Supplemental digital content is available in the text.
Key Words: cervical cancer screening; consensus management guidelines; HPV 16, HPV 18
Abstract
Introduction
The 2019 ASCCP Risk-Based Management Consensus Guidelines include recommendations for partial human papillomavirus (HPV) genotyping in management of abnormal cervical cancer screening results. The guidelines are based on matching estimates of cervical intraepithelial neoplasia (CIN) 3+ risk to consensus clinical action thresholds. In support of the guidelines, this analysis addresses the risks predicted by individual identification of HPV 16 and HPV 18.
Methods
Risk estimates were drawn from a subset of women in the Kaiser Permanente Northern California screening program, whose residual cervical specimens were HPV typed as part of the HPV Persistence and Progression study. We calculated risk of CIN 3+ to assess how identification of HPV 16, HPV 18, or 12 other “high-risk” HPV types would influence recommended clinical management of new abnormal screening results, taking into account current cytologic results and recent screening history. Immediate and/or 5-year risks of CIN 3+ were matched to clinical actions identified in the guidelines.
Results
Identification of HPV 16 at the first visit including HPV testing elevated immediate risk of diagnosing CIN 3+ sufficiently to mandate colposcopic referral even when cytology was Negative for Intraepithelial Lesions or Malignancy and to support a preference for treatment of cytologic high-grade squamous intraepithelial lesion. HPV 18 less clearly elevated CIN 3+ risk.
Conclusions
Identification of HPV 16 clearly mandated consideration in clinical management of new abnormal screening results. HPV 18 positivity must be considered as a special situation because of established disproportionate risk of invasive cancer. More detailed genotyping and use beyond initial management will be considered in guideline updates.
Cervical cancer screening strategies are shifting from cytology to human papillomavirus (HPV) testing. HPV testing is more sensitive and, when negative, offers greater reassurance against cancer. However, most infections are typically benign, clearing within 2 years of acquisition, and in most cases do not require invasive diagnostic procedures or treatment.1,2
Secondary “triage” testing of HPV-positive women is an important step in clinical management. Cytology is currently recommended as a triage strategy, with most nonnormal cytologic results among HPV-positive women leading to immediate colposcopy.3 Among HPV-positive women, HPV genotyping can be used in addition to cytology to effectively stratify the risk of precancer.4 In the 2012 guidelines and 2015 clinical guidance,3,5 partial genotyping was incorporated into management; women with HPV-positive, cytology of Negative for Intraepithelial Lesions or Malignancy (NILM) were referred to colposcopy if the highest-risk HPV types (HPV 16 or HPV 18) were detected.5
The Food and Drug Administration has approved several HPV tests that provide genotyping information. Commercially available clinical tests vary in the degree to which they can identify specific HPV types,4 from no individual typing to extended genotyping. Regardless of typing capability, the number of types actually reported by screening assays in the United States is currently restricted (as of the beginning of 2020) to the highest-risk types: HPV 16, HPV 18, and sometimes HPV 45.
The large National Cancer Institute/ Kaiser Permanente Northern California Persistence and Progression (PaP) study was initiated in 2006 partially to help guide the use of typing as a triage after positive HPV testing. This analysis uses data from the PaP study to fit risk estimates for partial HPV genotyping into the clinical action thresholds for the new 2019 ASCCP Risk-Based Management Consensus Guidelines.
METHODS
Study Population
This analysis uses data from routine HPV testing at the Kaiser Permanente Northern California (KPNC). HPV testing was used to triage the equivocal cytologic result of atypical squamous cells of undetermined significance (ASC-US, since 2001) and as a cotest with cytology in women 30 years and older (since 2003).6,7 The HPV PaP study was created at KPNC by banking residual discarded cervical specimens from 44,340 women aged 25–65 years who were HPV tested during the study enrollment window (2007–2011); the specimen collection focused on HPV-positive women. The opt-out provisions and other important details of the PaP study are described elsewhere.4
Women with known history of cervical intraepithelial neoplasia (CIN) 2+ or hysterectomy before baseline were excluded, as were women younger than 25 years or older than 65 years at baseline. We then restricted the analytic sample for type-specific analyses to all 18,624 infections found among a sample of positive HPV test results at the PaP study baseline.4 For 7,477 women, this HPV positive result was the first known HPV test. For 4,282 women, we had information from an immediate prior HPV test, either negative or positive. Subsequent screening visits and visits in nonscreening settings (postcolposcopy, posttreatment, etc.) were excluded.
Variables
The baseline HPV status was based on HC2 (Qiagen, Germantown, MD) testing performed on a cervical specimen at the KPNC regional laboratory. HC2 identified HPV results as negative or positive for infection with any of the 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and exhibited some cross-reactivity with other types.8
Screening history was defined using HC2 results from the last screening visit immediately before the Pap study baseline to permit crude estimation of length of infection. Unknown was assigned to visits with no prior information on HPV status, resulting in infections of unknown length. HPV negative had a preceding negative HPV test, referring to a potentially newly acquired and lower-risk infection. HPV–positive NILM had a preceding positive HPV test with NILM cytology (other cytology results would have been referred to colposcopy and not included in the study population), indicating potentially “older,” persistent, higher-risk infections.
HPV typing was performed at Roche Molecular Systems (Pleasanton, CA) by cobas and Linear Array or at BD Diagnostics (Sparks, MD) by Onclarity.4,9 Partial HPV genotyping was reported, grouping HPV genotypes in 3 hierarchical categories: HPV 16, HPV 18, and high-risk 12 (HR12, all the other HPV types identified by the 2 tests, specifically HPV 31, HPV 33, HPV 35, HPV 39, HPV 45, HPV 51, HPV 52, HPV 56, HPV 58, HPV 59, HPV 66, and HPV 68).
Cytology was performed at KPNC regional and local laboratories. Cytology results were reported based on the 2001 Bethesda System.10 Atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), atypical glandular cells (AGC, without subdivision), and high-grade squamous intraepithelial lesion or worse (HSIL+) were referred to as “high-grade cytologic result.”
Clinical outcomes were obtained by matching to KPNC cytology and histopathology electronic medical records. Precancer was defined as a histopathologic diagnosis of CIN 3+ (CIN 3/adenocarcinoma in situ/cancer). Although CIN 2+ is often used as a threshold for treatment, it generates distorted estimates that overemphasize the importance of less carcinogenic HPV genotypes.4
Management recommendations followed the decisions reached in the 2019 ASCCP Risk-Based Management Consensus Guidelines as described by Perkins et al.11 Based on risk of CIN 3+, possible clinical actions were as follows: (1) expedited treatment preferred (immediate CIN 3+ risk ≥60%); (2) expedited treatment or colposcopy acceptable (immediate CIN 3+ risk ≥25% and <60%); (3) colposcopy (immediate CIN 3+ risk ≥4.0% and <25%); (4) 1-year return (immediate CIN 3+ risk <4.0% and 5-year CIN 3+ risk ≥0.55%); (5) 3-year return (5-year CIN 3+ risk ≥0.15% and <0.55%); and (6) 5-year return (5-year CIN 3+ risk <0.15%).
Statistical Methods
We calculated risk of CIN 3+ to assess whether partial HPV genotyping would change the suggested clinical management given current cytology and medical history. We described the distribution of the study population using frequencies and proportions for all categories. We used prevalence-incidence models (a mixture of logistic regression for the events present at the current visit and proportional hazards for the events detected during the follow-up visits), as described by Cheung et al.,12–15 to estimate risk of CIN 3+ immediately (at the time of cotest visit) and at years 1, 2, 3, 4, and 5. The tables in this article only presents immediate and 5-year CIN 3+ risks; however, more complete results for CIN 2+, CIN 3+, and cancer risks can be found in the publication by Egemen et al.16 Of note, 5-year rescreening is not relevant to the current discussion, as no HPV-positive women fell into this lowest risk category.
Because the partial HPV genotyping test is applied only to a small portion of the KPNC study group, we estimated sampling weights for this sample to reconstitute the original KPNC populations: (1) the cohort of individuals that were new to HPV testing and (2) the cohort who had screening history with HPV testing and had not gone under colposcopy. Tables presented in this article display the actual sample sizes of the total that fell into each category (i.e., before applying the sampling weights). However, the percentages of each category were based on the weighted sample sizes.
RESULTS
Partial HPV genotyping stratified risk of CIN 3+ and affected recommended clinical management within groups defined by cytology results (see Table 1). Before consideration of partial HPV typing, HPV infections with NILM cytology were referred to 1-year follow-up based on their immediate risk of 2.1% and their 5-year risk of CIN 3+ of 4.8%. HPV partial genotyping permitted more precise management. Patients with NILM and HPV 16 would be referred to colposcopy based on their immediate CIN 3+ risk of 5.3%. Patients with NILM and HPV 18 were also judged to need colposcopic referral, as a previously established special situation based on their known high risk of cancer (not observable through immediate CIN 3+ risk estimates).
TABLE 1.
Risk of CIN 3+ by Partial HPV Genotyping and Cytology, for All Infections With No Known Prior HPV Testing History
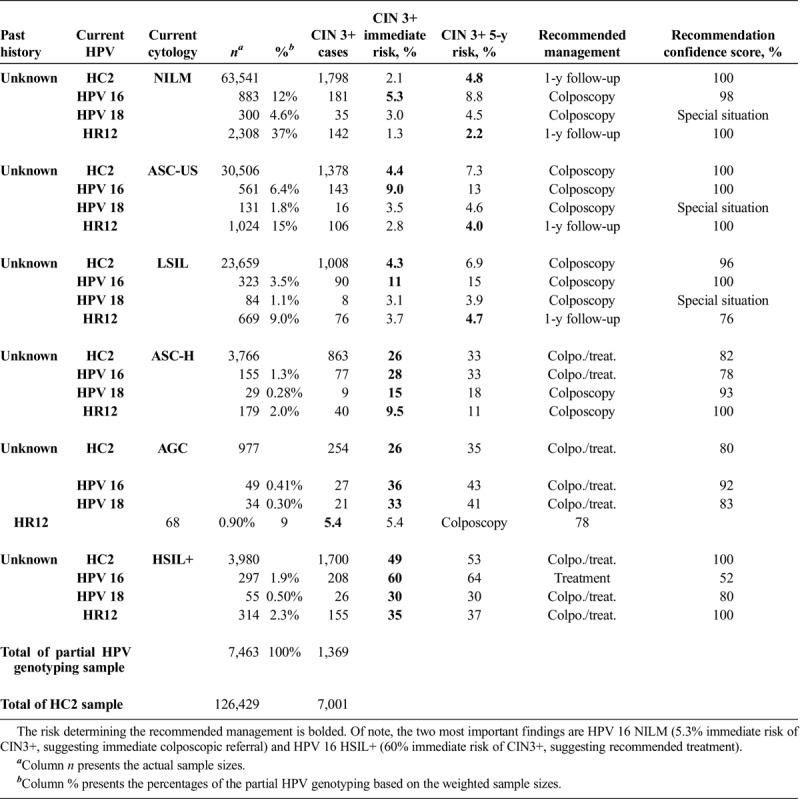
As a point of consideration for future revisions to the guidelines, recommendations for ASC-US and low-grade squamous intraepithelial lesion (LSIL) cytology could conceivably be downgraded from colposcopy referral among all HPV infections to 1-year follow-up for HR12 (i.e., no HPV 16 or HPV 18) based on risk of CIN 3+: immediate risk of 2.8% and 3.7%, 5-year risk of 4.0% and 4.7%, respectively. ASC-H and AGC again were judged to be special situations requiring colposcopy. Patients with HSIL+ cytology and HPV 16 had an immediate risk of 60% and would be recommended for treatment, rather than optional treatment or reliance on colposcopically directed biopsy diagnoses.
For future reference, we considered additional possible uses of HPV partial typing. For all type groups, repeat HPV positivity had higher risk than new infections (ancillary Tables 1–2, http://links.lww.com/LGT/A158, http://links.lww.com/LGT/A159). For new infections with known preceding HPV negative results and current normal or low-grade (NILM, ASC-US, or LSIL) cytology, untyped HPV-positive results might be managed with 1-year follow-up based on 5-year risk of 3.8% or lower (ancillary Table 1, http://links.lww.com/LGT/A158). In the same group, infections with known HPV 16 or HPV 18 were higher risk and would be referred to colposcopy, consistent with 2019 guidelines. High-grade cytology among new untyped or partially typed HPV positive infections had immediate risks of 10.0% or higher and would always be referred to colposcopy, with no change in clinical management based on genotype.
Persistent infections with a known preceding testing result of HPV-positive NILM had high risk of CIN 3+ and, in most cases, merited colposcopic referral, regardless of current cytology or HPV genotype (ancillary Table 2, http://links.lww.com/LGT/A159). An exception might be HR12 positivity with NILM cytology after HPV-positive NILM type unknown, where 1-year follow-up might be recommended based on immediate risk of 3.3% and 5-year risk of 4.7%.
DISCUSSION
HPV genotype stratified risk of CIN 3+ and influenced clinical management as defined by the 2019 ASCCP Risk-Based Management Consensus Guidelines. HPV papillomavirus 16 was particularly important to identify the need for more aggressive management for NILM and for HSIL+ cytology.
For the purpose of these guidelines, HPV 18 infections were handled as a “special situation,” with recommended colposcopic referral, regardless of whether the 4.0% immediate risk of CIN 3+ threshold was met. Immediate and 5-year cancer risk of HPV 18 infections were disproportionately high compared with other types, although the immediate CIN 3+ risk (especially for the low-grade cytology) did not exceed the 4.0% colposcopy threshold.11 We know from the direct typing of cancers from around the world as performed by International Agency for Research on Cancer and Institut Català d’Oncología that HPV 18 and HPV 45 are the second and third most important types, respectively, when cancer is the outcome.17,18 HPV 18 and 45 generally require integration into the host cellular genome to pose a risk for cancer.9,19,20 Our choices of CIN 3+ as our outcome and 5-year risk estimates do not permit observation of the cancer risk visible only in the longest cohorts spanning 15 or more years.6,21,22
Our analysis highlights the importance of partial HPV genotyping for clinical management of abnormal screening results. We still need to assess the value of genotyping for surveillance in different clinical settings (postcolposcopy and posttreatment) and the additional risk stratification of more detailed genotyping.
Supplementary Material
Footnotes
The NCI (M.D., D.E., L.C.C., X.C., J.C.G., N.W., and M.S.) has received cervical screening test results and supplies at no cost to conduct independent evaluations of methods and natural history research. The commercial donors have not participated in the decision to publish or the content. P.E.C. has received HPV tests and assays at a reduced or no cost from Roche, Becton Dickinson, Arbor Vita Corporation, and Cepheid for research.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jlgtd.com).
REFERENCES
- 1.Schiffman M, Wentzensen N. A suggested approach to simplify and improve cervical screening in the United States. J Low Genit Tract Dis 2016;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013;31(suppl 7):H1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Hyun N, Raine-Bennett TR, et al. A cohort study of cervical screening using partial HPV typing and cytology triage. Int J Cancer 2016;139:2606–15. [DOI] [PubMed] [Google Scholar]
- 5.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015;125:330–7. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman M, Glass AG, Wentzensen N, et al. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol Biomarkers Prev 2011;20:1398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castle PE, Shaber R, LaMere BJ, et al. Human papillomavirus (HPV) genotypes in women with cervical precancer and cancer at Kaiser Permanente Northern California. Cancer Epidemiol Biomarkers Prev 2011;20:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castle PE, Solomon D, Wheeler CM, et al. Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol 2008;46:2595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gage JC, Schiffman M, Solomon D, et al. Risk of precancer determined by HPV genotype combinations in women with minor cytologic abnormalities. Cancer Epidemiol Biomarkers Prev 2013;22:1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon D, Nayar R. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. 2nd ed New York: Springer; 2004. [Google Scholar]
- 11.Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun N, Cheung LC, Pan Q, et al. Flexible risk prediction models for left or interval-censored data from electronic health records. Ann Appl Stat 2017;11:1063–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung LC, Pan Q, Hyun N, et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat Med 2017;36:3583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landy R, Cheung LC, Schiffman M, et al. Challenges in risk estimation using routinely collected clinical data: the example of estimating cervical cancer risks from electronic health-records. Prev Med 2018;111:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung LC, Egemen D, Chen X, et al. 2019 ASCCP Risk-Based Management Consensus Guidelines: methods for risk estimation, recommended management, & validation. J Low Genit Tract Dis 2020;24:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The International Agency for Research on Cancer (IARC). Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed September 9, 2019.
- 18.Plummer M, Schiffman M, Castle PE, et al. , Group A. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis 2007;195:1582–9. [DOI] [PubMed] [Google Scholar]
- 19.Wentzensen N, Sun C, Ghosh A, et al. Methylation of HPV18, HPV31, and HPV45 genomes and cervical intraepithelial neoplasia grade 3. J Natl Cancer Inst 2012;104:1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wentzensen N, Wilson LE, Wheeler CM, et al. Hierarchical clustering of human papilloma virus genotype patterns in the ASCUS-LSIL triage study. Cancer Res 2010;70:8578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002;325:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HC, Schiffman M, Lin CY, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011;103:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


