Abstract
Staphylococcus aureus biofilms have a high tolerance to antibiotics, making the treatment of periprosthetic joint infection (PJI) challenging. From a clinical perspective, bacteria from surgical specimens are cultured in a planktonic state to determine antibiotic sensitivity. However, S. aureus exists primarily as established biofilms in PJI. To address this dichotomy, we developed a prospective registry of total knee and hip arthroplasty PJI S. aureus isolates to quantify the activity of clinically important antibiotics against isolates grown as biofilms. S. aureus planktonic minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were assessed using clinical laboratory standard index assays for 10 antibiotics (cefazolin, clindamycin, vancomycin, rifampin, linezolid, nafcillin, gentamicin, trimethoprim/sulfamethoxazole, doxycycline, and daptomycin). Mature biofilms of each strain were grown in vitro, after which biofilm MIC (MBIC) and biofilm MBC (MBBC) were determined. Overall, isolates grown as biofilms displayed larger variations in antibiotic MICs as compared to planktonic MIC values. Only rifampin, doxycycline, and daptomycin had measurable biofilm MIC values across all S. aureus isolates tested. Biofilm MBC observations complemented biofilm MIC observations; rifampin, doxycycline, and daptomycin were the only antibiotics with measurable biofilm MBC values. 90% of S. aureus biofilms could be killed by rifampin, 50% by doxycycline, and only 15% by daptomycin. Biofilm formation increased bacterial antibiotic tolerance nonspecifically across all antibiotics, in both MSSA and MRSA samples. Rifampin and doxycycline were the most effective antibiotics at killing established S. aureus biofilms.
Introduction
Staphylococcus aureus is a common organism responsible for orthopaedic related infections1,2. S. aureus in implant related infections regularly form and primarily exist as established biofilms3,4,5 These infections are difficult to treat because bacterial cells in biofilms have a high tolerance to traditional antibiotics2. This antibiotic tolerance of biofilms is also seen in many other types of bacteria6–8. A variety of drug tolerance mechanisms have been proposed, including impaired antibiotic penetration, quorum-sensing regulation, and altered metabolic states9.
Although biofilms are the primary state for bacterial cells in infections, standard antibiotic susceptibility testing uses bacteria grown as planktonic cultures10,11. This accurately quantifies antibiotic genetic resistance but fails to assess antibiotic activity against bacteria in a biofilm state. Antibiotic susceptibility and tolerance is much less understood when the bacteria are cultured as biofilms. There is a significant decrease in antibiotic sensitivity between planktonic bacteria and bacteria cells in biofilms because of the high tolerance of biofilms to antibiotics12,13. Therefore, testing of in vitro bacteria cultured as biofilms from patient isolates would provide a more accurate determination of antibiotic susceptibility by directly observing the bacteria phenotype that exists during infection.
There are limited studies that have evaluated the activity of antibiotics against S. aureus biofilms specifically using PJI clinical isolates. To address this, we developed a prospective clinical isolate registry of total knee arthroplasty (TKA) PJI samples to quantify the sensitivity of different antibiotics to clinical isolates of both methicillin sensitive S. aureus (MSSA) and methicillin resistant S. aureus (MRSA) in vitro cultured biofilms. Both planktonic and biofilm MIC and MBC of a panel of commonly administered antibiotics were quantified across all isolates. The objective was to determine the extent of variation in biofilm antibiotic sensitivity to clinically administered antibiotics.
Methods
Culture conditions and bacterial strains
All strains were inoculated from frozen stocks into 5 ml Tryptic Soy Broth (TSB, Bectin Dickinson and Company) in a 15 ml conical tube overnight at 37° C with shaking at 250 rpm. 16 hours later, all strains were diluted in Mueller Hinton Broth (MHB; Bectin Dickinson and Company) to a final concentration of 0.5×106 CFU/ml using the 0.5 MacFarland Standard (GFS Chemicals) and an Infinite M200 Spectrophotometer (Tecan). Assays were performed utilizing high throughput methods using sterile, tissue culture treated flat bottom 96 well plates (Thermo-Fisher Scientific). All experiments were performed at least in triplicate at three separate times with freshly inoculated cultures. Two lab strains, USA30014 and SH100015, as well as 10 MRSA clinical isolates and 8 MSSA clinical isolates were tested. Clinical isolates from PJI patients were obtained from the clinical microbiology laboratory which were cultured on TSB agar slants. S. aureus clinical isolates were expanded in TSB in a shaking overnight culture and banked in cryotubes at −80°C in TSB with 10% glycerol. Institutional Review Board guidelines and regulations were followed in completing this study, IRB approval # PRO15070263.
Planktonic Culture MIC and MBC
All lab strains and clinical isolates of S. aureus were cultured planktonically at a concentration of 0.5×106 CFU/ml in sterile, tissue culture treated flat bottom 96 well plates with fold dilutions of six antibiotics (vancomycin, rifampin, gentamicin, trimethoprim/sulfamethoxazole, doxycycline, and daptomycin) at a final volume of 100 ul. MSSA samples were additionally tested with cefazolin and nafcillin, while MRSA samples were additionally tested with clindamycin and linezolid. Antibiotic concentrations of 125, 62, 31, 16, 8, 4, 2, 1, 0.5, 0.25, 0.13 μg/ml as well as untreated controls were tested. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were assessed following clinical laboratory standard institute assays10,11. MIC was assessed by staining treated cultures with PrestoBlue viability assay (Thermo-Fisher Scientific)16 according to manufacturer’s instructions using a SynTek microplate reader. A clear and definitive color change occurred, and plates were quantified after 10 minutes of staining. MBC was determined by serial dilution of treated wells and plating on TSA II with 5% sheep blood CS100 plates. After an overnight incubation at 37° C, colony forming unit (CFU) analysis on plates was performed; a 99.9% reduction in CFU’s of the original plating density represented the MBC17.
Mature Biofilms MIC and MBC
For bacterial biofilms treatment assays, S. aureus strains were cultured planktonically at a concentration of 0.5×106 CFU/ml in sterile, tissue culture treated flat bottom 96 well plates at a volume of 100 ul. S. aureus strains were statically grown for 24 hours, at which time fresh MHB was exchanged. After another static incubation for 24 hours, wells were washed with dPBS to remove planktonic bacteria, and the 48-hour mature biofilms were treated with the same panel of antibiotics used in planktonic assays. Antibiotic concentrations were raised to 2000, 1000, 500, 250, 125, 62, 31, 16, 8, 4, 2 μg/ml and diluted in MHB. After 24 hours of treatment, the antibiotic supplemented MHB was removed, biofilms were washed with dPBS, and 100 μl of dPBS was added to biofilms in wells. The 96 well plates were then manually scrapped for 1 minute in the dPBS to homogenize biofilms for minimum biofilm inhibitory concentration (MBIC) analysis. For scraping, sterile and autoclaved 0.1–10 μl micropipette tips were kept in holder within the hinged box container and taped securely in place. Pipette tips were inserted simultaneously into all 96 wells and the wells were scraped vigorously. MBIC was assessed using PrestoBlue viability assay according to manufacturer’s instructions using a SynTek microplate reader. A clear and definitive color change took longer in biofilm wells and occurred after 1 hour of staining at which time plates were quantified. For minimum biofilm bactericidal concentration (MBBC), scrapped biofilms well contents were plated directly onto blood agar plates and CFU analysis was performed. MBBC was determined as 99.9% reduction of 48-hour biofilm CFU.
Statistical Analysis
Antibiotic susceptibility and tolerance between planktonic and established biofilms of PJI clinical isolates were collected and compared. All graphical and statistical analysis was performed using Prism 7.0 (GraphPad, La Jolla, CA). Data was analyzed using the D’Agostino & Pearson normality test. Since data was non-parametric, when comparing two groups, a Mann-Whitney test was performed. Matched sample analysis was performed using a Wilcoxon rank test, and multiple group variance testing was performed using a Kruskal-Wallis test with a Dunn’s multiple comparisons posttest.
Results
Variations in antibiotic activity against S. aureus planktonic cultures and mature biofilms
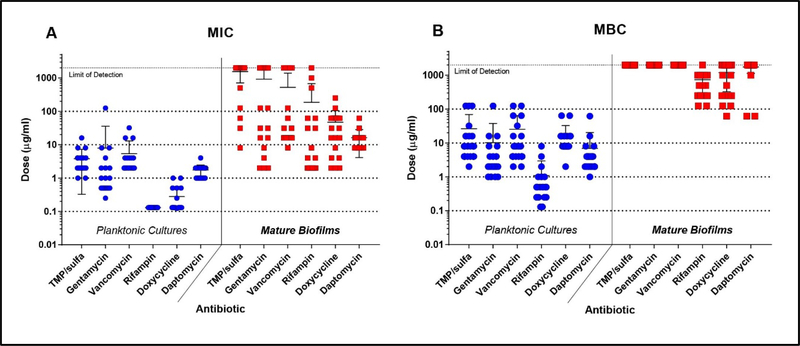
Clinical laboratory standard index protocol and a mature biofilm in vitro model was used to quantify variations in planktonic MIC, planktonic MBC, biofilm MIC, and biofilm MBC of different antibiotics across S. aureus isolates. Gentamicin, trimethoprim/sulfamethoxazole, and vancomycin displayed larger variations (~1.5 log spread) in planktonic MICs across all isolates, while rifampin, doxycycline and daptomycin displayed smaller variations (~0.5 log spread) (Fig 1A-blue). Alternatively, planktonic MBCs of all antibiotics displayed a large amount of variation (2 log spread) across all isolates (Fig 1B-blue). Rifampin showed superior antimicrobial action against planktonic cultures compared to other antibiotics, with MBC values ranging from 0.13 to 8 μg/ml. Next, we determined variations in MBIC and MBBC of antibiotics tested against all isolates. Most antibiotics showed a large variability (2–3 log spread) in MBIC across all isolates, except for daptomycin which displayed a variation of only one order of magnitude (Fig 1A-red). Many isolate biofilms showed no sensitivity to trimethoprim/sulfamethoxazole, gentamicin, or vancomycin despite normal sensitivity observed in planktonic cultures. Only rifampin, doxycycline, and daptomycin could kill the 48-hour mature biofilms, with biofilm MBCs ranging from 80 to 2000 μg/ml (Fig1B-red).
Figure 1. PJI S. aureus biofilms show decreased antibiotic sensitivity and increased tolerance to killing.
Across all clinical isolates and lab strains, antibiotic planktonic MICs (blue) and antibiotic mature biofilm MICs (red) were determined using a PrestoBlue viability assay (A). Antibiotic MICs of mature biofilms showed a much larger variation across all isolates compared to planktonic cultures. Across isolates, antibiotic planktonic MBCs (blue) and antibiotic mature biofilm MBCs (red) were determined by CFU analysis using blood agar plates (B). Clinical isolate biofilms and lab strains were all highly tolerant to antibiotics.
Both MSSA and MRSA biofilms display decreased sensitivity across all antibiotics
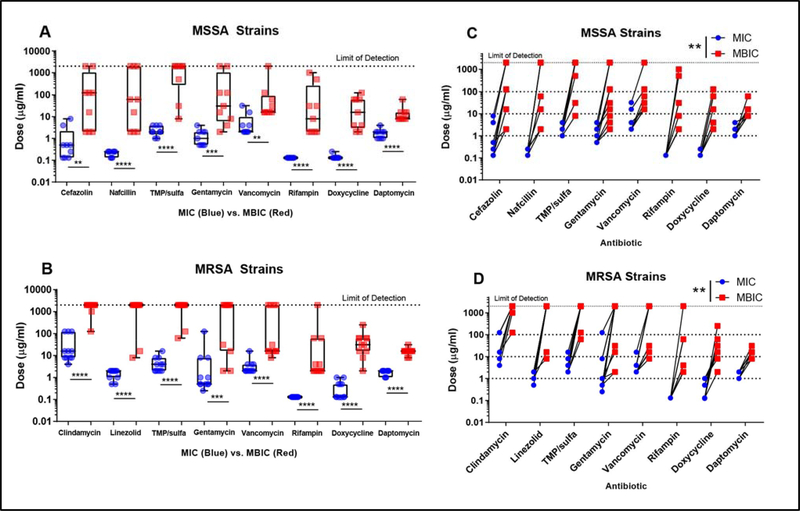
We assessed planktonic MIC and biofilm MIC (MBIC) of additional antibiotics cefazolin and nafcillin for MSSA isolates, as well as clindamycin and linezolid for MRSA isolates. 78% of MSSA isolate biofilms displayed sensitivity to cefazolin under our limit of detection (Fig 2A-red), while ~83% MRSA isolate biofilms displayed no sensitivity to clindamycin (Fig 2B-red). MIC values for established biofilms were more variable across isolates compared to planktonic cultures. In both MSSA and MRSA isolates, rifampin MIC for all 20 S. aureus strains was 0.125 μg/ml, while the MBICs ranged dramatically from 2–2000 μg/ml (Fig 2A and B). Daptomycin was the only antibiotic for which biofilm MIC did not display dramatic variation in sensitivity across all isolates. In every antibiotic tested, there was a significant increase in MBIC compared to planktonic MIC in both MSSA (Fig 2A) and MRSA (Fig 2B) isolates. Rifampin, doxycycline, and daptomycin could inhibit growth in all isolate biofilms at the doses tested. Lab strains SH1000 and USA300 also showed and statistically significant increase in 48- hour biofilm MIC compared to planktonic cultures. Matched sample analysis confirmed that the MIC for all strains did significantly increase in the biofilm phenotype compared to planktonic for both MSSA and MRSA (Fig 2C and D).
Figure 2. Both MSSA and MRSA biofilms demonstrate decreased sensitivity across all clinically used antibiotics.
MSSA and MRSA biofilms both show less antibiotic sensitivity with mature biofilm MICs (MBICs) significantly higher (p<0.001 ****, p<0.005*** p<0.01 **) than planktonic MIC in between every antibiotic tested using a Mann-Whitney Test (A-B). Matched analysis of S. aureus planktonic and biofilm MICs confirms a statistically significant increase (p<0.01 **) in MBIC compared to MIC in every clinical and laboratory strain tested using a Wilcoxon Test (C-D).
Cefazolin fails to eliminate MSSA biofilms and vancomycin fails to eliminate MRSA biofilms
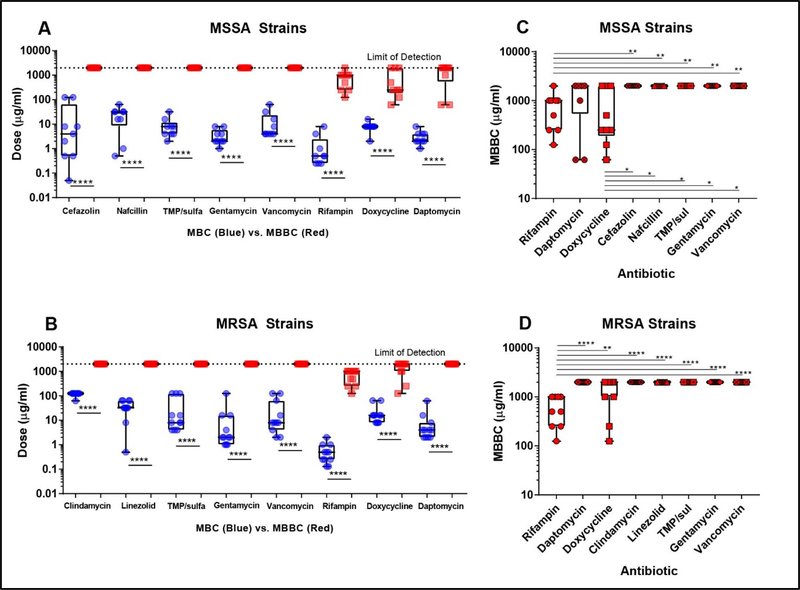
Planktonic MBC and biofilm MBC (MBBC) of isolates were determined. Cefazolin, nafcillin, clindamycin, linezolid, trimethoprim/sulfamethoxazole, gentamicin, and vancomycin could not eliminate any isolate biofilms up to our limit of detection (Fig 3A and B-red). In all antibiotics tested, there was a significant increase in MBBC compared to planktonic MBC in MSSA (Fig 3A) and MRSA (Fig 3B) isolates. Measurements of biofilm MBC complements MIC observations; rifampin, doxycycline, and daptomycin were best at killing biofilms in vitro. 90% of S. aureus biofilms could be eliminated by rifampin, 50% by doxycycline, and only 15% by daptomycin. MBBC values of each antibiotic against both MSSA (Fig 3A) and MRSA (Fig 3B) were compared to determine which antibiotic eliminated biofilms most effectively. Lab strain SH1000 and USA300 biofilms also showed similar tolerance to antibiotics compared to planktonic cultures. In MSSA biofilms, rifampin and doxycycline MBBC values were significantly lower than MBBCs of cefazolin, nafcillin, trimethoprim/sulfamethoxazole, gentamicin, and vancomycin (Fig 3C). In MRSA biofilms, only rifampin MBBC values were significantly lower across all antibiotics tested (Fig 3D).
Figure 3. Clinically important antibiotics are unable to effectively kill MSSA and MRSA S. aureus biofilms.
S. aureus planktonic minimum bactericidal concentration (MBC) was compared to minimum biofilm bactericidal concentration (MBBC) for MSSA (A) and MRSA (B). All clinical and laboratory strains show increased tolerance to all antibiotics tested in our panel with MBBC values all statistically significantly higher (p<0.0001****) than the MBC values (A-B). In MSSA isolate biofilms, the rifampin and doxycycline treatment groups had a significantly lower MBC (p<0.05 *, p<0.0001 ****) compared to the remaining antibiotics (C). In MRSA isolate biofilms, only the rifampin treatment group had significantly lower MBC (p<0.0001 ****) compared to remaining antibiotics (D).
Discussion
S. aureus is a virulent and extremely challenging pathogen to treat in orthopaedic implant associated infections. The major criterion for antibiotic selection in the treatment of infection is based on susceptibility testing of clinical isolates cultured in a planktonic state11. However, in a clinical infection, S. aureus primarily exists as established biofilms18. Based on this dichotomy, we were interested in determining variations in antibiotic sensitivity between clinical S. aureus isolates when cultured as established biofilms compared to the typical planktonic culture. We observed a remarkably large variation in biofilm MIC as compared to planktonic MIC in the clinically important antibiotics cefazolin, vancomycin, and rifampin.
The large decrease in antibiotic sensitivity of S. aureus biofilms as compared to planktonic cells across all classes of drugs tested demonstrated that this tolerance mechanism was nonspecific. Rifampin, daptomycin, and doxycycline demonstrated a superior ability to inhibit biofilm growth compared to other antibiotics tested based on relative comparison of MIC. This is in close agreement with other groups showing a clear tolerance to traditional antibiotics by bacterial biofilms12,19. Rifampin is the only traditionally administered antibiotic that has shown high and reliable anti-biofilm activity in S. aureus5. However, rifampin monotreatment is avoided clinically since S. aureus can develop rapid resistance to this drug. In periprosthetic joint infections, rifampin is only used in combination with another antibiotic such as cefazolin or vancomycin20,21. Daptomycin is a more recently developed lipopeptide which disrupts the cellular membrane of bacteria, rapidly lysing cells independent of metabolic activity. This antibiotic has previously been shown to be highly effective against a panel of MRSA clinical isolate biofilms19,22. Recently, suppressive doxycycline therapy in a small and high risk PJI cohort showed reasonable effectiveness and tolerability of the antibiotic for successful treatment23.To our knowledge, our work is the first-time doxycycline has been demonstrated to be as effective as rifampin or daptomycin at killing S. aureus mature biofilms from PJI patients.
Comparison of antibiotic activity in mature biofilms and planktonic cultures is complicated by intra-strain growth and biofilm characteristic differences. Our observations were likely limited due to possible differences in biophysical properties of the secreted extracellular matrix, metabolic output, and the population density within biofilms grown from each individual isolate, however these and other factors were not measured in this study. S. aureus bacterial biofilms have been shown to become more susceptible to antibiotics with increased exposure time from one to five days24. While this study did not address the effect of treatment time on biofilm drug susceptibility, we acknowledge this is an important factor to consider. Despite these limitations, we clearly show bacterial biofilms remained after treatment with extremely high doses of clinically important antibiotics. This study displays a clear loss of antibiotic activity against bacterial biofilms compared to planktonic cells. Rifampin, the optimal anti-biofilm antibiotic screened, could only effectively kill biofilms in vitro at doses not achievable in human patients secondary to overt toxicity.
Established bacterial biofilms have a remarkable tolerance to antibiotics. Our data suggests that antibiotic treatment in S. aureus knee and hip PJI and other orthopaedic infections are a critical but incomplete part of treatment. Surgical debridement and the host immune system play vital roles in successful treatment of orthopaedic related biofilm infections. Our results should not be interpreted in the context that standard planktonic antibiotic sensitivity testing should be replaced. Standard clinical laboratory standards institute testing for MIC provide invaluable clinical data on genetic antibiotic resistance. This work does suggest that there is a phenotypic and non-specific change in tolerance to antibiotics that occurs between planktonic and biofilm phenotypes of bacteria. Further, it suggests that from an in vitro perspective, rifampin, daptomycin, and doxycycline may have a stronger ability to control implant associated infections. Further clinical studies are warranted to confirm these results.
Acknowledgements
The study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS K08AR071494), the National Center for Advancing Translational Science (NCATS KL2TR0001856), the Orthopaedic Research and Education Foundation, and the Musculoskeletal Tissue Foundation.
Dr. Kenneth Urish is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS K08AR071494), the National Center for Advancing Translational Science (NCATS KL2TR0001856), the Orthopaedic Research and Education Foundation, and the Musculoskeletal Tissue Foundation.
This study was performed at the Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
Footnotes
All authors have been involved with the research design, acquisition, analysis, or interpretation of results. All authors either drafted or substantially revised manuscript and approved final submission.
References
- 1.Parvizi J, Azzam K, Ghanem E, Austin MS & Rothman RH Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res 467, 1732–1739, doi: 10.1007/s11999-009-0857-z (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urish KL et al. Antibiotic-tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin Orthop Relat Res 474, 1649–1656, doi: 10.1007/s11999-016-4720-8 [pii] (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoodley P et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am 90, 1751–1758, doi: 10.2106/JBJS.G.00838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoiby N et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21 Suppl 1, S1–25, doi: 10.1016/j.cmi.2014.10.024 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Zimmerli W Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 276, 111–119, doi: 10.1111/joim.12233 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Fux CA, Costerton JW, Stewart PS & Stoodley P Survival strategies of infectious biofilms. Trends Microbiol 13, 34–40, doi: 10.1016/j.tim.2004.11.010 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Nilsson M et al. The dlt genes play a role in antimicrobial tolerance of Streptococcus mutans biofilms. Int J Antimicrob Agents 48, 298–304, doi: 10.1016/j.ijantimicag.2016.06.019 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Rybtke M, Hultqvist LD, Givskov M & Tolker-Nielsen T Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J Mol Biol 427, 3628–3645, doi: 10.1016/j.jmb.2015.08.016 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Waters EM, Rowe SE, O’Gara JP & Conlon BP Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? PLoS Pathog 12, e1006012, doi: 10.1371/journal.ppat.1006012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. volumes (Clinical and Laboratory Standards Institute, Wayne, Pa., 2005). [Google Scholar]
- 11.Andrews JM Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48 Suppl 1, 5–16 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Pettit RK, Weber CA & Pettit GR Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob 8, 28, doi: 10.1186/1476-0711-8-28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard LP, Ceri H, Gibb AP, Olson M & Sepandj F MIC versus MBEC to determine the antibiotic sensitivity of Staphylococcus aureus in peritoneal dialysis peritonitis. Perit Dial Int 30, 652–656, doi: 10.3747/pdi.2010.00010 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Diep BA et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739, doi: 10.1016/S0140-6736(06)68231-7 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh MJ et al. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325–4. J Bacteriol 184, 5457–5467 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lall N, Henley-Smith CJ, De Canha MN, Oosthuizen CB & Berrington D Viability Reagent, PrestoBlue, in Comparison with Other Available Reagents, Utilized in Cytotoxicity and Antimicrobial Assays. Int J Microbiol 2013, 420601, doi: 10.1155/2013/420601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson LR & Shanholtzer CJ Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clin Microbiol Rev 5, 420–432 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto M Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64, 175–188, doi: 10.1146/annurev-med-042711-140023 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Smith K, Perez A, Ramage G, Gemmell CG & Lang S Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int J Antimicrob Agents 33, 374–378, doi: 10.1016/j.ijantimicag.2008.08.029 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Eisen DP & Denholm JS Recommendations for rifampicin therapy of staphylococcal infection in Infectious Diseases Society of America prosthetic Joint Infection Guidelines are not supported by available literature. Clin Infect Dis 57, 159–160, doi: 10.1093/cid/cit183 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Osmon DR et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56, e1–e25, doi: 10.1093/cid/cis803 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Meeker DG et al. Evaluation of Antibiotics Active against Methicillin-Resistant Staphylococcus aureus Based on Activity in an Established Biofilm. Antimicrob Agents Chemother 60, 5688–5694, doi: 10.1128/AAC.01251-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradier M et al. Suppressive antibiotic therapy with oral doxycycline for Staphylococcus aureus prosthetic joint infection: a retrospective study of 39 patients. Int J Antimicrob Agents 50, 447–452, doi: 10.1016/j.ijantimicag.2017.04.019 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Castaneda P, McLaren A, Tavaziva G & Overstreet D Biofilm Antimicrobial Susceptibility Increases With Antimicrobial Exposure Time. Clin Orthop Relat Res 474, 1659–1664, doi: 10.1007/s11999-016-4700-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]