Abstract
Context
According to the 2017 World Health Organization classification, pancreatic neuroendocrine neoplasms (PanNENs) include a new category of pancreatic neuroendocrine tumor, grade 3, which is often difficult to differentiate from pancreatic neuroendocrine carcinoma. However, pancreatic neuroendocrine tumor grade 3 and pancreatic neuroendocrine carcinoma are distinct entities with very different clinical presentation, prognosis, and therapeutic strategies. Recent discoveries on the molecular characteristics of pancreatic neuroendocrine tumors also play an essential role in the pathologic differential diagnosis of PanNENs. In addition, the histopathologic varieties of PanNENs bring in many differential diagnoses with other pancreatic neoplasms, especially acinar cell carcinoma, solid pseudopapillary neoplasm, and ductal adenocarcinoma.
Objective
To provide a brief update of the World Health Organization classification; the clinical, histopathologic, immunohistochemical, and molecular characteristics; and the differential diagnoses and biological behavior of PanNENs.
Data Sources
Analysis of the pertinent literature (PubMed) and authors’ clinical practice experience based on institutional and consultation materials.
Conclusions
The evolving clinical, histopathologic, immunohistochemical, and molecular features of PanNENs are reviewed. Important differential diagnoses with other neoplasms of the pancreas are discussed.
Pancreatic neuroendocrine neoplasms (PanNENs) account for approximately 1% to 5% of all pancreatic neoplasms; however, autopsy studies have shown that the prevalence may be as high as 10%.1–5 The incidence of PanNENs is increasing each year, largely because of the improvement of available diagnostic modalities, such as endoscopic ultrasound, somatostatin receptor scintigraphy, and positron emission tomography.6 During the past couple of decades, PanNENs have undergone multiple nomenclature and classification changes, resulting in confusion between pathologists and clinicians.
The 2017 World Health Organization (WHO) classification defines PanNENs as neoplasms with significant neuroendocrine differentiation and expression of neuroendocrine markers such as synaptophysin and chromogranin.40 The WHO divides PanNENs into well-differentiated neuroendocrine neoplasms (pancreatic neuroendocrine tumors [PanNETs]), poorly differentiated neuroendocrine neoplasms (pancreatic neuroendocrine carcinomas [PanNECs]), and mixed neuroendocrine-nonneuroendocrine neoplasms. The term neuroendocrine implies that these tumors are derived from neural crest cells.1,6 However, it is known that islet cells derive from endoderm, and the neoplastic cells in PanNENs presumably have the same origin.7 In fact, the WHO in 2000/2004 classified these neoplasms as endocrine tumor/carcinoma.6 Despite their embryologic derivation, PanNENs are histologically similar to neural cells. Electron microscopy shows that the neoplastic cells contain dense core granules, and the tumor cells express markers of neuroendocrine differentiation (eg, synaptophysin and chromogranin).1,6 Thus, in 2010, this group of tumors was reclassified as neuroendocrine.
The WHO 2017 classification made a number of changes to the classification of PanNENs. One of the most prominent changes was the introduction of well-differentiated PanNEN grade 3 (G3) to recognize that there have been an increasing number of cases of well-differentiated PanNENs with high proliferation indices (Ki-67 >20% and/or mitotic rate >20/10 high-power fields). Inevitably, this change has led to confusion about the terminology and how to best distinguish PanNET G3 and PanNEC. A second major change to PanNENs in the WHO 2017 classification was the Ki-67 cutoff for PanNET G1. Ki-67 less than 3% cutoff is now used for G1 PanNETs (changed from the previous ≤2% cutoff), rounded to the nearest whole number. A third change in the classification affects the nomenclature used for mixed neoplasms. Mixed neuroendocrine-nonneuroendocrine neoplasm is used to recognize that the mixed neoplasms may occasionally include well-differentiated components and nonneuroendocrine components beside adenocarcinoma. Lastly, recommendations were made for Ki-67 evaluation and reporting, which will be discussed later in this review.
Considering the number of classification changes that have occurred during the last couple of decades, there is confusion between pathologists and clinicians about the nomenclature and grading of PanNENs. Management and prognosis differ widely among these PanNENs, so it is imperative to accurately differentiate these neoplasms and communicate effectively with clinicians. This review will discuss the current classification update of PanNENs, their diagnostic challenges, and their clinical, histopathologic, ancillary, and molecular features.
CLINICAL FEATURES
The WHO 2017 classification divides PanNENs into PanNETs and PanNECs, which are genetically distinct entities with different clinical presentations, prognoses, and therapies (Table 1).8,9 Pancreatic neuroendocrine tumors affect patients of a wide age range, with most occurring in the third to sixth decade. The mean age at diagnosis is 50 to 56 years,9–11 and both sexes are affected equally.1,3,5 High-grade PanNETs, however, have a predilection for males, with a mean age of diagnosis of 52 years.11 The majority of PanNETs are sporadic, but about 10% to 20% of cases are found in association with hereditary syndromes.5 Pancreatic neuroendocrine neoplasms are present in 20% to 70% of patients with multiple endocrine neoplasia type 1 (MEN1), up to 20% of von Hippel–Lindau (VHL) syndrome patients, approximately 1% of tuberous sclerosis complex (TSC) patients, and up to 10% of neurofibromatosis type 1 (NF1) patients.12 Metastases are seen in 34% of low-grade PanNETs and 81% of high-grade PanNETs.11 Elevated serum chromogranin A (CgA) levels have been found in 50% to 100% of patients with PanNETs and have been shown to correlate with tumor burden and metastasis for PanNETs.13–15 However, because of CgA’s low specificity and low sensitivity, its use is limited in monitoring therapy response.4,14,16
Table 1.
Clinicopathologic Comparison of Pancreatic Neuroendocrine Tumors (PanNETs) and Pancreatic Neuroendocrine Carcinomas (PanNECs)
| PanNETs | PanNECs | |
|---|---|---|
| Age, mean, y | 50–56 | 59–65 |
| Gender, M:F | 1:1 | 7:5 |
| Distribution in pancreas | Tail > body | Head |
| Positive imaging modalities | Somatostatin receptor scintigraphy | Positron emission tomography |
| Serum findings | Elevated CgA | Elevated NSE |
| Metastasis, % | 34 (G1, G2) | 95–100 |
| 81% (G3) | ||
| Ki-67, % | 0–10.2 (G1, G2) | 50–70 |
| <55% (G3) | ||
| Clinical history | ||
| Course | Indolent | Aggressive |
| History of PanNET G1, G2 | May be present | Not present |
| Histology | ||
| Differentiation | Relatively well | Poor |
| Necrosis | Rare | Common |
| Mitosis and apoptotic cells | Few | Abundant |
| Non-NE components | No | May be present |
| Immunohistochemistry stains | Loss of DAXX/ATRX | Abnormal expression of TP53, loss of RB |
| Molecular findings | MEN1, DAXX/ATRX, mTOR mutations | TP53, RB1, KRAS, SMAD4 mutations |
| Recommended therapy | Somatostatin analogues, sunitinib/everolimus | Alkylating agent, antimetabolite, platinum-based chemotherapy |
Abbreviations: ATRX, a-thalassemia/mental retardation syndrome X-linked;CgA, chromogranin A;DAXX, death domain-associated protein;G1, grade 1;G2, grade 2; G3, grade 3;KRAS, Kirsten rat sarcoma;MEN1, multiple endocrine neoplasia type 1;mTOR, mammalian target of rapamycin; NE, neuroendocrine;NSE, neuron-specific enolase;RB, retinoblastoma protein;SMAD4, mothers against decapentaplegic homolog 4;TP53, tumor protein 53.
Pancreatic neuroendocrine carcinomas are incredibly rare, accounting for approximately 2% to 3% of all PanNENs.17 They are aggressive tumors, and 95% to 100% of patients develop metastatic disease.9,10,18,19 Because of the rarity of PanNECs, most of our understanding comes from case reports and small series studies. Review of the literature shows that there is a slight male predilection, affecting men mostly in their sixth to seventh decade of age, with a mean age of diagnosis of 59 to 65 years.5,9,11,18,19 Although CgA is a useful marker for PanNETs, it is less useful for PanNECs, especially small cell carcinoma.13,14 This may be because of the lower number of large dense-core granules within small cell carcinoma, leading to reduced release of CgA.13 Instead, neuron-specific enolase (NSE) has a better sensitivity for PanNECs; however, its use is still limited by its low specificity.13,15 A comparison of clinicopathologic characteristics between PanNETs and PanNECs is summarized in Table 1.
Pancreatic neuroendocrine neoplasms can be further divided into functional and nonfunctional neoplasms based on the presence of symptoms and clinical presentation. Functional PanNENs account for 10% to 30% of PanNENs and are defined by the production of inappropriate hormone and a clinical syndrome related to that hormone (Table 2).6,15,20 Because of the presence of a clinical syndrome, these PanNENs tend to be diagnosed earlier than nonfunctional PanNENs. The mean age of diagnosis is 55 years for functional and 59 years for nonfunctional PanNENs.3,21 The secreted hormone characterizes the clinical presentation, and the clinical diagnosis corresponds to the hormone produced as well (eg, insulinoma). Insulinomas (40%–60%) and gastrinomas (20%%–50%) are the most common, followed by VIPoma, glucagonoma, and somatostatinoma.22 Specific functional PanNETs carry prognostic complications. For example, insulinomas have a more indolent course.1
Table 2.
Clinical Syndromes of Functional Pancreatic Neuroendocrine Tumors
| Secreted Hormone | Syndrome | |
|---|---|---|
| Insulinoma | Insulin | Whipple triad: symptoms of hypoglycemia, low plasma glucose levels (<54 mg/mL), relief of symptoms with administration of glucose |
| Gastrinoma | Gastrin | Zollinger-Ellison syndrome: gastric or duodenal ulcers, diarrhea, vomiting |
| VIPoma | Vasoactive intestinal peptide | Verner-Morrison syndrome: voluminous watery diarrhea, electrolyte imbalances such as hypokalemia, achlorhydria, and metabolic acidosis |
| Glucagonoma | Glucagon | Skin rash that begins in the groin and spreads to the distal extremities (necrolytic migratory erythema), glucose intolerance, anemia, diarrhea |
| Somatostatinoma | Somatostatin | Diabetes mellitus, hypochlorhydria, cholelithiasis, diarrhea |
| Carcinoid | Serotonin | Carcinoid syndrome: flushing, diarrhea, nausea/vomiting, abdominal pain |
| ACTH-oma | ACTH | Cushing syndrome: weight gain, moon face, buffalo hump, easy bruising, slow healing, striae |
Abbreviation: ACTH, adrenocorticotropic hormone.
Nonfunctional PanNENs account for approximately 60% to 90% of all PanNENs.15,21 A critical consideration is that nonfunctional PanNENs are defined by lack of hormone-related symptoms rather than lack of hormone production. Thus, nonfunctional PanNENs can either produce or not produce hormones. The lack of symptoms in nonfunctional PanNENs that do release hormones has been attributed to unregulated secretion without intracellular buildup of larger levels of hormone.3 Because there is no clinical presentation, nonfunctional PanNENs are often found incidentally on imaging. Patients may present with symptoms due to the tumor mass, such as abdominal pain (40%–60%), weight loss, intestinal bleeding, obstruction, and jaundice.15,20 Because nonfunctional PanNENs are diagnosed later, they are often large (about 70% >5 cm) and found at an advanced stage (60%–85% with liver metastases).15,20 Nearly all PanNECs are nonfunctional.9,18
There are multiple available imaging modalities that are used to screen for PanNENs. The most common initial screening tool is contrast-enhanced computed tomography (CT). On CT, PanNENs appear as hypervascular masses that are often round and solid, though about 10% do present as cystic lesions with peripheral enhancement.4 Computed tomography has a sensitivity of about 63% to 82% and a specificity of about 83% to 100% for PanNETs larger than 2 cm.4 Magnetic resonance imaging is considered a second-line method for PanNEN detection. It has a higher sensitivity than CT and can detect PanNENs less than 2 cm as well as small liver metastases, but has a lower specificity.4
Somatostatin receptor scintigraphy is another imaging modality that is used, especially when a functional PanNEN is suspected but not seen on CT or magnetic resonance imaging. Many PanNENs, including nonfunctional PanNENs, express high levels of somatostatin receptors, and radiolabeled octreotide is used to visualize these neoplasms. Sensitivity can be as high as 75% to 100%; however, insulinomas and PanNECs have low levels of somatostatin receptor expression and are not usually detected.4 Positron emission tomography cannot detect most PanNETs because of their low metabolic activity, but PanNECs have increased metabolic rate with 18F-fluorodeoxyglucose uptake and can be visualized on positron emission tomography. Activity of 18F-fluorodeoxyglucose has been correlated with progression and increased mortality.4 A recently approved diagnostic tool is 68Ga-DOTATATE, a somatostatin analog labeled with a gallium tracer, which has been shown to have higher sensitivity than 18F-fluorodeoxyglucose for neuroendocrine tumors.23,24 A combination of positron emission tomography with 68Ga-DOTATATE and CT is becoming the preferred method over somatostatin receptor scintigraphy.4
Endoscopic ultrasonography can provide a high-resolution image of the pancreas. Studies have shown that it could detect tumors smaller than 2 cm that are often missed on CT. Pancreatic neuroendocrine tumors appear as well-defined hypoechoic homogenous lesions.4 The majority are solid, but they occasionally may be cystic. Peripancreatic abnormalities, such as local lymph node and vascular invasion, can be detected by endoscopic ultrasonography. Endoscopic ultrasonography may also be used to tattoo small lesions and for fine-needle aspiration to acquire tissue for histologic confirmation prior to surgery. Although endoscopic ultrasonography has high sensitivity that is equal to that of CT and magnetic resonance imaging, it is limited by the endoscopist and the limited window of the pancreatic tail.
HISTOPATHOLOGY
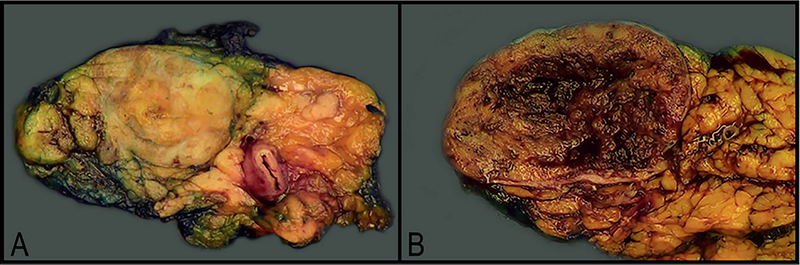
Grossly, PanNENs are usually solid tumors in the body or tail of the pancreas, although they may involve the entire pancreas.17 Tumors range from less than 0.5 to 30 cm and are often softer than the adjacent normal pancreas. Functional tumors are often found at a smaller size, typically less than 2 cm. Microadenomas (<0.5 cm), on the other hand, are nearly always nonfunctional.17 Tumors larger than 3 cm tend to be more aggressive, though severity of symptoms does not correlate with size. Pancreatic neuroendocrine tumors tend to be solitary, well-circumscribed tumors that vary in color from pink-red to tan-brown (Figure 1, A). Occasionally, they may be firm and sclerotic, appearing white-gray to yellow.17 About 5% of tumors are cystic (Figure 1, B); these also tend to be larger.10 Pancreatic neuroendocrine carcinomas are typically solid, relatively circumscribed, and lobulated tumors that are tan-red to yellow.9 They are often hemorrhagic and occasionally can be necrotic.3,9 One study showed that PanNECs occur more often in the head of the pancreas, unlike PanNETs, which often occur in the body and tail. Pancreatic neuroendocrine carcinoma sizes range from 2 to 18 cm (median, 4 cm).5,9
Figure 1.
Well-differentiated low-grade pancreatic neuroendocrine tumors (PanNETs), macroscopic: solitary tumors that are well circumscribed and range in color from pink-red to tan-brown. A, Most tumors are solid. B, A small fraction can have cystic degeneration and hemorrhage. These 2 tumors were microscopically consistent with grade 2 PanNETs.
Low-Grade Well-Differentiated PanNENs (PanNETs, G1 and G2)
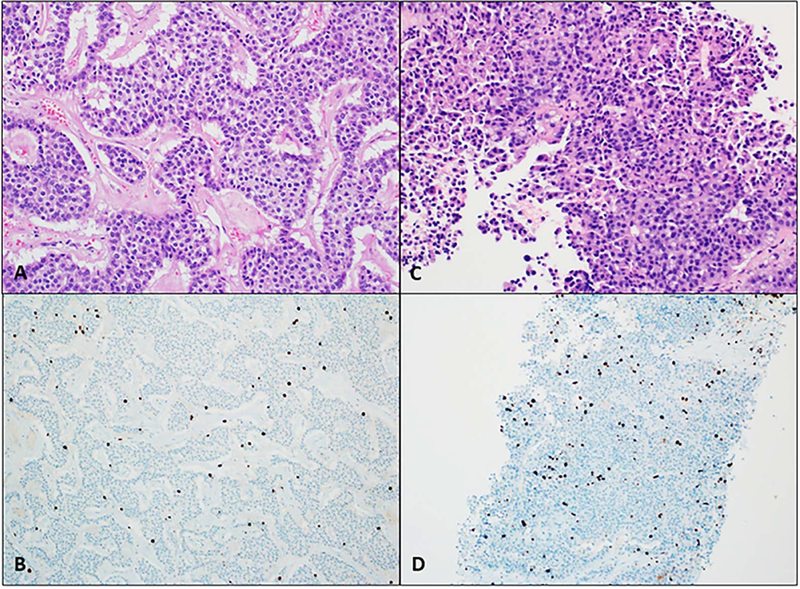
Well-differentiated PanNENs have a variety of histopathologic appearances. Differentiation refers to how well the tumor resembles the original structures, which for PanNETs are the islets of Langerhans. Well-differentiated PanNENs typically have an organoid architecture with patterns including solid, trabecular, glandular, and tubuloacinar (Figure 2).3 Cells are small to medium in size, with finely granular, eosinophilic cytoplasm. Nuclei are typically round or oval and are centrally located with finely stippled chromatin that gives the classic salt-and-pepper neuroendocrine appearance. The stroma is usually richly vascularized with variable fibrosis. There is minimal atypia and necrosis is not typically present in low-grade PanNETs (G1 and G2).
Figure 2.
Low-grade well-differentiated pancreatic neuroendocrine tumors (PanNETs). A, Grade 1 PanNET: small cells with finely granular cytoplasm and classic salt-and-pepper chromatin, arranged in a trabecular pattern. C, Grade 2 PanNET. B and D, Ki-67 less than 3% and 3% to 20% classifies these PanNETs into grades 1 and 2, respectively (hematoxylin-eosin, original magnification ×200 [A and C]; original magnification ×100 [B and D]).
Although the overall architecture and cytomorphologic features of functioning and nonfunctioning PanNETs are the same, there are a few features that can differentiate between types of functioning PanNETs. Amyloid is a unique finding that may be present in insulinomas. Somatostatinomas tend to have a more glandular architecture and will occasionally have psammoma bodies.3 Dense stromal fibrosis has also been seen in the rare serotonin-secreting PanNETs.25
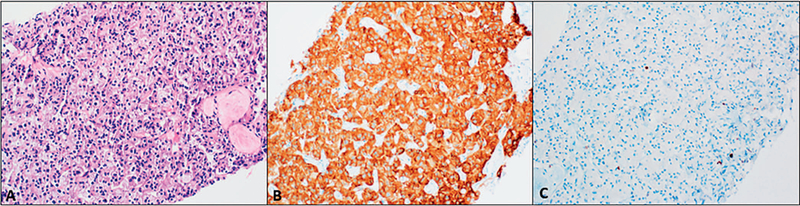
There are a few rare variants of PanNETs that often pose a diagnostic challenge. Clear cell variant can be found in up to 60% of PanNETs associated with VHL and is characterized by small to medium-sized cells with clear, multivacuolated cytoplasm and round nuclei with salt-and-pepper chromatin (Figure 3).6,26 These cells may comprise 30% to 95% of the tumor and are often accompanied by central hemorrhage that is separated by thin-walled vessels.26 This variant is particularly challenging as it resembles clear cell renal cell carcinoma, so metastasis to the pancreas needs to be ruled out.
Figure 3.
Clear cell variant of pancreatic neuroendocrine tumors (PanNETs). A, Small cells with clear, multivacuolated cytoplasm. Salt-and-pepper chromatin may be difficult to appreciate. B, Strong, diffuse synaptophysin staining supports neuroendocrine differentiation. C, Low Ki-67 less than 3% supports PanNET grade 1 (hematoxylin-eosin, original magnification ×200 [A]; original magnification ×200 [B and C]).
Lipid-rich variant is commonly mistaken for clear cell variant and is characterized by cells with foamy, micro-vesicular cytoplasm. The cells mimic adrenal cortical cells and foamy macrophages; however, electron microscopy confirms that the cytoplasmic vacuoles are filled with lipid. The nuclear features are typically similar to those of other PanNETs, with round nuclei, finely granular cytoplasm, and salt-and-pepper chromatin. However, some nuclei may be pyknotic and partially obscured by intracytoplasmic vesicles, and the classic neuroendocrine chromatin is not as evident. Most cases have vague compartmentalization by delicate vasculature. Unlike the clear cell variant, lipid-rich variant is not significantly associated with VHL.27
Other rare variants include oncocytic, rhabdoid, and pigmented black neuroendocrine neoplasm. The oncocytic variant is composed of large cells with granular, eosinophilic cytoplasm and is found in approximately 7% of PanNENs.17,28 The rhabdoid variant is characterized by cells with abundant cytoplasm and eosinophilic globular inclusions that displace the nuclei toward the periphery.29 Lastly, pigmented black neoplasms are PanNETs with neoplastic cells that contain brown-black pigment, and morphologically these neoplasms mimic metastatic melanoma.30 Pancreatic neuroendocrine tumor variants are difficult to diagnose on the basis of morphology alone, and immunohistochemistry is particularly beneficial for demonstrating their neuroendocrine differentiation.
High-Grade Well- and Poorly Differentiated PanNENs (PanNET G3 and PanNEC)
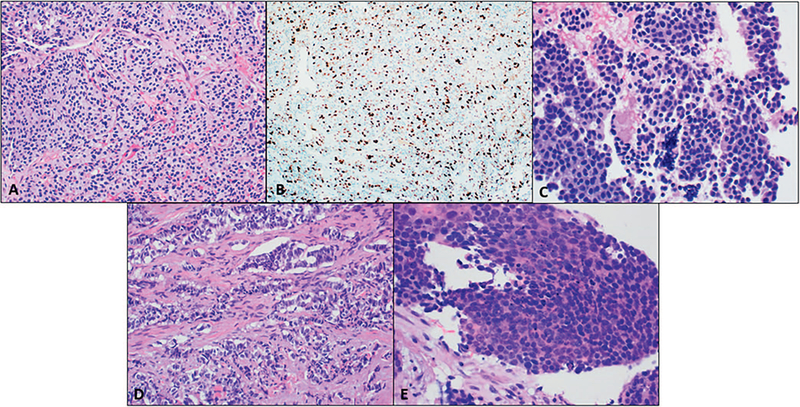
The new WHO 2017 classification acknowledges that well-differentiated PanNENs may be classified as high grade (G3) and are distinct from poorly differentiated PanNECs. Numerous studies have shown that PanNET G3 and PanNEC are distinct entities with different clinical outcomes.1,6,9,19 The entity that is now considered well-differentiated PanNET G3 has many morphologic similarities to low-grade PanNETs. They tend to have a similar organoid architecture with neoplastic cells that have abundant granular cytoplasm and salt-and-pepper chromatin (Figure 4, A through C).11 Studies have shown that high-grade PanNETs have a more indolent course and a poorer response to platinum-based chemotherapy compared with poorly differentiated PanNECs.1,6,11,19 To further support that these 2 entities are separate, distinct molecular pathways have been implicated, which will be discussed later. Because clinical management differs, it is important to accurately differentiate these neoplasms.
Figure 4.
Morphologic resemblance among high-grade pancreatic neuroendocrine neoplasms. A through C, Well-differentiated pancreatic neuroendocrine tumor (PanNET), grade 3. B, Ki-67 more than 20% classifies this PanNET into grade 3. D, Poorly differentiated pancreatic neuroendocrine carcinoma (PanNEC), large cell carcinoma. E, Poorly differentiated PanNEC, small cell carcinoma (hematoxylin-eosin, original magnification ×200 [A, C, D, and E]; original magnification ×100 [B]).
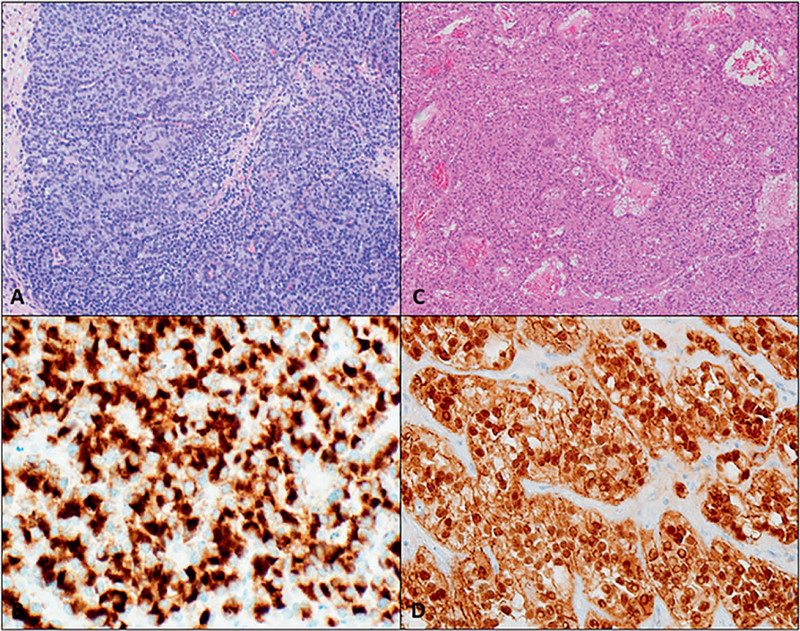
Poorly differentiated PanNENs are always considered high grade and are classified as PanNECs in the WHO 2017 classification. Pancreatic neuroendocrine carcinomas are composed of highly atypical neoplastic cells and can be further subclassified by their cell size into small cell and large cell carcinoma (Figure 4, D and E). Both the small cell and the large cell variants resemble respective carcinomas of the lung. The small cell variant is generally composed of diffuse sheets of small cells with molding and hyperchromatic nuclei with inconspicuous nucleoli and scant cytoplasm, whereas large cell has moderate to abundant eosinophilic cytoplasm with vesicular nuclei and prominent nucleoli. Large cell (61%) is more common than small cell (39%).9 In general, the neoplastic cells in PanNECs are pleomorphic and the classic salt-and-pepper chromatin is not as evident. Geographic necrosis as well as perineural and angiolymphatic invasion are often present in PanNECs.9,19
Histologically, high-grade PanNET G3 can be difficult to differentiate from PanNEC (Figure 4). Well-differentiated PanNET may rarely have marked nuclear pleomorphism, diffuse infiltrative patterns, as well as necrosis, making it difficult to separate from PanNECs.9 There are several critical factors to assess when distinguishing between high-grade well-differentiated and poorly differentiated PanNENs, including proliferative rate, organoid growth pattern, and extent of necrosis (Table 1).19 Some studies have shown that PanNECs on average have a significantly higher proliferative rate, with a Ki-67 rate typically about 50% to 70%.9 Pancreatic neuroendocrine carcinomas often have abundant mitosis and apoptotic bodies as well as tumor necrosis. Coexistence of a conventional carcinoma, such as adenocarcinoma, is also highly suggestive of PanNECs; well-differentiated PanNETs rarely combine with conventional carcinoma elements.1 On the other hand, coexistence of a low-grade PanNET supports a diagnosis of PanNET G3 because PanNECs do not arise from PanNETs. Additionally, a history of a low-grade PanNET on a previous biopsy also supports a high-grade PanNET over a PanNEC.1
Despite the aforementioned histologic clues to differentiate high-grade PanNETs G3 from PanNECs, Tang et al11,19 reviewed 33 cases of high-grade PanNENs and found that morphology alone was not enough to reach a definitive diagnosis in 61% of cases. Clinical presentation and diagnostic workup are critical to distinguish challenging high-grade PanNENs. High-grade PanNET G3 is more likely to be found incidentally and more likely to be functional than PanNEC. This can partly be attributed to the fact that PanNECs grow more rapidly and are more likely to present with obstructive symptoms like abdominal pain, jaundice, and weight loss. Pancreatitis and diabetes are also more common in PanNECs. Diagnostic imaging differs, as previously discussed. Genetic studies have shown that PanNETs have death domain–associated protein (DAXX) and α-thalassemia/mental retardation X-linked (ATRX) gene mutations, which can be demonstrated as a loss of DAXX/ATRX immunohistochemical staining in up to 43% of high-grade PanNETs.1,31,32 Pancreatic neuroendocrine carcinomas, on the other hand, can have mutations in TP53, RB1, KRAS, and SMAD4, and the abnormal expression of p53 and/or RB can be found in 92% of PanNECs.1,5
Large cell neuroendocrine carcinomas have variable growth patterns, including diffuse, organoid/nested, and trabecular. Occasionally, pseudopapillae may be seen, especially in the peripheries of necrotic areas, and peripheral palisading may occur.9,19 Rarely, they may have a glandular architecture. Apoptotic figures and mitotic figures are abundant, but tend to be less prominent than in small cell carcinomas. Cells are round to polygonal with nuclei with vesicular chromatin and prominent nucleoli.9,11,19
Small cell neuroendocrine carcinomas predominantly have diffuse, sheetlike growth patterns. Scattered tumor giant cells with hyperchromatic bizarre nuclei or rosettes have been reported. Tumor cells are relatively small, with high nucleus to cytoplasm ratio, hyperchromatic nuclei with inconspicuous nucleoli, gritty chromatin, and nuclear molding.9 There is often stromal desmoplasia, and background necrosis is invariably present, ranging from small foci to geographic necrosis. These tumors tend to have more than 50 mitoses per 10 high-power fields and often have higher mitotic rates and Ki-67 proliferative index compared with large cell carcinoma.
Mixed Neuroendocrine-Nonneuroendocrine Neoplasms
Mixed neuroendocrine-nonneuroendocrine neoplasm is defined as a mixed neoplasm with both neuroendocrine and nonneuroendocrine components, where each component must account for more than 30% of the cell population. Typically, the neuroendocrine component is mixed with a ductal adenocarcinoma, but it may occasionally be mixed with acinar cell carcinoma.33 Usually, all of the involved components are high-grade.5
Proliferation Indices
Because differentiating low-grade from high-grade PanNENs is largely dependent on the proliferation indices, reproducibility of counting mitoses and Ki-67–positive cells is necessary. The WHO 2017 classification made recommendations for Ki-67 and mitosis reporting, stating that the Ki-67 index should be based on counting at least 500 cells in higher nuclear labeling areas (“hot spots”) and that mitoses in 50 high-power fields should be counted. For discrepancies between Ki-67 index and mitotic index grading, the higher grade is assigned. Lastly, “eyeballing” is discouraged and manual counting of printed images is recommended.
Discordant mitotic and Ki-67 indices can occur in up to a third of cases.34 Many studies have demonstrated that assigning the higher grade in cases of discordant mitotic index and Ki-67 index does have a significant difference in prognoses.6,34 In the majority of these cases, the Ki-67 index is higher because mitoses tend to decrease over time from resection to tissue fixation. On the other hand, time to fixation does not affect Ki-67 as it labels cells from mid-G1 to S and G2 phases.6
Some issues that have made reproducibility difficult include identification of true mitoses from mimics, non-specificity of Ki-67 stain, and interlaboratory and intralaboratory variation. The biggest mitotic mimicker is apoptotic debris and pyknotic nuclei, and Ki-67 can be positive in intratumoral lymphocytes as well as endothelial cells.6,34 Some authors recommend using phosphohistone H3 immunohistochemical stain to separate pyknotic nuclei from true mitoses and identify hot spots.1,6 Lastly, fixation and processing affects the staining quality, and currently, there is no standardization of staining.34
Although the WHO 2017 classification has recommendations, the method of Ki-67 counting is still a subject of much discussion. Counting at least 500 cells is time consuming and not entirely practical in busy services. However, eyeballing leads to poor reproducibility.6,34 Automated systems have been explored, but limitations include high costs and operator experience. As the WHO 2017 classification suggests, many studies have opted in favor of manual counting of a printed image as the most reproducible and accessible method.6
Metastases
Metastatic PanNENs are common. Metastases are seen in more than 50% of PanNETs and up to 100% of PanNECs, and most often involve the liver and lymph nodes.1,9,10,11 One study has shown that grade discrepancies between the primary tumor and metastasis occur in up to 55% of cases, and patients have a poorer prognosis when the liver metastasis has a higher grade than the primary tumor. Therefore it is recommended to regrade metastatic deposits with mitotic counts and Ki-67.6,35
It is challenging to identify the primary tumor in neuroendocrine metastases because there is significant morphologic overlap among neuroendocrine neoplasms from the pancreas, other gastrointestinal sites, and pulmonary sites. Immunohistochemical stains are recommended for diagnosis, especially in the context of a pulmonary nodule or no clinical history. A recent study found that a 3-marker panel (TTF1, CDX2, and ISL1) has relatively high accuracy (82%) in separating metastatic NETs into 3 main primary sites: pancreas/rectum, small intestine, and lung.36 Another study has shown that a combination of Pax8, NESP55, Islet 1, and PDX1 is the most specific for PanNENs.6 TTF1 positivity in isolation is a fairly specific marker that favors pulmonary primary, but it is not sensitive.6 Molecular methods have also been used to confirm the primary tumor, such as reverse-transcription polymerase chain reaction molecular cancer classifier (92-gene assay); however, this is a costly method and fresh-frozen tissue is not always available.6 Fluorescence in situ hybridization is another possible tool, as studies6 have shown that PanNEN liver metastases were positive for alternative lengthening of telomeres in 56% of cases, whereas other gastrointestinal neuroendocrine neoplasms were positive for alternative lengthening of telomeres in only 4% of cases.
Differential Diagnosis
Because of the variable architecture and cytomorphologic features that are possible in PanNENs, there is a large differential, and here we will address some of the neoplasms that are more commonly mistaken for PanNENs. The most common entities with overlapping histopathologic features include acinar cell carcinoma, solid pseudopapillary neoplasm, and pancreatic ductal adenocarcinoma (Figure 5).
Figure 5.
Morphologic mimickers of pancreatic neuroendocrine neoplasms. A and B, Acinar cell carcinoma: relatively uniform cells forming acinar architecture with granular, eosinophilic cytoplasm. B, Trypsin immunohistochemical stain is strongly positive. C and D, Solid pseudopapillary neoplasm: sheets of polygonal cells with degenerative changes. D, The tumor cells are diffusely positive for nuclear β-catenin (hematoxylin-eosin, original magnification ×100 [A and C]; original magnification ×400 [B and D]).
Acinar cell carcinomas are most commonly associated with an acinar architectural pattern, but like PanNENs, they can have a variety of patterns that overlap with PanNENs, including trabecular, glandular, and solid. Furthermore, PanNENs may also display acinar formation.17 Microscopically, most acinar cell carcinomas have a loose, acinar to clustered arrangement (Figure 5, A). The neoplastic cells have round nuclei with prominent, cherry-red nucleoli, and significant granular, eosinophilic cytoplasm. However, some acinar cell carcinomas can have inconspicuous nucleoli and focal expression of neuroendocrine markers, and thus they are probably the most relevant mimickers of PanNENs. More importantly, acinar cell carcinomas are positive for trypsin (Figure 5, B), BCL10, and chymotrypsin, which are negative in PanNENs.10,33,37
Solid pseudopapillary neoplasms are most commonly seen in young females and will not produce a clinical hormone syndrome. They are usually large tumors, found in the body and tail of the pancreas. Microscopically, they have a degenerative papillary appearance, with foamy histiocytes and broad, hyalinized to myxoid septa. The neoplastic cells typically have oval nuclei with grooves, fold, or clefts (Figure 5, C). Blood and necrosis are common. Solid pseudopapillary neoplasms are positive for CD56, NSE, and synaptophysin, which can lead to a misdiagnosis of neuroendocrine tumor. However, they are positive for β-catenin and are negative for chromogranin (Figure 5, D).17
Some PanNENs have marked nuclear atypia, and can sometimes be mistaken for pancreatic ductal adenocarcinomas.17 Microscopically, pancreatic ductal adenocarcinomas often have glandular architecture, but can have single-cell infiltration, and the neoplastic cells have irregular nuclear chromatin and prominent nucleoli. Overall, pancreatic ductal adenocarcinoma cells tend to be more pleomorphic than those in PanNENs. Pancreatic ductal adenocarcinomas often have necrosis and a high mitotic rate, which can make them difficult to distinguish from PanNECs.9
IMMUNOHISTOCHEMISTRY AND MOLECULAR STUDIES
Immunohistochemical stains are useful for demonstrating the neuroendocrine features of PanNENs. Pancreatic neuroendocrine neoplasms are positive for synaptophysin, chromogranin, NSE, CD56, and CD57. More than 95% of PanNENs express synaptophysin and/or CgA.17 Of these neuroendocrine markers, CgA is the most sensitive and will stain apically and focally; however, it is not specific. Synaptophysin stain is generally diffuse and strong, but will be weaker in PanNECs compared with PanNETs.3 Other useful stains include CK, especially CK8/18/CAM5.2, and E-cadherin.
Specific hormone markers are available to demonstrate hormone production, which may be useful for functional PanNENs. However, utility in patients without a clinical syndrome is uncertain. Hormone production can occur in patients without resulting in a clinical syndrome, but the PanNEN is not classified as a functional tumor in these cases. Nevertheless, hormone markers are available for insulin, glucagon, somatostatin, gastrin, VIP, polypeptide, and serotonin, and the staining and distribution of these markers are unrelated to symptom severity. Staining can vary from focal to diffuse, and multiple hormones or peptides are frequently detected within the same tumor.
There have been 3 main molecular pathways implicated in PanNETs: MEN1 inactivation (44%), DAXX/ATRX mutation and/or loss (43%), and mammalian target of rapamycin (mTOR) pathway alterations (14%), which include PTEN, TSC2, and PIK3CA.6 Those with MEN1 and DAXX/ATRX alterations carry a better prognosis overall, whereas those with mTOR mutations have a worse prognosis.15,38 MEN1 is the most common mutation found in hereditary PanNETs. VHL, NF1, and TSC1 diseases have a poorer prognosis compared with MEN1 tumors.38
MEN1 gene is a tumor-suppressor gene, located on chromosome 11q13, that encodes for menin, which promotes the expression of cell cycle inhibitors and prevents β-cell proliferation.6,15 MEN1 mutation is found in approximately 21% of sporadic PanNETs.6,15,38 Some studies15 suggest that there may be a tumor suppressor gene distal to the menin gene involved with tumorigenesis of PanNETs. Recent studies8 have also shown that disruption of tumor suppression in MEN1 is dependent on the PI3K-Akt-mTOR pathway, and is mediated by PHLDA3, an inhibitor of Akt. DAXX/ATRX, on the other hand, is important for telomeric chromatin stabilization, and dysregulation results in unlimited cell cycling of neoplastic cells.6
VHL gene impairment is also associated with nearly 25% of sporadic PanNETs and is associated with a worse prognosis.38 Previous literature38 states that somatic mutations of the VHL gene are rare. However, Schmitt et al38 found that 6% (n=37) of PanNETs had hypermethylation in the VHL promoter, and fluorescence in situ hybridization demonstrated that 18% (n = 78) of their tumors had a VHL gene deletion. It has been proposed that VHL, as well as NF1 and TSC1, is important in hypoxia signaling, which has been shown to have a role in sporadic PanNETs. The pVHL protein is found in a protein complex that marks hypoxia inducible factor 1 (HIF1) for proteasomal degradation, so a decrease of pVHL results in a buildup of HIF1 and subsequently increased transcription of hypoxia-inducible genes.38
The genes implicated in PanNETs are distinct from those associated with PanNECs and mixed neuroendocrine-nonneuroendocrine neoplasms. The molecular alterations in PanNECs and mixed neuroendocrine-nonneuroendocrine neoplasms are more similar to adenocarcinomas, which are associated with microsatellite instability, and TP53, KRAS, APC, BRAF, and RB mutations.6 Conversely, DAXX/ATRX mutations are not found in PanNECs. TP53 and RB are tumor suppressor genes that are important for cell division and apoptosis, and whereas inactivation of the TP53 and Rb/p16 pathways has been implicated in PanNECs, no such relationship has been found in PanNETs.1,9,19 Abnormal TP53 and RB gene expression can be demonstrated by immunohistochemical stains in PanNECs, either as a strong nuclear expression of TP53 due to nuclear accumulation caused by the gene mutation or as a loss of RB expression.5
Finally, whole-genome sequencing is continuously discovering new gene mutations that may be involved with tumorigenesis of PanNETs. Novel germline mutations in DNA repair genes such as MUTYH, CHECK2, and BRCA2 have been discovered in sporadic PanNETs.15 Studies on promoter hypermethylation, resulting in silencing of tumor suppressor genes, have shown that RASSF1A, p16/INK4A, and O6-MGMT may also be involved with PanNETs tumorigenesis.15 Despite an increased understanding of PanNETs genetics, there is no evidence that identifying specific genetic changes impacts management. Thus, genetic analysis is not currently recommended.15
PROGNOSIS AND TREATMENT
Most PanNENs follow an indolent course, and adverse prognostic factors include tumor size, functional status, metastasis, and tumor grade. A Ki-67 greater than 5% has also been associated with a worse prognosis.39 For low-grade PanNETs, the overall 10-year survival is 60% to 70%.10 Small, low-grade PanNETs that are less than 2 cm in size have a 10-year survival of more than 95%.10 High-grade PanNENs, however, carry a poor prognosis. High-grade (G3) PanNETs have a 5-year survival of 22%, compared with the 5-year survival of 61% in G2 PanNETs. Pancreatic neuroendocrine carcinomas have a 5-year survival of 17%.10,18 Because of the wide spectrum of presentation and prognoses, a multidisciplinary approach is recommended for every case.
The standard treatment approach in all PanNET cases is surgery, either exploratory laparotomy or a laparoscopic or robotic approach. It is especially recommended for cases associated with tumor complications such as bleeding and bowel obstruction.15 For small (<2 cm), nonfunctional, low-grade PanNETs, surgery may be delayed for a wait-and-see approach because of their indolent nature. However, some studies have shown that nodal metastases occur in up to 30% of these patients, so surgical resection has been advocated regardless.15 An important consideration prior to surgery is the functionality of the neoplasm. Surgery is recommended for all functional tumors, and it is also recommended to reduce symptoms via medical management prior to surgery.15 Additional drug therapy is recommended in PanNENs with aggressive and extensive disease.
There are multiple drug therapies available for PanNETs. Long-acting somatostatin analogues, such as octreotide, lanreotide, and pasireotide, have been considered the mainstay therapy for PanNETs. The majority of PanNETs have a high expression of somatostatin receptors, and somatostatin both inhibits the release of neuroendocrine hormones and has a direct antiproliferative effect on the tumors.6,14,15 However, the tumor response to somatostatin analogues decreases in higher-grade PanNETs, and a significant reduction in effectiveness has been noted in PanNETs with Ki-67 more than 5%.6 Somatostatin analogues are not recommended for PanNECs.6,14,15
For more aggressive PanNETs, cytotoxic chemotherapy and molecular therapy are also available. A combination of streptozotocin and fluorouracil is recommended in PanNETs with Ki-67 more than 10%, as well as for tumors that progressed while treated with somatostatin analogues.15 Temozolomide has also been found to be beneficial for advanced PanNETs, especially when combined with other agents.15 Molecular therapies including everolimus, an mTOR inhibitor, and sunitinib, a tyrosine kinase inhibitor, have been found to be effective against advanced PanNETs G1 and G2.6,14,15,20 Well-differentiated PanNENs with a Ki-67 less than 55% have a decent response to cytotoxic chemotherapy and molecular therapy, whereas PanNET G3 with Ki-67 more than 55% is less likely to respond and may be more likely to response to platinum-based chemotherapy.6,15 However, well-differentiated PanNENs, including high grade, have a poor response to platinum-based chemotherapy. In fact, one review found that only an average of 9% of PanNET G3 responded to platinum-based therapy.23
Unlike PanNETs, PanNECs have a favorable response to platinum-based chemotherapy.1,6,9,15 A combination of alkylating agents and antimetabolites along with the platinum-based chemotherapy is recommended, especially in tumors with Ki-67 more than 55%.1 At this time, both small cell and large cell PanNECs are managed similarly, but some data suggest that large cell carcinomas do not respond to platinum-based chemotherapy as well as small cell carcinomas.18 An optimal chemotherapy regimen has not been determined for large cell PanNECs.
Metastases of PanNENs are common in the liver. Localized metastases are handled surgically, but unresectable cases may need additional radio/chemotherapies.1 Somatostatin analogues are recommended in advanced PanNETs, especially those with multiple liver metastases.1,15 Metastatic PanNECs are commonly treated with platinum-based chemotherapy.6
CONCLUSION
Pancreatic neuroendocrine neoplasm continues to be a challenging entity to diagnose. The WHO 2017 classification added well-differentiated high-grade PanNETs G3 to acknowledge that there is a distinct entity with high proliferation indices separate from PanNECs. Pancreatic neuroendocrine tumors G3 have the same molecular mutations as low-grade PanNETs and are clinically and molecularly distinct from PanNECs. They respond to the same chemotherapy regimen as low-grade PanNETs, but carry a worse prognosis than PanNETs G1 and G2 and a better prognosis than PanNECs. Because the prognoses and therapeutic strategies differ so drastically between PanNET G3 and PanNEC, it is imperative to accurately distinguish the 2 for proper clinical care.
Acknowledgments
This manuscript was supported by the National Cancer Institute of the National Institutes of Health under award number K08CA234222 (Dr Shi) and funding from the Department of Pathology at the University of Michigan.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72(1):168–177. [DOI] [PubMed] [Google Scholar]
- 2.Sun J Pancreatic neuroendocrine tumors. Intractable Rare Dis Res. 2017; 6(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukushima N Neuroendocrine neoplasms of the pancreas: the pathological viewpoint. JOP. 2017;S3:328–334. [Google Scholar]
- 4.Lee DW, Kim MK, Kim HG. Diagnosis of pancreatic neuroendocrine tumors. Clin Endosc. 2017;50(6):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilmette JM, Nose V. Neoplasms of the neuroendocrine pancreas: an update in the classification, definition, and molecular genetic advances. Adv Anat Pathol. 2019;26(1):13–30. [DOI] [PubMed] [Google Scholar]
- 6.Chai SM, Brown IS, Kumarasinghe MP. Gastroenteropancreatic neuroendocrine neoplasms: selected pathology review and molecular updates. Histopathology. 2018;72(1):153–167. [DOI] [PubMed] [Google Scholar]
- 7.Pictet RL, Rall LB, Phelps P, Rutter WJ. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976;191(4223):191–192. [DOI] [PubMed] [Google Scholar]
- 8.Ohki R, Saito K, Chen Y, et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A. 2014;111:E2404–E2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancraes: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014;25(1):65–79. [DOI] [PubMed] [Google Scholar]
- 11.Tang LH, Untch BR, Reidy DL, et al. Well differentiated neuroendocrine tumors with a morphologically apparent high grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22(4):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol. 2012;9(4):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korse CM, Taal BG, Vincent A, et al. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade: a marker study of chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48(5):662–671. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(6): 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinik A, Perry RR, Casellini C, Hughes MS, Feliberti E. Pathophysiology and treatment of pancreatic neuroendocrine tumors (PNETs): new developments In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. –. https://www.ncbi.nlm.nih.gov/books/NBK279074/ Updated June 12, 2018 Accessed June 1, 2019. [Google Scholar]
- 16.Tseng CM, Cheng TY, Chen TB, et al. Low accuracy of chromogranin A for diagnosing early-stage pancreatic neuroendocrine tumors. Oncol Lett. 2018;15: 8951–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimstra D Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20(1s):94–112. [DOI] [PubMed] [Google Scholar]
- 18.Basturk O, Yang Z, Tang LH, et al. The high grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogeneous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang LH, Basturk O, Sue JJ, Klimstra DS. A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol. 2016;40(9):1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krampitz GW, Norton JA. Pancreatic neuroendocrine tumors. Curr Probl Surg. 2013;50(11):509–545. [DOI] [PubMed] [Google Scholar]
- 21.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19(10):1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: advances in diagnosis and management. World J Gastroenterol. 2015; 21(32):9512–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hijioka S, Hosoda W, Morizane C, Mizuno N, Hara K, Okusaka T. The diagnosis and treatment of pancreatic NEN-G3—a focus on clinicopathological difference of NET-G3 and NEC G3. JOP. 2018;S3:346–353. [Google Scholar]
- 24.Sadowski SM, Neychev V, Millo C, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCall CM, Shi C, Klein AP, et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum Pathol. 2012;43(8):1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang M, Hruban R, Albores-Saavedra J. Clear cell endocrine pancreatic tumor mimicking renal cell carcinoma: a distinctive neoplasm of von Hippel-Lindau disease. Am J Surg Pathol. 2001;25(5):602–609. [DOI] [PubMed] [Google Scholar]
- 27.Singh R, Basturk O, Klimstra DS, et al. Lipid-rich variant of pancreatic endocrine neoplasms. Am J Surg Pathol. 2006;30(2):194–200. [DOI] [PubMed] [Google Scholar]
- 28.Carlo S, Zaitoun A, Fielding D, Dhanny G. Oncocytic neuroendocrine tumour of the pancreas and duodenum: two case reports with review of the literature. JOP. 2015;16(4):380–383. [Google Scholar]
- 29.Miyazaki T, Aishima S, Fujino M, et al. Neuroendocrine tumor of the pancreas with rhabdoid feature. Virchows Arch. 2019;473(2):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AE, Levi AW, Nadasdy T, Campbell KA, Fishman EK, Hruban RH. The pigmented “black” neuroendocrine tumor of the pancreas: a question of origin. Cancer. 2001;92(7):1984–1991. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011; 331(6021):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JK, Paik WH, Lee K, Ryu JK, Lee SH, Kim YT. DAXX/ATRX and MEN1 genes are strong prognostic markers in pancreatic neuroendocrine tumors. Oncotarget. 2017;8(30):49796–49806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Rosa S, Sessa F, Capella C. Acinar cell carcinoma of the pancreas: overview of clinicopathologic features and insights into the molecular pathology. Front Med (Lausanne). 2015;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37(11):1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS. Discordance of histologic grade between primary and metastatic neuroendocrine carcinomas. Ann Surg Oncol. 2015;22(suppl 3):S817–S821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Klimstra DS, Hruban RH, Tang LH. Immunohistochemical characterization of the origins of metastatic well-differentiated neuroendocrine tumors to the liver. Am J Surg Pathol. 2017;41(7):915–922. [DOI] [PubMed] [Google Scholar]
- 37.La Rosa S, Franzi F, Marchet S, et al. The monoclonal anti-BCL10 antibody (clone 331.1) is a sensitive and specific marker of pancreatic acinar cell carcinoma and pancreatic metaplasia. Virchows Arch. 2009;454(2):133–142. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt AM, Schmid S, Rudolph T, et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer. 2009;16(4):1219–1227. [DOI] [PubMed] [Google Scholar]
- 39.Genç CG, Falconi M, Partelli S, et al. Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol. 2018; 25:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. 4th ed Lyon, France: IARC Press; 2017 [Google Scholar]