1.49 (2.96) | +0.98 (−0.54, +2.50) | --- | --- |
| Central nuclei (all % of total) | ||||||
| Cocoa | 11 | 9.29 (4.69) | 9.59 (3.70) | +0.25 (−3.14, +3.63) | −7.10 (−∞, −2.33) | 0.033 |
| Placebo | 6 | 15.64 (9.04) | 22.88 (5.95) | +7.34 (+2.55, +12.14) | --- | --- |
| Nitrotyrosine abundance (arbitrary units) | ||||||
| Cocoa | 10 | 1.00 (0.28) | 1.14 (0.32) | +0.16 (+0.05, +0.27) | +0.13 (−∞, +0.28) | 0.86 |
| Placebo | 6 | 0.99 (0.29) | 1.05 (0.42) | +0.03 (−0.12, +0.18) | --- | --- |
| 4-HNE abundance (arbitrary units) | ||||||
| Cocoa | 10 | 1.07 (0.35) | 1.10 (0.41) | −0.02 (−0.27, +0.23) | −0.07 (−∞, +0.28) | 0.40 |
| Placebo | 6 | 1.03 (0.23) | 0.99 (0.42) | +0.05 (−0.30, +0.39) | --- | --- |
| Capillary density | ||||||
| Cocoa | 11 | 1.42 (0.76) | 1.44 (0.83) | +0.07 (−0.08, +0.21) | +0.38 (+0.17, +∞) | 0.014 |
| Placebo | 6 | 1.66 (0.15) | 1.43 (0.22) | −0.31 (−0.52, −0.11) | --- | --- |
Data adjust for smoking, body mass index and race. Baseline and six-month follow-up values do not sum to the within-group change value, due to adjustment for smoking, body mass index, and race.
Results are based on log-transformed data.
Figure 2. Immunohistochemistry of muscle from a participant with PAD.
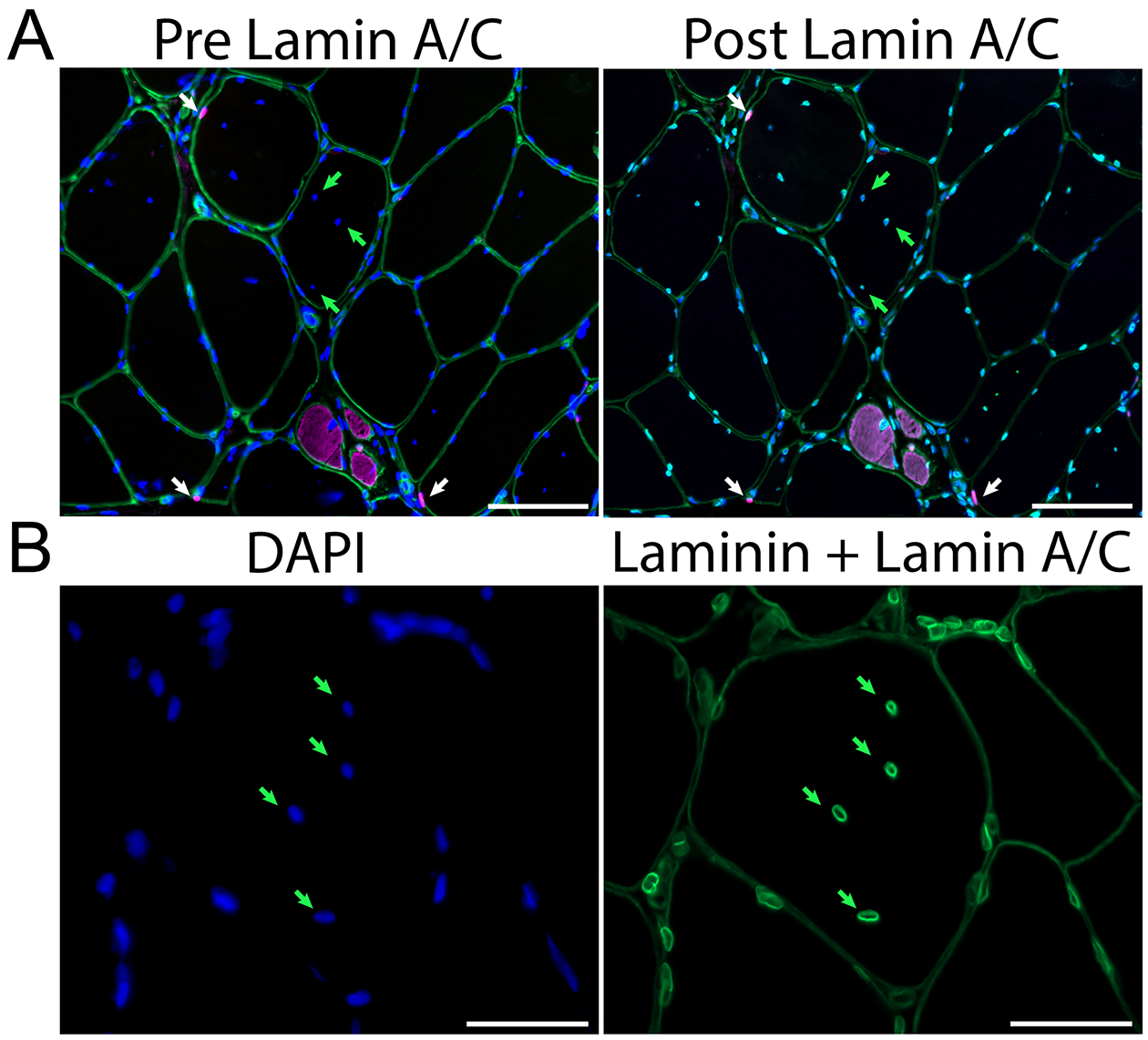
A. Left: Representative image showing red, Pax7+ satellite cells (white arrows), DAPI+ (blue) labeled DNA, laminin (green) demarcating muscle fiber borders and embryonic myosin heavy chain (eMyHC, pink) identifying small, regenerating myofibers. The abundance of centrally located DAPI+ myonuclei (green arrows) were changed with cocoa. Right: The same sample showing the nuclear envelope marker lamin A/C (bright green) surrounding each DAPI+ event. Image acquired at 200x, scale bars = 100 μm. B. Higher magnification showing the presence of lamin A/C nuclear envelope (bright green) surrounding each centrally located DAPI+ nucleus (green arrows). Laminin demarcating fiber borders is also shown (dim green). Image acquired at 400x, scale bar = 50 μm.
Post-hoc analyses.
To determine whether the centrally located myofiber nuclei were actually cytoplasmic chromatin fragments, a marker of senescent cells (31), muscle sections were stained for lamin A/C, nuclear envelope proteins (Figures 2A and B). DAPI+DNA was surrounded by a nuclear envelope, verifying that these were central nuclei.
Adverse events.
There was no significant difference in BMI change between the cocoa vs. placebo groups (26.6 to 26.4 kg/M2 vs. 30.7 to 30.8 kg/M2, respectively, mean difference=−0.20 kg/M2, 95% CI:−0.82,+0.41, P=0.50). In the cocoa group, there was one death due to myocardial infarction, two hospitalizations for lower extremity revascularizations and one hospitalization each for ischemic stroke, foot infection, urinary difficulty, and depression. In the placebo group, there were two hospitalizations, one for atrial fibrillation and one for cough with chest pain. No serious adverse event was considered likely related to study participation.
DISCUSSION
In this pilot double blind randomized clinical trial, participants randomized to cocoa for six months walked 42.6 meters further in the six-minute walk at 6-month follow-up, 2.5 hours after beverage consumption, and 18 meters further at 6-month follow-up, 24 hours after beverage consumption, compared to those randomized to placebo. The difference in change at 6-month follow-up between the cocoa vs. the placebo groups was statistically significant at the 2.5 hour time point but not at the 24 hour time point. Results did not substantially change but became statistically significant at the 24 hour time point when analyses were repeated using multiple imputation to account for missing data and when analyses were repeated, excluding the two participants randomized to cocoa who underwent revascularization during the trial. Among people with PAD, a small meaningful change in six-minute walk distance has been defined as 12.0 meters and a large meaningful change has been defined as 34.0 meters (18). Cocoa significantly improved calf muscle perfusion and calf muscle COX enzyme activity, capillary density, and abundance of central nuclei, compared to placebo. The effects on calf muscle suggest a durable benefit from the cocoa beverage. To our knowledge, no prior randomized trials have tested the effects of chronically administered cocoa on change in walking performance, calf muscle biopsy characteristics, or calf muscle perfusion in people with PAD. Results reported here are consistent with a recent 8 week randomized trial of 74 older men and women without PAD (mean age 75.9), in which participants randomized to a cocoa flavanol beverage had a 35.7 meter greater improvement in six-minute walk distance at 8-week follow-up, compared to those randomized to a placebo beverage (p=0.02) (32).
A prior study of 20 participants with PAD demonstrated an acute effect of cocoa on treadmill walking time and serum nitrite levels in people with PAD (14). To our knowledge, no prior trials have reported the effects of chronic daily cocoa intake on change in walking performance in people with PAD. The current pilot trial was designed in part to test an acute (i.e. immediate) effect vs. chronic (i.e. durable after the intervention is discontinued) effect of chronic cocoa on walking performance in PAD. Results reported here showing significant benefits of cocoa on calf muscle COX enzyme activity (an indicator of mitochondrial activity), capillary density, and central myofiber nuclei compared to placebo, suggest that cocoa may have a durable beneficial effect on calf muscle in people with PAD, in addition to an acute effect previously reported (14). If daily cocoa has both an acute and chronic benefit in people with PAD, these combined benefits may explain the greater improvement in six-minute walk distance at the 2.5 hour time point (which reflected both acute and chronic effects) than at the 24 hour time point (which reflected only chronic effects), relative to placebo.
In this trial, the 42.6 meter improvement in six-minute walk distance at the 6-month follow-up 2.5 hour time point in the cocoa group relative to placebo was due to a combination of improvement in six-minute walk distance in the cocoa intervention group (+18.4 meters) and decline in the placebo group (−24.2 meters). The decline in six-minute walk distance among participants randomized to placebo at the 2.5 hour time point was consistent with the decline in six-minute walk distance observed previously in longitudinal studies of people with PAD who were not randomized to an effective therapy (3–6,15,30). For example, prior randomized trials of people with PAD reported declines of −18.0 meters, −15.0 meters, and −11.4 meters, in the control groups, respectively (15,30,33).
An unexpected finding was that among participants randomized to placebo, six-minute walk distance declined at six-month follow-up by 24.2 meters at the 2.5 hour time point compared to baseline, but only declined by 2.9 meters at the 24 hour time point compared to baseline. In contrast, among participants randomized to cocoa, six-minute walk distance improved at six-month follow-up by 18.4 meters at the 2.5 hour time point compared to baseline and by 15.1 meters at the 24 hour time point, compared to baseline. The greater benefit of cocoa relative to placebo at the 2.5 hour time point compared to the 24 hour time point may have been due in part to a learning effect between the 2.5 hour and the 24 hour time points for the six-minute walk among participants randomized to placebo. It is possible that a learning effect between the 2.5 hour and 24 hour time points in the cocoa group was obscured by an acute effect of cocoa on six-minute walk distance at the 2.5 hour time point. Specifically, the six-minute walk distance performed 2.5 hours after cocoa ingestion in the cocoa group may have been enhanced by the acute benefits of cocoa at the 2.5 hour time point. The loss of this acute benefit from cocoa at the 24 hour time point in the cocoa group may have been compensated for by a learning effect in the cocoa group. Because the placebo had no acute effect on 6-minute walk distance at the 2.5 hour time point, a learning effect at 24 hours would be more apparent in the placebo group, since the 2.5 hour six-minute walk distance was not enhanced by any acute effect 2.5 hours after the placebo drink. In support of this hypothesis, the mean net difference in six-minute walk distance between the 2.5 hour and the 24 hour measurement was 0 meters in the cocoa group and 13.0 meters in the placebo group (Online supplement Figures I and II). However, other possibilities include that the six-minute walk distance measured at the 2.5 hour time point was influenced by a learning effect in both groups, since all participants had completed a baseline six-minute walk six months previously or that those randomized to cocoa did not experience an acute benefit at the 2.5 hour time point but that instead a chronic benefit affected the six-minute walk distance at both the 2.5 hour and the 24 hour time points. While previous work suggested no learning effect of the six-minute walk in people with PAD (17), to our knowledge, no studies have assessed whether a learning effect exists when participants with PAD perform two six-minute walk tests 24 hours apart. It is also possible that fatigue from the six-minute walk test 24 hours previously may have reduced the six minute walk distance achieved at the 24 hour 6-month time point.
The higher rate of hospitalizations or death in the cocoa group compared to placebo was unexpected but may have been due to the substantially higher rate of current smoking among those randomized to cocoa compared to placebo (48% vs. 14%). Of the seven serious adverse events in the cocoa group, four were cardiovascular in nature. Of the four participants in the cocoa group with a cardiovascular event, two (50%) smoked cigarettes.
This study has limitations. First, this was a pilot study and the sample size was small. Second, because of the small sample size, randomization resulted in imbalance of BMI, sex, and race. Results adjusted for these characteristics, but this adjustment may not have been sufficient to overcome potential confounding by baseline differences, resulting in residual confounding and bias toward the null. Third, there were multiple outcomes. It is possible that some significant findings were due to chance. Fourth, the study did not include people with PAD and an ABI > 1.40. There are no biologic reasons to anticipate that results would have differed in people with PAD and ABI > 1.40. Fifth, the absence of significant improvement in treadmill walking performance among participants randomized to cocoa may be because the treadmill test at 6-month follow-up was performed 48 hours after study beverages. Sixth, dietary data were not collected.
Conclusions.
These preliminary results suggest a therapeutic effect of chronically administered cocoa on walking performance in people with PAD. Further study is needed to definitively determine whether cocoa significantly improves walking performance in people with PAD.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
People with lower extremity peripheral artery disease (PAD) have a faster decline in a six-minute walk than people without PAD.
Few therapies improve six-minute walk or prevent decline in people with PAD.
Pre-clinical and preliminary human evidence suggested that cocoa flavanols may increase limb perfusion, skeletal muscle regeneration, and mitochondrial activity.
What New Information Does This Article Contribute?
Among 44 participants with PAD randomized to either a cocoa flavanol or placebo beverage for six months, cocoa flavanols significantly improved six-minute walk distance at 6-month follow-up, compared to placebo.
PAD participants randomized to cocoa flavanols had significant improvement in limb perfusion compared to placebo.
PAD participants randomized to cocoa flavanols improved several skeletal muscle measures, including in mitochondrial activity and capillary abundance, compared to placebo.
Patients with PAD have poorer lower extremity perfusion, reduced calf muscle mitochondrial activity, and faster decline in walking performance than people without PAD. Few medical therapies improve walking ability in people with PAD. Pre-clinical and preliminary human evidence suggested that cocoa flavanols may increase limb perfusion, promote skeletal muscle regeneration, and improve mitochondrial activity. Therefore, in this pilot double blinded clinical trial, 44 participants with PAD were randomized to either a cocoa flavanol beverage or placebo beverage taken three times daily for six months. At six-month follow-up, participants with PAD who were randomized to cocoa flavanols had significant improvement in six-minute walk distance, compared to those randomized to placebo. Participants randomized to cocoa flavanols also had significant improvement in limb perfusion, calf muscle mitochondrial activity, and calf muscle capillary abundance, compared to those randomized to placebo. Further study with a larger sample size is needed to definitively assess the effects of cocoa flavanols on walking ability in people with PAD.
ACKNOWLEDGEMENTS
The authors acknowledge Reedmond Y. Fong, MS; Department of Nutrition, University of California Davis (One Shields Av, Davis, CA 95616) and Javier I. Ottaviani, PhD; Mars, Incorporated (6885 Elm St, McLean, VA 22101) for performing plasma measures of epicatechin metabolites, valerolactones, and theobromine. J.I.O. is employed by Mars, Inc., a company engaged in flavanol research and flavanol-related commercial activities.
SOURCES OF FUNDING
Funded by the National Institute on Aging (R21-AG050897) and by the intramural office of the National Institute on Aging. Funded in part by the Office of Dietary Supplements, National Institutes of Health. The cocoa beverage and matching placebo were provided by The Hershey Company. Mars Incorporated performed measures of epicatechin metabolites, valerolactones, and theobromine.
Nonstandard Abbreviations And Acronyms:
- IC
intermittent claudication
- FMD
flow-mediated dilation
- PGC-1α
peroxisome proliferator activated receptor-γ co-activator 1α
- COX
cytochrome c oxidase
- 4-HNE
nitrotyrosine and 4-hydroxynonenal
- DAPI
4’6-diaminidino-2-phenylindole
- eMyHC
myosin heavy chain
- SREM
structurally related (–)-epicatechin metabolites
- gVLM
5-(3ʹ,4ʹ-dihydroxyphenyl)-γ-valerolactone metabolites
Footnotes
DISCLOSURES
Dr. Villarreal is a co-founder and stockholder of Cardero Therapeutics, Inc.
Dr. McDermott has received other research support from Helixmith, Reserveage, Chromadex, and ArtAssist. This other research support is not related to the current study.
No other co-authors have disclosures.
REFERENCES
- 1.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61 [DOI] [PubMed] [Google Scholar]
- 4.Gardner AW, Montgomery PS, Killewich LA. Natural history of physical function in older men with intermittent claudication. J Vasc Surg. 2004;40:73–8. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Ferrucci L, Tian L, Guralnik JM, Lloyd-Jones D, Kibbe MR, Polonsky TS, Domanchuk K, Stein JH, Zhao L, Taylor D, Skelly C, Pearce W, Perlman H, McCarthy W, Li L, Gao Y, Sufit R, Bloomfield CL, Criqui MH. Effect of Granulocyte-Macrophage Colony-Stimulating Factor With or Without Supervised Exercise on Walking Performance in Patients With Peripheral Artery Disease: The PROPEL Randomized Clinical Trial. JAMA. 2017;318:2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Leeuwenburgh C, Guralnik JM, Tian L, Sufit R, Zhao L, Criqui MH, Kibbe MR, Stein JH, Lloyd-Jones D, Anton SD, Polonsky TS, Gao Y, de Cabo R, Ferrucci L. Effect of Resveratrol on Walking Performance in Older People With Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol. 2017;2:902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hüttemann M, Lee I, Perkins GA, Britton SL, Koch LG, Malek MH. (−)-Epicatechin is associated with increased angiogenic and mitochondrial signaling in the hindlimb of rats selectively bred for innate low running capacity. Clin Sci (Lond). 2013;124:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez-Salmean G, Ciaraldi TP, Nogueira L, Barboza J, Taub PR, Hogan MC, Henry RR, Meaney E, Villarreal F, Ceballos G, Ramirez-Sanchez I. Effects of (−)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J Nutr Biochem. 2014;25(1):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol. 2011;589:4615–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe N, Inagawa K, Shibata M, Osakabe N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis. 2014;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Perkins G, Murphy AN, Naviaux R, Hogan M, Maisel AS, Henry RR, Ceballos G, Villarreal F. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci. 2012;5:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Gonzalez-Basurto S, Coral-Vazquez R, Perkins G, Hogan M, Maisel AS, Henry RR, Ceballos G, Villarreal F. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and type 2 diabetes: restorative effects of (−)-epicatechin-rich cocoa. Clin Sci (Lond). 2013;125:383–389. [DOI] [PubMed] [Google Scholar]
- 13.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients: A double-masked, randomized, controlled trial. J Am Coll Cardiol. 2008;51:2141–2149. [DOI] [PubMed] [Google Scholar]
- 14.Loffredo L, Perri L, Catasca E, Pignatelli P, Brancorsini M, Nocella C, De Falco E, Bartimoccia S, Frati G, Carnevale R, Violi F. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J Am Heart Assoc. 2014;3(4):e001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Spring B, Berger JS, et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease: The HONOR Randomized Clinical Trial. JAMA. 2018;319:1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg 2000;32:1164–1171. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-Minute Walk Is a Better Outcome Measure Than Treadmill Walking Tests in Therapeutic Trials of Patients With Peripheral Artery Disease. Circulation. 2014;130:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med. 2018;23:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 20.Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, Dimaria JM, Christopher JM, Kramer CM. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging. 2012;5:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly LA, McMillan DG, Anderson A, Fippinger M, Fillerup G, Rider J. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Peterson CA, Sufit R, Ferrucci L, Guralnik JM, Kibbe MR, Polonsky TS, Tian L, Criqui MH, Zhao L, Stein JH, Li L, Leeuwenburgh C. Peripheral artery disease, calf skeletal muscle mitochondrial DNA copy number, and functional performance. Vasc Med. 2018;23:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C. The impact of aging on mitochondria function and biogenesis pathways in skeletal muscles of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012;7:1235–1246. [DOI] [PubMed] [Google Scholar]
- 25.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fiber type-specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592:2625–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White SH, McDermott MM, Sufit RL, Kosmac K, Bugg AW, Gonzalez-Freire M, Ferrucci L, Tian L, Zhao L, Gao Y, Kibbe MR, Criqui MH, Leeuwenburgh C, Peterson CA. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: an observational study. J Transl Med. 2016;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, Schroeter H. The metabolome of [2-(14)C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep. 2016;6:29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottaviani JI, Fong R, Kimball J, Ensunsa JL, Britten A, Lucarelli D, Luben R, Grace PB, Mawson DH, Tym A, Wierzbicki A, Khaw KT, Schroeter H, Kuhnle GGC. Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies. Sci Rep. 2018;8:9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottaviani JI, Fong R, Kimball J, Ensunsa JL, Gray N, Vogiatzoglou A, Britten A, Lucarelli D, Luben R, Grace PB, Mawson DH, Tym A, Wierzbicki A, Smith AD, Wareham NJ, Forouhi NG, Khaw KT, Schroeter H, Kuhnle GGC. Evaluation of (−)-epicatechin metabolites as recovery biomarker of dietary flavan-3-ol intake. Sci Rep. 2019;9:13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd-Jones D, Kibbe M, Tao H, Zhao L, Liao Y, Rejeski WJ. Home-based walking exercise intervention in people with peripheral arterial disease: a randomized clinical trial. JAMA. 2013;310:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Z, Ghosh, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Smithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jian T, Shoshkes-Carmel M, Tanim KMAA, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017;550:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munguia L, Rubio-Gayosso I, Ramirez-Sanchez I, Ortiz A, Hidalgo I, Gonzalez C, Meaney E, Villarreal F, Najera N, Caballos G. High flavnonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: A double-blind randomized clinical trial. Journals of Gerontology Medical Science 2019;74:1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: A randomized controlled trial. J Am Geriatr Soc 2001;49:755–762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


