Abstract
Background -
Atrial fibrillation (AF) may occur after an acute precipitant and subsequently resolve. Management guidelines for AF in these settings are unclear, since the risk of recurrent AF and related morbidity is poorly understood. We examined the relations between acute precipitants of AF and long-term recurrence of AF in a clinical setting.
Methods -
From a multi-institutional longitudinal electronic medical record database, we identified patients with newly diagnosed AF between 2000-14. We developed algorithms to identify acute AF precipitants (surgery, sepsis, pneumonia, pneumothorax, respiratory failure, myocardial infarction, thyrotoxicosis, alcohol, pericarditis, pulmonary embolism, and myocarditis). We assessed risks of AF recurrence in individuals with and without a precipitant and the relations between AF recurrence and heart failure, stroke, and mortality.
Results -
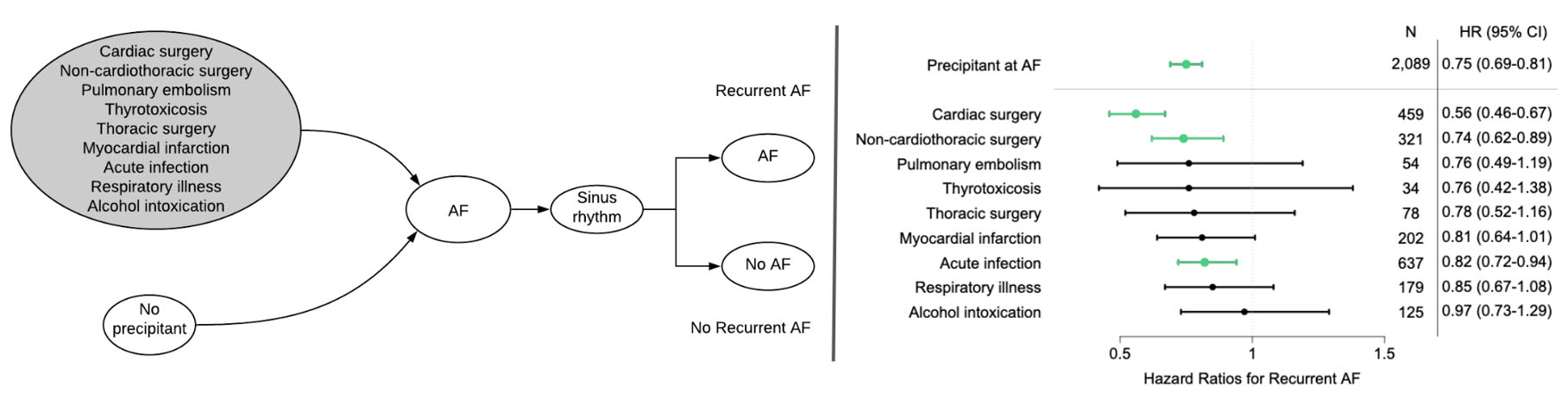
Among 10,723 patients with newly diagnosed AF (67.9 ± 9.9 years, 41% women), 19% had an acute AF precipitant, the most common of which were cardiac surgery (22%), pneumonia (20%), and non-cardiothoracic surgery (15%). The cumulative incidence of AF recurrence at 5 years was 41% among individuals with a precipitant compared to 52% in those without a precipitant (adjusted hazard ratio [HR]: 0.75, 95% confidence interval [CI]: 0.69-0.81, p<0.001). The lowest risk of recurrence among those with precipitants was postoperative AF (5-year incidence 32% in cardiac surgery and 39% in non-cardiothoracic surgery). Regardless of the presence of an initial precipitant, recurrent AF was associated with increased adjusted risks of heart failure (HR: 2.74, 95% CI: 2.39-3.15, p<0.001), stroke (HR: 1.57, 95% CI: 1.30-1.90, p<0.001) and mortality (HR: 2.96, 95% CI: 2.70-3.24, p<0.001).
Conclusions -
AF after an acute precipitant frequently recurs, although the risk of recurrence is lower than among individuals without an acute precipitant. Recurrence is associated with substantial long-term morbidity and mortality. Future studies should address surveillance and management after newly diagnosed AF in the setting of an acute precipitant.
Keywords: atrial fibrillation, risk factor, heart failure, stroke, mortality, Epidemiology, Ischemic Stroke, Mortality/Survival
Graphical Abstract
Introduction
Atrial fibrillation (AF) is a common arrhythmia, with a lifetime risk exceeding 30% in individuals of European ancestry, and 20% in those of African ancestry.1–4 Newly-diagnosed AF may be triggered by acute, potentially reversible precipitants, including surgery, infection, acute myocardial infarction, and thyrotoxicosis, or it can occur in the absence of an identifiable acute precipitant.5–11 Previous guidelines have classified AF occurring in the context of an acute precipitant as secondary and potentially “self-limited” after treatment of the underlying cause.12 Recent data suggest that risk of recurrent AF and related morbidity may in fact be substantial, however these studies are small or include patients from prior decades before advances in AF therapy.5,13–17
Studies show that clinicians often manage patients with AF in the setting of an acute precipitant differently from those who develop AF without an identifiable precipitant. Patients with an acute AF trigger are less likely to be prescribed anticoagulation for stroke prophylaxis, even in the presence of elevated stroke risk.18–20 In one study, 10% of patients with “newly” detected AF at time of stroke had evidence of a prior episode of AF that occurred in the setting of a transient precipitant, which was not carried forward in their health records and for which they were not anticoagulated.21 Almost half of patients with newly-diagnosed AF during sepsis who later had an ischemic stroke did not receive another AF diagnosis before the stroke.22 Thus, there is a critical need to understand the long-term course of AF occurring in the setting of an acute precipitant, and to assess how recurrences of AF are associated with morbidity and mortality.
We sought to ascertain acute triggers of newly diagnosed AF using a large and contemporary longitudinal electronic health record (EHR) database. We quantified the frequency of acute precipitants in patients with newly diagnosed AF and examined the risk of long-term AF recurrence. We then examined the relations between recurrent AF and the risks of heart failure, stroke, and mortality in patients with and without an acute precipitant.
Methods
Reasonable requests to access the data that support the findings of this study may be sent to the corresponding author.
Study sample
We collected data from the Partners HealthCare EHR using the Research Patient Database Query Tool, which contains longitudinal records of more than 6.5 million patients across 7 hospitals in Massachusetts. Details of the Partners HealthCare EHR have been previously described.23,24 We assembled detailed longitudinal medical record information in a database including 921,733 patients 30 to 90 years of age with at least one outpatient visit in each of two consecutive calendar years between January 2000 and December 2014. The beginning of the follow-up period began after this two-year window (Supplemental Figure 1). Longitudinal follow-up data in this analysis were acquired through September 19, 2016. The use of EHR data for research purposes was approved with a waiver of informed consent by the Partners HealthCare Institutional Review Board.
We ascertained the presence of AF or atrial flutter using a previously described electronic algorithm23 comprised of diagnostic and procedure codes, text from electrocardiogram (ECG) readings, and medication prescriptions. We defined ECGs as demonstrating a non-AF rhythm when they did not meet our criteria for AF. The positive predictive value of the algorithm using chart review as the gold standard was estimated to be ≥88%.23 Individuals with AF identified in their longitudinal medical records prior to or during the two-year calendar window were considered to have prevalent AF and were omitted from the analysis. In total, we identified 26,806 patients with newly diagnosed AF during follow-up. We eliminated patients in whom the first documented non-AF rhythm occurred greater than three months after newly diagnosed AF, as we considered these patients to have sustained AF (n=5,232). We also eliminated patients with sustained AF throughout the entire follow-up period (n=1,832) since they were not at risk for recurrence. Individuals with death or last follow-up within 30 days of a non-AF rhythm (n=2,901) were omitted, since we sought to determine relations between precipitants and long-term recurrence and morbidity. We eliminated patients with indeterminate patterns (only 1 ECG) after the start of person-time accrual (n=5,968). Individuals with indeterminate data had a median follow-up time of 1.0 [interquartile range [IQR]: 0.15, 2.9] years. Finally, we eliminated individuals with missing follow-up data (n=150). Consequently, 10,723 individuals remained eligible for analysis (Supplemental Figure 2).
Development of AF precipitant algorithms
The selection of candidate acute AF precipitants was informed by prior American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines.11,12 Precipitants included surgery (classified as cardiac, thoracic non-cardiac, and non-cardiothoracic), acute myocardial infarction, sepsis, pneumonia, pulmonary embolism, pneumothorax, respiratory failure due to other causes, acute alcohol consumption, thyrotoxicosis, pericardial disease (pericarditis and tamponade), and myocarditis.
We developed electronic algorithms to identify whether patients had a precipitant. Algorithm components included International Classification of Disease (ICD) -9 and -10 codes, Current Procedural Terminology (CPT) codes, laboratory values, and free text extracted from discharge summaries. In iterations of each algorithm, we reviewed charts for samples of 50 patients whom the algorithm identified as having the precipitant. We evaluated the positive predictive values of each algorithm, and evaluated the mean absolute date discrepancy between the algorithm-determined date and manually adjudicated date of precipitant occurrence. We reviewed a test set of 650 charts (50 charts x 13 precipitants) after running our initial algorithms. We then iteratively revised algorithm components to achieve a positive predictive value of ≥80% for each precipitant. We subsequently determined sensitivity and specificity of final algorithms by manually adjudicating 13 random independent samples in the EHR database. The samples were assembled using a targeted sampling technique with 50 potential cases and 50 potential referents for each precipitant, and were reviewed in a blinded fashion.25 Algorithms and test characteristics for identification of each precipitant are summarized in Supplemental Tables 1–3.
After validation, we applied the acute precipitant algorithms to the sample of patients with newly diagnosed AF to determine which cases of AF were associated with a precipitant. We defined newly diagnosed AF as having occurred with a precipitant if at least one acute precipitant had been documented within 30 days prior to the onset of AF. For cases with multiple precipitants (n= 320, 15%), we assigned a primary precipitant according to the following sequence: cardiac surgery, thoracic non-cardiac surgery, non-cardiothoracic surgery, myocardial infarction, sepsis, pulmonary embolism, pneumonia, respiratory failure, thyrotoxicosis, pneumothorax, myocarditis, pericarditis, alcohol intoxication. The hierarchical sequence gave priority to the precipitant most likely to have the greatest impact of triggering AF, as defined in a prior study.5 We then randomly selected 100 independent patient charts for which the presence of an identifiable AF precipitant was manually validated. Sensitivity, specificity, and positive predictive value were 83%, 82%, and 92%, respectively. We further examined pairwise agreement between two independent adjudicators (E.Y.W, S.K.) using the Cohen’s κ, which was 0.73.
Ascertainment of recurrent AF, stroke, heart failure, and mortality
The primary outcome was AF recurrence. We defined recurrence as either the presence of an ECG showing AF or atrial flutter following intercurrent non-AF rhythm or documentation of a cardioversion. AF or flutter rhythms were abstracted from ECG readings using text search methods.23 We identified ablations and cardioversions via CPT codes for respective procedures.
Secondary outcomes included incident ischemic stroke, heart failure, and mortality after newly diagnosed AF. Stroke and heart failure were defined using ICD codes on two or more dates specifying each outcome (Supplemental Table 4). Stroke codes were informed by previously published data.26 Stroke and heart failure algorithms have been validated previously to have positive predictive values >85%. The date of last follow-up was defined as the last date of a recorded ICD code in each patient chart, or death. Death was determined via regular updates within the Research Patient Database Query Tool based on the hospital register and Social Security Death Index.
Ascertainment of covariates
Demographic and vital status were obtained from the Research Patient Database Query Tool. Additional covariates included admission status at AF diagnosis (i.e., inpatient, outpatient, emergency department, not recorded), history of coronary heart disease, diabetes mellitus, heart failure, myocardial infarction, hypertension, valvular disease, stroke or transient ischemic attack, peripheral arterial disease, chronic kidney disease, smoking, and alcohol use. Covariates were ascertained from the EHR using previously validated methods and had sensitivities ≥ 84% and specificities ≥ 88% on chart review (Supplemental Table 4). Covariates were considered prevalent at AF if they occurred at or before the time of AF diagnosis.
Statistical analysis
We determined the cumulative risk of AF recurrence using the Kaplan Meier method. We also accounted for death as a competing risk by using the Fine-Gray proportional subdistribution hazards model.27,28 For individuals with first documented non-AF rhythm within 30 days of AF, person-time began 30 days after non-AF rhythm (to account for possible transitions in and out of AF in the setting of a precipitant); otherwise, person-time began at the first documented non-AF rhythm. We censored person-time at ablation, last billing code, or death. We verified the proportional hazards assumption by examining scaled Schoenfeld residuals. Models were adjusted for established predictors of AF prevalent at diagnosis, which were determined a priori.29 Covariates included age, sex, race (white vs non-white), hypertension, diabetes, history of myocardial infarction, heart failure, valvular disease, prior stroke or transient ischemic attack, peripheral artery disease, chronic obstructive pulmonary disease, malignancy, tobacco use, and alcohol use. Effect estimates were stratified by admission status at time of AF diagnosis (i.e., inpatient, outpatient, emergency department, not-recorded).
In precipitant subgroup analyses, we stratified precipitants into broader categories including acute infection (sepsis, pneumonia, pericarditis, myocarditis), and acute respiratory illness (respiratory failure and pneumothorax). We determined adjusted hazard ratios for AF recurrence in the context of each precipitant using AF without any precipitant as the referent category.
In secondary analyses we examined the relations between recurrent AF and stroke, heart failure, and mortality by fitting Cox proportional hazards models in which we specified the first recurrence of AF as a time-dependent covariate, with start of person-time determined as above. We excluded individuals with the outcome of interest prevalent at the time of newly-diagnosed AF or before the start of person-time (Supplemental Figures 3–4). Analyses of heart failure, stroke, and death were censored at the last follow-up date. Models for outcomes were adjusted for the aforementioned covariates as well as the presence of an AF precipitant. We again verified the proportional hazards assumption in each model by examining scaled Schoenfeld residuals. We performed a sensitivity analysis of AF recurrence in the subset of patients who were inpatients at newly diagnosed AF.
A two-sided p value <0.05 was considered significant. Analyses were performed using R version 3.4.0.30
Results
Among the 10,723 individuals with newly diagnosed AF in our overall sample of 921,733 patients, the mean age was 67.9 ± 9.9 years, 41% were women, and 86% were white. At least one acute precipitant was identifiable in 2,089 (19%), of whom 320 (15%) had multiple precipitants. The most commonly identified precipitants were cardiac surgery (n=459, 22%), pneumonia (n=415, 20%), and non-cardiothoracic surgery (n=321, 15%) (Figure 1).31 At onset of AF, patients with acute precipitants were more likely to be older and inpatients, with a history of comorbidity, including hypertension, heart failure, myocardial infarction, valvular heart disease, peripheral artery disease, stroke, diabetes mellitus, chronic lung disease, and cancer. They were also more likely to have documentation of behaviors associated with AF, including tobacco use and alcohol consumption (Table 1).32 The cumulative incidences of death at 1, 5, and 10 years after newly-diagnosed AF were 3%, 9%, and 13% in patients without an AF precipitant, and 6%, 17%, and 22% in those with an AF precipitant. At least one week prior to AF diagnosis, a total of 1,863 (89%) of individuals with an acute precipitant and 7,103 (82%) of individuals without an acute precipitant had one or more ECGs confirming the absence of AF. The median follow-up time among all patients after newly diagnosed AF was 2.5 [IQR: 0.8, 5.4] years.
Figure 1.
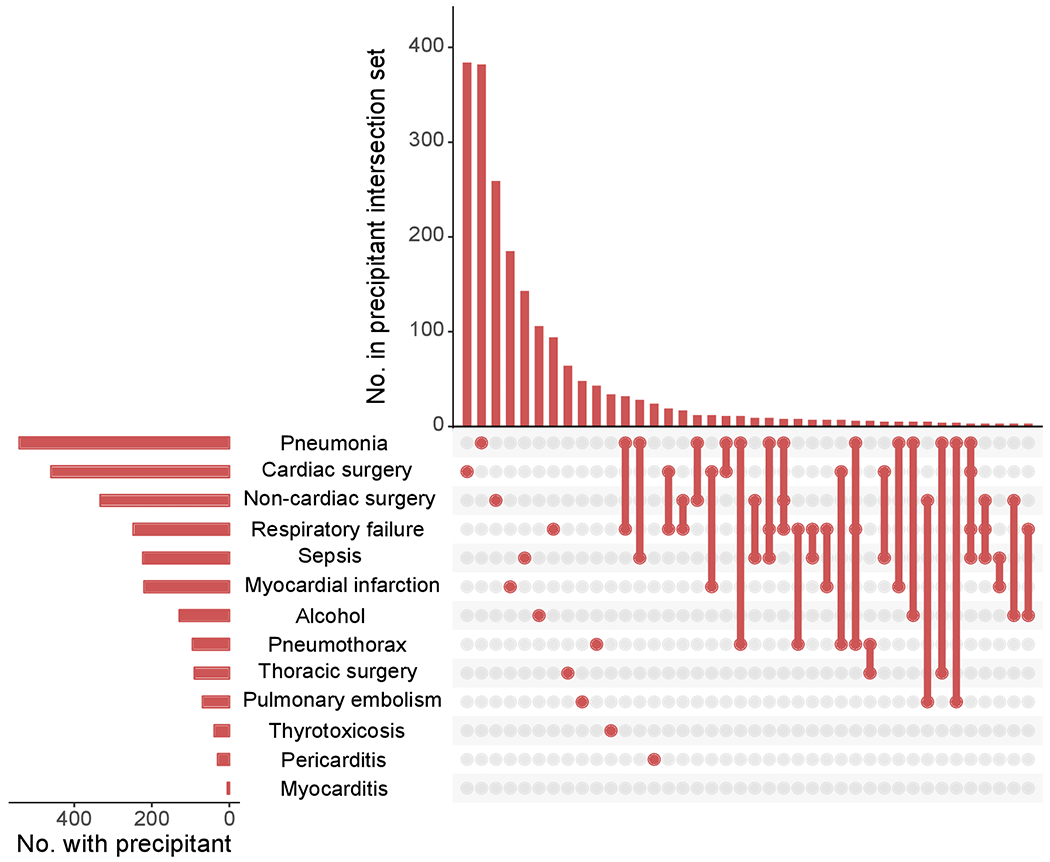
Distribution of precipitants in 2,089 patients with newly diagnosed atrial fibrillation and an acute precipitant. Since precipitants may coexist, the figure displays both the number of individuals with AF with a precipitant as well as the number of individuals with AF with overlapping precipitants. The side plot shows overall number of individuals with each precipitant. The main plot demonstrates the number of individuals with only one precipitant type (illustrated by individual bubbles), as well as number of individuals with multiple concurrent precipitants (intersection sets are denoted by connected bubbles).
Table 1.
Baseline characteristics of 10,723 patients with newly diagnosed atrial fibrillation in the presence and absence of an identifiable precipitant.
| Precipitant Absent (n=8,634) | Precipitant Present (n=2,089) | p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 66.9 ± 9.8 | 67.0 ± 9.7 | 0.69 |
| Male | 4,997 (58%) | 1,276 (61%) | 0.008 |
| White | 7425 (86%) | 1775 (85%) | 0.24 |
| Admission status at time of first-detected AF | <0.001 | ||
| Inpatient | 3,603 (42%) | 1,634 (78%) | |
| Outpatient | 3,866 (45%) | 269 (13%) | |
| Outpatient-Emergency | 45 (0.005%) | 2 (0.001%) | |
| Not recorded | 1,120 (13%) | 184 (0.09%) | |
| Comorbid Conditions | |||
| Hypertension | 6,471 (75%) | 1,689 (81%) | <0.001 |
| Heart failure | 2,632 (30%) | 885 (42%) | <0.001 |
| Myocardial infarction | 1,909 (30%) | 766 (43%) | <0.001 |
| Valvular heart disease | 1,061 (12%) | 455 (22%) | <0.001 |
| Peripheral artery disease | 1,430 (17%) | 519 (25%) | <0.001 |
| Stroke/transient ischemic attack | 1,265 (15%) | 375 (18%) | <0.001 |
| Diabetes mellitus | 2,440 (28%) | 748 (36%) | <0.001 |
| Chronic lung disease | 2,389 (28%) | 844 (40%) | <0.001 |
| Cancer | 2,281 (26%) | 773 (37%) | <0.001 |
| CHA2DS2-VASc score* | 3 [2, 4] | 4 [2, 5] | <0.001 |
| Health-Related Behaviors | |||
| Tobacco use | 1,999 (23%) | 695 (33%) | <0.001 |
| Alcohol use | 395 (5%) | 230 (11%) | <0.001 |
Characteristics displayed as n (%), mean ± SD, or median [25th, 75th percentile]
Characteristics are determined at the date of newly diagnosed AF.
Calculated by assigning 1 point to the presence of heart failure, hypertension, diabetes, vascular disease (prior myocardial infarction, peripheral artery disease, or aortic atherosclerosis), female sex, or age between 65 and 74, and 2 points to age greater than 75 years or history of stroke/transient ischemic attack.
In analyses of AF recurrence, the median number of ECGs in the follow-up period starting 30 days after a non-AF rhythm was 10 [IQR: 5, 21] in patients with AF and no precipitant, with a median follow-up time of 3.2 [IQR: 1.5, 5.7] years, and 10 [IQR: 5, 22] ECGs in individuals with AF and a precipitant, with a median follow-up time of 2.5 [IQR: 1.1, 4.9] years. Kaplan-Meier curves for the cumulative risk of recurrent AF are shown in Figure 2. Adjusting for the competing risk of death, the cumulative incidences of recurrent AF at 1, 5, and 10 years after newly diagnosed AF were 26%, 52%, and 65% in individuals without a precipitant, and 20%, 41%, and 55% in patients with a precipitant. Individuals with recurrent AF had their first recurrence documented a mean of 1.7 years after the incident episode (median 0.9 [IQR: 0.2, 2.5]). In competing risks analyses with adjustment for clinical AF risk factors, newly diagnosed AF with a precipitant was associated with a lower risk of recurrence than AF without a precipitant (HR 0.75, 95% CI 0.69-0.81, p<0.001).
Figure 2.
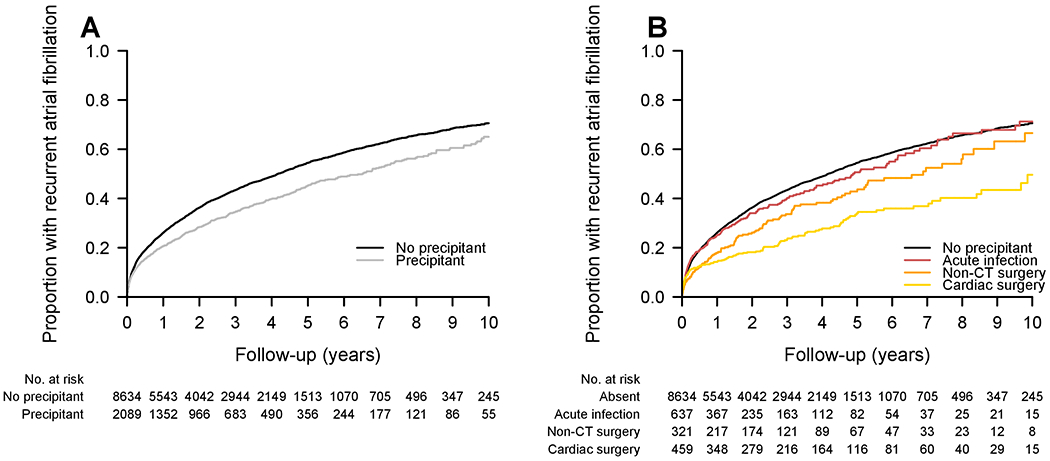
Unadjusted cumulative risk of atrial fibrillation recurrence. Unadjusted curves displaying cumulative risk of recurrent AF, generated using Kaplan-Meier method. Panel A) overall risk of recurrent AF among individuals with and without acute precipitants. Panel B) overall risk of recurrent AF among individuals with infection, cardiac surgery, and non-cardiothoracic surgery, as compared to no precipitant. These three precipitants were selected for display because the risk of recurrent AF was significantly reduced as compared to the referent group without precipitants in multivariable adjusted models. Individuals with other AF precipitants excluded from this plot for clarity. CT = cardiothoracic.
In our sensitivity analysis among the subset of individuals who were inpatients at time of newly diagnosed AF, the cumulative incidence of AF recurrence and hazard ratios for association between AF precipitants and AF recurrence were similar to the overall sample (Supplemental Figures 5 and 6).
Factors prevalent at AF diagnosis associated with higher hazards of AF recurrence in multivariable models included age (HR 1.01 per year, 95% CI 1.01-1.02, p<0.001), male sex (HR 1.15, 95% CI 1.08-1.21, p<0.001), alcohol use (HR 1.20, 95% CI 1.07-1.36, p=0.003), and heart failure (HR 1.30, 95% CI 1.22-1.39, p<0.001). In our multivariable adjusted precipitant subgroup analysis, patients undergoing cardiac surgery (HR 0.56, 95% CI 0.46-0.67, p<0.001), non-cardiothoracic surgery (HR 0.74, 95% CI 0.62-0.89, p=0.001), and those with acute infection (HR 0.82, 95% CI 0.72-0.94, p=0.005) as AF triggers were significantly less likely to have an AF recurrence than those without a precipitant. Compared to those without a precipitant, no other precipitant subgroup was associated with a statistically significant difference in the rate of recurrence, although point estimates indicated a reduced risk of AF recurrence across all precipitant categories (Figure 3). The competing risk of death at 5 years was highest in the thoracic surgery, acute infection, and pulmonary embolism categories (cumulative incidence of 28%, 23%, and 23%, respectively).
Figure 3.
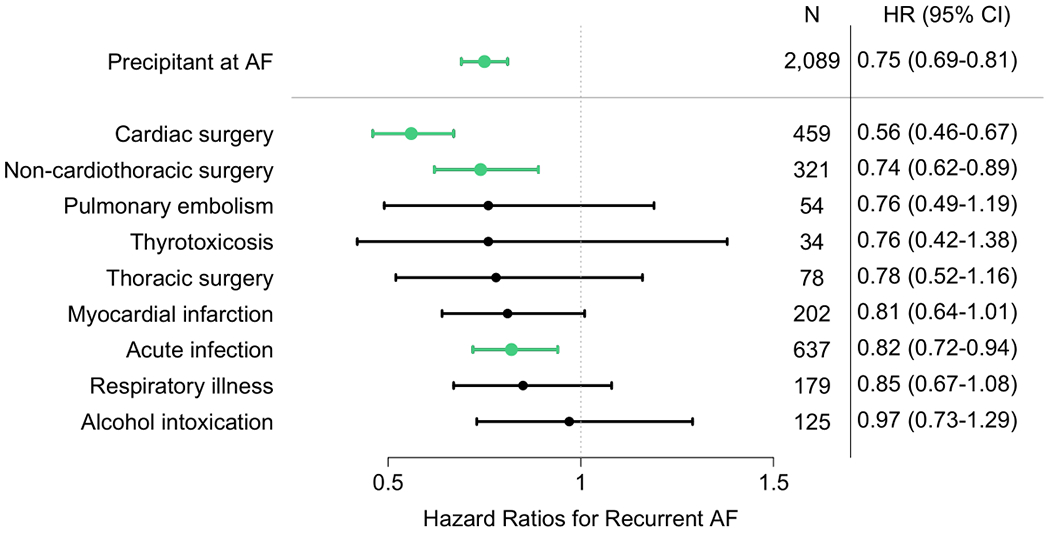
Multivariable-adjusted association between acute atrial fibrillation precipitants and atrial fibrillation recurrence. Multivariable models taking the competing risk of death into account and adjusted for age, sex, race, hypertension, diabetes mellitus, myocardial infarction, heart failure, valvular disease, stroke/transient ischemic attack, peripheral artery disease, chronic lung disease, cancer, tobacco use, alcohol use, and stratified by admission status at AF diagnosis. Green bars signify statistically significant lower risk of association with AF recurrence, comparing to newly diagnosed AF without a precipitant. Data are number of AF cases with acute precipitant and subdistribution hazard ratio (95% confidence interval). AF = Atrial fibrillation.
Of patients at risk for recurrent AF in the sample of 10,723 patients, and without the relevant outcome at baseline, the incidence of heart failure occurred at a rate of 4.1 events per 100 person-years (crude n = 995 events / 6,646 at risk), stroke at 1.4 events per 100 person-years (crude n = 520 events / 9,648 at risk), and mortality at 5.0 events per 100 person-years (crude n = 2,072 events / 10,723 at risk). Recurrent AF was associated with an increased risk of heart failure (HR 2.74, 95% CI 2.39-3.15, p<0.001), stroke (HR 1.57, 95% CI 1.30-1.90, p<0.001), and mortality (HR 2.96, 95% CI 2.70-3.24, p<0.001) in time-varying models adjusted for the presence of an acute precipitant (Table 2). Recurrent AF also was significantly associated with heart failure, stroke, and mortality in analyses stratified by the presence or absence of an initial AF precipitant (Supplemental Table 5).
Table 2.
Incidence of outcomes and association with atrial fibrillation recurrence.
| N outcomes / N at risk* | Incidence (per 100 person-years) | Hazard ratio (95% CI) † | p-value | |
|---|---|---|---|---|
| Heart Failure | 995/6,646 | 4.1 | 2.74 (2.39-3.15) | <0.001 |
| Ischemic Stroke | 520/9,648 | 1.4 | 1.57 (1.30-1.90) | <0.001 |
| All-cause death | 2,072/10,723 | 5.0 | 2.96 (2.70-3.24) | <0.001 |
N patients at risk for recurrent AF in the sample of 10,723 patients, and without the relevant outcome at baseline
Hazard ratio for association of AF recurrence with outcome. Referent category is individuals without AF recurrence
Covariates were ascertained at the date of newly-diagnosed AF; Models are adjusted for the presence of a precipitant at the date of newly-diagnosed AF, age, sex, race, hypertension, diabetes mellitus, myocardial infarction, heart failure, valvular disease, stroke/transient ischemic attack, peripheral artery disease, chronic lung disease, malignancy, tobacco use, alcohol use, and stratified by admission status at newly-diagnosed AF.
Discussion
In a multi-institutional academic medical center sample comprising over 10,000 individuals with initial resolution of newly diagnosed AF, we observed that 19% had newly diagnosed AF occurring in the context of an acute precipitant. The most common precipitants were cardiac surgery and pneumonia. AF recurrence rates were substantial after newly diagnosed AF with and without an acute precipitant, with a 5-year cumulative incidence of 41% and 52%, respectively. Since reports indicate that providers are less likely to prescribe anticoagulation to patients with newly-diagnosed AF in the setting of an acute precipitant on the basis of commonly held assumptions that recurrent AF risk is low,18,21 our observation that AF recurrence is common among patients with AF occurring in the context of a precipitant may have significant implications for clinical practice.
In 1,409 patients with newly-diagnosed AF followed between 1949 and 2012 from the Framingham Heart Study, cumulative recurrence rates of AF were 42% and 59% at 5 years in those with and without a precipitant, respectively.5 Our findings complement and extend these observations by confirming that in a much larger and more contemporary sample derived from an academic medical center, recurrence rates remain high. Given the large sample size, our study enabled examination of recurrence rates stratified by acute precipitants. Other studies also have observed substantial risks of AF recurrence, but have largely focused on specific clinical settings such as cardiac surgery, myocardial infarction, or sepsis.13,15,33–37 Studies using large claims or administrative data have identified AF precipitants via ICD-9 codes or Diagnosis-Related Group codes, without validation for diagnosis or disease date accuracy.13,15,38 Our use of an EHR database enabled ascertainment of patients with an extensive range of validated precipitants, and demonstrated considerable long-term risks of recurrence across precipitants. We also extend previous findings by demonstrating that recurrent AF is strongly associated with increased morbidity.
Our study provides several important contributions to the understanding of newly diagnosed AF with an acute precipitant. First, our results counter the common assumption that newly-diagnosed AF in the acute care setting is self-limiting and not associated with a high rate of recurrence.12 Although AF may occur as a secondary process in this context, resolution of the initial trigger does not preclude further episodes of AF. Recurrence after newly diagnosed AF may occur due to a combination of traditional clinical risk factor accumulation over time, predisposition to the arrhythmia, or perhaps a long-term impact of the initial AF episode or precipitant itself. Furthermore, we submit that recurrent AF episodes are underestimated in our analysis due to probable occurrence of additional subclinical events and receipt of care outside the studied healthcare system. Therefore, our findings underscore the potential need to follow-up for arrhythmia recurrence after newly diagnosed AF in the context of an acute precipitant, particularly if the initial episode resolves and discontinuation of thromboembolism prophylaxis is being considered. Given the multitude of possible methods for surveillance of AF (e.g., pulse palpation, adhesive and handheld electrocardiograms, smart watches, and implantable monitors), prospective comparative and cost-effectiveness studies are warranted to determine the optimal mode of follow-up.
Creating a distinction between newly diagnosed AF with and without an identifiable precipitant may obscure the risk and adverse prognosis of recurrent AF. Although the causal mechanisms linking AF to outcomes among individuals with an acute AF precipitant are not fully elucidated, we observed that AF recurrence was consistently associated with heart failure, ischemic stroke, and mortality. Given the high probability of AF recurrence irrespective of a precipitant and the risk associated with recurrent AF, differentiating newly-diagnosed AF with an acute precipitant from AF that develops without a precipitant may adversely affect management choices, including therapeutic decision making regarding oral anticoagulation 21. Although we observed that AF recurrence rates were lowest among those with AF occurring in the context of cardiac and non-cardiothoracic surgery, our data suggest that reliance on a specific precipitant to discriminate prognosis for patients with AF should be performed with caution until we can more accurately determine the mechanisms of recurrent AF and morbidity, perform surveillance for recurrent AF, or definitively identify subsets of patients without risk for recurrent AF and morbidity.
Limitations
Our results must be interpreted in the context of our study design. In adjusted analyses, we were unable to include certain important potential confounding variables, such as height, weight, and ejection fraction. We were unable to ascertain longitudinal anti-arrhythmic and anticoagulation exposure, which may influence analyses of recurrence and morbidity. Our sample consisted primarily of white patients, limiting generalizability of our outcomes to other races/ethnicities. There are inherent biases of disease ascertainment and misclassification within the EHR. For instance, we could not ascertain conditions documented solely in episodes of care outside the studied healthcare system. Reliance solely on AF episodes captured by ECG or leading to cardioversion likely leads to underestimation of recurrent AF, as episodes may be asymptomatic or undetected, both prior to and following AF diagnosis. Furthermore, AF recurrence may have been more likely to be recorded in patients with poorer health, who returned to the hospital in the setting of a subsequent illness. Individuals with AF precipitants on average were likely in poorer health at baseline than those without, and there may be residual confounding from factors not included in our multivariable models and sensitivity analyses. We also acknowledge that there is likely differential follow up depending upon the acute precipitant diagnosis. For instance, individuals with cancer or cardiac surgery may be more closely followed than individuals after pneumonia; caution should be exercised in comparing the recurrence rate by precipitant. In addition, we did not assess the duration of initial AF episodes, which may impact outcomes. We were also unable to assess severity of exposure within each precipitant subgroup, and we did not examine whether recurrence occurred in the setting of a subsequent precipitant. Finally, we cannot establish a causal relation between recurrent AF and outcomes after AF.
Conclusions and future research
In conclusion, our findings in a multicenter healthcare system challenge the perception that AF occurring with an acute precipitant is a transiently occurring phenomenon with little risk of AF recurrence and associated morbidity. Future studies are needed to identify the mechanisms of stroke, heart failure, and mortality after initial resolution of AF occurring with precipitants. Further investigation should be performed to define markers of higher and lower risk for AF recurrence after an acute precipitant. Studies using continuous cardiac rhythm monitoring are warranted to prospectively and precisely examine the rates, timing, and clinical associations between recurrent AF and long-term outcomes. Precipitant-specific comparative effectiveness trials of anticoagulation should address strategies to risk-stratify and reduce morbidity and mortality in patients with newly diagnosed AF in the setting of acute precipitants.
Supplementary Material
What is known:
Newly diagnosed atrial fibrillation may be triggered by acute, potentially reversible precipitants, including surgery, infection, acute myocardial infarction.
Previous guidelines have classified atrial fibrillation occurring in the context of an acute precipitant as self-limited. Recent data suggest that the risk of recurrent atrial fibrillation may in fact be substantial.
What the study adds:
In a multi-institutional health system, new-onset atrial fibrillation occurring in the setting of an acute precipitant recurred in about 40% of individuals by 5 years. Recurrence varied by precipitant.
Recurrent atrial fibrillation was associated with increased risks of heart failure, stroke, and mortality whether or not an acute precipitant was present.
Prospective studies are warranted to identify optimal methods of cardiac rhythm surveillance and treatment following new-onset AF occurring in the context of an acute precipitant.
Acknowledgments
Sources of Funding: This work was supported by a Doris Duke Charitable Foundation (New York City, NY) Clinical Research Mentorship Grant 2017039 (Wang and Lubitz), NIH (Bethesda, MD) grants R01HL139731 (Lubitz), 2R01HL092577 (Ellinor, Benjamin), 1R01HL128914 (Ellinor and Benjamin), 1P50HL120163 (Benjamin), R01HL104156 and K24HL105780 (Ellinor), U54HL143541, R01HL126911, R01HL137794, 1R01HL141434, R01HL137734, R01HL136660, R01HL135219, 1R21AG060529 (McManus), T32HL007208 (Khurshid), a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 (Lubitz), American Heart Association (Dallas, TX) grant 18SFRN34250007 (Lubitz), 18SFRN34110082 (Benjamin), 18SFRN34110082 (Ellinor), 18SFRN34150007 (Trinquart), and an American Heart Association postdoctoral fellowship 17POST33660226 (Weng).
Disclosures: Dr. Lubitz receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, and Boehringer Ingelheim. Dr. Lubitz has received consulting support from Bristol-Myers Squibb and Bayer AG. Dr. Ellinor is a principal investigator on a Bayer AG grant to the Broad Institute related to the development of new therapeutics for atrial fibrillation. Dr. Ellinor has received consulting support from Bayer AG, Novartis and Quest Diagnostics. Dr. McManus has received research support from Apple, Samsung, Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, and Philips. Dr. McManus has received consulting support from Bristol-Myers Squibb, Pfizer, Samsung, and FlexCon. Dr. Singer has received research support from Boehringer Ingelheim and Bristol-Mers Squibb. Dr. Singer has received consulting support and is on advisory boards for Boehringer Ingelheim, Bristol-Myers Squbb, Johnson and Johnson, Merck, and Pfizer.
Non-standard Abbreviations and Acronyms:
- AF
Atrial fibrillation
- EHR
electronic health record
- ECG
electrocardiogram
- ICD
International Classification of Disease
- CPT
Current Procedural Terminology
References:
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng L-C, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, et al. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation. 2017;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LR, Soliman EZ, Alonso A. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status. Circ Arrhythmia Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnussen C, Niranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njolsta I, Vartiainen E, Snas S, Pasterkamp G, Hughes M et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts. Circulation. 2017;136:1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubitz SA, Yin X, Rienstra M, Schnabel RB, Walkey AJ, Magnani JW, Rahman F, McManus DD, Tadros TM, Levy D, et al. Long-term outcomes of secondary atrial fibrillation in the community the framingham heart study. Circulation. 2015;131:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2015;164:1675–1678. [DOI] [PubMed] [Google Scholar]
- 7.Bielecka-Dabrowa A, Mikhailidis DP, Rysz J, Banach M. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res. 2009;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barra SNC, Paiva LV, Providência R, Fernandes A, Marques AL. Atrial fibrillation in acute pulmonary embolism: Prognostic considerations. Emerg Med J. 2014;31:308–312. [DOI] [PubMed] [Google Scholar]
- 9.Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: An unknown relationship. J Cardiol. 2017;69:699–705. [DOI] [PubMed] [Google Scholar]
- 10.Mayosi BM. Pericarditis-associated atrial fibrillation. Heart. 2015;101:1439–1440. [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann SW, Joseph F, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Huezey J-Y, Kay GN, Lowe JE, et al. 2011 ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2011;57:e101–e198. [DOI] [PubMed] [Google Scholar]
- 13.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. Jama. 2011;306:2248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. Jama. 2014;312:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwich P, Buth KJ, Legare J-F. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28:8–13. [DOI] [PubMed] [Google Scholar]
- 17.El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New-Onset Atrial Fibrillation Predicts Long-Term Mortality After Coronary Artery Bypass Graft. J Am Coll Cardiol. 2010;55:1370–1376. [DOI] [PubMed] [Google Scholar]
- 18.Lubitz SA, Khurshid S, Weng L-C, Doros G, Keach JW, Gao Q, Gehi AK, Hsu JC, Reynolds MR, Turakhia MP, et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt JH, Xian Y, Peterson ED, Olsen PS, Rorth R, Gundlund A, Olesen JB, Gislason GH, Torp-Pedersen C, Kober L, et al. Long-term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburner JM, Atlas SJ, Khurshid S, Weng L, Hulme OL, Chang Y, Singer DE, Ellinor PT, Lubitz SA. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. J Gen Intern Med. 2017;2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borowsky LH, Regan S, Chang Y, Ayres A, Greenberg SM, Singer DE. First Diagnosis of Atrial Fibrillation at the Time of Stroke. Cerebrovasc Dis. 2017;43:192–199. [DOI] [PubMed] [Google Scholar]
- 22.Walkey AJ, Hogarth DK, Lip GYH. Optimizing Atrial fibrillation management from ICU and beyond. Chest. 2015;148:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khurshid S, Keaney J, Ellinor PT, Lubitz SA. A simple and portable algorithm for identifying atrial fibrillation in the electronic medical record. Am J Cardiol. 2016;117:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc 2006;1044. [PMC free article] [PubMed] [Google Scholar]
- 25.Spratt SE, Pereira K, Granger BB, Batch BC, Phelan M, Pencina M, Miranda ML, Boulware E, Lucas JE, Nelson CL, et al. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus. J Am Med Inform Assoc. 2017;24:e121–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein LB. Accuracy of ICD-9-CM Coding for the Identification of Patients With Acute Ischemic Stroke. Stroke. 1998;29:1602–1604. [DOI] [PubMed] [Google Scholar]
- 27.Gray B Cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-7. 2014. https://CRAN.R-project.org/package=cmprsk. [Google Scholar]
- 28.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 31.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of Intersecting Sets, IEEE Transactions on Visualization and Computer Graphics. InfoVis ’14. 20:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, Eberly LE, Alonso A. Smoking and incidence of atrial fibrillation : Results from the Atherosclerosis Risk in Communities (ARIC) Study. HRTHM. 2011;8:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funk M, Richards SB, Desjardins J, Bebon C, Wilcox H. Incidence, Timing, Symptoms, and Risk Factors for Atrial Fibrillation After Cardiac Surgery. Am J Crit Care. 2003;12:424–433. [PubMed] [Google Scholar]
- 34.Siu CW, Jim MH, Zhang X, Chan YH, Pong V, Kwok J, Kung AWC, Lau CP, Tse HF. Comparison of Atrial Fibrillation Recurrence Rates After Successful Electrical Cardioversion in Patients With Hyperthyroidism-Induced Versus Non-Hyperthyroidism-Induced Persistent Atrial Fibrillation. Am J Cardiol. 2009;103:540–543. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Kang DR, Uhm JS, Shim J, Sung JH, Kim JY, Pak HN, Lee MH, Joung B. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am Heart J. 2014;167:593–600. [DOI] [PubMed] [Google Scholar]
- 36.Ariyarajah V, Malinski M, Khadem A, Harizi R, Wolfe K, Spodick DH. Relation of Recurrence of Atrial Fibrillation After Non-ST-Elevation Acute Myocardial Infarction to Left Atrial Abnormality. Am J Cardiol. 2008;101:30–34. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. [DOI] [PubMed] [Google Scholar]
- 38.Quon MJ, Behlouli H, Pilote L. Anticoagulant Use and Risk of Ischemic Stroke and Bleeding in Patients With Secondary Atrial Fibrillation Associated With Acute Coronary Syndromes, Acute Pulmonary Disease, or Sepsis. JACC Clin Electrophysiol. 2017; 386–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


