Abstract
Background:
Anal cancer incidence increased markedly in people living with HIV (PLWHIV) after the introduction of HAART, but in a few setting settings, recent declines have been reported. We report the incidence and time trends of anal cancer in PLWHIV in Australia.
Study design:
A data linkage study between the National HIV Registries and the Australian Cancer Database.
Methods:
Cases of anal squamous cell carcinoma (ASCC) in Australians aged 16 years and above diagnosed with HIV between 1982 and 2012 were identified. Standardized incidence ratios (SIRs) were calculated to compare incidence with that of the general population. Poisson regression models were developed to describe the time trends of ASCC over time and to compare ASCC risk within subgroups of PLWHIV.
Results:
Among 28 696 individuals, a total of 129 cases of ASCC were identified. The crude incidence was 36.3 per 100 000 person-years and it increased sharply from 14.8 to 62.1 per 100 000 person-years between 1982–1995 and 2009–2012 (P trend <0.001). The SIR was 35.3 (95% confidence interval 29.5–42.0), and there was an inverse association between SIR and increasing age (P trend <0.001). In multivariate analyses, ASCC incidence was significantly higher in recent years (P trend <0.001), in those who acquired HIV through male homosexual contact (P=0.002), and in those who had a history of AIDS (P < 0.001).
Conclusion:
PLWHIV in Australia are at markedly higher risk of anal cancer. Unlike in some industrialized countries with a mature HIV epidemic, the incidence of anal cancer is still increasing in this population in Australia.
Keywords: anal cancer, data linkage, health registry, homosexuality, male, people living with HIV
Introduction
Persistent human papillomavirus (HPV) infection causes squamous cell cancer of the anus (anal cancer) [1,2]. The incidence of anal cancer is highest in gay and bisexual men (GBM) who live with HIV [3], who have high rates of exposure to HPV infection in the anal canal through sexual transmission and may have impaired control of HPV infection because of immune dysregulation [4].
The introduction of HAART in 1996 led to substantial improvements in life expectancy of those living with HIV [5,6]. However, after widespread HAART implementation anal cancer incidence in people living with HIV (PLWHIV) increased markedly, with crude annual rates of more than 100 per 100 000 person-years being reported in some HIV-positive cohorts in the mid-2000s [7,8]. In countries where GBM constitute the majority of those living with HIV, including Australia, anal cancer has become the most common non-AIDS defining cancer in HIV-infected individuals [9].
Although the association between anal cancer and immune deficiency is complex [10,11], it appears that a low CD4+ cell count nadir may be predictive [12]. If this is correct, then the era of vastly improved HIV therapies and early treatment initiation before the onset of severe immune deficiency may lead to reductions in anal cancer incidence [13]. Early evidence suggests that this may have begun to occur in the United States and the Netherlands with incidence starting to decrease in the late 2000s [14,15], while anal cancer incidence remained high and stable in France [16]. We report the incidence and time trends of invasive anal cancer in PLWHIV in Australia, where over 70% of those infected are GBM and over 90% of those diagnosed have achieved HIV viral suppression [17].
Methods
Study cohort
Australia has commenced nationwide disease notification systems for both HIV and invasive cancers since 1982. The study cohort included all individuals aged 16 years and above who were diagnosed with HIV in Australia between 1982 and 2012 (n = 33 561) and were notified to the Australian National HIV Registries. Individuals (n = 4178, 12.4%) who had missing information on both first and last names were excluded from the study. The study was approved by Human Research Ethics Committees of the University of New South Wales, Australian Institution of Health and Welfare, and health departments of all states and territories.
Anal cancer diagnoses and deaths
Incident invasive anal cancer diagnoses were ascertained using probabilistic data linkage with the Australian Cancer Database, which collects data on all incident invasive cancers diagnosed in Australian residents since 1 January 1982, with the exception of nonmelanoma skin cancer. Only primary cases are included in the analysis. The linking algorithm has been described in detail in previous studies [9]. For each matched record, the date of anal cancer diagnosis and international classification of diseases (ICD)-O-3 and ICD10 codes were obtained. Only cases with an ICD-O-3 topography code C21 (anus and anal canal, including C21.0–C21.8) and a histology code of squamous cell carcinoma (SCC) were accepted as incident anal cancer. In addition, SCCs (n = 7) of the rectum (C20.9) were assumed to be anal cancers miscoded to the rectum or cancers of overlapping anorectal sites and thus included as anal cancer cases [18]. The HIV registries were also probabilistically linked with the population-based National Death Index to ascertain time of deaths.
Statistical analysis
Statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas, USA).
Anal cancer incidence
Incidence (per 100 000 person-years) was calculated as the total number of confirmed anal cancer cases over total person-years accumulated. Consistent with previous studies [14], follow-up started 90 days after the date of HIV diagnosis (to limit detection bias), and ended at the date of anal cancer diagnosis, death, or 31 December 2012, whichever came earlier. This resulted in the exclusion of those whose HIV diagnosis within 90 days from 31 December 2012 (n = 313), those who died within 90 days of their HIV diagnosis (n = 367), and those whose anal cancer diagnosis preceded, or was within 90 days of, their HIV diagnosis (n = 7). Standardized incidence ratios (SIRs) with 95% exact confidence intervals (CIs) were computed as the number of observed cases divided by that expected based on the application of 5-year age-specific, sex-specific, and calendar-year-specific general Australian population anal SCC incidence rates, assuming a Poisson distribution.
The mean age at the time of anal cancer diagnosis over time and mean time from HIV diagnosis to anal cancer diagnosis by the age at HIV diagnosis were compared using generalized linear regression with a logarithmic link function and P values for trend were reported.
Risk factors
Univariate and multivariate Poisson regression models were developed to estimate incidence rate ratios (IRR), comparing anal cancer incidence in subgroups of individuals with HIV infection. Data examined from the National HIV Registries included attained age (<30, 30–44, 45–59, and ≥60 years), whether HIVacquisition was due to male homosexual contact, CD4+ cell count once only at the time of HIV diagnosis (<50, 50–199, 200–499, and ≥500 cells/μl), and a history of diagnosis of AIDS defining illness between 1982 and 2012. For privacy reasons, sex at birth (cisgender) was not considered in the risk factor analyses as small numbers of anal cancer were identified in women, and female cases were included in HIV transmission routes other than male homosexual contact. Cancer incidence was also compared across five time periods broadly representative of HIV treatment advances, 1982–1995 (pre-HAART), 1996–1999 (early-HAART), 2000–2004 (coformulated protease inhibitors became available), 2005–2008 (advent of fusion inhibitor), and 2009–2012 (advent of integrase and CCR5inhibitor) [9,14]. As a sensitivity analysis, the same analyses were repeated after excluding those seven cases that were coded as rectal SCC, and the results were similar.
Time trends
Time trends in the characteristics of individuals diagnosed with HIV were compared using logistic regression, and P values for trend were reported.
Time trends in anal cancer incidence rates and SIRs over the five time periods and different age groups were analysed using generalized linear models with a logarithmic link function and were expressed as percentage change in incidence.
Nonparametric cumulative anal cancer incidence curves were presented by treating death as a competing risk [19]. Cumulative incidence curves were compared for anal cancer cases diagnosed before (1982–1995) and after (1996–2012) the advent of HAART.
Results
A total of 28 696 individuals diagnosed with HIV were included in the analysis. Over time, there was a trend towards an increasing age at HIV diagnosis [proportion aged 45 years and above increased from 13.1% in 1982–1995 to 25.0% in 2009–2012 (P trend <0.001, Table 1)]. There were decreasing trends in the proportion of HIV diagnoses in men, from 94.9% in 1982–1995 to 86.7% in 2009–2012 (P trend <0.001). However, among men, the proportion acquiring HIV through male homosexual contact remained stable at above 78% since 1996 (P trend = 0.553). There was a substantial reduction in PLWHIV who were ever diagnosed with AIDS, from 61.8 to 2.5% (P trend <0.001).
Table 1.
Characteristics of people diagnosed with HIV in Australia, 1982–2012.
| 1982–1995 | 1996–1999 | 2000–2004 | 2005–2008 | 2009–2012 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Total | 13 453 | 100.0 | 3057 | 100.0 | 4090 | 100.0 | 3969 | 100.0 | 4127 | 100.0 |
| Age (years) | ||||||||||
| 16–29 | 5245 | 39.0 | 919 | 30.1 | 1076 | 26.3 | 985 | 24.8 | 1165 | 28.2 |
| 30–44 | 6440 | 47.9 | 1528 | 50.0 | 2162 | 52.9 | 2026 | 51.1 | 1930 | 46.8 |
| 45–59 | 1527 | 11.4 | 522 | 17.1 | 724 | 17.7 | 798 | 20.1 | 865 | 21.0 |
| ≥60 | 240 | 1.2 | 88 | 2.9 | 128 | 3.1 | 160 | 4.0 | 167 | 4.1 |
| Cisgender | ||||||||||
| Male | 12 767 | 94.9 | 2746 | 89.8 | 3633 | 88.8 | 3463 | 87.3 | 3578 | 86.7 |
| Female | 686 | 5.1 | 311 | 10.2 | 457 | 11.2 | 506 | 12.8 | 549 | 13.3 |
| Male homosexual contacta | ||||||||||
| No | 2261 | 17.7 | 589 | 21.5 | 769 | 21.2 | 753 | 21.7 | 782 | 21.9 |
| Yes | 10 506 | 82.3 | 2157 | 78.6 | 2864 | 78.8 | 2710 | 78.3 | 2796 | 78.1 |
| CD4+ cell count at diagnosis (cells/μl) | ||||||||||
| <50 | 489 | 3.6 | 242 | 7.9 | 261 | 6.4 | 233 | 5.9 | 242 | 5.9 |
| 50–199 | 586 | 4.4 | 347 | 11.4 | 452 | 11.1 | 418 | 10.5 | 449 | 10.9 |
| 200–499 | 863 | 6.4 | 803 | 26.3 | 1109 | 27.1 | 1288 | 32.5 | 1488 | 36.1 |
| ≥500 | 820 | 6.1 | 771 | 25.2 | 1341 | 32.8 | 1307 | 32.9 | 1372 | 33.2 |
| Missing | 10 695 | 79.5 | 894 | 29.2 | 927 | 22.7 | 723 | 18.2 | 576 | 14.0 |
| Mean CD4+ cell countb | 359.3 | 414.3 | 455.1 | 453.5 | 445.6 | |||||
| History of AIDS | ||||||||||
| No | 5138 | 38.2 | 2289 | 74.9 | 3463 | 84.7 | 3558 | 89.6 | 4026 | 97.6 |
| Yes | 8315 | 61.8 | 768 | 25.1 | 627 | 15.3 | 411 | 10.4 | 101 | 2.5 |
Excluding females.
Excluding those with missing CD4+ cell counts.
Over a total of 355 407 person-years of follow-up (median 10.8 person-years), 129 cases of invasive anal SCC were identified, an overall crude incidence of 36.3 per 100 000 person-years (95% CI 30.5–43.1). Among the 129 cases, in 102 (79.1%) anal SCC (ASCC) were the first primary diagnoses, and in 27 (20.9%) were second or higher order primary ASCC. Between 1982–1995 and 2009–2012, there was a four-fold increase in anal cancer incidence from 14.8 to 62.1 per 100 000 person-years (P trend <0.001), equivalent to an increase of 43.7% per time period (95% CI 37.6–50.3). This increasing trend was only significant in those who acquired HIV through male homosexual contact (P trend <0.001), and not in those who acquired HIV through other means (P trend = 0.335, Fig. 1).
Fig. 1. Time trends of crude incidence of anal squamous cell carcinoma in people with HIV in Australia, 1982–2012, stratified by HIV acquisition route.

The mean age at anal cancer diagnosis in people with HIV was 49.9 years (SD: 10.7) and increased over the time periods under study (P trend = 0.001). The mean age at anal cancer diagnosis increased from 40.3 years in 1996–1999, to 48.2 in 1999–2004, 49.3 in 2005–2008, and 52.5 in 2009–2012.
The mean time from HIV diagnosis to anal cancer diagnosis was 13.2 years (SD: 6.5). It was 16.0 years in those aged 16–29 at the time of HIV diagnosis, 12.3 years in those aged 30–44, 11.7 years in those aged 45–59, and 9.3 years in those aged 60 years and above (P trend = 0.002). HIV exposure route was not associated with mean time to cancer diagnosis (13.6 years in GBM and 11.2 years in all other, P = 0.159).
Compared with the incidence of anal cancer in the general population in Australia, the SIR of anal SCC in PLWHIV was 35.3 (95% CI 29.5–42.0, Table 2). SIRs were highest in those aged 16–29 years and markedly decreased with increasing age (P trend <0.001).
Table 2.
Standardized incidence ratio of anal squamous cell carcinoma in people living with HIV in Australia from 1982 to 2012, by calendar period and by age at cancer diagnosis.
| Observed cases | Expected cases | SIR | 95% CI | |
|---|---|---|---|---|
| Time period | ||||
| 1982–1995 | 11 | 0.26 | 43.02 | 21.47–76.97 |
| 1996–1999 | 9 | 0.27 | 33.77 | 15.44–64.11 |
| 2000–2004 | 24 | 0.72 | 33.35 | 21.37–49.62 |
| 2005–2008 | 31 | 0.98 | 31.73 | 21.56–45.04 |
| 2009–2012 | 54 | 1.43 | 37.70 | 28.32–49.19 |
| Age | ||||
| 16–29 | 2 | 0.0071 | 198.10 | 33.93–1012.02 |
| 30–44 | 42 | 0.62 | 68.29 | 49.22–92.31 |
| 45–59 | 62 | 1.94 | 31.97 | 24.51–40.99 |
| ≥60 | 23 | 1.09 | 21.10 | 13.38–31.67 |
| Overall | 129 | 3.65 | 35.33 | 29.50–41.98 |
CI, confidence interval; SIR, standardized incidence ratio.
In unadjusted models, anal cancer incidence increased significantly with older age (P trend <0.001, Table 3). There was a 10-fold increase in those aged above 60 years compared with those aged less than 30 years (63.9 vs. 6.3 per 100 000 person-years). Risk was also significantly elevated in men who acquired HIV through homosexual contact compared with other means (IRR = 2.18, 95% CI 1.31–3.63) and those who were ever diagnosed with AIDS (IRR = 2.12, 95% CI 1.48–3.02). In those who had CD4+ cell count recorded at the time of their HIV diagnosis, a higher CD4+ cell count was associated with a lower incidence of anal cancer (P trend = 0.003). In multivariate analyses, age and CD4+ cell count at the time of HIV diagnosis were no longer significant, but the association of anal cancer with time period, male homosexual exposure and a history of AIDS remained (Table 3). The association between increasing trend of anal cancer over time was heightened after adjustment for confounding factors (P trend <0.001).
Table 3.
Incidence of anal squamous cell carcinoma in people living with HIV in Australia, 1982–2012, by time period, attained age, HIV exposure route, and CD4+ cell count at HIV diagnosis, and history of an AIDS-defining diagnosis.
| Person years | Cases | Incidence (per 100 000) | IRR | 95% CI | P value | Adjusted IRRa | 95% CI | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Time period | <0.001 | <0.001 | |||||||
| 1982–1995 | 74 236.6 | 11 | 14.82 | 1 | – | 1 | – | ||
| 1996–1999 | 48 109.0 | 9 | 18.71 | 1.26 | 0.52–3.05 | 1.41 | 0.58–3.47 | ||
| 2000–2004 | 73 425.6 | 24 | 32.69 | 2.21 | 1.08–4.50 | 2.63 | 1.23–5.58 | ||
| 2005–2008 | 72 622.7 | 31 | 42.69 | 2.88 | 1.45–5.73 | 3.62 | 1.65–7.92 | ||
| 2009–2012 | 87 012.7 | 54 | 62.06 | 4.19 | 2.19–8.01 | 5.53 | 2.65–11.52 | ||
| Attained age (years) | <0.001 | 0.229 | |||||||
| 16–44 | 19 6858.4 | 44 | 22.35 | 1 | – | 1 | – | ||
| 45–59 | 122556.6 | 62 | 50.59 | 2.26 | 1.54–3.33 | 1.37 | 0.88–2.12 | ||
| ≥60 | 35 970.8 | 23 | 63.94 | 2.86 | 1.73–4.74 | 1.37 | 0.75–2.52 | ||
| HIV exposure route | 0.003 | 0.002 | |||||||
| Other routes | 88 343.5 | 17 | 19.24 | 1 | – | 1 | – | ||
| Male homosexual | 26 7063.2 | 112 | 41.94 | 2.18 | 1.31–3.63 | 2.25 | 1.35–3.76 | ||
| CD4+ cell count at HIV diagnosis | 0.003b | 0.569b | |||||||
| <50 | 14 121.5 | 7 | 49.57 | 1 | – | 1 | – | ||
| 50–199 | 20 644.2 | 13 | 62.97 | 1.27 | 0.51–3.18 | 1.49 | 0.59–3.75 | ||
| 200–499 | 46 933.3 | 14 | 29.83 | 0.60 | 0.24–1.49 | 0.92 | 0.36–2.40 | ||
| ≥500 | 49 200.5 | 9 | 18.29 | 0.37 | 0.14–0.99 | 0.58 | 0.20–1.68 | ||
| Missing | 22 4507.1 | 86 | 38.31 | 0.77 | 0.36–1.67 | 1.16 | 0.53–2.51 | ||
| History of AIDS | <0.001 | <0.001 | |||||||
| No | 20 0604.3 | 49 | 24.43 | 1 | – | 1 | – | ||
| Yes | 15 4802.3 | 80 | 51.68 | 2.12 | 1.48–3.02 | 2.25 | 1.45–3.48 |
CI, confidence interval; IRR, incidence rate ratio.
Adjusted for time period, attained age, HIV exposure route, CD4+ cell count at time of HIV diagnosis, a history of AIDS.
Excluding those who had missing CD4+ at time of HIV diagnosis.
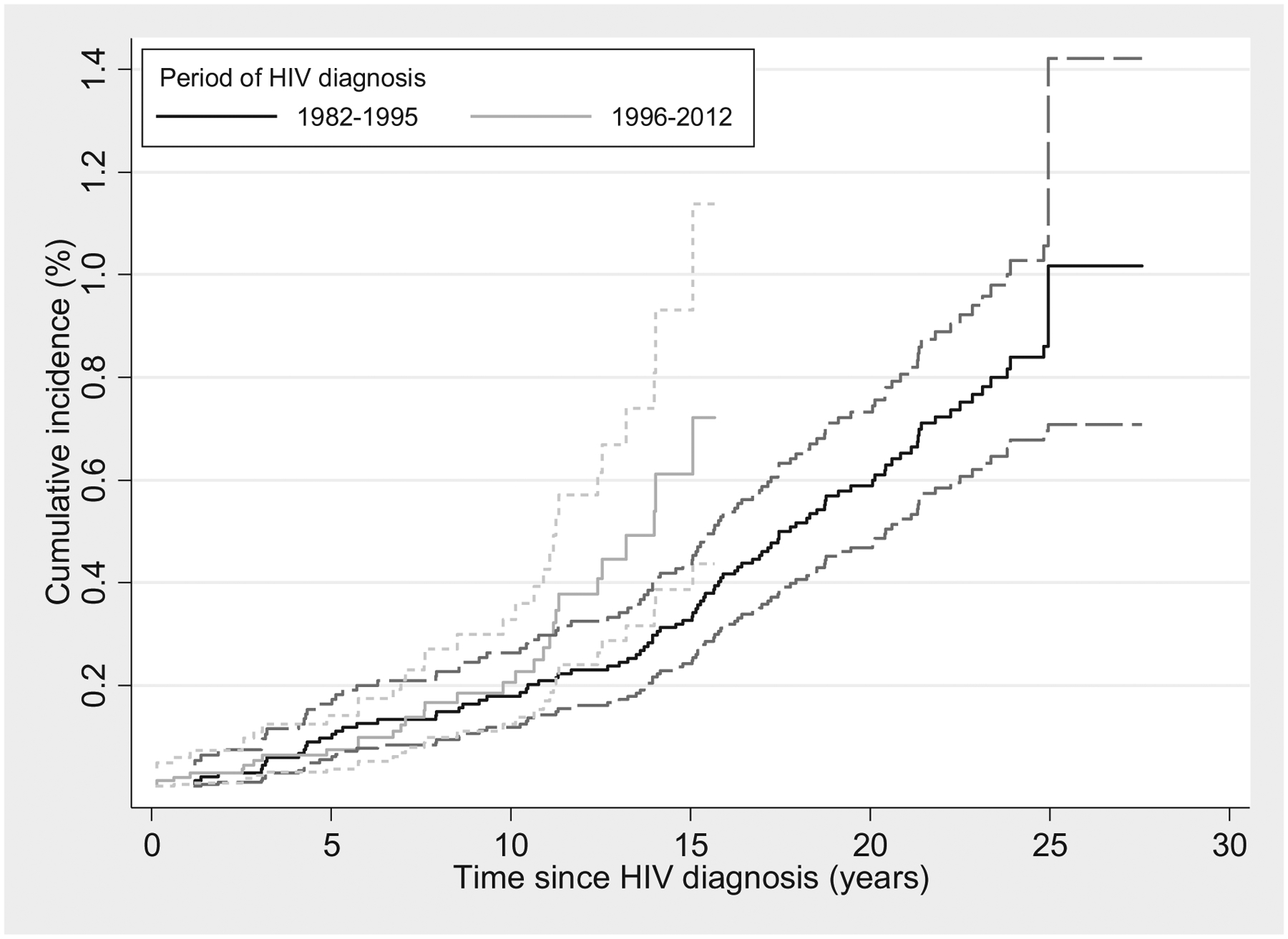
The cumulative anal SCC incidence at 10 and 15 years after HIV diagnosis was 0.17% (95% CI 0.13–0.24) and 0.38% (95% CI 0.30–0.47), respectively. At 10 years after HIV diagnosis, the cumulative anal SCC incidence was higher in those who were diagnosed with HIV in the HAART era than those who were diagnosed in the pre-HAART era (1996–2012, Fig. 2).
Fig. 2. Cumulative incidence of anal squamous cell carcinoma in people living with HIV in Australia, 1982–2012, stratified by period of HIV diagnosis.
Discussion
In PLWHIV in Australia during 1982–2012, the crude incidence of anal SCC was 36.3 per 100 000 person-years, more than 30 times higher than that of the general population. Anal cancer incidence continuously increased over the last 30 years by four-fold and reached 62.1 per 100 000 person-years in 2009–2012. This increase remained significant after adjusting for the increasing age of the cohort. Anal cancer diagnoses occurred an average of 13.2 years after HIV diagnosis at a mean age of 49.9 years. Those who acquired HIV infection through male homosexual contact and those who had a history of AIDS were at significantly elevated risk of anal cancer. The cumulative anal cancer incidence after 10 years since HIV diagnosis was higher in men diagnosed with HIV in the HAART era compared with those diagnosed prior to 1996, but remained less than 1%.
There is consistent evidence that PLWHIV experience the highest incidence of anal cancer, a risk more than 30 times higher than that of the general population [20]. It is also established that among PLWHIV, GBM have the highest incidence [3,6,14,21]. The extraordinarily high incidence of anal cancer in PLWHIV has become more apparent after the wide availably of HAART due to much improved survival in PLWHIV [22,23]. The Multicenter AIDS Cohort Study in the US reported a nearly six-fold increase in the incidence of anal cancer in the HAARTera compared with the pre-HAART era [5], and a 2.5-fold increase was also observed in a French cohort study [6]. Anal cancer has become the most common non-AIDS defining cancer in people with HIV in Australia [9], a finding also being reported in other developed countries with similar HIV epidemics [7]. The high burden of anal cancer in PLWHIV is not limited to developed countries. In emerging economies like Brazil and India, high rates of anal cancer in those with HIV/AIDS have also been reported in recent years [24,25].
The evidence is not consistent, however, in relation to anal cancer trends in recent years. Long-term HAART use may be associated with a lower prevalence of the anal squamous intraepithelial lesions [26,27]. Cohort studies on time trends of anal cancer incidence have reported diverging results. A study in the US and Canada included 34 189 HIV-infected individuals and reported a crude incidence of 87.1 per 100 000 for anal cancer. The authors found that rates for anal cancer were stable between 1996 and 2007, although there was a nonsignificant declining trend over time among MSM [28]. In a Californian cohort study of 20 277 HIV-infected individuals, the crude incidence for anal cancer was 130.2 per 100 000, and there was a significant decline at an average of 9% each year from 1996 to 2007 [8]. In a French cohort that included 109 771 HIV-infected patients, incidence remained high and stable between 1997 and 2008 [16]. More recently, another data linkage study which included a total of 447 953 HIV-infected people with HIV in the United States between 1996 and 2012 reported a crude anal cancer incidence of 50.7 per 100 000 person-years. The authors noted a significant decrease in anal cancer incidence since 2008 by 7.2% each year thereafter [14]. Similarly, a Dutch study also reported a slow decline in crude anal cancer incidence among 20 765 people with HIV after a peak in 2005–2006 from 114 to 72 per 100 000 person-years in 2011–2012 [15]. These data suggest that the incidence of anal cancer in PLWHIV may have reached a peak late in the first decade of this century in some industrialized countries with a mature HIV epidemic, and started to decline thereafter. Nevertheless, this trend is not observed in all settings with similar HIV epidemics. There are no population-based anal cancer screening programs existing in Australia, and only a very few referral services for high-risk populations in major cities. It is unlikely that the recent increase in anal cancer diagnosis is influenced by early cancer diagnosis due to screening.
The small decline, or absence of decline, demonstrated for anal cancer stands in complete contrast to the rapid and marked declines which occurred for the AIDS-defining cancers, including Kaposi’s sarcoma and non-Hodgkin lymphoma [9]. Reversal of immune deficiency appears to have a limited effect on anal cancer risk. A Swiss nested case–control study indicated that immuno-deficiency even at modest levels 6–7 years prior was an important risk factor for anal cancer development [12]. Preventing a person with HIV from ever becoming immune deficient may be more likely to reduce anal cancer risk. In the last decade, evidence on HIV treatment on reducing onward transmission and improving long-term health outcomes if commenced at HIV diagnosis led to early initiation of HIV treatment [29,30].
Some strengths and limitations should be taken into consideration when interpreting the results. Australia has nationwide disease notification systems for both HIV and invasive cancers, with almost complete capture of both conditions since 1982. This has allowed us to delineate anal cancer incidence through the entire timespan of the HIV epidemic. All incident anal cancers have been coded by the jurisdictional cancer registries based on multiple notifications of the case, including detailed histopathology reports. These cancer registries operate to a high standard internationally with respect to both classification and completeness [31]. Our analysis focused on anal SCC, for which the causal role of HPV is well established, and excluded anal adenocarcinoma [32]. Just over 10% of those notified to the National HIV Registries had missing information for both their first and last names, thus could not be included in the analysis. Most were diagnosed in the pre-HAART era (97.7%) due to concerns of confidentiality at that time. Therefore, our study probably underestimated anal cancer diagnoses in the early stage of the HIV epidemic. Australian National HIV Registries only record the name code (first two letters of last and first names) of those notified for enhanced privacy protection, which may have affected the linkage accuracy for anal cancer ascertainment. A previous validation study indicated that although the sensitivity is lower at 65.3% using name codes compared with using full names, the specificity is high at 99.9% [33]. Therefore, anal cancer estimates generated in the current study might be conservative. AIDS is no longer a notifiable condition in Australia. Although historically collected diagnoses of AIDS defining illnesses remain in the National HIV Register, the date of AIDS diagnoses is no longer available. Hence, we were not able to adjust the total follow-up time in those who were notified with AIDS diagnoses but not HIV infection to allow 5 extra years which has been a common practice in previous studies [9]. Lastly, we did not have individual-level data on antiretroviral therapy [14].
GBM experience much higher incidence of anal cancer largely due to extremely high prevalence of anal HPV infection [34]. In Australia, a recent cohort study has reported that anal HPV detection was nearly universal in GBM recruited from the communities in Sydney, in whom the prevalence of anal HPV16 infection in HIV-positive participants was nearly a third [35]. These are comparable rates with those of studies conducted in similar settings in other industrialised countries [34]. In 2007, Australia’s HPV vaccination program commenced for 12–13-year-old girls, with a widely implemented catch-up program for young women up to the age of 26. In 2013, the HPV vaccination program was extended to include 12–13-year-old boys with a catch-up program up to the age of 15 over 2 years. Recent clinical trial results demonstrating high efficacy of the quadrivalent HPV vaccine against anal intraepithelial lesions offer great promise for future prevention of anal cancer [36]. Australia’s HPV vaccination program should lead to substantial declines in anal cancer in the future. However, the HPV vaccine has no therapeutic efficacy in those already infected with those HPV types. In those already infected with HPV, alternative anal cancer prevention strategies are required [37].
Incidence of anal cancer in PLWHIV is still increasing in Australia. Although anal cancer screening programs have been advocated by some clinicians, its impact on anal cancer risk is uncertain, and the efficacy of treating precancerous lesions to prevent progression to invasive cancer is unknown [38]. The US-based ANCHOR randomized trial study (clinicaltri-als.gov identifier: NCT02135419) is currently examining whether anal cancer screening can decrease anal cancer incidence. In the interim, annual digital anorectal examinations have been recommended in some clinical guidelines to enable the early detection and treatment of anal cancer, and to reduce anal cancer morbidity and mortality [39].
Acknowledgements
The authors are indebted to people living with HIV in Australia and thank the Data Linkage Unit of the Australian Institute of Health and Welfare for their assistance in the project.
The HIV Cancer Data Linkage Study was funded by the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The IeDEA Asia-Pacific Research Collaboration: Cancer Studies is an initiative of TREATAsia, a program of amfAR, the Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907).
The Kirby Institute is affiliated with the Faculty of Medicine, University of New South Wales and funded by the Australian Government of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens – Part B: Biological agents. Lancet Oncol 2009; 10:321–322. [DOI] [PubMed] [Google Scholar]
- 2.Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin HR, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 2015; 136:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melbye M, Cote TR, Kessler L, Gail M, Biggar RJ. High incidence of anal cancer among AIDS patients. The AIDS/Cancer Working Group. Lancet 1994; 343:636–639. [DOI] [PubMed] [Google Scholar]
- 4.Mooij SH, van Santen DK, Geskus RB, van der Sande MA, Coutinho RA, Stolte IG, et al. The effect of HIV infection on anal and penile human papillomavirus incidence and clearance: a cohort study among MSM. AIDS 2016; 30:121–132. [DOI] [PubMed] [Google Scholar]
- 5.Seaberg EC, Wiley D, Martinez-Maza O, Chmiel JS, Kingsley L, Tang Y, et al. Cancer incidence in the multicenter AIDS cohort study before and during the HAART era: 1984 to 2007. Cancer 2010; 116:5507–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS 2008; 22:1203–1211. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, Cranston RD, Jacobson LP. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2008; 48:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23:2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Leeuwen MT, Vajdic CM, Middleton MG, McDonald AM, Law M, Kaldor JM, Grulich AE. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. AIDS 2009; 23:2183–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stebbing J, Duru O, Bower M. Non-AIDS-defining cancers. Curr Opin Infect Dis 2009; 22:7–10. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS 2009; 23:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schöni-Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol 2013; 178:877–884. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colon-Lopez V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018; 36:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richel O, Van Der Zee RP, Smit C, De Vries HJ, Prins JM. Brief report: anal cancer in the HIV-positive population: slowly declining incidence after a decade of cART. J Acquir Immune Defic Syndr 2015; 69:602–605. [DOI] [PubMed] [Google Scholar]
- 16.Piketty C, Selinger-Leneman H, Bouvier AM, Belot A, Mary-Krause M, Duvivier C, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the French Hospital Database on HIV. J Clin Oncol 2012; 30:4360–4366. [DOI] [PubMed] [Google Scholar]
- 17.The Kirby Institute HIV. viral hepatitis and sexually transmissible infections in Australia: Annual Surveillance Report 2017. Sydney: The Kirby Institute, UNSW; 2017. [Google Scholar]
- 18.Jin F, Stein AN, Conway EL, Regan DG, Law M, Brotherton JM, et al. Trends in anal cancer in Australia, 1982–2005. Vaccine 2011; 29:2322–2327. [DOI] [PubMed] [Google Scholar]
- 19.Lin DY. Nonparametric inference for cumulative incidence functions in competing risks studies. Stat Med 1997; 16:901–910. [DOI] [PubMed] [Google Scholar]
- 20.Grulich AE, Poynten IM, Machalek DA, Jin F, Templeton DJ, Hillman RJ. The epidemiology of anal cancer. Sex Health 2012; 9:504–508. [DOI] [PubMed] [Google Scholar]
- 21.Legarth R, Helleberg M, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Anal carcinoma in HIV-infected patients in the period 1995–2009: a Danish nationwide cohort study. Scand J Infect Dis 2013; 45:453–459. [DOI] [PubMed] [Google Scholar]
- 22.McManus H, O’Connor CC, Boyd M, Broom J, Russell D, Watson K, et al. Long-term survival in HIV positive patients with up to 15 years of antiretroviral therapy. PLoS One 2012; 7:e48839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013; 26:17–25. [DOI] [PubMed] [Google Scholar]
- 24.Godbole SV, Nandy K, Gauniyal M, Nalawade P, Sane S, Koyande S, et al. HIV and cancer registry linkage identifies a substantial burden of cancers in persons with HIV in India. Medicine (Baltimore) 2016; 95:e4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka LF, Latorre M, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from Sao Paulo, Brazil. Int J STD AIDS 2017; 28:1190–1198. [DOI] [PubMed] [Google Scholar]
- 26.de Pokomandy A, Rouleau D, Ghattas G, Trottier H, Vézina S, Coté P, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis 2011; 52:1174–1181. [DOI] [PubMed] [Google Scholar]
- 27.van der Snoek EM, van der Ende ME, den Hollander JC, Schutten M, Neumann HA, van Doornum GJ. Use of highly active antiretroviral therapy is associated with lower prevalence of anal intraepithelial neoplastic lesions and lower prevalence of human papillomavirus in HIV-infected men who have sex with men. Sex Transm Dis 2012; 39:495–500. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray F, Colombet M, Mery L (Eds): Cancer incidence in five continents. Lyon: International Agency for Research on Cancer; 2017 [Google Scholar]
- 32.Grulich AE, Jin F, Poynten IM. Anal cancer In: Schottenfeld D, Fraumeni J,editors. Cancer epidemiology prevention. 4th ed. London: Oxford Press; 2017. [Google Scholar]
- 33.Swart A, Meagher NS, van Leeuwen MT, Zhao K, Grulich A, Mao L, et al. Examining the quality of name code record linkage: what is the impact on death and cancer risk estimates? A validation study. Aust N Z J Public Health 2015; 39:141–147. [DOI] [PubMed] [Google Scholar]
- 34.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and`1 associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 35.Poynten IM, Tabrizi SN, Jin F, Templeton DJ, Machalek DA, Cornall A, et al. Vaccine-preventable anal human papillomavirus in Australian gay and bisexual men. Papillomavirus Res 2017; 3:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585. [DOI] [PubMed] [Google Scholar]
- 37.Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, Darragh TM. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 14:415–422. [DOI] [PubMed] [Google Scholar]
- 38.Macaya A, Munoz-Santos C, Balaguer A, Barberà MJ. Interventions for anal canal intraepithelial neoplasia. Cochrane Database Syst Rev 2012; 12:CD009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: a review. World J Gastrointest Surg 2016; 8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


