Abstract
Understanding the relationship between physical environments and nonhuman primate behavior is a key element for effective care and management in a range of settings. The physical features of the captive environment, including not only gross useable space but also environmental complexity, can have a significant influence on primate behavior and ultimately, animal welfare. But despite this connection, there remains relatively little conclusive data on how captive primates, especially great apes, use the spaces provided to them, especially in modern, indoor-outdoor enclosures that have become more prevalent in recent years. In this study, we used four years of detailed data on where 23 great apes (chimpanzees and gorillas) positioned themselves within a modern, indoor-outdoor zoo enclosure to determine not only how the apes utilized their space but also how access to outdoor areas affected their spatial selectivity. We found that both species used relatively little of their available space: chimpanzees and gorillas spent half their time in only 3.2 and 1.5% of their useable three-dimensional space, respectively. Chimpanzees utilized the outdoor space more than gorillas, but access to the outdoors did not affect space selectivity in the indoor area for either species. Although both species of ape were highly selective in their space use, consideration should be given to the importance of providing the choice to locate in a variety of spaces, including outdoor areas. These data represent an extremely detailed account of space selectivity by great apes in an indoor-outdoor environment and have substantial implications for future facility design and captive primate management.
Keywords: chimpanzee, gorilla, space use, management, behavior, welfare, selectivity, facility design
INTRODUCTION
Understanding how animals utilize their captive environments is fundamental to address critical issues related to animal welfare, captive management and enclosure design. The characteristics of the space provided for housing nonhuman primates (NHPs) dictate not only the ability of the animals to perform species-typical behaviors but also factors such as the optimal group size, the space available for environmental enrichment and the ability to manage the group to promote individual welfare [Joint Working Group on Refinement, 2009]. For several decades, scientists have examined the interrelationships among primate behavior, health, wellbeing and physical environments in a range of settings, primarily laboratories and zoos. While earlier evaluations focused on the mere quantity of space, more recent assessments have investigated other environmental characteristics, such as complexity and naturalism [Badihi, 2006; Davey, 2006; Jensvold et al., 2001; Kozorovitskiy et al., 2005; Sambrook & Buchanan-Smith, 1997; Schapiro et al., 1997]. Although quantity of space is important, especially in relation to minimum requirements to promote health and wellbeing, the functionality of the space is critical, as even large enclosures can be of limited value to the animals, if they are unable to make good use of the space available [Kerl & Rothe, 1996; Paulk et al., 1977].
In order to improve existing environments, it is important first to document what primates do in their enclosures and understand how proximate conditions influence their behavior therein [Seidensticker & Doherty, 1996]. Several studies have taken such an approach with captive great ape species. For instance, Wilson [1982] measured the activity of gorillas and orangutans in 41 different zoos. She found that the presence of objects, rather than gross enclosure size or useable area, predicted activity levels of the apes. Likewise, Perkins [1992] found that the provision of enclosures with a variety of moveable objects promoted higher activity levels in orangutans. Bloomsmith et al. [1999] reported that chimpanzees housed in large outdoor corrals spent equivalent proportions of time off the ground compared to those housed in much smaller indoor/outdoor runs.
Fewer attempts have been made to quantify how much use animals make of the space they are provided. Stoinski et al. [2001] found that while zoo-housed gorillas in their study entered 80–98% of the quadrats in their habitat, individuals spent 50% of their time in less than 15% of the available space. This uneven enclosure use seemed to be driven by an attraction to areas immediately adjacent to the off-exhibit holding building. In a study of chimpanzees in a more modest, indoor laboratory environment, adults actually preferred the smaller of the rooms available to them and showed distinctly habitual use of particular sites [Traylor-Holzer & Fritz, 1985].
Clearly, captive apes show a range of environmental preferences, some of which may be consistent across very different environments. Ross et al. [2009] found that chimpanzees and gorillas showed strong preferences for the relatively small areas around doorways, both in a smaller, indoor, hardscape exhibit and in a larger, naturalistic, indoor-outdoor space. While the fact that captive apes show preferences for particular elements in their enclosures is now well established, it does raise questions about what an uneven distribution of use means in terms of exhibit efficiency. Given the increasing costs of building contemporary enclosures, it is likely that managers would not favor designs in which the inhabitants rarely visit proportionately large sections of expensive space provided to them. In zoo exhibits especially, the creation of environments in which animals make use of all available space has potentially positive ramifications for visitor experience, animal activity levels and wear and tear on exhibit elements. Understanding the factors that influence space utilization is paramount to making enclosures more effective and maximizing animal welfare in the space available.
One such factor that may influence space use is access to outdoor areas. Despite the fact that a variety of animal care and management policies are specifically supportive of the provision of outdoor access, there is mixed scientific evidence of specific beneficial effects of this practice. There are several studies in which animals displayed positive behavioral changes following a move to an outdoor enclosure [Clarke et al., 1982; Jensvold et al., 2001; Macedonia, 1987; O’Neill et al., 1991; Ross et al., 2009], but there is much less known about the animals’ preferences for outdoor access when given the choice. Choice is often cited as an important factor in captive animal environments and though the empirical evidence to support this is relatively scarce, the provision of a range of options can provide valuable insight into animal preferences. For instance, in one study, six pairs of common marmosets were given the choice between a large, enriched indoor room and a smaller outdoor cage. When given free access to move between their home cage and outdoors, the marmosets spent 70% of their day in the outdoor cage and showed a strong, long-lasting preference for that space [Pines et al., 2007]. Similar effects have been reported for sifakas [Pereira et al., 1989] and chimpanzees [Baker & Ross, 1998; Bloomsmith et al., 1999; Howell et al., 2002]; however, another study reported that gentle lemurs spent very little time outdoors when given a choice [Bellingham, 1998]. These conflicting results are problematic as claims are made for and against the value of giving primates access to outdoor housing.
In this study, we used detailed three-dimensional space use data from zoo-housed gorillas and chimpanzees to address the following questions:
How do apes utilize their three-dimensional space in a modern, naturalistic, indoor-outdoor enclosure?
When given the opportunity, to what degree do great apes utilize their outdoor enclosures?
Does provision of outdoor access affect how apes utilize their indoor enclosure?
Does space utilization differ between their indoor and outdoor enclosures?
Together, these data will bolster efforts to better understand interactions between modern enclosures and animal behavior, and will subsequently be of use to managers seeking to improve animal welfare and affect space use selectivity.
METHODS
Subjects, Housing and Management
Table I lists the 23 great apes (Pan troglodytes, Gorilla gorilla gorilla) on which data were collected. All subjects were housed at the Regenstein Center for African Apes (RCAA) at the Lincoln Park Zoo (Chicago, IL). The Lincoln Park Zoo is an accredited member of the Association of Zoos and Aquariums and all management and data collection procedures herein reported complied with all state and federal animal care and welfare regulations and the standards set forth in the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates.
TABLE I.
Study Subjects
| Subject | Genus | Sex | Age (conclusion of study) | Number of data points per condition | |
|---|---|---|---|---|---|
| Access | Locked in | ||||
| Cashew | Pan | F | 23 | 9,121 | 4,610 |
| Chuckie | Pan | F | 8 | 8,937 | 4,925 |
| Donna | Pan | F | 43 | 9,101 | 7,992 |
| Hank | Pan | M | 17 | 9,776 | 4,780 |
| June | Pan | F | 42 | 9,754 | 4,243 |
| Keo | Pan | M | 49 | 8,313 | 3,966 |
| Kibale | Pan | F | 27 | 9,406 | 3,583 |
| Kipper | Pan | M | 8 | 10,470 | 4,065 |
| Kathy | Pan | F | 17 | 8,986 | 4,905 |
| Nana | Pan | F | 14 | 8,428 | 4,844 |
| Optimus | Pan | M | 9 | 9,737 | 4,863 |
| Vicky | Pan | F | 43 | 9,468 | 3,326 |
| Azizi | Gorilla | M | 4 | 8,126 | 7,232 |
| Bahati | Gorilla | F | 17 | 8,706 | 7,625 |
| Bulera | Gorilla | F | 21 | 8,650 | 5,518 |
| Jojo | Gorilla | M | 27 | 9,545 | 7,252 |
| Kowali | Gorilla | F | 30 | 8,703 | 6,642 |
| Kwan | Gorilla | M | 19 | 9170 | 6,968 |
| Madini | Gorilla | F | 13 | 8,703 | 6,195 |
| Makari | Gorilla | F | 20 | 8,221 | 7,253 |
| Rollie | Gorilla | F | 11 | 9,194 | 7,322 |
| Susie | Gorilla | F | 3 | 8,532 | 7,877 |
| Tabibu | Gorilla | F | 15 | 8,959 | 7,350 |
Two chimpanzee groups (n = 7 and 5) and two gorilla groups (n = 7 and 4) lived as stable social units for the duration of the study.
Each group was housed in one of the four semi-naturalistic enclosures that were composed of an indoor dayroom (mean area: 101.4 m2) and an outdoor yard (mean area: 585.5 m2) separated by a 6 m high sliding glass window that opened to provide outdoor access. The dayrooms were very similar to each other in that they contained a deep mulch substrate, 8 m high meshed ceilings, elevated platforms that supported multiple individuals and artificial climbing structures, ropes and vines. Most elements were provided in multiple locations to prevent dominant animals from monopolizing a particular resource. The four outdoor yards were also very similar to one another, with a grass substrate, varied topography and a combination of natural and artificial climbing apparatus. Three of the outdoor yards were enclosed with wire mesh that was climbable by the apes. The remaining outdoor yard had no cover, but zoo visitors were separated from the animals by an approximately 6 m wide dry moat. Three of the four enclosures were viewable by the visiting public through large glass walls. Apes in the other enclosure were not visible to the public, but they had regular visual access to carestaff, researchers and hosted guests. Apes in all exhibits were able to interact with care staff through mesh partitions. As part of the regular husbandry routine, ape groups were moved between the exhibits periodically. Data from all four exhibits were considered equivalent and were combined for analyses.
All apes were provided with fresh produce and biscuits in the morning, as well as supplemental food and enrichment in the afternoon. Apes received additional scattered enrichment and structured training sessions on a daily basis. The apes lived in their indoor-outdoor enclosures throughout the day and night, only transferring to the basement level holding areas from 0800 to 1000 hr each day so that the exhibits could be cleaned [see Ross et al., 2010; in press for more details]. Nesting materials were provided to the apes on a daily basis and opportunities to build ground nests (on the mulch substrate) or elevated nests (on platforms) was always available.
Apes were generally provided access to their outdoor yards when daytime temperatures exceeded 5°C. If this temperature criterion was not met, or weather conditions were poor, access to the outdoors was prevented. The two key conditions for this study included (1) the “restricted access” condition, in which animals were only able to occupy and utilize the space in their indoor dayroom, and (2) the “free access” condition, in which the apes could locate themselves anywhere in the indoor dayroom or the outdoor yard. In both of these conditions, indoor conditions were relatively consistent (22°C) and as such we do not consider outdoor conditions to be a confounding variable.
Data
Ten observers collected data on the apes between the 1000 and 1700 hr during weekdays over a period of four years (July 2004-July 2008). Data were collected using ten-minute focal observations with samples recorded at 20-s intervals. At each sample, the location of the focal animal was recorded on a map interface using a Lenovo X61 touch-screen tablet computer. The map interface used a grid overlay in which each cell represented a space of approximately 1 m2. With each sample, the observer also noted the placement of the subject within a set of five vertical tiers. Level 0 represented the ground level and level 1 represented subjects situated off the ground, but within 2 m of the ground. The remaining levels were in 2 m intervals and level 4 was recorded when individuals were located at a height above 6 m. As such, we effectively utilized a three-dimensional map of each exhibit, with each cell representing a space of 1 × 1 × 2m (2 m3).
All observers were reliability-tested and demonstrated greater than 85% average agreement with an experienced researcher. A total of 1,896 hr of data were collected (mean = 14,840 data points per subject).
Analysis
In order to determine how selectively each subject used their space, we calculated a spread of participation index (SPI) [Dickens, 1955] with the following formula:
where M is the mean frequency of observations in all sites, Na the number of useable sites above mean, Nb the number of useable sites below mean, Fa the number of observations in Na, Fb the number of observations in Nb and N the total number of observations.
The index, varying between 0 (maximum enclosure use, all zones used equally) and 1 (minimum use, only one zone used), is a simple descriptor of how evenly the available space is used by a captive animal. We calculated SPI values for each vertical tier to address how selection of space use might differ across heights in the exhibit. Cells in which there were no supporting structures on which an ape could position itself were deducted from the total number of cells. Cells directly adjacent to the ceiling mesh from which apes could suspend were included.
There were two primary comparisons: An “access test” compared the apes’ indoor space use when they were confined to their indoor space and when they had access to their outdoor yard. An “indoor/outdoor test” compared the ape’s indoor space use to their outdoor space use when they had full access to both areas. Both of these tests were conducted using two measures: percentage time use (the proportion of time spent at each level in each condition) and SPI (the measure of clustering within that space). We used analysis of variance (ANOVA) to assess the effect of three factors (species, vertical tier and condition) on space use. Because of the repeated nature of the analysis, we used a standard Bonferonni correction resulting in a critical α value of 0.05/4 = 0.0125. Systat 11 was used for all analyses.
RESULTS
Figure 1 shows the distribution of space use by chimpanzees and gorillas for one of the exhibits across the five vertical tiers. Space use patterns were similar for the other three exhibits, although all exhibits differed slightly in shape, size and layout. Over the course of the four years of the study, chimpanzees were recorded in 56.5% of the possible three-dimensional locations in the exhibits. Gorillas were recorded in 28.5% of the possible locations.
Fig. 1.
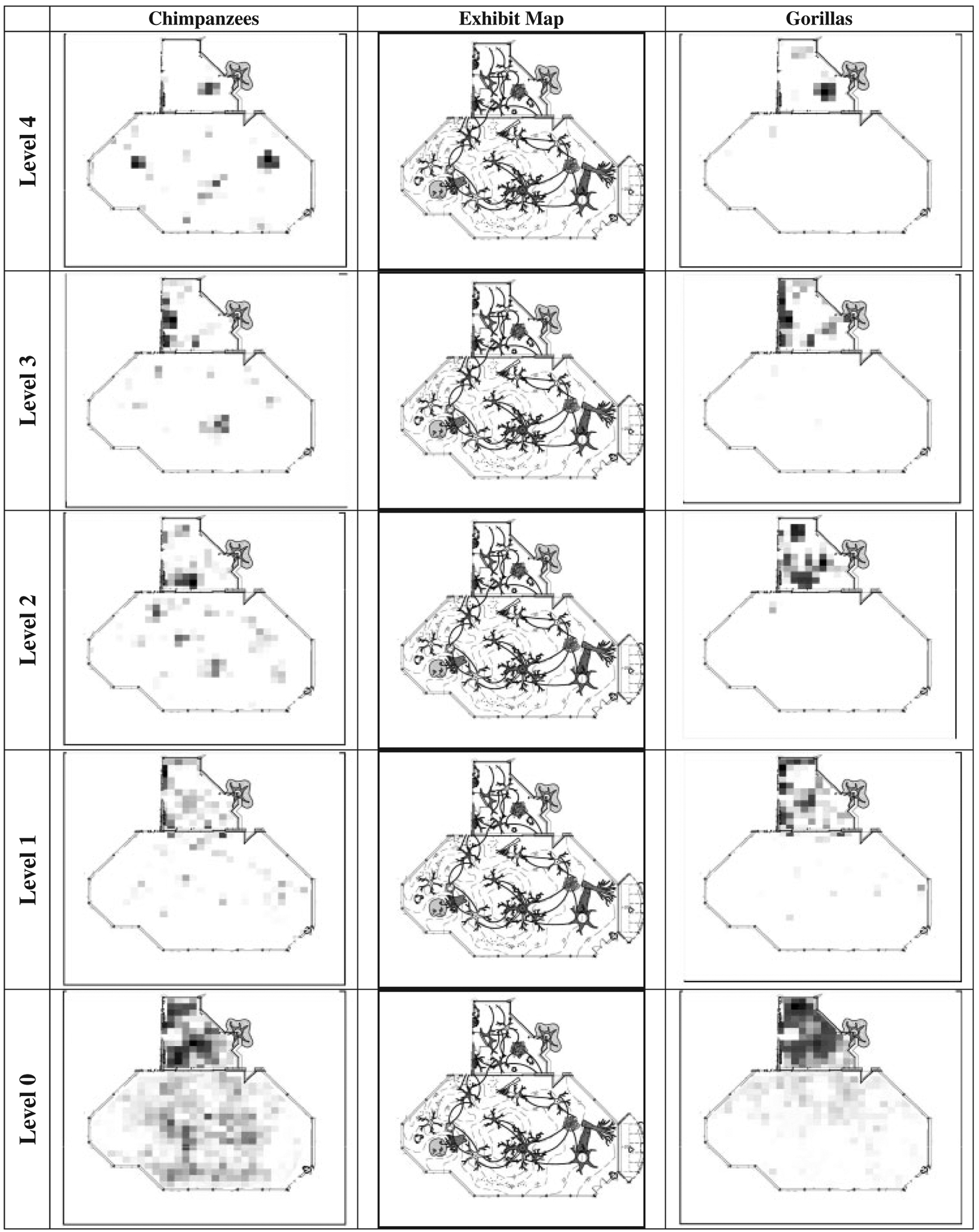
Distribution of space use for chimpanzees and gorillas at five vertical tiers of an indoor-outdoor enclosure during periods in which the apes had free access to outdoor areas. Darker areas indicate a higher frequency of use. Level 0 represents the ground level and each subsequent level is approximately 2 m in height.
Both species were proportionately less selective when only indoor spaces were quantified. Chimpanzees and gorillas used 74.4 and 76.1% of their indoor space, respectively. The percentage of total ground level space that the apes utilized was 91.4 and 59.0% for chimpanzees and gorillas, respectively. Use of indoor ground space was 98.6% for chimpanzees and 97.9% for gorillas.
Both species were highly habitual in their spatial selections. Chimpanzees spent 50% of their total time in only 3.2% of their total space. Similarly, gorillas spent half their time in only 1.5% of their available space. Space selection indoors was only slightly more distributed; chimpanzees spent 50% of their time in 4.2% of their indoor space, and gorillas spent 50% of their time in 4.5% of their indoor space.
When provided access to their outdoor yard, chimpanzees spent an average of 33.2% of their observed time outdoors, while gorillas spent only 7.1%. This was a significant species difference (t = 7.01, P<0.001).
Table II shows the percentage of time spent in each of the vertical tiers for both species in each condition. Overall, both chimpanzees and gorillas spent more than half their time off the ground.
TABLE II.
Percentage of Time That Chimpanzees and Gorillas Spent in Each of the Five Vertical Tiers Under Different Conditions of Access to Outdoor Space
| Chimpanzees (n = 12) | Gorillas (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|
| Level | Restricted access (%) | Free access (indoors) (%) | Free access (outdoors) (%) | Overall (%) | Restricted access (%) | Free access (indoors) (%) | Free access (outdoors) (%) | Overall (%) |
| 4 | 4.7 | 4.5 | 4.7 | 4.6 | 0.83 | 1.8 | 1.7 | 1.5 |
| 3 | 29.8 | 40.4 | 11.0 | 30.5 | 29.9 | 40.5 | 0.75 | 35.4 |
| 2 | 5.5 | 6.5 | 11.2 | 7.5 | 11.4 | 12.9 | 1.5 | 11.9 |
| 1 | 8.2 | 10.1 | 17.4 | 11.6 | 9.5 | 8.2 | 10.6 | 8.7 |
| 0 | 51.8 | 38.5 | 55.8 | 45.8 | 48.4 | 36.5 | 85.5 | 42.6 |
Level 0 represents the ground level and each subsequent level is approximately 2m in height.
Indoor Space Use and the Effects of Outdoor Access
We examined the effects of outdoor access on where the apes positioned themselves in the indoor dayroom. We found significant differences in how the apes distributed their time between vertical levels (F = 89.46, df = 4,210, P<0.001). There was a significant level by access effect (4.68, df = 4,210, P<0.001), which showed that provision of outdoor access affected the percentage of time spent at different levels. Specifically, when provided outdoor access, both species of ape reduced the percentage of indoor time on the ground (level 0) and increased the percentage of time at level 3. There were no statistically significant main effects of species or access.
SPI values were used to examine differences in space use selectivity in the indoor space when the apes had access to their outdoor yard and when they were locked inside. There were neither species differences nor any effect of outdoor access on the distribution of indoor space use. There was a significant level effect (F = 102.29, df = 4,208, P<0.001), which showed the highest SPI values (increased clustering of space use) in the highest vertical tiers of their indoor space (Fig. 2).
Fig. 2.
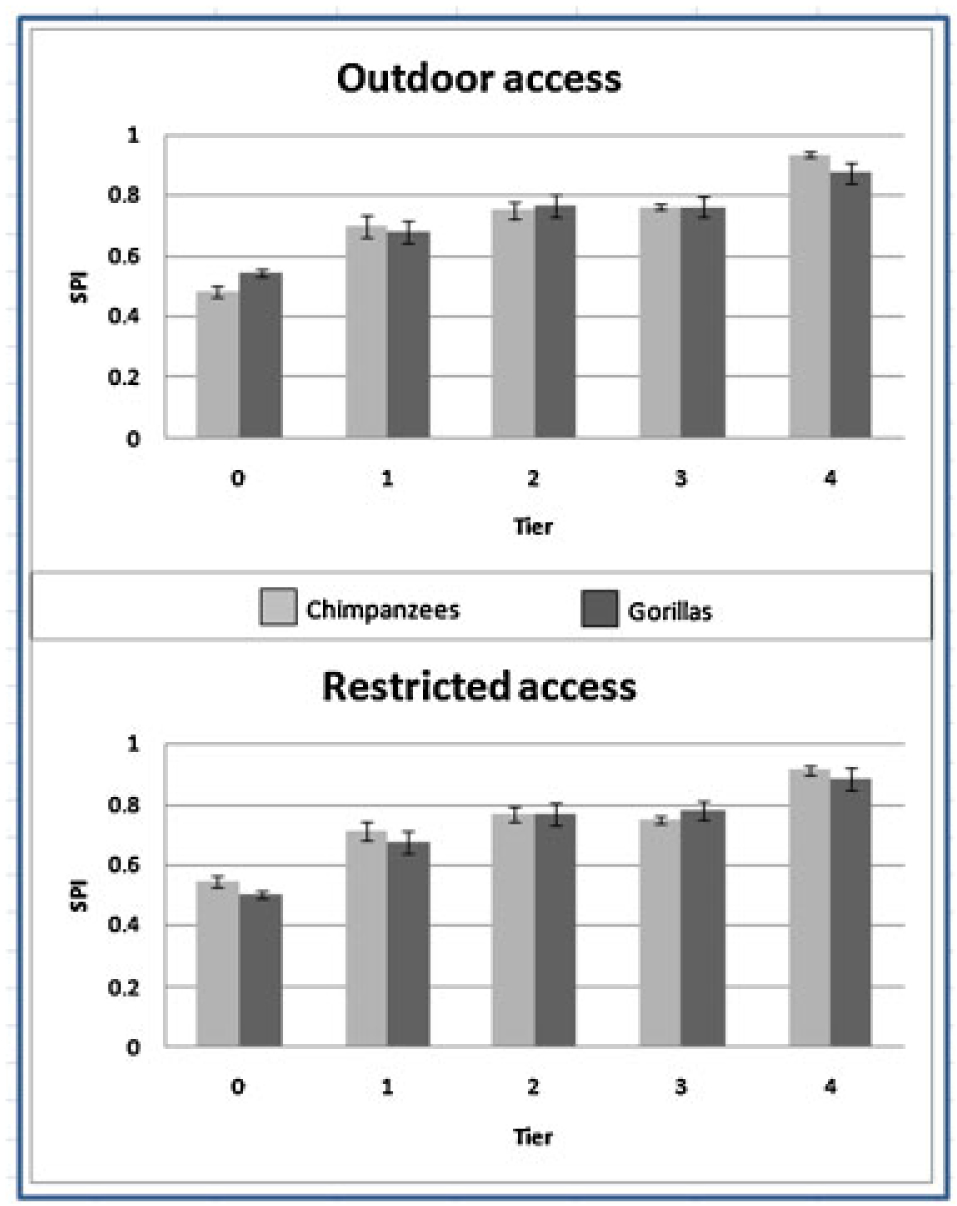
Spread of participation index (SPI) for chimpanzees and gorillas at five vertical tiers of an indoor enclosure. The top graph shows the space selectivity during periods in which the apes had free access to outdoor areas. The bottom graph shows space selectivity during periods in which the apes were restricted only to indoor space. Level 0 represents the ground level and each subsequent level is approximately 2 m in height.
Space use indoors versus outdoors
Comparing space use data on indoor and outdoor use when subjects had free access to both areas (space effect) revealed a significant level effect (F = 201.78, df= 4,428, P<0.001) showing preferential use of the substrate and level 3. There was also a significant level by space interaction effect (F = 63.91, df = 4,428, P<0.001) suggesting that there was differential selection of the ground level and tier three depending on whether the apes were inside or outside. There were no main effects of species or space (indoors versus outdoors).
The apes were significantly more clustered in their use of outdoor space compared to indoors (F = 198.38, df = 1,192, P<.001). There was a significant species effect (F = 32.31, df = 1,192, P<0.001) demonstrating that chimpanzees were less clustered than gorillas in their overall space use and there was a significant effect of level (F = 103.77, df = 4,192, P<0.001) showing that the apes were more clustered in their space use at higher levels than at lower levels. There were several significant interaction effects as well. A species by level interaction (F = 4.56, df = 4,192, P = 0.01) showed that chimpanzees were less clustered than gorillas only at the lower levels. A species by space interaction (F = 30.37, df = 1,192, P<0.001) showed that chimpanzees were more clustered than gorillas in the outside space, but much less so in the indoor space. Finally, a significant level by space interaction (F = 4.159, df = 4,192, P = 0.003) demonstrated that ape clustering was more strongly affected by level in the indoor space than outdoors.
DISCUSSION
These results clearly demonstrate that great apes make very selective use of their available space in captive settings. This clustered distribution of highly used areas has been reported in other studies of captive apes [Bettinger et al., 1994; Hedeen, 1982; Ogden et al., 1990; Stoinski et al., 2001; Traylor-Holzer & Fritz, 1985], but this study is the first to investigate great ape spatial distribution in an indoor-outdoor environment.
The SPI was used to determine the degree of spatial selectivity and to help elucidate the degree to which the apes made efficient use of their enclosures. When Traylor-Holzer and Fritz [1985] observed chimpanzees in relatively small indoor spaces (66.3 m2) they reported adult SPI scores that ranged widely (from 0.32 to 0.75; mean = 0.61). In this study, chimpanzee SPI scores in the free-access condition were much higher (0.71–0.85; mean = 0.78) suggesting a much more selective distribution of space use. One might conclude from this comparison that the captive environment studied by Traylor-Holzer and Fritz [1985] promoted a more even distribution of space use. However, the large difference in space use selectivity between studies is likely attributable to key methodological differences, specifically the density of measurements. Plowman [2003] used a series of theoretical models to demonstrate how smaller zones give a more accurate spread of participation estimates. In this study, an average of 1,822 locations, each measuring approximately 2 m3, were used, whereas Traylor-Holzer and Fritz [1985] only utilized 18 larger and unequally sized location sites. As Plowman [2003] demonstrated (1) spread of participation is severely overestimated by large grid sizes, and (2) an increase in the number of possible locations increases the accuracy of estimates of spatial clumping. Although we are unable to statistically compare the efficiency of space use between datasets utilizing different methods, we are confident that this study provides extremely accurate measures of space use efficiency and we hope that it will stimulate additional detailed investigations of these behavioral patterns in other modern captive environments.
Vertical Space Use
Gorillas and chimpanzees in this study were remarkably similar in their use of the vertical tiers, despite traditional characterization of these species as terrestrial (primarily observed on the ground) and arbo-terrestrial (using both ground and elevated areas), respectively [Reynolds, 1965]. Although recent research has clarified that wild lowland gorillas spend significant time in the trees [Tutin et al., 1995], the arboreality of this species (especially large silverbacks) is constrained primarily by biomechanical factors; specifically the paucity of tree limbs that can support an adult gorilla’s weight [Remis, 2005]. Captive environments, such as that at RCAA, may eliminate these restrictions through the use of robust artificial materials that can support even the heaviest ape (Jojo was approximately 500 lbs and spent 40% of his time above ground level). The fact that gorillas spent more than half their time off the ground reinforces the importance of providing sufficient climbing opportunities for this species. This is especially relevant given that the natural motivation for climbing by wild apes (to access fruit in trees) did not exist in this captive situation where food was almost always provided at ground level.
Space (In)efficiency
Chimpanzees were less selective in their space use compared to gorillas overall, but this was especially true in the outdoor space and at the lower levels, where gorillas tended to be more narrow in their spatial selections. Over the course of the four-year study, chimpanzees were seen in 56.5% of the available quadrants and gorillas in only 28.5%. Even more striking was the fact that chimpanzees and gorillas spent half of their total time in just 3.2 and 1.5% of their space, respectively. This is in stark contrast to results from Stoinski et al. [2001], who found a much higher percentage of quadrants (80–98%) and a higher (though still modest) distribution of space use (50% of time in 15% of the space). These differences again may be attributed to disparities in methodology. Stoinski et al. [2001] utilized much larger (36 m2) grid sizes, and had only 14–67 possible locations in which the animals could be scored. Additionally, these measurements were done on a two-dimensional scale for species that typically spend significant time above the ground. Nonetheless, our results show that almost half of the available three-dimensional spaces were rarely, if ever, used by the chimpanzees, and over 70% of available space was never used by the gorillas. While these findings may have negative implications for some managers, they do underscore the importance of the relatively small percentage of the exhibit space that is utilized regularly.
There is a widespread assertion that providing more space for captive animals will improve their welfare [Paulk et al., 1977]. These assertions are tightly linked to the idea that the spatial constraints of a captive environment result in motivational frustration for species that have evolved to roam over large areas in the wild. For instance, Clubb and Mason [2003] presented data that suggest that widely ranging species are prone to problems in captivity, including poor health, breeding difficulties and behavioral stereotypies. However, other investigations have served to clarify the relationship between enclosure size and the wellbeing of captive animals. For instance, available space does not correlate with activity levels for gorillas [Wilson, 1982]. The fact that the apes in this study were highly selective in the use of their enclosure underscores the importance of the quality of the space over the quantity of space. These findings do not negate calls for larger spaces to improve captive wellbeing. Indeed we are unaware of any reports that have empirically determined that providing too much space is detrimental to captive primate welfare. However, we advocate an additional emphasis on the importance of environmental complexity and animal preferences over simple gross quantity augmentation. Given that captive primates may be utilizing only a fraction of the space available to them, managers are especially challenged to quantify the factors that influence why captive animals choose to occupy certain spaces and choose not to occupy others.
Outdoor Access
One such factor purported to influence captive primate behavior is access to outdoor areas. Many studies have addressed this question across a range of primate species [reviewed in Badihi, 2006]. Unfortunately, the results of these studies are surprisingly variable, due in part to the frequency of potentially confounding factors, such as increased size and complexity of the outdoor space. Despite the ambiguity of these results, many regulatory bodies specifically emphasize the importance of this feature in environments for captive primates [Council of Europe, 2004; Farmer et al., 2009; IPS, 1989; Ross, 2010; USDA, 1991]. There are several pragmatic reasons to suggest that outdoor access holds at least the potential for behavioral benefits [Badihi, 2006]. One fundamental advantage of free access to outdoor areas is simply the expansion of available space for the animals, thereby providing more room for species-typical behavior patterns, such as locomotion, foraging and social activities. Second, there may be additional advantages directly related to the partitioning of the available space into two separate areas. This physical division of space may serve to reduce stress by providing increased opportunities for the avoidance of aggressive encounters [Coe et al., 2001; Novak & Suomi, 1988]. A third potential benefit of outdoor enclosures is the inherent exposure to seasonal fluctuations in light, temperature, humidity and climate outdoors, which may differ substantially from the more carefully regulated environments of indoor settings. Exposure to these more variable climates may result in behavioral and physiological effects with positive welfare outcomes [Buchanan-Smith, 1994, 1998; Novak & Suomi, 1988; Poole, 1991]. Finally, outdoor enclosures provide the animals with more sensory stimulation (novel sights and smells) and unique organic qualities (grass, live plantings, indigenous wildlife, etc.) that may provide greater opportunities for exploration and exhibit use [Honess & Marin, 2006; O’Neill et al., 1991]. Given these potential benefits, one would expect that apes should utilize outdoor spaces and that access to these spaces should affect their spatial distribution patterns.
In this study, the chimpanzees and gorillas made substantially different use of their outdoor yards when provided with the opportunity to use them. Chimpanzees spent a third of their daytime outside, but there are very few comparable long-term accounts of free-access outdoor space use with which to compare these rates. Bloomsmith et al. [1999] reported that laboratory-housed chimpanzees spent 45% of their time outside and in another study, young chimpanzees used a novel and enriched outdoor “play yard” 72% of the time [Brent et al., 1991]. In both of these studies, the environmental complexity of the outdoor yard was considerably more than the indoor space. The 11 gorillas observed in this study spent only 7% of their day away from their indoor dayroom and we are aware of no published accounts with which to compare this. Although we are unsure of the degree to which these outdoor use patterns are typical of captive apes, there are many potential explanations for the relatively modest use of the outdoor space, especially by the gorillas. In some cases, individual animal histories and experience may play an important role in determining the propensity to use or avoid outdoor areas. Individuals who have previously experienced the outdoors and its accompanying environmental stimuli (sunshine, grass, wind, noise, etc.) may be more comfortable in those spaces. The adult gorillas in this study spent most of their lives in indoor facilities and as such, they may have favored the familiarity of an indoor space. The two groups of chimpanzees had widely varying backgrounds; seven subjects were born and raised in a completely outdoor environment, while the four older individuals lived almost exclusively indoors for their entire lives. In this light, it is perhaps not surprising that the younger, outdoor-reared group averaged a higher percentage of time outdoors (41%) than did the older group (22%).
Species-typical attributes may also contribute to the use of outdoor space, as only one gorilla (Rollie = 17%) spent more time outside than any of the 12 chimpanzees. Chimpanzees, who typically have broader ranging patterns [Yamagiwa, 1999] than gorillas in the wild, may be predisposed to make use of the larger outdoor yards. Likewise, gorillas, who tend to associate closely with the silverback male of the group in the wild, may prefer to stay within viewing distance of their group leader in captivity [Nakamichi & Kato, 2001]. Behavioral research on these two species in captive settings suggests that chimpanzees are more exploratory and active in nature [Ross et al., 2010; in press] and a preliminary comparison of personality factors between these species suggests that gorillas score lower in “Openness,” a factor that is associated with the characteristics of inquisitiveness and curiosity [Inoue-Murayama et al., 2010].
There are also proximate explanations for the demonstrated preference for indoor spaces. Specific characteristics of the indoor dayroom, such as the relatively stable temperature range, may have better met the behavioral needs of the apes. Studies of captive apes’ environmental preferences have demonstrated strong preferences for structural elements, such as corners, doorways and proximity to keeper areas [Ross & Lukas, 2006; Ross et al., 2009; Stoinski et al., 2001]. All these structural elements occur in higher frequency in the indoor dayroom than in the outdoor yard. Given that the dayrooms were specifically designed to match the spatial preferences of the apes in this facility, it is possible that these characteristics are able to buffer any attraction to the outdoors. Interestingly, other characteristics that distinguish the indoor dayroom from the outdoor yard are the proximity and scope of the visiting public. Although much literature has been dedicated to exploring the potentially negative aspects of zoo visitors on animal behavior and welfare, the data from this study suggest that the apes may be choosing to locate themselves nearer to the potentially engaging stimulus of the public [see Bloomfield et al., 2010]. Indoors, a thick glass barrier allows visitors to get within inches of the apes, but prevents most of the sound transmission. On the other hand, the outdoor yards are surrounded by mesh netting and/or maintain a larger distance (2–6 m) between apes and people, but the noise from crowds is easily heard by the apes in the yard. Apes in this facility showed preferences for spaces near the glass barrier [Ross et al., 2009], but they spent relatively little time near mesh barriers outside. While visual contact and proximity to the visiting public may not necessarily act as negative stimuli in this environment, the accompanying acoustic stimuli from large crowds may be aversive.
Access Effects: The Choice of Going Outdoors
Although the outdoors was relatively underutilized, we do not feel that the lack of outdoor space use is a reason to de-emphasize its importance for primate housing. Although several of the previously mentioned studies have sought to explain the behavioral effects of using outdoor areas, it is more difficult to determine the degree to which the opportunity to go outdoors is a significant influence on behavior, and ultimately, wellbeing. The potential benefits of providing opportunities for choice and control in captive environments has been of interest to scientists and managers for several decades [Chamove & Anderson, 1989; Markowitz, 1982; Novak & Drewsen, 1989; Sambrook & Buchanan-Smith, 1997; Schapiro & Lambeth, 2007; Snowdon & Savage, 1989; Videan et al., 2005] and before one interprets the relatively low frequency of outdoor use to mean that access to outdoor space is unnecessary for apes, one must consider the potential benefits of simply being able to make the choice to go outside.
Many animals seem to prefer the option to make choices. Badihi [2006] found that providing a choice of access to outside runs resulted in substantial welfare improvement for marmosets of all ages. In a study of captive polar bears, subjects showed a significant decrease in stereotyped pacing behavior when provided access to their off-exhibit holding areas, although the bears did not utilize those off-view dens when given free access [Ross, 2006]. In this case, it appeared that the choice to use those dens resulted in a substantial behavioral change indicative of improved wellbeing. The analogous characterization in this study is that even though many of the apes did not use the outdoor space much at all, having the opportunity to use it may be an important aspect of their environment. When provided the choice to go outdoors, the apes in this study tended to shift their vertical use of space from the ground (when they were locked inside) to a much higher tier when the doors to the outside were opened. One possible explanation for this reaction is that during colder months, when the outside air was cooler than the inside air, the apes may have sought the warmer air at higher levels indoors when the doors were open. Another possibility is that the apes preferred a higher vantage point when the outside was accessible to enhance their view of their entire indoor-outdoor range. This may have been less important when the groups were restricted to the smaller indoor space only. Further behavioral research is needed to specifically determine whether the opportunity to use outdoor areas, rather than the outdoors itself, enhances the welfare of primates.
In addition to measuring the apes’ gross use of indoor and outdoor space, this study focused on measures of selectivity of space use and their relationship to outdoor access. Both species were equally selective with their space utilization of the indoor space, regardless of whether or not they had access to their outdoor yards. While this may not be surprising for the gorillas that only rarely utilized the outdoor space, chimpanzees maintained their space selectivity with lowering frequencies of use. That is, although they were indoors less, they distributed themselves in the space similarly in both conditions.
The other comparison in which we were interested was the difference in space use between the indoor and outdoor yards. While interpretation of these results is somewhat confounded by the different physical structures in the two spaces, understanding how the apes utilized these spaces can be informative. Both species of apes, but especially gorillas, tended to select the ground level more and markedly decreased their time at level three when outdoors. Jensvold et al. [2001] found different results in their evaluation of indoor and outdoor space use in a group of captive chimpanzees at the Chimpanzee Human and Communication Institute (CHCI). Indoors, the CHCI chimpanzees were recorded on the ground at near identical frequencies to chimpanzees at RCAA (39%). Outdoors, however, the CHCI chimpanzees spent significantly less time on the ground level, which is in contrast to the results of this study. It is difficult to determine whether these inter-facility differences in space use are attributable to substrate differences (grass substrate is not reported at CHCI but is a favored substrate in the outdoor yard at RCAA), different climbing opportunities, or simply differences in individual or group preferences.
Applied Consequences
The results from the present four-year investigation emphasize the importance of careful attention to the functional composition of designed spaces for captive animals. The current trend toward creating more naturalistic and enriched environments in zoos and laboratories may be associated with an unintended reduction in emphasis on providing functional space that caters to species-specific needs. Indoor spaces, especially in zoological settings, are traditionally more structured and controllable than are modern outdoor yards favoring a more natural and diffuse composition. Although there is some evidence that naturalistic environments can have impacts on animal behavior [Clarke et al., 1982; Maple & Finlay, 1986], these older findings are confounded by factors of environmental complexity and temporal change. As such, it remains unclear whether these changes can be attributed directly to outdoor access per se or to a combinative effect of environmental enhancement. While recent zoo exhibit design may be resulting in more natural-looking exhibits, there may be reason to question the ability of these enclosures to promote natural behavior patterns. We advocate functional naturalism in which functional elements are provided within the naturalistic aesthetic. This approach was used in the design of RCAA, and behavioral benefits of this facility have been demonstrated for both species [Ross et al., 2010; in press]. However, this study also identified potential design weaknesses of RCAA. For instance, although both the indoor and outdoor areas provide substantial climbing opportunities for the apes, there are relatively few elevated resting sites outdoors compared to indoors. This likely influenced outdoor spatial distribution for both species. Gorilla behavior was primarily transitory in the outdoor yard and individuals were primarily observed simply gathering food to bring back indoors, regardless of weather conditions [personal observation]. Similarly, the chimpanzees, which used the outdoor yard more frequently than gorillas, tended to use the substrate much more than indoors, perhaps because there were relatively few large, stable and elevated options outside. Given that resting and inactivity constitute a large proportion of these apes’ waking hours, the facilitation of these behaviors should probably be considered an important design element [Pruetz & McGrew, 2001].
These findings raise questions about if and how enclosure size for captive primates should be regulated. Currently, there is a tremendous range of enclosure size guidelines for species such as chimpanzees; from the USDA minimum requirements [2.33 m2; USDA, 1991]], to the recommendations from the Association of Zoos and Aquarium’s Chimpanzee Species Survival Plan [185 m2 for groups of 5 or less; Ross, 2010], up to the standards of the Pan African Sanctuary Alliance [250 m2 per individual; Farmer et al., 2009]. While each of these documents specifically notes the importance of other considerations such as vertical height and environmental complexity, it is clear that there is very little consensus on how much space is necessary to provide to this and other species. Given the push to formulate scientifically based management standards, further research that accounts for a range of environmental variables is necessary, especially studies that help elucidate the value of all the space that captive primates are not using.
The data in this study represent an important contribution to the evaluation of captive primate space use, both in terms of the fine-scale measures used and the opportunity to study effects of free-choice outdoor access. However, the lack of comparable published studies makes the interpretation of these results somewhat difficult and it remains unclear whether this selectivity of space use is typical of apes in captive settings. Nevertheless, these data should have substantive consequences for future exhibit designs for chimpanzees, gorillas and related species. In an analysis of captive gorilla space use, Stoinski et al. [2001] suggested that habitual choice of particular areas indicated a high degree of dependence on functionality and that increasing habitat use might be augmented by increasing the functionality. We agree that the provision of additional functional elements in an enclosure may increase the selectivity of space use, but one must consider the ultimate objectives of the captive environment. There is little reason to predict a direct relationship between maximization of space use efficiency and augmentation of psychological wellbeing. The degree to which captive animals are seen throughout their enclosures may be important to managers of zoological parks, where visitor interest and visibility is important to achieve educational objectives, but most managers seek to prioritize environments in which captive animal welfare is maximized. Heterogeneous distribution of space use may indicate the successful provision of desirable resources, such as an elevated viewing point, resting opportunities or visual access to keeper areas [Ross et al., 2009]. Given that the provision of functional elements is likely to be restricted by resources (e.g. construction costs), we suggest that increasing the distribution of space use by primates in captive environments might be achieved by distributing the functional space as evenly as possible across the enclosure. Additionally, techniques such as positive reinforcement training might be utilized to encourage captive animals to use a broader range of their environment and allow them benefit from that experience. Whether these interventions would result in a broader distribution of desirable sites in the environment or an overall weakening of animal preferences for specific locations is difficult to determine with the existing data. The results presented from this study augment our understanding of the ways in which captive primates utilize the space provided to them, but further research that investigates the relationships between captive environments and animal welfare are still very much in need.
ACKNOWLEDGMENTS
The authors thank the numerous Fisher Center interns that collected data for this study and Megan Ross, Kathy Wagner and Marissa Milstein for providing substantial feedback on earlier drafts. Thanks also to Maureen Leahy and the carestaff at RCAA for their logistical support of the study.
Contract grant sponsor: Leo S. Guthman Foundation.
REFERENCES
- Badihi I 2006. The effects of complexity, choice and control on the behaviour and the welfare of captive common marmosets (Callithrix jacchus). Ph.D. Dissertation, University of Stirling, Stirling, UK. [Google Scholar]
- Baker KC, Ross SK. 1998. Outdoor access: the behavioral benefits to chimpanzees. American Journal of Primatology 45:166. [Google Scholar]
- Bellingham L 1998. Behavioural adaptation of a group of Alaotran gentle lemurs Hapalemur griseus alaotrensis to a large, naturalistic enclosure at the Jersey Wildlife Preservation Trust. Dodo 34:167–168. [Google Scholar]
- Bettinger T, Wallis J, Carter T. 1994. Spatial selection in captive adult female chimpanzees. Zoo Biology 13:167–176. [Google Scholar]
- Bloomfield RC, Gillespie G, Hemsworth PH. 2010. The effects of zoo visitors on the behaviour of orang-utans. Proceedings of the 2010 ARAZPA Conference, Healesville, Victoria, Australia. [Google Scholar]
- Bloomsmith MA, Lambeth SP, Haberstroh MD. 1999. Chimpanzee use of enclosures. American Journal of Primatology 49:36. [Google Scholar]
- Brent L, Lee DR, Eichberg J. 1991. Evaluation of a chimpanzee enrichment enclosure. Journal of Medical Primatology 20:29–34. [PubMed] [Google Scholar]
- Buchanan-Smith HM. 1994. Environmental enrichment in captive marmosets and tamarins. Human Innovations and Alternatives in Animal Experimentation 8:559–564. [Google Scholar]
- Buchanan-Smith HM. 1998. Enrichment of marmosets and tamarins—considerations for the care of captive callitrichids In: Field DA, editor Guidelines for environmental enrichment. Bristol, UK: Top Copy; pp 183–201. [Google Scholar]
- Chamove AS, Anderson JR. 1989. Examining environmental enrichment In: Segal EF, editor Housing, care and psychological well-being of captive and laboratory primate. New Jersey: Noyes Publications; pp 183–202. [Google Scholar]
- Clarke S, Juno C, Maple TL. 1982. Behavioral effects of a change in the physical environment: a pilot study of captive chimpanzees. Zoo Biology 1:371–380. [Google Scholar]
- Clubb R, Mason G. 2003. Animal welfare: captivity effects on wide-ranging carnivores. Nature 425:473–474. [DOI] [PubMed] [Google Scholar]
- Coe JC, Fulk R, Brent L. 2001. Chimpanzee facility design In: Brent L, editor The care and management of captive chimpanzees. San Antonio, TX: American Society of Primatologists. [Google Scholar]
- Council of Europe. 2004. European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Final draft of the revision of Appendix A, Guidelines on accommodation and care, (ETS 123), Strasbourg. [Google Scholar]
- Davey G 2006. Relationships between exhibit naturalism, animal visibility and visitor interest in a Chinese zoo. Applied Animal Behaviour Science 96:93–102. [Google Scholar]
- Dickens M 1955. A statistical formula to quantify the “spread of participation” in group discussion. Speech Monographs 22:28–31. [Google Scholar]
- Farmer KH, Unwin S, Cress D, Cox D, Lucas D, Cartwright B, Tooze Z. 2009. Pan African Sanctuary Alliance (PASA) operations manual, 1st ed Portland, OR: PASA. [Google Scholar]
- Hedeen SF. 1982. Utilization of space by captive groups of lowland gorillas (Gorilla g. gorilla). Ohio Journal of Science 82:27–30. [Google Scholar]
- Honess PE, Marin CM. 2006. Enrichment and aggression in primates. Neuroscience and Biobehavioral Reviews 30:413–436. [DOI] [PubMed] [Google Scholar]
- Howell S, Schwandt M, Fritz J, Walker S. 2002. From laboratory to more natural enclosures: maintaining the well-being of captive chimpanzees (Pan troglodytes). Laboratory Primate Newsletter 41:5–9. [Google Scholar]
- Inoue-Murayama M, Weiss A, Morimura N, Tanaka M, Yamagiwa J, Idani G. 2010. Molecular behavioral research in great apes In: Inoue-Murayama M, Kawamura S, Weiss A, editors From genes to behavior. Tokyo: Springer. [Google Scholar]
- International Primatological Society. 1989. IPS International guidelines for the acquisition, care and breeing of nonhuman primates. Primate Report 25:3–27. [Google Scholar]
- Jensvold MLA, Sanz CM, Fouts RS, Fouts DH. 2001. Effect of enclosure size and complexity on the behaviours of captive chimpanzees (Pan troglodytes). Journal of Applied Animal Welfare Science 4:53–69. [Google Scholar]
- Joint Working Group on Refinement. 2009. Refinements in husbandry, care and common procedures for non-human primates: ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Animal 43:S1:1–S1:47. [DOI] [PubMed] [Google Scholar]
- Kerl J, Rothe H. 1996. Influence of cage size and cage equipment on physiology and behavior of common marmosets (Callithrix jacchus). Laboratory Primate Newsletter 35:10–13. [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. 2005. Experience induces structural and biochemical changes in the adult primate brain. Proceedings of the National Academy of Sciences of the United States of America 102:17478–17482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedonia JM. 1987. Effects of housing differences upon activity budgets in captive sifakas (Propithecus verreauxi). Zoo Biology 6:55–67. [Google Scholar]
- Maple TL, Finlay TW. 1986. Evaluating the environments of captive non-human primates In: Benirschke K, editor Primates: the road to self-sustaining populations. New York: Springer. [Google Scholar]
- Markowitz H 1982. Behavioral enrichment in the zoo. New York: Van Nostrand Reinhold Company. [Google Scholar]
- Nakamichi M, Kato E. 2001. Long-term proximity relationships in a captive social group of western lowland gorillas (Gorilla gorilla gorilla). Zoo Biology 20:197–209. [DOI] [PubMed] [Google Scholar]
- Novak MA, Suomi SJ. 1988. Psychological well-being of primates in captivity. American Psychologist 43:765–773. [DOI] [PubMed] [Google Scholar]
- Novak MA, Drewsen KH. 1989. Enriching the lives of captive primates: issues and problems In: Segal EF, editor Housing, care and psychological well-being of captive and laboratory primates. Park Ridge, NY: Noyes Publications; pp 161–182. [Google Scholar]
- Ogden JJ, Finlay TW, Maple TL. 1990. Gorilla adaptations to naturalistic environments. Zoo Biology 9:107–121. [Google Scholar]
- O’Neill P, Novak MA, Suomi SJ. 1991. Normalizing laboratory-reared rhesus macaques (Macaca mulatta) behavior with exposure to complex outdoor enclosures. Zoo Biology 10:237–245. [Google Scholar]
- Paulk HH, Dienske H, Ribbens LG. 1977. Abnormal behavior in relation to cage size in rhesus monkeys. Journal of Abnormal Psychology 86:87–92. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Macedonia JM, Haring D, Simons EL. 1989. Maintenance of primates in captivity for research: the need for naturalistic environments In: Segal EF, editor Housing, care and psychological well-being of captive and laboratory primates. Park Ridge, NY: Noyes Publications. [Google Scholar]
- Perkins LA. 1992. Variables that influence the activity of captive orangutans. Zoo Biology 11:177–186. [Google Scholar]
- Pines MK, Kaplan G, Rogers LJ. 2007. A note on indoor and outdoor housing preferences of common marmosets (Callithrix jacchus). Applied Animal Behaviour Science 108:348–353. [Google Scholar]
- Plowman AB. 2003. A note on a modification of the spread of participation index allowing for unequal zones. Applied Animal Behaviour Science 83:331–336. [Google Scholar]
- Poole TB. 1991. Criteria for the provision of captive environments In: Box HO, editor Primate responses to environmetal changes. London: Chapman and Hall; pp 357–374. [Google Scholar]
- Pruetz JDE, McGrew WC. 2001. What does a chimpanzee need? Using behavior to guide the care and management of captive populations In: Brent L, editor The care and management of captive chimpanzees. San Antonio, TX: American Society of Primatologists. [Google Scholar]
- Remis M 2005. Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. Physical Anthropology 97:413–433. [DOI] [PubMed] [Google Scholar]
- Reynolds V 1965. Some behavioral comparisons between the chimpanzee and the mountain gorilla in the wild. American Anthropologist 67:691–706. [Google Scholar]
- Ross SR. 2006. Issues of choice and control in a pair of captive polar bears (Ursus maritimus). Behavioural Processes 73: 117–120. [DOI] [PubMed] [Google Scholar]
- Ross SR. 2010. Chimpanzee (Pan troglodytes) care manual. Silver Spring, MD: Association of Zoos and Aquariums. [Google Scholar]
- Ross SR, Lukas KE. 2006. Use of space in a non-naturalistic environment by chimpanzees (Pan troglodytes) and lowland gorillas (Gorilla gorilla gorilla). Applied Animal Behaviour Science 96:143–152. [Google Scholar]
- Ross SR, Schapiro SJ, Hau J, Lukas KE. 2009. Space use as an indicator of enclosure appropriateness: a novel measure of captive animal welfare. Applied Animal Behaviour Science 121:42–50. [Google Scholar]
- Ross SR, Wagner KE, Schapiro SJ, Hau J. 2010. Ape behavior in two alternating environments: Comparing exhibit and short-term holding areas. American Journal of Primatology 72:951–959. [DOI] [PubMed] [Google Scholar]
- Ross SR, Wagner KE, Schapiro SJ, Hau J. in press. Transfer and acclimation effects on the behavior of two species of African great apes moved to a novel and naturalistic zoo environment. International Journal of Primatology. [Google Scholar]
- Sambrook TD, Buchanan-Smith HM. 1997. Control and complexity in novel object enrichment. Animal Welfare 6:207–216. [Google Scholar]
- Schapiro SJ, Lambeth SP. 2007. Control, choice and assessments of the value of behavioral management to nonhuman primates in captivity. Journal of Applied Animal Welfare Science 10:39–47. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez SA, Porter LM. 1997. A comparison of the effects of simple versus complex environmental enrichment on the behaviour of group-housed, subadult rhesus macaques. Animal Welfare 6:17–28. [Google Scholar]
- Seidensticker J, Doherty JG. 1996. Integrating animal behavior and exhibit design In: Kleiman DG, Allen ME, Thompson KV, Lumpkin S, editors Wild mammals in captivity: principles and techniques. Chicago, IL: University of Chicago Press. [Google Scholar]
- Snowdon CT, Savage A. 1989. Psychological well-being of captive primates: general considerations and examples from callitrichids In: Segal EF, editor Housing, care and psychological well-being of captive and laboratory primates. Park Ridge, NY: Noyes Publications. [Google Scholar]
- Stoinski TS, Hoff MP, Maple TL. 2001. Habitat use and structural preferences of captive western lowland gorillas (Gorilla gorilla gorilla): effects of environmental and social variables. International Journal of Primatology 22:431–447. [Google Scholar]
- Traylor-Holzer K, Fritz P. 1985. Utilization of space by adult and juvenile groups of captive chimpanzees (Pan troglodytes). Zoo Biology 4:115–127. [Google Scholar]
- Tutin CG, Parnell RJ, White LJT, Fernandez M. 1995. Nest building by lowland gorillas in the Lope Reserve, Gabon: environmental influences and implications for censusing. International Journal of Primatology 16:53–76. [Google Scholar]
- USDA, United States Department of Agriculture. 1991. Animal Welfare Act as Amended (7 USC, 2131–2156) Federal Register. [Google Scholar]
- Wilson SF. 1982. Environmental influences on the activity of captive apes. Zoo Biology 1:201–209. [Google Scholar]
- Videan EN, Fritz J, Schwandt ML, Smith HF, Howell S. 2005. Controllability in environmental enrichment for captive chimpanzees (Pan troglodytes). Journal of Applied Animal Welfare Science 8:117–130. [DOI] [PubMed] [Google Scholar]
- Yamagiwa J 1999. Socioecological factors influencing population structure of gorillas and chimpanzees. Primates 40: 87–104. [DOI] [PubMed] [Google Scholar]


