Abstract
Purpose of Review:
This review article focuses on recent advances in the approach to the diagnosis and treatment of human herpesvirus 6B (HHV-6B) in hematopoietic cell and solid organ transplant recipients.
Recent Findings:
Over the past few years, key studies have broadened our understanding of best practices for the prevention and treatment of HHV-6B encephalitis after transplantation. Moreover, important data have been reported that support a potential role of HHV-6B reactivation in the development of acute graft-versus-host disease and lower respiratory tract disease in transplant recipients. Finally, increasing recognition of inherited chromosomally integrated HHV-6 (iciHHV-6) and an expanding array of diagnostic tools have increased our understanding of the potential for complications related to viral reactivation originating from iciHHV-6 in donor allografts and recipients.
Summary:
Recent advances in diagnostic tools, disease associations, and potential treatments for HHV-6B present abundant opportunities for improving our understanding and management of this complex virus in transplant recipients.
Keywords: herpes, HHV, HHV-6, transplant, organ
Introduction
Human herpesvirus 6B (HHV-6B) is a betaherpesvirus that infects most individuals within the first 3 years of life and is the cause of Roseola.[1] Like other herpesviruses, HHV-6B results in a chronic infection, and most adults are considered to be infected. Unlike other human herpesviruses, HHV-6B can integrate into chromosomes as a mechanism of latency, which may result in the unique condition of inherited chromosomally integrated HHV-6 (iciHHV-6).[2] HHV-6B accounts for the majority, if not all, of HHV-6 species reactivation and disease after hematopoietic cell transplantation (HCT) and solid organ transplantation (SOT); the closely related species HHV-6A is rarely detected. Systemic reactivation of HHV-6B based on detection of HHV-6B DNA in blood is frequent after HCT and SOT.[3–8] While HHV-6B is the most frequent infectious cause of encephalitis after HCT,[9] its role in other post-transplant diseases is less clear. The role of iciHHV-6 (species A or B) in graft rejection and other diseases after transplantation is an emerging area of investigation. In this review, I will focus on recent advances in the diagnosis and treatment of HHV-6B and iciHHV-6 after transplantation.
HHV-6 reactivation and associated diseases in transplant recipients
Definitions
Throughout this review, HHV-6 will be specified as species A or B when relevant. Furthermore, I will distinguish between HHV-6 acquired through post-natal infection versus iciHHV-6. Notably, iciHHV-6 results from vertical transmission of HHV-6A or HHV-6B that has integrated its viral genome into the chromosome of a germ cell after primary infection.[10] As a result, every nucleated cell will contain ≥1 copy HHV-6 DNA, which can confound diagnostic and treatment strategies.
Epidemiology
HHV-6A reactivation is rarely identified after transplantation, and no disease has been causally linked with HHV-6A.[11,12] When HHV-6A is detected, this is typically in the context of iciHHV-6A. Conversely, HHV-6B reactivation is frequent after transplantation, affecting approximately 40% of subjects within the first few months.[4,5] HHV-6B is the most frequent infectious cause of encephalitis after HCT.[9] HHV-6B encephalitis develops in approximately 1% of allogeneic HCT recipients but is even more frequent in recipients of umbilical cord blood or selective T-cell depleted HCT.[13,14] Patients who develop HHV-6B encephalitis typically have concurrent detection of HHV-6B in the plasma with viral loads ≥10,000 copies/mL.[15,16] HHV-6B is also associated with other central nervous system (CNS) symptoms and diseases, but it is important to note that HHV-6B DNA can be found in cerebrospinal fluid (CSF) in patients without CNS symptoms.[17,18]
Evidence for a causal association of HHV-6B or iciHHV-6 with other diseases post-transplantation are limited. Recent data implicate HHV-6B in the pathogenesis of acute graft-versus-host disease[8,19] and post-transplant pneumonia syndromes,[20–22] although definitive evidence from controlled treatment trials are lacking. As recognition of iciHHV-6 increases, more cases of potential HHV-6 reactivation from the integrated virus in donor allograft products or from the recipient are being reported. Population-based studies have identified iciHHV-6 in approximately 1% of individuals, and HHV-6B accounts for approximately one-third of these cases.[2] Associated manifestations have included fever, rash, and organ rejection.[23–26] One large study of allogeneic HCT recipients demonstrated an increased risk for grades 2–4 acute graft-versus-host disease in transplants involving either donor or recipient iciHHV-6.[27]
Diagnostic Strategies
Diagnostic assays for HHV-6 and iciHHV-6
The primary approach to diagnosis of HHV-6 infections relies on quantitative polymerase chain reaction (PCR) assays to identify HHV-6 A or B DNA in clinical specimens, and a variety of tests are available. Most clinically available assays do not differentiate between HHV-6A and HHV-6B, and inter-assay variability remains large.[28] As laboratories begin to adopt the newly available World Health Organization international standard for HHV-6B, generalizability of viral load results and establishment of treatment thresholds may improve.[29] A multiplex qualitative PCR panel including HHV-6 for acute meningitis/encephalitis, the FilmArray Meningitis/Encephalitis (ME) panel (Biofire), was approved by the Food and Drug Administration for diagnostic use on cerebrospinal fluid (CSF) and has been widely adopted. However, there is concern for a high false-positive rate for HHV-6B encephalitis when the test is used in clinical contexts with a low pre-test probability for this condition.[18]
Interpretation of HHV-6 PCR results must be considered in light of the possibility of iciHHV-6 in the patient or the donated allograft. In individuals with iciHHV-6, latent viral DNA will typically be detected by PCR testing in all clinical samples. However, this may not indicate viral replication, and as such, antivirals will not reduce the viral load. In cellular samples such as whole blood, HHV-6 viral loads are typically >106 copies/ml representing 1 copy of HHV-6 per cell, although the quantification will vary by sample type and cellularity.[2] The level of DNA detected in acellular samples such as serum or plasma is variable depending on the timing of separation from whole blood but typically more than 10–100 fold lower than in whole blood.[30,31] A patient who receives an HCT from a donor with iciHHV-6 will have persistently detectable HHV-6 DNA in blood after hematopoietic cell engraftment, and the viral load will correlate with absolute lymphocyte count.[32,33] If the HCT recipient has iciHHV-6, HHV-6 DNA will be detectable in blood before HCT and will decrease in parallel with recipient lymphocytes after HCT. However, HHV-6 DNA will continue to be detected at high levels in non-hematopoietic tissue throughout the body and may be found in blood or other fluid samples (e.g. CSF) if there is organ damage. In the context of SOT using an organ from an individual with iciHHV-6, high levels will be detected in the organ with possible detection in blood samples.[25]
Droplet digital PCR has been validated as a well-suited test to identify iciHHV-6 in cellular samples. This technique uses the same methodology as real-time PCR but partitions the reaction into thousands of individual droplets, which are each read as positive or negative for DNA template to provide absolute quantitation of target DNA without the use of a standard curve. This allows the comparison of the number of HHV-6 A or B copies to the number of human cells in the sample: a ratio of ~1 copy of HHV-6 per human cell suggests iciHHV-6.[31,34,35] Comparison of two quantitative PCR results (one for HHV-6 and one for a human gene) may also suffice but may have a larger margin of error due to assay imprecision. The optimal sample to test depends on the suspected source of iciHHV-6 (i.e. donor or recipient).
The use of PCR to detect viral DNA to establish viral replication has important limitations, because HHV-6B can establish latency in diverse cell types throughout the body. [36] This is particularly problematic when testing cellular samples and/or in the context of donor or recipient iciHHV-6, where detection of latent viral DNA confounds interpretation of active versus inactive infection. Molecular detection of HHV-6B gene transcripts using reverse transcription real-time qPCR (RT-qPCR) has potential to improve the specificity of diagnostic studies for HHV-6B reactivation in highly cellular samples (e.g. tissue biopsies) and in individuals with iciHHV-6B. This method may have better accuracy for identifying active infection, and studies using this approach have demonstrated relatively high sensitivity and specificity for viral replication in vivo.[37–40] However, these assays are generally restricted to research laboratories and are not yet widely available. Use of RT-qPCR for prognostic or treatment purposes for HHV-6 will require optimization and establishment of actionable thresholds. Additional research-based methods of immunohistochemistry and in situ hybridization are in development and will play an important role for identifying active infection,[40,41] although clinical adoption of these techniques is currently limited by their complexity, expense, and variable sensitivity.
Clinical implementation of HHV-6 testing
Routine surveillance testing of blood for HHV-6B, and testing of transplant donors or recipients for iciHHV-6, is generally not recommended due to the lack of clearly actionable preemptive treatment thresholds and the poor efficacy of preemptive therapy as detailed below. Rather, given the low positive predictive value of HHV-6B detection in blood for end-organ disease, testing should be based on symptoms in appropriate clinical contexts. Quantitative PCR for HHV-6 DNA is the preferred method for testing blood (plasma preferred) and other fluid samples for viral reactivation. Positive results from PCR identifying HHV-6 DNA in tissue samples are challenging to interpret as detailed above, although quantitation may help. Ideally, HHV-6 PCR assays should distinguish between species A and B. If treatment is initiated and HHV-6 DNA detection in plasma or CSF does not decrease by >1 log10 copies/mL after >2 weeks of antiviral treatment therapy, or if HHV-6 DNA is persistently detected for ≥3 consecutive weeks, testing for iciHHV-6 should be considered. Detection of HHV-6A species DNA in any sample should also prompt consideration of iciHHV-6. Figure 1 provides an approach to the clinical evaluation of patients with suspected HHV-6-associated symptoms.
Figure 1. Evaluation for, and management of, suspected HHV-6 disease after hematopoietic cell or solid organ transplantation.

qPCR indicates quantitative PCR; CSF, cerebrospinal fluid; ID, infectious diseases.
AHHV-6B DNA is typically detected in blood by PCR in patients with HHV-6B encephalitis. If the plasma or CSF HHV-6B viral load does not decrease >1 log10 copies/ml after ≥2 weeks of therapy or is persistently positive for ≥3 consecutive weeks, test for iciHHV-6.
BAny detection of HHV-6 species A or B DNA in CSF is considered abnormal. However, HHV-6 DNA can be detected in the CSF in the absence of clinical symptoms[17] or when the pre-test probability for HHV-6B encephalitis is low.[18] Close monitoring without treatment can be considered in select cases with the guidance of an ID consult. These recommendations are based on expert opinion and adapted from the 7th European Conference on Infections in Leukaemia (ECIL) 2017 guidelines.[58]
Treatment strategies
Anti-HHV-6 therapies
Foscarnet was recently approved for the treatment of HHV-6B encephalitis by Japan’s Ministry of Health, Labor and Welfare.[42] This is the only treatment with approval from a Regulatory Authority for HHV-6. Foscarnet and ganciclovir both remain the first-line recommended treatments for HHV-6B[43] based on their in vitro activity[44] and evidence of in vivo effectiveness.[45] There are insufficient data to recommend foscarnet over ganciclovir for treatment of HHV-6B, and the choice of antivirals should be guided by the clinical context and potential drug toxicities. The largest study to date demonstrated similar response rates of neurologic symptoms (~70–80%) with foscarnet or ganciclovir treatment in a retrospective analysis of a Japanese registry of 145 HCT recipients.[13] In this study, induction dosing with foscarnet at 90 mg/kg every 12 hours or ganciclovir at 5 mg/kg every 12 hours was associated with a significantly higher response rate than treatment with lower doses. Of 10 patients receiving combination therapy, all responded, but there are insufficient data to support the routine use of combination therapy. It is important to note that despite initial responses, neuropsychological sequelae remained in 57% of 121 evaluated patients, and patients with HHV-6B encephalitis had significantly increased mortality by 100 days post-HCT. Cidofovir and brincidofovir (CMX001), an experimental prodrug of cidofovir conjugated to a lipid to allow for higher intracellular and lower plasma concentrations of cidofovir,[46] have the lowest EC50 values for HHV-6B in vitro (Figure 2).[44] Brincidofovir is a promising drug for HHV-6B prophylaxis or treatment but is not currently available for clinical use. Given the substantial risk for side effects from currently available antivirals, including myelosuppression (ganciclovir) and nephrotoxicity (foscarnet, cidofovir) among others, treatment should typically be reserved for patients with a high level of suspicion for end-organ disease and virologic evidence of active infection with HHV-6B as detailed above.
Figure 2. In vitro activity of currently available and investigational antiviral agents for HHV-6B.
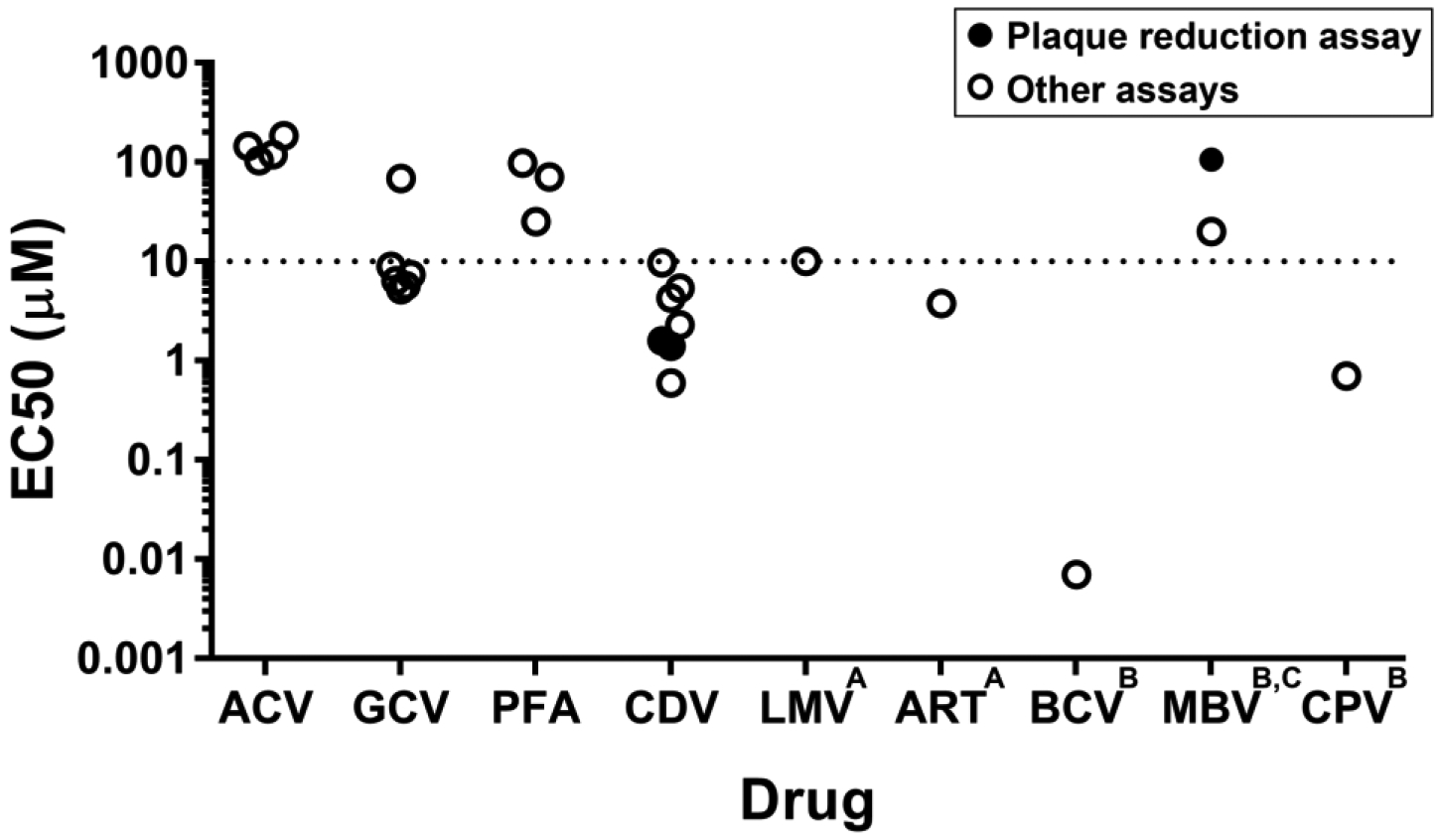
ACV indicates acyclovir; GCV, ganciclovir; PFA, foscarnet; CDV, cidofovir; LMV, letermovir; ART, artesunate; BCV, brincidofovir; MBV, maribavir; CPV, cyclopropavir.
This figure shows the range of in vitro activity reported for antiviral compounds that are currently available or in development. The data represent the half-maximal effective concentration (EC50) of the compounds in vitro, or the concentration at which inhibition of viral growth is 50% of the maximum response within the specified exposure time. For the purposes of categorizing the in vitro activity data, an EC50 value of 10 μM was selected as a threshold for determining the relative strength. A variety of methods were used in different studies to generate these data; data generated by a plaque reduction assay, which is generally considered the gold standard, are in solid circles.
These data are adapted from Chemaly et al, Antiviral Res. 2019 Mar;163:50–58.44
AThe reported EC50 values for LMV and ART are from studies using HHV-6A; data for HHV-6B are unavailable.
BNot clinically available.
CValues shown represent the minimum detected value (primary data reported as >20 μM and >106 μM).
Adoptive immunotherapy with virus-specific T-cells is an exciting new therapeutic approach to treat viruses after HCT and SOT.[47,48] HHV-6-specific T cells appear to be safe and potentially effective after HCT for refractory HHV-6 viremia in a small, uncontrolled study.[47] In this study, one patient with HHV-6 encephalitis that was unresponsive to antiviral therapy was successfully treated with HHV-6-specific T cells without any reported adverse events.
Is there a role for prophylactic or preemptive therapy for HHV-6B?
Prophylactic or preemptive strategies to prevent HHV-6B encephalitis after HCT have been unsuccessful to date. Multiple studies demonstrate that a plasma HHV-6B viral load ≥10,000 copies/ml has up to 100% sensitivity and 81% specificity for the subsequent development of HHV-6B encephalitis.[15,16] However, in two prospective, non-randomized studies in HCT recipients with an HHV-6B plasma viral load ≥10,000 copies/ml, preemptive ganciclovir or foscarnet did not significantly reduce the risk for HHV-6B encephalitis.[49,50] Additionally, in three prospective, non-randomized studies in HCT recipients (pre- or post-engraftment), prophylactic foscarnet did not significantly reduce the rate of HHV-6B encephalitis, although the incidence and severity of HHV-6B DNA detection in plasma was significantly lower in patients receiving prophylactic antivirals.[51–53]. Failure of preemptive and prophylactic approaches have been attributed to the rapid onset of end-organ disease after first detection of plasma viremia,[54] inadequate dosing due to concerns about drug toxicity, and the possibility of poor drug penetration into the CSF. In a post-hoc analysis of a randomized, double-blind, placebo-controlled trial of oral brincidofovir for CMV prophylaxis after allogeneic HCT,[55] HHV-6B DNA detection in plasma was significantly reduced in a subgroup of participants who were randomized within 2 weeks of HCT and who received at least 6 doses of brincidofovir within the first 3 weeks of randomization compared to those receiving placebo.[56] An intravenous formulation of brincidofovir is in development that achieves high levels in the CNS and may be more effective at preventing encephalitis than foscarent, but brincidofovir is not clinically available at this time. Thus, screening for HHV-6B viremia, and prophylactic or preemptive therapy, should not be routinely performed given the toxicities of currently available treatments and a lack of evidence to support the efficacy of early intervention with these treatments.
Treatment for HHV-6B encephalitis
In transplant recipients with signs or symptoms of encephalitis without another clear etiology, empiric treatment for HHV-6B should be considered while workup is initiated (Figure 1). The recommended antiviral dosing regimens for HHV-6B encephalitis are ganciclovir 5 mg/kg IV every 12 hours or foscarnet 90 mg/kg IV every 12 hours.[43] Antiviral treatment for HHV-6B encephalitis in transplant recipients should generally be administered for at least 3 weeks and until clearance of HHV-6B from the blood and CSF. Repeat CSF testing for HHV-6B should be performed, if feasible, to guide antiviral treatment and duration. Reduction of immunosuppression should also be attempted if feasible.
There are insufficient data to recommend treatment of other HHV-6B-associated end-organ diseases aside from encephalitis. However, this should be considered on a case-by-case basis and guided by clinical and diagnostic findings. Provocative data are emerging about the potential role of HHV-6B in other post-transplant complications such lower respiratory tract disease,[20–22] but the efficacy of HHV-6B-targeted therapy outside of HHV-6B encephalitis remains to be established.
Conclusion
HHV-6B reactivation is frequent in transplant recipients, and HHV-6B encephalitis can result in substantial morbidity. Determining the etiologic role of HHV-6B reactivation, or the condition of iciHHV-6, in other complications after transplant requires additional study. Near-ubiquitous infection with HHV-6B early in life, coupled with HHV-6B latency in diverse cell types and the unique condition of iciHHV-6, complicate interpretation of molecular diagnostic assays such as PCR for viral genomic DNA, which do not necessarily identify actively replicating virus. Given the toxicity of currently available antiviral treatments, and the lack of a reduction in the incidence of HHV-6B encephalitis when prophylactic or preemptive treatment with ganciclovir or foscarnet were studied, neither routine testing for HHV-6B in blood nor preemptive therapy are indicated. As treatment and supportive care for transplant recipients evolve, we must continue to monitor for potential changes in the epidemiology of infectious complications such as HHV-6B. For example, implementation of letermovir for CMV prophylaxis after allogeneic HCT may substantially reduce the use of broader-spectrum antivirals such as ganciclovir and foscarnet,[57] but letermovir has low in vitro activity against HHV-6. Further study is needed to establish the role of HHV-6B and iciHHV-6 in associated post-transplant complications, and the development of safe and effective strategies to mitigate HHV-6B reactivation warrant continued attention.
Key Points.
HHV-6B reactivation is frequent in transplant recipients, and HHV-6B encephalitis can result in substantial morbidity.
The etiologic role of HHV-6B, HHV-6A, or the condition of iciHHV-6 in other complications after transplant require further study.
Recognition of iciHHV-6 in allograft products or transplant recipients is important for interpretation of HHV-6 test results and clinical management.
Routine testing for HHV-6B in blood, and prophylactic or preemptive treatment approaches, are not supported by the data for currently available antiviral agents.
Financial support and sponsorship:
This work was supported by a grant from the NIAID (National Institute of Allergy and Infectious Diseases; K23 AI119133).
Footnotes
Conflicts of interest:
J.A.H. has served as a consultant for Nohla Therapeutics, Inc. and Amplyx and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire), all unrelated to the material in this publication.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, (18 months/ 2017–2019) have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang M-L, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, et al. : A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005, 352:768–76. [DOI] [PubMed] [Google Scholar]
- 2.Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, Flamand L, Gautheret-Dejean A, Hall CB, Kamble RT, et al. : Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 2012, 22:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellett Madan R, Hand J: Human herpesvirus 6, 7, and 8 in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019, doi: 10.1111/ctr.13518. ••Updated guidelines for the management of HHV-6 after solid organ transplantation.
- 4.Hill JA, Mayer BT, Xie H, Leisenring WM, Huang M-L, Stevens-Ayers T, Milano F, Delaney C, Sorror ML, Sandmaier BM, et al. : The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017, 129. •Demonstration of the pattern of HHV-6B reactivation after multiple types of allogeneic HCT regimens.
- 5.Huang Y-T, Kim SJ, Lee YJ, Burack D, Nichols P, Maloy M, Perales M-A, Giralt SA, Jakubowski AA, Papanicolaou GA: Co-Infections by Double-Stranded DNA Viruses after Ex Vivo T Cell–Depleted, CD34+ Selected Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2017, 23:1759–1766. •Demonstration of the pattern of HHV-6B reactivation after T cell-depleted allogeneic HCT.
- 6.Balsat M, Pillet S, Tavernier E, Cacheux V, Escuret V, Moluçon-Chabrot C, Augeul-Meunier K, Mirand A, Regagnon C, Tinquaut F, et al. : Human herpesvirus 6 infection after autologous stem cell transplantation: A multicenter prospective study in adult patients. J Infect 2019, 79:36–42. [DOI] [PubMed] [Google Scholar]
- 7.de Koning C, Admiraal R, Nierkens S, Boelens JJ: Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv 2018, 2:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Admiraal R, de Koning C, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, Wolfs TFW, Nierkens S, Boelens JJ: Viral Reactivations and Associated Outcomes in Context of Immune Reconstitution after Pediatric Hematopoietic Cell Transplantation. J Allergy Clin Immunol 2017, doi: 10.1016/j.jaci.2016.12.992. •Insight into the association between HHV-6 reactivation and acute GVHD.
- 9.Abidi MZ, Hari P, Chen M, Kim S, Battiwala M, Dahi PB, Diaz MA, Gale RP, Ganguly S, Gergis U, et al. : Virus detection in the cerebrospinal fluid of hematopoietic stem cell transplant recipients is associated with poor patient outcomes: a CIBMTR contemporary longitudinal study. Bone Marrow Transplant 2019, doi: 10.1038/s41409-019-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellett P, Ablashi D: Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 2012, doi: 10.1002/rmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zerr DM, Boeckh M, Delaney C, Martin PJ, Xie H, Adler AL, Huang M-L, Corey L, Leisenring WM: HHV-6 Reactivation and Associated Sequelae after Hematopoietic Cell Transplant. Biol Blood Marrow Transplant 2012, 18:1700–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razonable RR: Human herpesviruses 6, 7 and 8 in solid organ transplant recipients. Am J Transplant 2013, 13:67–78. [DOI] [PubMed] [Google Scholar]
- 13.Ogata M, Oshima K, Ikebe T, Takano K, Kanamori H, Kondo T, Ueda Y, Mori T, Hashimoto H, Ogawa H, et al. : Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017, 52:1563–1570. ••Largest study to date of the clinical characteristics of, risk factors for, and management of HHV-6 encephalitis.
- 14.Perruccio K, Sisinni L, Perez-Martinez A, Valentin J, Capolsini I, Massei MS, Caniglia M, Cesaro S: High Incidence of Early Human Herpesvirus-6 Infection in Children Undergoing Haploidentical Manipulated Stem Cell Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant 2018, 24:2549–2557. [DOI] [PubMed] [Google Scholar]
- 15.Hill J a, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, Armand P, Alyea EP, Baden LR, Antin JH, et al. : Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant 2012, 18:1638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogata M, Satou T, Kadota J-I, Saito N, Yoshida T, Okumura H, Ueki T, Nagafuji K, Kako S, Uoshima N, et al. : Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis After Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clin Infect Dis 2013, 57:671–81. [DOI] [PubMed] [Google Scholar]
- 17.Hill JA, Boeckh MJ, Sedlak RH, Jerome KR, Zerr DM: Human herpesvirus 6 can be detected in cerebrospinal fluid without associated symptoms after allogeneic hematopoietic cell transplantation. J Clin Virol 2014, 61:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green DA, Pereira M, Miko B, Radmard S, Whittier S, Thakur K: Clinical Significance of Human Herpesvirus 6 Positivity on the FilmArray Meningitis/Encephalitis Panel. Clin Infect Dis 2018, 67:1125–1128. ••Insight into the high false-positivity rate of the FilmArray meningitis/encephalitis panel for HHV-6 detection in CSF when used in patients with a low pre-test probability.
- 19.Phan TL, Carlin K, Ljungman P, Politikos I, Boussiotis V, Boeckh M, Shaffer ML, Zerr DM: Human Herpesvirus-6B Reactivation Is a Risk Factor for Grades II to IV Acute Graft-versus-Host Disease after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transpl 2018, 24:2324–2336. ••Systematic review and meta-analysis demonstrating a significant association between HHV-6B and subsequent grades II to IV acute GVHD.
- 20.Zhou X, O’Dwyer DN, Xia M, Miller HK, Chan PR, Trulik K, Chadwick MM, Hoffman TC, Bulte C, Sekerak K, et al. : First Onset Herpesviral Infection and Lung Injury in Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med 2019, doi: 10.1164/rccm.201809-1635OC. ••Demonstration of an association between HHV-6 reactivation and idiopathic pneumonia syndrome.
- 21.Seo S, Renaud C, Kuypers JM, Chiu CY, Huang M-L, Samayoa E, Xie H, Yu G, Fisher CE, Gooley TA, et al. : Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015, 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J, Vande Vusse L, Xie H, Chung EL, Yeung C, Seo S, Stevens-Ayers T, Fisher C, Huang ML, Stewart FM, et al. : Human Herpesvirus 6B and Lower Respiratory Tract Disease after Hematopoietic Cell Transplantation. J Clin Oncol 2019, in press. ••Large study showing that HHV-6B DNA detection in bronchoalveolar lavage fluid after HCT was associated with increased overall mortality and death due to respiratory failure.
- 23.Endo A, Watanabe K, Ohye T, Suzuki K, Matsubara T, Shimizu N, Kurahashi H, Yoshikawa T, Katano H, Inoue N, et al. : Molecular and virological evidence of viral activation from chromosomally integrated human herpesvirus 6A in a patient with X-linked severe combined immunodeficiency. Clin Infect Dis 2014, 59:545–8. [DOI] [PubMed] [Google Scholar]
- 24.Komaroff AL, Phan T, Flamand L, Pellett PE: Summary of the 9th international conference on human herpesviruses 6 and 7 (HHV-6A, HHV-6B, and HHV-7). J Med Virol 2016, 88:2038–2043. ••Demonstration of likely reactivation of HHV-6A from a liver allograft with iciHHV-6A.
- 25.Bonnafous P, Marlet J, Bouvet D, Salamé E, Tellier A-C, Guyetant S, Goudeau A, Agut H, Gautheret-Dejean A, Gaudy-Graffin C: Fatal outcome after reactivation of inherited chromosomally integrated HHV-6A (iciHHV-6A) transmitted through liver transplantation. Am J Transplant 2018, 18:1548–1551. [DOI] [PubMed] [Google Scholar]
- 26.Politikos I, McMasters M, Bryke C, Avigan D, Boussiotis VA: Possible reactivation of chromosomally integrated human herpesvirus 6 after treatment with histone deacetylase inhibitor. Blood Adv 2018, 2:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JA, Magaret AS, Hall-Sedlak R, Mikhaylova A, Huang M-L, Sandmaier BM, Hansen JA, Jerome KR, Zerr DM, Boeckh M: Outcomes of hematopoietic cell transplantation using donors or recipients with inherited chromosomally integrated HHV-6. Blood 2017, 130. ••Large study demonstrating an association between iciHHV-6 and acute GVHD after HCT.
- 28.de Pagter PJ, Schuurman R, de Vos NM, Mackay W, van Loon AM: Multicenter external quality assessment of molecular methods for detection of human herpesvirus 6. J Clin Microbiol 2010, 48:2536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govind S, Hockley J, Morris C. World Health Organization & WHO Expert Committee on Biological Standardization. (2017). Collaborative study to establish the 1st WHO international standard for human herpes virus 6B (HHV-6B) DNA for nucleic acid amplification technique (NAT)-based assays. World Health Organization https://apps.who.int/iris/handle/10665/260259
- 30.Ward K, Leong H: Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol 2006, 44:1571–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedlak RH, Cook L, Huang M-L, Magaret A, Zerr DM, Boeckh M, Jerome KR: Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin Chem 2014, 60:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purev E, Winkler T, Danner RL, Fahle GA, Cook L, Zerr DM, Jerome KR, Childs RW: Engraftment of donor cells with germ-line integration of HHV6 mimics HHV6 reactivation following cord blood/haplo transplantation. Blood 2014, 124:1198–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada Y, Osumi T, Imadome K-I, Takahashi E, Ohye T, Yoshikawa T, Tomizawa D, Kato M, Matsumoto K: Transmission of chromosomally integrated human herpesvirus 6 via cord blood transplantation. Transpl Infect Dis 2017, 19:e12636. [DOI] [PubMed] [Google Scholar]
- 34.Sedlak RH, Hill JA, Nguyen T, Cho M, Levin G, Cook L, Huang M-L, Flamand L, Zerr DM, Boeckh M, et al. : Detection of human herpesvirus 6B (HHV-6B) reactivation in hematopoietic cell transplant recipients with inherited chromosomally integrated HHV-6A by droplet digital PCR. J Clin Microbiol 2016, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellucci A, Leibovitch EC, Jacobson S: Using droplet digital PCR to detect coinfection of human herpesviruses 6a and 6b (HHV-6a and HHV-6b) in clinical samples. In Methods in Molecular Biology. . 2018:99–109. ••Detailed description of a ddPCR procedure for multiplexed detection of HHV-6A and HHV-6B.
- 36.Hill JA, Zerr DM: Roseoloviruses in transplant recipients: clinical consequences and prospects for treatment and prevention trials. Curr Opin Virol 2014, 9:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ihira M, Enomoto Y, Kawamura Y, Nakai H, Sugata K, Asano Y, Tsuzuki M, Emi N, Goto T, Miyamura K, et al. : Development of quantitative RT-PCR assays for detection of three classes of HHV-6B gene transcripts. J Med Virol 2012, 84:1388–95. [DOI] [PubMed] [Google Scholar]
- 38.Bressollette-Bodin C, Nguyen TVH, Illiaquer M, Besse B, Peltier C, Chevallier P, Imbert-Marcille B-M: Quantification of two viral transcripts by real time PCR to investigate human herpesvirus type 6 active infection. J Clin Virol 2014, 59:94–9. [DOI] [PubMed] [Google Scholar]
- 39.Hill JA, Ikoma M, Zerr DM, Basom RS, Peddu V, Huang M-L, Hall Sedlak R, Jerome KR, Boeckh M, Barcy S: RNA Sequencing of the In Vivo Human Herpesvirus 6B Transcriptome To Identify Targets for Clinical Assays Distinguishing between Latent and Active Infections. J Virol 2018, 93. •Unbiased RNA sequencing approach to identify diagnostic targets for HHV-6B replication.
- 40.Prusty BK, Gulve N, Chowdhury SR, Schuster M, Strempel S, Descamps V, Rudel T: HHV-6 encoded small non-coding RNAs define an intermediate and early stage in viral reactivation. npj Genomic Med 2018, 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford JR, Santi MR, Thorarinsdottir HK, Cornelison R, Rushing EJ, Zhang H, Yao K, Jacobson S, Macdonald TJ: Detection of human herpesvirus-6 variants in pediatric brain tumors: association of viral antigen in low grade gliomas. J Clin Virol 2009, 46:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinigen’s Foscavir gains approval for HHV-6 encephalitis in Japan | Clinigen. https://www.clinigengroup.com/news/news-container/2019/clinigen-s-foscavir-gains-approval-for-hhv-6-encephalitis-in-japan/ 2019,
- 43.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young J-AH, Boeckh MJ, Boeckh MA: Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009, 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chemaly RF, Hill JA, Voigt S, Peggs KS: In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antiviral Res 2019, 163:50–58. •Detailed summary of the in vitro activity of antiviral compounds for HHV-6A and HHV-6B.
- 45.Zerr DM, Gupta D, Huang M-L, Carter R, Corey L: Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002, 34:309–17. [DOI] [PubMed] [Google Scholar]
- 46.Florescu DF, Keck MA: Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther 2014, 12:1171–1178. [DOI] [PubMed] [Google Scholar]
- 47.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, et al. : Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2017, doi: 10.1200/JCO.2017.73.0655. ••Demonstration of the feasibility of adoptive immunotherapy with HHV-6-specific T cells for treating HHV-6B after HCT.
- 48.Smith C, Beagley L, Rehan S, Neller MA, Crooks P, Solomon M, Holmes-Liew C-L, Holmes M, McKenzie SC, Hopkins P, et al. : Autologous Adoptive T-cell Therapy for Recurrent or Drug-resistant Cytomegalovirus Complications in Solid Organ Transplant Recipients: A Single-arm Open-label Phase I Clinical Trial. Clin Infect Dis 2019, 68:632–640. [DOI] [PubMed] [Google Scholar]
- 49.Ogata M, Satou T, Kawano R, Goto K, Ikewaki J, Kohno K, Ando T, Miyazaki Y, Ohtsuka E, Saburi Y, et al. : Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant 2008, 41:279–85. [DOI] [PubMed] [Google Scholar]
- 50.Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S: Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant 2011, 46:863–9. [DOI] [PubMed] [Google Scholar]
- 51.Ishiyama K, Katagiri T, Ohata K, Hosokawa K, Kondo Y, Yamazaki H, Takami A, Nakao S: Safety of pre-engraftment prophylactic foscarnet administration after allogeneic stem cell transplantation. Transpl Infect Dis 2012, 14:33–39. [DOI] [PubMed] [Google Scholar]
- 52.Ogata M, Satou T, Inoue Y, Takano K, Ikebe T, Ando T, Ikewaki J, Kohno K, Nishida A, Saburi M, et al. : Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transpl 2013, 48:257–264. [DOI] [PubMed] [Google Scholar]
- 53.Ogata M, Takano K, Moriuchi Y, Kondo T, Ueki T, Nakano N, Mori T, Uoshima N, Nagafuji K, Yamasaki S, et al. : Effects of Prophylactic Foscarnet on Human Herpesvirus-6 Reactivation and Encephalitis in Cord Blood Transplant Recipients: A Prospective Multicenter Trial with an Historical Control Group. Biol Blood Marrow Transplant 2018, 24:1264–1273. ••Prospective study of prophylactic foscarnet to prevent HHV-6 reactivation and encephalitis after cord blood HCT.
- 54.Hill JA, Mayer BT, Xie H, Leisenring WM, Huang M-L, Stevens-Ayers T, Milano F, Delaney C, Jerome KR, Zerr DM, et al. : Kinetics of Double-Stranded DNA Viremia after Allogeneic Hematopoietic Cell Transplantation. Clin Infect Dis 2018, 66. •Demonstration of risk factors for persistent HHV-6B viremia and the temporal association between HHV-6B plasma detection and HHV-6B encephalitis.
- 55.Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, Chittick G, Brundage TM, Wilson C, Morrison ME, et al. : A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2019, 25:369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill JA, Zerr D, Nichols G, Brundage TM, Lanier R, Boeckh MJ: Oral Brincidofovir Decreased HHV-6 Viremia in Hematopoietic Cell Transplant Recipients: Results from the Suppress Study. Biol Blood Marrow Transplant 2019, 25:S358. •Demonstration of the efficacy of prophylactic brincidofovir to prevent HHV-6B reactivation after allogeneic HCT.
- 57.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, et al. : Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med 2017, doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 58.Ward K, Hill J, Hubacek P, de la Camara R, Crocchiolo R, Einsele H, Navarro D, Robin C, Cordonnier C, Ljungman P: Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematological malignancies and after hematopoietic stem cell transplantation. Haematologica 2019, in press ••Guidelines for the management of HHV-6 in hematological malignancies.


