Abstract
Purpose of review:
Primary central nervous system lymphoma (PCNSL) is a rare but aggressive variant of non-Hodgkin lymphoma. The diagnostic gold standard remains the pathologic review of tumor tissue, mainly collected though biopsies. The majority of PCNSL are diffuse large B cell lymphoma (DLBCL). Biopsies are invasive procedures, and there have been efforts to develop minimally invasive diagnostic testing using serum and cerebral spinal fluid (CSF). This article reviews multiple markers that potentially could serve as future diagnosis tools and predictors of treatment response.
Recent findings:
Many studies have attempted to classify DLBCL into different subtypes for prognostic purposes using methods such as immunohistochemistry. PCNSL often falls under the activated B-cell-like subgroup, and further genomic sequencing has identified genomic alterations in genes within the B-cell receptor signaling axis, e.g. MYD88 or CD79B, at increased frequencies in PCNSL. MYD88 and CD79B implicate the involvement of the NF-kB pathway, and targeted agents to this pathway are currently being used in the treatment of relapsed/refractory PCNSL.
Summary:
Although recent genomic profiling of PCNSL has increased the understanding of drivers in this disease and has also led to the introduction of targeted inhibitors, these markers have not yet been used for diagnostic and/or prognostic purposes. Further studies will need to evaluate if they hold great diagnostic potential.
Keywords: PCNSL, B-cell receptor, MYD88, CD79B, activate B-cell subtype
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare but aggressive type of extranodal non-Hodgkin lymphoma found only in the central nervous system. Treatment regimens vary according to geographic location and physician practice, but all include the use of high-dose methotrexate. Despite this, the rate of relapse is high, and there are ongoing studies as to which treatment regimen is best for this patient population. The gold standard for diagnosis is the pathologic review of biopsy material. Stereotactic biopsies are an invasive procedure with multiple associated risks such as brain edema, hemorrhage and permanent neurologic deficits. With the advent of genomic sequencing and other technology, efforts have been made to identify additional biomarkers for diagnosis and their prognostic and predictive value. This review aims to identify current work on these potential markers.
Background
PCNSL is found only in the central nervous system, which includes the brain, spine, cerebrospinal fluid (CSF), and eyes [1]. It represents 3% to 4% of all newly diagnosed intracranial neoplasms with an overall incidence rate of 0.5 per 100,000-person years. PCNSL represent 4% to 6% of all extranodal lymphomas. [2].
PCNSL presents as solitary or multiple intracranial lesions, diffuse leptomeningeal or periventricular lesions, vitreous/uveal deposits, and rarely as intradural spinal cord lesions [3-7]. The clinical presentation varies depending on the location of the lesion. Patients may develop mental status and behavioral changes (32% to 43%), seizures (11% to 14%), and signs of elevated intracranial pressure such as headache, nausea, vomiting, and papilledema (32% to 33%) [1, 8, 9].
To diagnose and assess the extent of disease, the International PCNSL Collaborative Group (IPCG) recommends baseline staging to include magnetic resonance imaging of the brain and spine (if symptoms localizing to the spine are present), an ophthalmologic examination to evaluate for intraocular lymphoma, and CSF evaluation to determine the presence of lymphoma in the CSF [10]. A body positron emission tomography/computed tomography scan and bone marrow biopsy should also be performed to detect the presence of non-CNS disease as this would change management strategies. To establish a tissue diagnosis of PCNSL, the diagnostic procedure of choice is a stereotactic biopsy. If ocular or CSF involvement is present, then a vitrectomy or CSF cytology may be collected instead. Molecular markers have not been included in the diagnostic recommendations.
There is currently no standard of care treatment for PCNSL, but expert consensus recommends treating patients with a high-dose methotrexate-based multimodal regimen. Methotrexate was initially added to whole brain radiation therapy, but there was significant long-term neurotoxicity from chemoradiation [11, 12]. This led to the development of chemotherapy-only regimens such as rituximab, methotrexate, vincristine, and procarbazine [13, 14]; rituximab, methotrexate, and temozolomide [15]; methotrexate, cytarabine, thiotepa, and rituximab [16]; and rituximab, methotrexate, carmustine, teniposide, and prednisone [17]. No head-to-head comparisons have been conducted to determine which regimen is the most efficacious; thus, the regimen used often depends on geographic location and physician preference. Ongoing trials will hopefully add to this literature and provide further evidence for the optimal first line treatment. Agents targeting tumor specific alterations have not been introduced into the first-line setting.
Pathology, Pathophysiology and the Tumor Microenvironment
Approximately 90% of PCNSL are diffuse large B cell lymphomas (DLBCL) with a small subset of patients diagnosed with T cell, Burkitt, lymphoblastic, and marginal zone lymphomas [18]. DLBCL are characterized by diffuse proliferation of mature B cells that are usually larger than twice the normal size of macrophages or lymphocytes [19]. It is thought that B-cell lymphomas arise from B cells that arrest at specific stages of differentiation when malignant transformation takes place [20].
Expression profiling data [21] was used to establish three major DLBCL subtypes: 1) germinal center B-cell-like (GCB), 2) activated B-cell-like (ABC), and 3) type 3. The type 3 subgroup is not well defined, but both the type 3 and ABC subtypes appear to have a poor outcome and are often grouped together. For easier clinical application, an immunohistochemical classification scheme for systemic DLBCL was developed by Hans et al subdividing tumors into germinal center B-cell-like (GCB) and non-germinal center like (non-GC) based on the expression pattern for CD10, BCL6, and MUM1 [22].
Further genomic sequencing revealed that the pattern of somatic mutations in DLBCL varies depending on the cell of origin: GCB tumors were more likely to have mutations in EZH2, GNA13, and translocations in BCL2, whereas ABC tumors were associated with mutations in MYD88, CD79A, CARD 11, and TNFAIP3, all of which are involved in B-cell receptor signaling activating NF-kB [23].
The majority of PCNSL are of the non-GC subtype [24-26]. In comparison to the ABC subtype in systemic DLBCL, mutations in MYD88 and CD79B are identified in higher frequencies [26-32] (Figure 1) and even found in tumors of the GC subtype in PCNSL, which is not typically observed in systemic DLBCL [26, 33, 34].
Figure 1. Frequent Mutations affecting the B-cell receptor signaling axis in PCNSL.
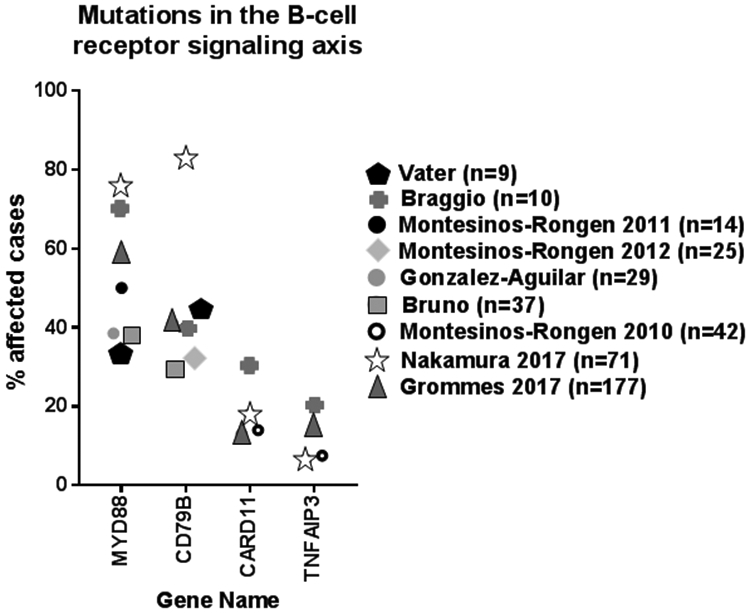
Mutations in MYD88, CD79B, CARD11, and TNFAIP3 were found to be present in cases of PCNSL. The percentage of cases with mutations in these genes is delineated here with the majority of case studies reporting the presence of mutations within MYD88.
The NF-kB pathway plays a key role in DNA transcription and cell survival. Constitutively active NF-kB leads to cell proliferation and prevention of cellular apoptosis and sustains the viability of ABC subtype DLBCL [33, 35]. Bruton tyrosine kinase (BTK) links B-cell receptor (BCR) and toll-like receptor (TLR) signaling pathways to downstream NF-kB activation. In over 90% of PCNSL tissue samples, BCR, TLR, or NF-kB pathways are altered [28]. Fifty-five percent of PCNSL patients have mutations identified within the Toll/IL-1 receptor domain of MYD88, an adaptor protein that activates the exchange of leucine to proline at position 265 leading to the development of DLBCL [36]. In addition, 40% of PCNSL cases have mutations in the immunoreceptor tyrosine-based activation motif of CD79B located at Y196, which leads to chronic activation of BCR signaling and subsequent activation of the NF-kB pathway [37]. This is in contrast to systemic DLBCL cases where MYD88 and CD79B mutations are seen less frequently in ABC subtypes (8-37% MYD88 and 12-22% CD79B) [38-41]. NF-kB activity can also be further amplified through deletions or mutations in tumor necrosis factor alpha induced protein 3 (TNFAIP3) [42].
The BCL6 gene is a proto-oncogene expressed on normal B-cells in the germinal center that produces the transcriptional repressor protein BCL6, which regulates its own expression by binding to its promotor. BCL6 has many functions, one of which is to represses microRNA expression (ie miR155) leading to increased expression of genes needed for germinal center reactions to produce antibody diversity [43]. BCL6 also allows for the rapid proliferation of germinal center B-cells in response to T-cell dependent antigens without inducing a p53/TP53-dependent apoptotic response to the physiologic DNA breaks needed for immunoglobulin class switch recombination and somatic hypermutation [44]. Chromosomal translocations of BCL6 occurring in 30% to 40% of DLBCL or acquired mutations in the BCL6 promotor occurring in 73% of DLBCL lead to overexpression of BCL6 and subsequent downregulation of the TP53 tumor suppression gene, resulting in continuous activation and cell proliferation [19, 45]. A subset of ABC DLBCL activates the transcription factor STAT3 (a BCL-6 target) through JAK kinase signaling, which synergizes with NF-kB and promotes cell survival [46, 47]. JAK/STAT signaling pathway activators such as interleukin-4 (IL-4) and IL-10 were found to be upregulated in the PCNSL microvascular environment and in the vitreous and CSF [48].
Given that the CNS is a site that does not have resident lymphoid tissue, it is unknown which cell of origin PCNSL arise from. The brain is immunologically quiet under physiologic conditions, but biopsies from PCNSL patients show an inflammatory response with reactive T cells and infiltrating activated macrophages. In addition, there are highly proliferative tumor cells that diffusely infiltrate the CNS in an angiographic growth pattern migrating in the perivascular space [18, 49]. The tumor microenvironment is not fully characterized, but in vitro studies have found that large B-cell lymphomas respond to chemokines CXCL12 and CXCL13 [50]. The presence of tumor-infiltrating CD14+ macrophages may portend a better treatment response as they provide complement and Fc receptors needed for rituximab to be effective [51]. Macrophages in the PCNSL tumor environment were also found to overexpress programmed death-1 (PD-1), indoleamine 2,3-dioxygenase (IDO1), and several other cytokines in response to in vitro PCNSL cell-line derived soluble factors [52]. Genomic studies of PCNSL samples have identified a frequent 9p24.1/PDL1/PDL-2 copy number gain that is associated with increased PDL1/PDL2 protein. Taken together, this suggests that the expression of immunosuppressive molecules like PD-1 may be involved in immune evasion of lymphoma cells [53].
Another transcription factor that is often upregulated in DLBCL due to translocation is MYC, which drives cell proliferation, regulates cell growth and differential, and apoptosis through downregulation of BCL2 [54, 55]. MicroRNAs associated with the MYC pathway have been identified in PCNSL patients where putative tumor-suppressor microRNAs such as miR-199a, miR-214, miR-193b, and miR-145 were downregulated [56].
Diagnostic Markers
Diagnostic delay is often an issue for PCNSLs given the rapidly progressive nature of the disease, and the gold standard for diagnosis involves a stereotactic biopsy of the lesion [57]. This is invasive and is associated with a complication rate of 8.5% including brain edema, hematomas, or seizures [58]. To minimize this risk, efforts have been made to identify other less invasive methods for diagnosis. Cytology and flow cytometry from the CSF or vitreous fluid are often tested, however the diagnostic yield is low and often only positive when there is significant leptomeningeal or vitreal involvement. Moreover, conventional CSF cytology and flow cytometry testing often does not produce corresponding results. A prospective study of 123 B-cell lymphoma patients reports that flow cytometry identified neoplastic B cells in 27 patients (22%) while conventional cytology was only positive or suspicious in 10 patients [59].
Additional CSF markers have been assessed. On a systematic review performed by van Westrhenen et al, CXCL-13, B2M, and neopterin isolated from the CSF appear to have the most potential to become a diagnostic marker in PCNSL [60]. Table 1 lists additional CSF markers investigated as potential diagnostic markers for PCNSL [50, 61-77]. To date, none of these markers has been established in the clinical routine diagnostic use.
Table 1. Potential diagnostic markers for primary central nervous system lymphoma (PCNSL).
AUC: area under the curve; CSF: cerebral spinal fluid; ddPCR: droplet digital PCR; ctDNA: circulating tumor DNA; NGS: next generation sequencing; PCR: polymerase chain reaction; ELISA: enzyme-linked immunosorbent assay; CBA: cytometric bead array; ECLIA: electrochemiluminescence immunoassay; CLEIA: chemiluminescent enzyme immune assay.
| First Author (year) | No. Cases | Marker | Serum or CSF | Technology | AUC (95% CI) | Sensitivity | Specificity | ||
|---|---|---|---|---|---|---|---|---|---|
| DNA | |||||||||
| Hiemcke-Jiwa (2018) | 32 | MYD88 p.(L265P) | CSF | ddPCR | |||||
| Hattori (2018) | 14 | MYD88 p.(L265P) | CSF | ddPCR | |||||
| Fontanille (2017) | 25 | ctDNA | Serum | NGS | |||||
| MYD88 | Serum | NGS | 24.0% | 100.0% | |||||
| RNA | |||||||||
| Baraniskin (2011) | 23 | miR-21 + miR-19b + miR-92a | CSF | PCR | 95.7% | 96.7% | |||
| miR-21 | CSF | PCR | 95.7% | 83.3% | |||||
| miR-19b | CSF | PCR | 95.7% | 83.7% | |||||
| miR-92a | CSF | PCR | 95.7% | 80.0% | |||||
| Mao (2014) | 56 | miR-21 | serum | PCR | 0.93 (0.88-0.98) | ||||
| Baraniskin (2016) | 72 | RNU2-1f | CSF | PCR | 0.91 | 68.1% | 91.4% | ||
| RNU2-1f + miR-21 | CSF | PCR | 0.99 | 91.7% | 95.7% | ||||
| Chemokine | |||||||||
| Rubenstein (2013) | 55 | CXCL13 | CSF | ELISA | 71.0% | 95.0% | |||
| CXCL13 + IL-10 | CSF | ELISA | 50.0% | 99.3% | |||||
| Mabray (2016) | 43 | CXCL13 | CSF | ELISA | 0.83 (0.74-0.90) | 76.7% | 90.9% | ||
| Cytokine | |||||||||
| Rubenstein (2013) | 55 | IL-10 | CSF | ELISA | 64.0% | 94.1% | |||
| Mabray | 43 | IL-10 | CSF | ELISA | 0.79 (0.69-0.87) | 62.8% | 95.5% | ||
| Nguyen-Them (2016) | 112 | IL-10 | CSF | CBA | 0.88 | 88.6% | 88.9% | ||
| Sasagawa (2015) | 19 | IL-10 | CSF | ELISA | 0.97 | 94.7% | 100.0% | ||
| Song (2016) | 22 | IL-10 | CSF | ECLIA | 0.96 (0.90-1.00) | 95.5% | 96.1% | ||
| IL-6 | CSF | ECLIA | 0.61 (0.48-0.74) | 54.6% | 70.1% | ||||
| IL-8 | CSF | ECLIA | 0.56 (0.42-0.69) | 31.8% | 83.1% | ||||
| TNFa | CSF | ECLIA | 0.66 (0.41-0.68) | 59.1% | 57.1% | ||||
| Sasayama (2012) | 31 | IL-10 | CSF | ELISA | 0.92 (0.84-0.99) | 71.0% | 100.0% | ||
| IL-6 | CSF | CLEIA | 0.68 (0.57-0.79) | 77.0% | 63.0% | ||||
| Protein Receptor | |||||||||
| Ikeguchi (2017) | 12 | sIL-2R | CSF | ELISA | 0.87 | 83.3% | 90.0% | ||
| Sasagawa (2015) | 19 | sIL-2R | CSF | ELISA | 0.91 | 94.7% | 84.6% | ||
| Sasayama (2012) | 31 | sIL-2R | CSF | ELISA | 0.85 (0.75-0.96) | 81.0% | 56.7% | ||
| Thaler (2017) | 33 | sTACI | CSF | ELISA | 0.94 (0.88-0.99) | 87.9% | 88.3% | ||
| sBCMA | CSF | ELISA | 0.76 (0.66-0.86) | 72.7% | 71.8% | ||||
| Protein | |||||||||
| Strehlow (2016) | 37 | Osteopontin | CSF | ELISA | 0.93 (0.87-1.00) | 87.0% | 86.0% | ||
| Sasagawa (2015) | 19 | B2M | CSF | Latex agglutination- turbidimetric immunoassay | 0.81 | 89.4% | 88.5% | ||
| Sasayama (2012) | 31 | B2M | CSF | Latex agglutination- turbidimetric immunoassay | 0.93 (0.87-1.00) | 88.0% | 90.3% | ||
| Kuusisto (2015) | 41 | AT III | CSF | ELISA | 0.79 | ||||
| Roy (2008) | 24 | AT III | CSF | ELISA | 0.91 | 75.0% | 98.1% | ||
| Murase (1998) | 12 | sCD27 | CSF | ELISA | 100.0% | 100.0% | |||
| Kersten (1996) | 7 | sCD27 | CSF | ELISA | 100.0% | 98.0% | |||
| Viaccoz (2015) | 28 | Neopterin | CSF | Liquid chromatography with fluorimetric detection | 96.0% | 92.0% | |||
The identification of circulating tumor DNA (ctDNA) in the CSF and/or blood might be a possibly more promising diagnostic biomarker for PCNSL. Fontanilles et al investigated ctDNA changes in serum and primary tumors in 25 PCNSL. Eight patients (32%) had detectable somatic mutations in the blood. The sensitivity was determined as 24%, with a specificity of 100% [63]. In a study of 9 relapsed/refractory PCNSL patients, tumor specific ctDNA was found in the CSF in all patients [78]. Of note, in 3 patients, no CSF involvement could be detected using conventional MRI or CSF cytology and flow cytometry. At the time of tumor recurrence, between 11%-37% of single nucleotide variants found in the CSF were shared with the original tumor. The frequency of shared mutations (60%) was higher for mutations belonging to BCR pathway participants (e.g. MYD88 L265P, CD79B Y196, CARD11). CSF was also collected through the course of treatment for ctDNA testing, and 7 out of 9 patients with repeated collections were observed to clear their CSF of ctDNA, which corresponded to response on brain imaging. One patient with early disease progression after initial tumor response had persistence of ctDNA in the CSF suggesting that ctDNA in the CSF may be a potential marker for minimal residual disease [78]. 1/3 cases with CD79B
Prognostic Markers
Two prognostic scoring systems have been used: 1) Memorial Sloan Kettering Cancer Center (MSKCC) prognostic score and 2) International Extranodal Lymphoma Study Group (IELSG) score. The MSKCC score separates patients into 3 groups stratified by age and Karnofsky performance status (KPS). Age ≤50 years correlated with a median overall survival (OS) of 8.5 years, age >50 years plus KPS ≥70 correlated with OS of 3.2 years, and age >50 years plus KPS <70 correlated with OS 0.9 years [79]. The IELSG score, on the other hand, scores patients based on age, Eastern Cooperative Oncology Group (ECOG) performance score, lactase dehydrogenase level, CSF protein concentration, and deep brain involvement. A score of 0-1 corresponds to a 2-year survival rate of 80%. A score of 2-3 corresponds to a 2-year survival rate of 48%, and a score of 4-5 corresponds to a 2-year survival rate of 15% [80].
In systemic DLBCL, the ABC (or non-GC) subtype has been associated with poor clinical outcome. Multiple studies have suggested that classifying PCNSL into GCB or non-GC does not predict a survival benefit. Raoux et al determined no significant survival difference between GCB and non-GC patients in 39 cases where 13 tumors were classified as GCB and 26 patients as non-GCB [81]. Similarly, Liu et al analyzed 89 cases with 18 tumors classified as GCB and 71 as non-GCB without a difference in overall survival or PFS between the two groups [82]. Table 2 delineates multiple other studies that have examined survival difference between GCB and non-GC groups in PCNSL, all of which did not report a significant difference in overall survival [24, 81-86]. Limitations of these studies include small sample sizes, retrospective nature, and incomplete treatment information that could impact overall survival.
Table 2. Survival difference between diffuse large B-cell lymphoma subgroups.
GCB: germinal center B-cell-like; non-GC: non-germinal center; NR: Not reported; HD-MTX: high-dose methotrexate (>3g/m2); Ara-C: cytosine arabinoside; TMZ: temozolomide; RT: radiotherapy; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone
| First author (year) | No. cases | Median OS (months) | Median age (range) |
Treatment |
|---|---|---|---|---|
| Liu (2017) | 89 | 45.3 | 56 (11-85) | HD-MTX + Ara-C or HD-MTX + TMZ |
| GCB | 18 | *NR | ||
| non-GC | 71 | *NR | ||
| Aki (2013) | 35 | NR | 52 (21-85) | Unspecified: some were treated with chemotherapy (NR) + RT or chemotherapy alone |
| GCB | 6 | 19 | ||
| non-GC | 29 | 17 | ||
| Hattab (2010) | 31 | NR | 62 (13-81) | Most were treated with HD-MTX and steroids, but given the retrospective nature, some treatment regimens were unknown |
| GCB | 5 | 2.5 | ||
| non-GC | 26 | 7.4 | ||
| Raoux (2010) | 39 | 5.3 | 67 (49-83) | 19pts with chemotherapy and RT, 10pts with RT alone, 6pts with chemotherapy alone, 4pts untreated |
| GCB | 13 | *NR | ||
| non-GC | 26 | *NR | ||
| Momota (2010) | 27 | Not reached | 63 (26-78) | All pts received HD-MTX; 25/27 pts received RT |
| GCB | 1 | N/A | ||
| non-GC | 22 | N/A | ||
| Lin (2006) | 51 | 13.7 | 62 (16-82) | 29 pts with HD-MTX, 1 pt with CHOP, 15 pts with RT, 6 pts with supportive care |
| GCB | 7 | 34.5 | ||
| non-GC | 22 | 11 | ||
| Camilleri-Broet (2006) | 82 | 42 | 60 (23-80) | HD-MTX |
| GCB | 3 | *NR | ||
| non-GC | 79 | *NR |
There has also been conflicting evidence regarding the prognostic significance of BCL6 protein detection by immunohistochemistry. A recent metanalysis of 22 studies involving 3037 DLBCL patients suggested that BCL6 rearrangement portends a worse overall survival but does not seem to affect progression free survival (PFS) [87]. For PCNSL, the CALGB 50202 trial suggested that high BCL6 expression correlated with shorter PFS, which was supported by a post-hoc analysis of the G-PCNSL-SG1 prospective trial [15, 88]. Other smaller studies, however, have suggested that BCL6 expression is associated with a better survival [89, 90].
CXCL13 has also been implicated as a poor prognostic marker in PCNSL patients perhaps due to its function of mediating pro-survival signals through B cell activation [91]. Rubenstein et al noticed that newly diagnosed patients with low CXCL13 levels in the CSF at time of diagnosis had a longer progression free survival with standard treatment compared to patients with high levels of CXCL13 [50]. CXCL13 has often been studied with IL-10 where the combination of elevated CXCL13 levels and IL-10 levels in the CSF provided increased specificity but decreased sensitivity for PCNSL detection. Nguyen-Them et al showed that serial CSF IL-10 measurements during treatment correlated with the course of the disease where the patients with a detectable level of IL-10 at the end of treatment despite imaging response were more likely to relapse in the first year compared to patients who had undetectable levels [68].
The recent genomic sequencing data has also not significantly contributed to novel prognostic biomarkers. Frequently mutated genes, like MYD88 or CD79B do not have prognostic value yet in PCNSL in contrast to systemic DLBCL where response to ibrutinib, a selective Bruton tyrosine kinase inhibitor, depends on the mutational status of these two genes. In systemic DLBCL, an overall response rate to ibrutinib was seen in 37% of ABC DLBCL and only 5% of GCB DLBCL, but the response was particularly high in those ABC DLBCL with a mutation in the BCR signaling pathway (55%) and coexisting mutations in both MYD88 and CD79B (80%). Those with MYD88 mutations but wild-type CD79b were unresponsive to ibrutinib [33]. These results however were not observed in PCNSL patients. Responses to ibrutinib were seen in both GCB and ABC PCNSL and also in tumors without mutations in the BCR pathway. Incomplete tumor responses were associated with mutations in the B-cell antigen receptor-associated protein CD79B. Based on the current data, only PCNSL patients with CARD11 mutations, which confer upfront resistance to BTK inhibition, should not be treated with ibrutinib [26].
Conclusion
There has been significant progress made in understanding the molecular pathogenesis of PCNSL leading to the use of targeted agents such as ibrutinib, a BTK inhibitor, for the treatment of PCNSL as well as agents targeting the activation of NF-kB, e.g. immunomodulatory drugs such as lenalidomide or pomalidomide [92]. A stereotactic biopsy and pathologic review will represent the diagnostic gold standard in PCNSL. Different markers have been evaluated, particularly in the CSF but have not yet been evaluated rigorously to warrant routine clinical use. Next generation sequencing of ctDNA isolated from CSF samples might represent a promising diagnostic biomarker but need to be evaluated in a more stringent setting.
Key Points.
Stereotactic biopsy and pathologic review remain the diagnostic gold standard in PCNSL
Clinical parameters (mainly age and performance status) are still the most significant prognostic parameters in PCNSL
Subgroups as defined by the Hans immunohistochemical staining algorithm no not have a different clinical outcome in PCNSL
Different CSF markers have been evaluated but not yet applied to routine clinical use
Next generation sequencing of ctDNA isolated from CSF samples might represent a promising diagnostic biomarker
Acknowledgements
This research was supported by a NIH/NCI Cancer Center Support Grant (P30-CA008748) and supported by grants from Cycle for Survival Equinox (CG) and the Leukemia & Lymphoma Society (CG).
Footnotes
Disclosures:
K. Grace Ho: no relationships to disclose
Christian Grommes: Consulting for BTG International and Kite
References
- 1.Grommes C and DeAngelis LM, Primary CNS Lymphoma. J Clin Oncol, 2017. 35(21): p. 2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villano JL, et al. , Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer, 2011. 105(9): p. 1414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Baumgarten L, et al. , The Diagnosis and Treatment of Primary CNS Lymphoma. Dtsch Arztebl Int, 2018. 115(25): p. 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrlinger U, et al. , Clinical presentation and therapeutic outcome in 26 patients with primary CNS lymphoma. Acta Neurol Scand, 1998. 97(4): p. 257–64. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, et al. , Limited efficacy of high-dose methotrexate in patients with neurolymphomatosis. Int J Hematol, 2019. 109(3): p. 286–291. [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente MI, et al. , Bilateral radiation therapy followed by methotrexate-based chemotherapy for primary vitreoretinal lymphoma. Am J Hematol, 2019. 94(4): p. 455–460. [DOI] [PubMed] [Google Scholar]
- 7.Do AS, et al. , Primary spinal intradural extramedullary lymphoma: A novel management strategy. J Clin Neurosci, 2017. 35: p. 122–126. [DOI] [PubMed] [Google Scholar]
- 8.Fine HA and Mayer RJ, Primary central nervous system lymphoma. Ann Intern Med, 1993. 119(11): p. 1093–104. [DOI] [PubMed] [Google Scholar]
- 9.Bataille B, et al. , Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg, 2000. 92(2): p. 261–6. [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, et al. , Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol, 2005. 23(22): p. 5034–43. [DOI] [PubMed] [Google Scholar]
- 11.Gavrilovic IT, et al. , Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol, 2006. 24(28): p. 4570–4. [DOI] [PubMed] [Google Scholar]
- 12.Omuro AM, et al. , Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol, 2005. 62(10): p. 1595–600. [DOI] [PubMed] [Google Scholar]
- 13.Morris PG, et al. , Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol, 2013. 31(31): p. 3971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omuro A, et al. , R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood, 2015. 125(9): p. 1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein JL, et al. , Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol, 2013. 31(25): p. 3061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreri AJ, et al. , Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol, 2016. 3(5): p. e217–27. [DOI] [PubMed] [Google Scholar]
- *17.Bromberg JEC, et al. , Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol, 2019. 20(2): p. 216–228.This is a Phase 3 trial studying the benefit of adding rituximab to a common induction regimen composed of methotrexate, carmustine, teniposide, and prednisone. There is controversy over whether adding rituximab, a large molecule that does not normally cross the blood-brain barrier, to methotrexate-based regimens is beneficial to patients with PCNSL. This study reports no additional benefit, but the dose used in the study is lower than some current protocols published in the literature.
- 18.Camilleri-Broet S, et al. , Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d'etude des Leucenies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol, 1998. 110(5): p. 607–12. [DOI] [PubMed] [Google Scholar]
- 19.Gouveia GR, Siqueira SA, and Pereira J, Pathophysiology and molecular aspects of diffuse large B-cell lymphoma. Rev Bras Hematol Hemoter, 2012. 34(6): p. 447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe ES, et al. , Burkitt's lymphoma: a single disease with multiple variants. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Blood, 1999. 93(3): p. 1124. [PubMed] [Google Scholar]
- 21.Alizadeh AA, et al. , Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature, 2000. 403(6769): p. 503–11. [DOI] [PubMed] [Google Scholar]
- 22.Hans CP, et al. , Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood, 2004. 103(1): p. 275–82. [DOI] [PubMed] [Google Scholar]
- 23.Pasqualucci L and Dalla-Favera R, The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol, 2015. 52(2): p. 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri-Broet S, et al. , A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood, 2006. 107(1): p. 190–6. [DOI] [PubMed] [Google Scholar]
- 25.Preusser M, et al. , Primary central nervous system lymphoma: a clinicopathological study of 75 cases. Pathology, 2010. 42(6): p. 547–52. [DOI] [PubMed] [Google Scholar]
- **26.Grommes C, et al. , Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov, 2017. 7(9): p. 1018–1029.Bruton tyrosine kinase (BTK) links BCR and toll-like receptors with NF-kB and is known to play a role in systemic B cell lymphoma. Its role in the pathogenesis of B cell lymphoma within the CNS was unknown. This study showed that ibrutinib, a BTK inhibitor, has substantial activity in patients with relapsed or refractory B-cell lymphoma of the CNS to a degree that is higher than that seen in B cell lymphomas outside the CNS, suggesting a divergent molecular pathogenesis.
- 27.Vater I, et al. , The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia, 2015. 29(3): p. 677–85. [DOI] [PubMed] [Google Scholar]
- 28.Braggio E, et al. , Genome-Wide Analysis Uncovers Novel Recurrent Alterations in Primary Central Nervous System Lymphomas. Clin Cancer Res, 2015. 21(17): p. 3986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montesinos-Rongen M, et al. , Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol, 2012. 124(6): p. 905–6. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Aguilar A, et al. , Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res, 2012. 18(19): p. 5203–11. [DOI] [PubMed] [Google Scholar]
- 31.Bruno A, et al. , Mutational analysis of primary central nervous system lymphoma. Oncotarget, 2014. 5(13): p. 5065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T, et al. , Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol, 2016. 42(3): p. 279–90. [DOI] [PubMed] [Google Scholar]
- 33.Wilson WH, et al. , Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med, 2015. 21(8): p. 922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis RE, et al. , Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med, 2001. 194(12): p. 1861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlahopoulos SA, Aberrant control of NF-kappaB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode. Cancer Biol Med, 2017. 14(3): p. 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Knittel G, et al. , B-cell-specific conditional expression of Myd88p.L252P leads to the development of diffuse large B-cell lymphoma in mice. Blood, 2016. 127(22): p. 2732–41.MYD88 is an adaptor protein needed to relay the activation of toll-like receptor signaling to NF-kB activation. Mutations in MYD88 have been described in many B-cell malignancies. This study reports the generation of a novel mouse model with conditional expression of MYD88 leading to the developing of a lymphoproliferative disease with morphologic and immunophenotypical characteristics of activated B-cell type diffuse large B cell lymphoma, suggesting that MYD88 plays a role in the development of DLBCL.
- 37.Rubenstein JL, Biology of CNS lymphoma and the potential of novel agents. Hematology Am Soc Hematol Educ Program, 2017. 2017(1): p. 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngo VN, et al. , Oncogenically active MYD88 mutations in human lymphoma. Nature, 2011. 470(7332): p. 115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasqualucci L, et al. , Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet, 2011. 43(9): p. 830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morin RD, et al. , Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood, 2013. 122(7): p. 1256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohr JG, et al. , Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A, 2012. 109(10): p. 3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honma K, et al. , TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood, 2009. 114(12): p. 2467–75. [DOI] [PubMed] [Google Scholar]
- 43.Basso K, et al. , BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med, 2012. 209(13): p. 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan RT and Dalla-Favera R, The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature, 2004. 432(7017): p. 635–9. [DOI] [PubMed] [Google Scholar]
- 45.Migliazza A, et al. , Frequent somatic hypermutation of the 5' noncoding region of the BCL6 gene in B-cell lymphoma. Proc Natl Acad Sci U S A, 1995. 92(26): p. 12520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam LT, et al. , Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood, 2008. 111(7): p. 3701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding BB, et al. , Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood, 2008. 111(3): p. 1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubenstein JL, et al. , Gene expression and angiotropism in primary CNS lymphoma. Blood, 2006. 107(9): p. 3716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponzoni M, et al. , Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol, 2007. 138(3): p. 316–23. [DOI] [PubMed] [Google Scholar]
- 50.Rubenstein JL, et al. , CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood, 2013. 121(23): p. 4740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadoch C, et al. , Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin Cancer Res, 2014. 20(4): p. 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyasato Y, et al. , The expression of PD-1 ligands and IDO1 by macrophage/microglia in primary central nervous system lymphoma. J Clin Exp Hematop, 2018. 58(2): p. 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapuy B, et al. , Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood, 2016. 127(7): p. 869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nosrati A, et al. , MYC, BCL2, and BCL6 rearrangements in primary central nervous system lymphoma of large B cell type. Ann Hematol, 2019. 98(1): p. 169–173. [DOI] [PubMed] [Google Scholar]
- 55.Kalkat M, et al. , MYC Deregulation in Primary Human Cancers. Genes (Basel), 2017. 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer L, et al. , Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol, 2011. 13(10): p. 1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nayak L, Pentsova E, and Batchelor TT, Primary CNS lymphoma and neurologic complications of hematologic malignancies. Continuum (Minneap Minn), 2015. 21(2 Neuro-oncology): p. 355–72. [DOI] [PubMed] [Google Scholar]
- 58.Khatab S, Spliet W, and Woerdeman PA, Frameless image-guided stereotactic brain biopsies: emphasis on diagnostic yield. Acta Neurochir (Wien), 2014. 156(8): p. 1441–50. [DOI] [PubMed] [Google Scholar]
- 59.Quijano S, et al. , Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin's lymphoma: improved sensitivity of flow cytometry. J Clin Oncol, 2009. 27(9): p. 1462–9. [DOI] [PubMed] [Google Scholar]
- 60.van Westrhenen A, et al. , Diagnostic markers for CNS lymphoma in blood and cerebrospinal fluid: a systematic review. Br J Haematol, 2018. 182(3): p. 384–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Hiemcke-Jiwa LS, et al. , The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol Oncol, 2018. 36(2): p. 429–435.This study suggests that droplet digital PCR is successful in detecting MYD88 in CSF even when the amount of DNA is limited. It may help in diagnosing PCNSL (diffuse large B cell type) in the CSF in the future.
- 62.Hattori K, et al. , Clinical significance of disease-specific MYD88 mutations in circulating DNA in primary central nervous system lymphoma. Cancer Sci, 2018. 109(1): p. 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fontanilles M, et al. , Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget, 2017. 8(29): p. 48157–48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baraniskin A, et al. , Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood, 2011. 117(11): p. 3140–6. [DOI] [PubMed] [Google Scholar]
- 65.Mao X, Sun Y, and Tang J, Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol Sci, 2014. 35(2): p. 233–8. [DOI] [PubMed] [Google Scholar]
- 66.Baraniskin A, et al. , Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for primary central nervous system lymphoma. Neuro Oncol, 2016. 18(3): p. 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mabray MC, et al. , The Combined Performance of ADC, CSF CXC Chemokine Ligand 13, and CSF Interleukin 10 in the Diagnosis of Central Nervous System Lymphoma. AJNR Am J Neuroradiol, 2016. 37(1): p. 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen-Them L, et al. , The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer, 2016. 61: p. 69–76. [DOI] [PubMed] [Google Scholar]
- 69.Sasagawa Y, et al. , Diagnostic value of interleukin-10 in cerebrospinal fluid for diffuse large B-cell lymphoma of the central nervous system. J Neurooncol, 2015. 121(1): p. 177–83. [DOI] [PubMed] [Google Scholar]
- 70.Sasayama T, et al. , Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol, 2012. 14(3): p. 368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thaler FS, et al. , Soluble TACI and soluble BCMA as biomarkers in primary central nervous system lymphoma. Neuro Oncol, 2017. 19(12): p. 1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strehlow F, et al. , Osteopontin in cerebrospinal fluid as diagnostic biomarker for central nervous system lymphoma. J Neurooncol, 2016. 129(1): p. 165–71. [DOI] [PubMed] [Google Scholar]
- 73.Kuusisto ME, et al. , Antithrombin III is probably not a suitable biomarker for diagnosis of primary central nervous system lymphoma. Ann Hematol, 2015. 94(7): p. 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy S, et al. , Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol, 2008. 26(1): p. 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murase S, et al. , Increased levels of CSF soluble CD27 in patients with primary central nervous system lymphoma. Cancer Lett, 1998. 132(1-2): p. 181–6. [DOI] [PubMed] [Google Scholar]
- 76.Kersten MJ, et al. , Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood, 1996. 87(5): p. 1985–9. [PubMed] [Google Scholar]
- 77.Viaccoz A, et al. , CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol, 2015. 17(11): p. 1497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *78.Grommes C, et al. , Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood, 2019. 133(5): p. 436–445.This study uses irbutinib, a first in class inhibitor of Bruton tyrosine kinase, in combination with high-dose methotrexate and rituximab in patients with recurrent or refractory PCNSL. Within this paper, next generation sequencing of circulating tumor DNA (ctDNA) in cerebral spinal fluid was used as a possible biomarker for CNS disease.
- 79.Abrey LE, et al. , Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol, 2006. 24(36): p. 5711–5. [DOI] [PubMed] [Google Scholar]
- 80.Ferreri AJ, et al. , Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol, 2003. 21(2): p. 266–72. [DOI] [PubMed] [Google Scholar]
- 81.Raoux D, et al. , Primary central nervous system lymphoma: immunohistochemical profile and prognostic significance. Neuropathology, 2010. 30(3): p. 232–40. [DOI] [PubMed] [Google Scholar]
- 82.Liu J, et al. , Immunohistochemical profile and prognostic significance in primary central nervous system lymphoma: Analysis of 89 cases. Oncol Lett, 2017. 14(5): p. 5505–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aki H, et al. , Primary central nervous system lymphoma in immunocompetent individuals: a single center experience. Int J Clin Exp Pathol, 2013. 6(6): p. 1068–75. [PMC free article] [PubMed] [Google Scholar]
- 84.Hattab EM, et al. , Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: a retrospective analysis of 31 cases. Mod Pathol, 2010. 23(2): p. 235–43. [DOI] [PubMed] [Google Scholar]
- 85.Momota H, et al. , Prognostic value of immunohistochemical profile and response to high-dose methotrexate therapy in primary CNS lymphoma. J Neurooncol, 2010. 98(3): p. 341–8. [DOI] [PubMed] [Google Scholar]
- 86.Lin CH, et al. , Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res, 2006. 12(4): p. 1152–6. [DOI] [PubMed] [Google Scholar]
- 87.Li S, et al. , BCL6 Rearrangement Indicates Poor Prognosis in Diffuse Large B-cell Lymphoma Patients: A Meta-analysis of Cohort Studies. J Cancer, 2019. 10(2): p. 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kreher S, et al. , Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro Oncol, 2015. 17(7): p. 1016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braaten KM, et al. , BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res, 2003. 9(3): p. 1063–9. [PubMed] [Google Scholar]
- 90.Levy O, et al. , Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer, 2008. 112(1): p. 151–6. [DOI] [PubMed] [Google Scholar]
- 91.Chunsong H, et al. , CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J Immunol, 2006. 177(10): p. 6713–22. [DOI] [PubMed] [Google Scholar]
- 92.Grommes C, et al. , Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol, 2019. 21(3): p. 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]


