Abstract
The neurons of the retina can be affected by a wide variety of inherited or environmental degenerations that can lead to vision loss and even blindness. Retinal Ganglion Cell (RGC) degeneration is the hallmark of glaucoma and other optic neuropathies that affect millions of people worldwide. Numerous strategies are being trialed to replace lost neurons in different degeneration models and, in the recent years, stem cell technologies have opened promising avenues to obtain donor cells for retinal repair. Stem cell-based transplantation has been most frequently used for the replacement of rod photoreceptors but the same tools could potentially be used for other retinal cell types including RGCs. However, RGCs are not abundant in stem cell-derived cultures and, in contrast to the short-distance wiring of photoreceptors, RGC axons take a long and intricate journey to connect with numerous brain nuclei. Hence, a number of challenges still remain such as the ability to scale-up the production of RGCs and a reliable and functional integration into the adult diseased retina upon transplantation. In this review, we discuss the recent advancements in the development of replacement therapies for RGC degenerations and the challenges that we need to overcome before these technologies can be applied to the clinic.
Introduction
Vision is a complex task that begins as soon as light enters the eyes through the cornea and lens and leads to the vivid images that allow us to understand and navigate the world. The brain is ultimately responsible for painting a visual picture of our surrounding environment, but the first steps of visual processing take place in the retina. The retina is a thin sheet of neural tissue located at the back of the eye that translates the inputs of focused light into patterns of action potentials that are then transmitted to the brain for visual recognition.
The retina contains six main neural populations organized in three well-defined nuclear layers: the outer nuclear layer (ONL), the inner nuclear layer (INL) and the retinal ganglion cell layer (GCL) (Rodieck, 1973; Kolb et al., 1992), (Fig.1A). The ONL contains the light-sensitive neurons: the rod and cone photoreceptors, the INL includes three different populations of interneurons: the bipolar cells, the horizontal cells and the amacrine cells, and the innermost layer of the retina, the GCL, is where the Retinal Ganglion Cells (RGCs) reside. The RGCs are the sole output of the retina; on average, RGCs are larger than most of the other retinal neurons, they have intricate dendritic arbors that receive inputs from the preceding retinal circuitry and their large-diameter axons are capable of transmitting these signals to the recipient areas of the brain, many centimeters away from the soma. All RGCs project their axons towards the center of the retina, where they converge as the optic nerve collects all the axons at the optic nerve head (Fig.1B–C). Then, this bundle of thousands of axonal fibers passes information to the next relay stations in the brain, mainly the lateral geniculate nuclei of the thalamus, but also the suprachiasmatic nuclei, the superior colliculi and other pretectal nuclei (Berson, 2008; Dhande and Huberman, 2014). These highly complex connections are established during development as RGC axons grow along precise paths to recognize and form synapses with the appropriate target cells (McCabe et al., 1999; Mann and Holt, 2001; Sernagor et al., 2001; Erskine and Herrera, 2007).
Figure 1.
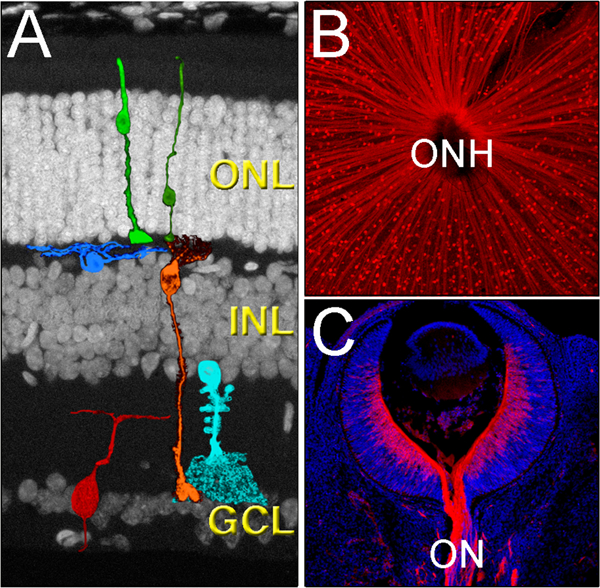
Cytoarchitecture of the mammalian retina. A) The retina is organized in three main layers, the outer nuclear layer (ONL), the inner nuclear layer (INL) and the ganglion cell layer (GCL), and contains six main neuronal cell types, the cone and rod photoreceptors (light and dark green, respectively), the horizontal cells (blue), the bipolar cells (orange), the amacrine cells (light blue), and the retinal ganglion cells (red). B) All RGCs (red) send their axons to the center of the eye, a region called the optic nerve head (ONH). The image shows the RGCs of an adult flat-mounted retina. C) As axons leave the eye, they bundle to form the optic nerve (ON). The image shows a section of a E13.5 mouse retina immunolabeled with Tuj1 (red) and co-stained with DAPI (blue).
Many diseases can lead to degeneration of the optic nerve and apoptotic RGC death, including glaucoma, Leber’s hereditary optic neuropathy, ischemic optic neuropathy, and optic neuritis, among others (Rucker et al., 2004; Toosy et al., 2014; Weinreb et al., 2014; Carelli et al., 2017; Jonas et al., 2017). Glaucomatous optic neuropathy is one of the leading causes of irreversible blindness and it has been estimated that over 64 million people are affected by this disease worldwide (Quigley and Broman, 2006; Weinreb et al., 2014). In our aging society, glaucoma is an especially critical issue because unless the disease can be effectively treated or prevented, the number of affected patients will increase dramatically; it has been predicted that by 2040, the rates of vision loss due to glaucoma will nearly double (Tham et al., 2014). Considering the personal, social and economic burden of glaucoma, there is a pressing need for novel therapeutic approaches.
While the primary site of injury in glaucoma is not well understood, this disease leads to a progressive degeneration of the RGCs and subsequent visual field loss (Kerrigan et al., 1997; Levin, 1997; Kerrigan-Baumrind et al., 2000; Almasieh et al., 2012; Medeiros et al., 2013; You et al., 2013). Most of the available therapeutic strategies for glaucoma are aimed at limiting the progression of vision loss. Currently, the only effective treatment is lowering the intraocular pressure (IOP), as elevated IOP is the only well-known modifiable risk factor for glaucoma (Nickells et al., 2012). However, glaucoma can occur at normal IOP, and increasing evidence indicates that lowering IOP may be insufficient to prevent vision loss in some patients (Realini et al., 2002; Sit, 2014). While better strategies for early diagnosis, identification of other modifiable risk factors and neuroprotection will be crucial to manage glaucoma in the near future (Calkins et al., 2017), none of these strategies is effective to regenerate lost RGCs to reverse vision loss. Since the mammalian retina has no spontaneous regenerative capabilities, the only possibilities to recover vision loss after RGC death are either prosthetics or cell replacement by transplantation or endogenous repair.
In this review, we discuss the past and current methods to generate and transplant RGCs, and identify the key outstanding challenges that must be met before RGC transplantation can be translated to clinical settings. It is important to note that the retina is an excellent model to investigate the ability of donor cells to functionally integrate with central nervous system tissue. The retina is easily accessible with minimal surgery, can be imaged in vivo, and has been studied at a cellular level in excruciating detail for over a century. Therefore, investigating RGC transplants will hopefully lead to optic neuropathy treatments, but also will serve as proof-of-principle studies that will help determine the feasibility of cell replacement throughout the central nervous system.
Stem cell strategies to generate RGCs in vitro
Given the vast number of people that could benefit from RGC transplant therapies (Tham et al., 2014), a reliable and cost-effective source of RGCs will need to be established before RGC transplants can be feasibly used in human clinical trials.
In the past few decades, stem cell technologies have undergone astonishing progression from the isolation and culture methods of mouse and human embryonic stem cells (mESCs and hESCs (Evans and Kaufman, 1981; Thomson et al., 1998; Thomson and Marshall, 1998), and the techniques to reprogram somatic cells into induced-pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006; Yu et al., 2007; Pang et al., 2011), to the significant advancement of the methods to differentiate pluripotent cells into retinal lineages in vitro (Table1). Over a decade ago, groundbreaking studies from several laboratories developed the basic protocols to differentiate mouse and human ESCs into retinal neurons (Zhao et al., 2002; Lamba et al., 2006; Meyer et al., 2009; Kayama et al., 2010; Eiraku et al., 2011; La Torre et al., 2012; Nakano et al., 2012; Zhong et al., 2014), Table1). Most of these protocols were designed to mimic the normal environment that the developing eye encounters in an embryo. During the early stages of development, a region of the anterior neural plate becomes specified for eye fates, this area that contains the earliest retinal progenitors has been termed the “eye field” (Chow and Lang, 2001; Zuber et al., 2003; Zuber, 2010). This unique region is then divided in two separate regions that evaginate bilaterally to form two optic vesicles (Chiang et al., 1996; Li et al., 1997). Subsequently, complex morphogenetic movements lead to the formation of two optic cups that contain an external layer that will form the retinal pigment epithelium (RPE) and an internal layer (neural retina) of multipotent retinal progenitors that will develop into all the layers of the adult retina (Adler and Canto-Soler, 2007; Fuhrmann, 2010).
Table 1.
Summary of differentiation protocols
| Publication | Method | Markers | Timing | Species | Ref | |
|---|---|---|---|---|---|---|
| 2D | Zhao, 2002 | RA or FGF2 induction. Ectopic Crxexpression | None of RGCs | NA | mouse | 38 |
| Lamba, 2006 | IGF, Dkk1, Noggin | PAX6, HUC/D | NA | human | 32 | |
| Osakada, 2008 | Dkk1, LeftyA, Activin, DAPT | PAX6, ISL1 | 28 days | mouse | 62 | |
| Kayama, 2010 | ectopic Pax6 transfection | PAX6, ISL1, BRN3, THY1, OPN4, NFM | 14 days | mouse | 39 | |
| La Torre, 2012 | IGF, Dkk1, Noggin | BRN3, TUJ1 | 21 days | mouse and human | 34 | |
| Boucherie, 2013 | Matrigel, N2, B27 | None of RGCs | NA | human | 63 | |
| Riazifar, 2014 | Dkk1, LeftyA, Activin, DAPT | BRN3, TUJ1, ISL1, gSYN, THY1 | 21 days | human | 64 | |
| Sluch, 2015 | Matrigel, N2, B27 | BRN3, TUJ1, NEUN, THY1, MAP2, SNC, ISL1, RBPMS, PAX6 | 25 days | human | 66 | |
| Teotia, 2016 | SHH, FGF8, DAPT, Follistatin, Cyclopamine | BRN3, TUJ1, THY1 | 15 days | mouse and human | 65 | |
| Chao, 2017 | IGF, Dkk1, Noggin and DAPT | BRN3, PAX6, HUC/D, TUJ1 | 60 days | human | 67 | |
| Daniszewski, 2018 | IGF, Dkk1, Noggin | cRNAseq | 36 days | human | 68 | |
| 3D | Eiraku, 2011a | 2% Matrigel, 1.5% KSR | BRN3, PAX6, CALRET | 10 days | mouse | 35 |
| Eiraku, 2011b | 2% Matrigel, 1.5% KSR | BRN3, PAX6, CALRET | 13 days | mouse | 60 | |
| Nakano, 2012 | 2% Matrigel, 10% KSR, IWR1e | BRN3 | 30 days | human | 36 | |
| La Torre, 2015 | 2% Matrigel, 1.5% KSR | BRN3, PAX6, TUJ1 | 13 days | mouse | 50 | |
| Decembrini, 2014 | 2% Matrigel, 1.5% KSR | PAX6 | 20 days | mouse | 69 | |
| Volkner, 2016 | 2% Matrigel, 1.5% KSR, DAPT | BRN3, ELAVL3/4) | 14 days | mouse | 70 | |
| Aparicio, 2017 | 0.5% Matrigel, 10% KSR, IWR1e | BRN3, RBPMS | 27 days | human | 71 | |
| Yokoi, 2017 | 2% Matrigel, FBS, BDNF | BRN3, Tuj1 | 30 days | human | 72 | |
| Kobayashi, 2018 | 2% Matrigel, 1.5% KSR, RA | BRN3, THY1, ISL1, RBPMS | 21 days | human | 73 | |
| DiStefano, 2018 | 2% Matrigel, 1.5% KSR, RA | BRN3a | 15 days | mouse | 74 | |
| iPSCs | Meyer, 2009 | N2, B27 | None of RGCs | NA | human | 33 |
| Parameswaran, 2010 | Noggin, FGF2 | PAX6, BRN3b, RPF1 | 15 days | mouse | 75 | |
| Tucker, 2011 | IGF, Dkk1, Noggin, bFGF, DAPT | NF200 | 33 days | mouse | 76 | |
| Tucker, 2013 | IGF, Dkk1, Noggin, bFGF, DAPT | NF200, BRN3B | 60 days | human | 77 | |
| Zhong, 2014 | Blebbistatin, N2, B27, RA | BRN3, HUC/D | 35 days | human | 37 | |
| Riazifar, 2014 | bFGF, DAPT | BRN3A, BRN3B, THY1, TUJ1, ISL1, SNC | 40 days | human | 64 | |
| Tanaka, 2015 | 2% Matrigel, FBS, BDNF | BRN3b, TUJ1, ISL1, SNCG, NFLM | 34 days | human | 79 | |
| Teotia, 2016 | SHH, FGF8, DAPT, Follistatin, Cyclopamine | BRN3, TUJ1, THY1 | 15 days | mouse and human | 65 | |
| Vergara, 2017 | Blebbistatin, N2, B27, RA | BRN3b | 35 days | human | 80 | |
| Yokoi, 2017 | 2% Matrigel, FBS, BDNF | BRN3, Tuj1 | 30 days | human | 72 | |
| Langer, 2018 | N2, B27 | RNAseq | 40 days | human | 81 | |
Several extracellular signaling molecules regulate eye field patterning, including Wnt (Lewis et al., 2008; Alldredge and Fuhrmann, 2016), Insulin-like growth factors (IGFs) (Pera et al., 2001; La Torre et al., 2015), fibroblast growth factors (FGFs) (Pittack et al., 1997; Picker and Brand, 2005), Notch (Nelson et al., 2006; Riesenberg et al., 2009), Bone morphogenetic proteins (BMPs) (Sakuta et al., 2006), Retinoic acid (RA) (Marsh-Armstrong et al., 1994), and Sonic hedgehog (SHH)(Wang et al., 2005; Sakagami et al., 2009; Wall et al., 2009). Many of these signaling cues reappear at different stages of development to regulate a wide array of different developmental events.
When confronted with similar stimuli, pluripotent stem cells can commit to neural lineages in a step-wise manner. At early time points, the pluripotent cells down-regulate many pluripotency markers and form a polarized neuroepithelium that expresses many of the normal markers of a developing neural plate/tube (e.g. Sox2, Nestin, Otx2); subsequently, these cells become early retinal progenitors and express eye field transcription factors (e.g. Rax, Lhx2, Six3, Six6, Pax6, Chx10/Vsx2) and finally, these cultures can differentiate into postmitotic retinal neurons, including RGCs (Figure 2).
Figure 2.
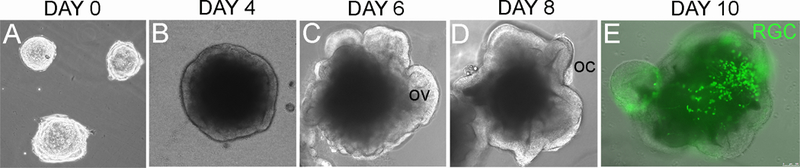
Upon differentiation, undifferentiated pluripotent stem cells (A) can be directed towards retinal cell fates following several steps that resemble normal developmental stages. First, they develop into a neural epithelium (B) that evaginates to form optic vesicle-like structures (arrows in C), and finally optic cup-like structures (arrows in D). The retinal progenitors can then differentiate into RGCs (E). The arrows in E show fluorescently-labeled RGCs (green) in a 10-day retinal organoid. Abbreviations: OV: optic vesicles, OC: optic cups, RGC: Retinal Ganglion Cells. Scale: 100 microns
Our knowledge of the signaling pathways involved in eye field specification aided in the development of one of the first protocols to successfully differentiate hESCs into retinal progenitor cells, which was introduced by Lamba and Reh in 2006 (Lamba et al., 2006). By using Noggin, a BMP inhibitor, and Dickkopf-1, a Wnt1 inhibitor the authors were able to promote forebrain development in human stem cell cultures. Additionally, IGF-1 was added to the differentiation media, since IGF-1 mRNA injection into Xenopus embryos promoted eye formation (Pera et al., 2001). Using this method, Lamba et al. observed that 80% of ESCs were directed towards a retinal progenitor fate, and a few weeks later some of these cells became responsive to glutamate and NMDA stimuli suggesting that these cultures can yield functional RGCs.
In 2011, Yoshiki Sasai revolutionized the technologies to generate retina cells from pluripotent cells using three-dimensional organoid cultures (Eiraku and Sasai, 2011; Eiraku et al., 2011; Nakano et al., 2012). This method is based on plating dissociated pluripotent cells onto special non-adherent U or V-shaped plates. Since the cells cannot attach to the bottom of the plate, they attach to each other forming three-dimensional aggregates called embryoid bodies (EBs). While other methods also use EBs, Sasai’s protocol enables a very tight control of the number of cells in each EB and it is easily scalable for high-throughput applications. High concentrations of extracellular matrix proteins (Matrigel or Laminin) are added to the media to promote differentiation and polarity. Consistent with the proposed neural-default model for ESCs (Munoz-Sanjuan and Brivanlou, 2002), these cultures do not rely on other inductive signals or serum. Hence, these cultures are also called serum-free floating culture of embryoid body-like aggregates with quick reaggregation or SFEBq. Remarkably, inductive signals secreted by different cell populations within each EB can lead to the spatially and temporally defined events that stimulate complex morphogenesis. Accordingly, retinal organoids not only express markers of retinal progenitor cells, but these cultures undergo morphogenetic changes to first develop optic vesicle-like structures, optic cups and finally fully laminated retinas that can contain the entire repertoire of retinal cell types present in a normal retina.
Other protocols use a combination of 2D and 3D techniques, initially generating cellular aggregates that are then plated on Laminin-coated plates to allow for the development of neural tubelike structures. Subsequently, the 2D rosettes are manually isolated and cultured in suspension where they develop into 3D retinal tissue. This method was first introduced by Meyer and Gamm (Meyer et al., 2009) and while it may be more laborious than other procedures, it generates high yields of all the different retinal cell types organized in well-defined layers. Canto-Soler (Zhong et al., 2014) elegantly refined this method by changing the coating strategy to Matrigel and using sharpened Tungsten needles to manually detach the neuroretinas from the 2D cultures. Interestingly, these cultures achieved high degrees of maturation with the photoreceptors exhibiting outer-segment discs and photosensitivity.
These different protocols have been the basis for many others that followed, and they have been replicated using mESCs, hESCs and iPSCs (mouse and human, Table 1 (Osakada et al., 2008; Parameswaran et al., 2010; Tucker et al., 2011a; Tucker et al., 2011b; La Torre et al., 2012; Nakano et al., 2012; Boucherie et al., 2013; Tucker et al., 2013; Decembrini et al., 2014; Riazifar et al., 2014; Zhong et al., 2014; La Torre et al., 2015; Sluch et al., 2015; Tanaka et al., 2015; Volkner et al., 2016; Aparicio et al., 2017; Chao et al., 2017; Teotia et al., 2017; Vergara et al., 2017; Yokoi et al., 2017; Daniszewski et al., 2018; DiStefano et al., 2018; Kobayashi et al., 2018; Langer et al., 2018)). Retinal organoids have also proven useful as platforms to study different aspects of development not only using mouse cells, but also human cells (Capowski et al., 2014; Phillips et al., 2014; Ohlemacher et al., 2016; Takata et al., 2017; Eldred, 2018). The latter is especially crucial, given that human developmental studies are often unfeasible given the limited availability of tissue and the difficulties to perform genetic manipulations. While we now benefit from a wide array of methods to generate bona fide retinal neurons, most of these methods are still inefficient for generating large numbers of specific retinal neurons other than rod photoreceptors. This is not surprising since in vitro stem cell culture methods mimic what is occurring during normal retinal development in vivo, and rod photoreceptors greatly outnumber RGCs in mouse and human retinas (Curcio and Allen, 1990; Jeon et al., 1998). Similarly, the average yield of stem cell-derived RGCs has been estimated to be 0.1–30% of the cells in the culture, which is similar to the normal percentage of RGCs in the retina at different developmental stages. Additionally, it has been shown that the number of RGCs decreases over time in 3D cultures (Aparicio et al., 2017). This is also expected, given that during normal development, newly born RGCs undergo two waves of programmed cell death as they become critically dependent on trophic support from their synaptic targets (Isenmann et al., 2003). Since the brain target cells (e.g. lateral geniculate nucleus) are likely not present in the organoid cultures, Bax-mediated apoptosis is probably responsible for the decline in the number of RGCs over time. This phenomenon is less severe in monolayer cultures because, similar to endogenous RGCs (Harris et al., 2002), stem-cell derived RGCs form synaptically-coupled networks in 2D settings. Several factors, including BDNF, NT4, CNTF and Forskolin have been suggested to improve RGC survival and, as shown before in normal RGCs (Goldberg et al., 2002a), some of these factors also promote axogenesis (Teotia et al., 2017; Yokoi et al., 2017).
In order to minimize the number of cultures required to obtain enough donor cells and ease any purification process, the current protocols must be further improved to generate higher yields of the neurons of interest before we can scale-up these methods as a source of donor cells for transplant purposes.
Purification of Stem cell-derived RGCs
Techniques that have existed for decades may prove useful for generating nearly-pure cultures of SC-derived RGCs and eliminate any residual proliferative cells. Immunopanning is a technique for purifying RGCs which exploits the RGC-specific expression of Thy-1, a surface antigen expressed by RGCs. Using a two-step protocol, >99.5% pure RGC cultures can be produced from postnatal rat retinas (Barres et al., 1988). Immunopanning has since become a standard for isolating RGCs from a heterogeneous population of retinal neurons (Goldberg et al., 2002a; Goldberg et al., 2002b; Hertz et al., 2014; Venugopalan et al., 2016). Gill et al. successfully adapted this immunopanning protocol to separate RGCs from other retinal neurons and cells produced in hESC-derived organoids using magnetically activated cell sorting (Gill et al., 2016). By sorting RGCs from other retinal neurons made in vitro from stem cells, nearly pure suspensions of SC-derived RGCs were produced; a likely requirement for single-cell suspension transplant methods. However, while Thy-1 is specifically expressed in RGCs within the retina, this marker is expressed by myriads of other neuronal types across the nervous system (Kelley et al., 1994), and other Thy-1+ neurons might also be generated in stem cell-derived cultures. Future improvements to this method may include identifying different surface antigens that are unique to RGCs, which may improve RGC isolation specificity from organoid cultures. In this direction, Aparicio et al. (Aparicio et al., 2017) identified two surface antigens, CD184 and CD171, as potential markers to obtain enriched RGCs or RGC precursors.
Another potential method for specifically isolating RGCs from stem cell cultures involves RGC- specific expression of fluorescent proteins followed by fluorescence-activated cell sorting (FACS). Sluch and collaborators used CRISPR to engineer a hESC line to express mCherry under an RGC- specific promoter (POU4F2) (Sluch et al., 2015) and were able to monitor RGC production in hESC- derived cultures and purify the cells by FACS (Sluch et al., 2015). In these experiments, the authors observed mCherry+ cells from day 25 and importantly, these cells expressed many markers of normal RGCs, including Brn3, Isl1, Thy1, MAP2, RBPMS and Tuj1. Also, these cells showed electrophysiological and ultrastructural properties consistent with RGC properties in vivo. Currently, CRISPR technologies can be applied to generate reporter lines in combination with platforms that enable automated reporter quantification to facilitate the detection and monitoring of RGCs (Vergara et al., 2017).
The ability to purify SC-derived RGCs from other cell types is useful to produce highly pure RGC cultures but, unfortunately, does not address the low yield of RGCs produced in vitro. Furthermore, these methods are expensive, time-consuming and quite challenging to scale-up for clinical purposes.
Scaling-up the production of RGCs from stem cell cultures: clued-in by developmental studies
In all mammals, retinal neurons are born from multipotent retinal progenitor cells (RPCs) in a highly conserved, temporal sequence; in which RGCs are the first cells generated. For a more in-depth discussion of neurogenesis in the developing retina, see (Bassett and Wallace, 2012; Kohwi and Doe, 2013; Cepko, 2014). RPCs progress through a series of competence states, in which they have the ability to produce only specific populations of retinal neurons at different developmental times. Thus, once a progenitor has passed the competence window to generate early cell types (e.g. RGCs), it is no longer able to produce these cells, and instead will generate later cell types (e.g. rod photoreceptors). Several intrinsic temporal factors are known to contribute to RPC competence states and could potentially be exploited to regulate the temporal progression of stem cell-derived cultures and increase the yields of specific cell types.
One important transcription factor in the regulation of neural progenitor competence is Ikaros (Elliott et al., 2008; Alsio et al., 2013). Ikaros mutant mice exhibited decreased numbers of early-born retinal neurons relative to control littermates, suggesting that Ikaros plays a role in conferring RGCs with the ability to produce early-born retinal neurons. Viral vectors were used to misexpress Ikaros in later stages in development and interestingly, these experiments generated retinal neurons that are normally only produced embryonically, suggesting that Ikaros may be able to circumvent temporal changes in competence.
MicroRNAs (miRNAs) have also been shown to play a role in RPC competence during retinal development. miRNAs are short, non-coding RNAs that bind to the 3’ untranslated region (UTR) of mRNAs to inhibit translation and/or stimulate mRNA decay. The first study to link a lack of miRNAs in and a shift in RPC temporal competence was conducted in 2010 by Georgi et al (Georgi and Reh, 2010). The authors observed that in the absence of mature functional miRNAs, an excess of RGCs were produced and RPCs continued to generate RGCs beyond the normal competence window. In a later study, La Torre et al. identified the miRNAs responsible for this effect. In concert, miR-125b, miR- 9 and Let-7 play a significant role in orchestrating progenitor competence (La Torre et al., 2013). Overexpression of these three miRNAs in mouse retinas resulted in temporal changes and precocious rod photoreceptor genesis, while specific inhibition of these miRNAs using AntagomiRs resulted in increased production of early cell types -including RGCs; but, this effect was only achieved during a relatively short developmental window.
While both Ikaros overexpression and miRNA inhibition resulted in increased early cell types, including RGCs and cone photoreceptors, these approaches have not yet been translated to in vitro systems to boost RGC production. The manipulation of temporal progression in SC-derived RPCs could potentially provide a very convenient method to increase the number of RGCs without scaling-up the number of cultures.
Transplant methods
Tissue transplants have been performed in the retina for decades, with early transplant studies focusing on engrafting sheets of retina into host animal eyes or onto brain regions to analyze the morphology of RGCs and the ability of the donor cells to grow axons towards the appropriate brain regions (Hankin and Lund, 1990; Lund and Hankin, 1995), and various methods have been used by different groups to assess the ability of RGCs to survive and integrate with host tissue.
In a series of studies, Hankin and Lund transplanted embryonic day 13 (E13) CD-1 mouse retinae into the midbrain of postnatal day 1 (P1) Sprague-Dawley rats (Hankin and Lund, 1990; Lund and Hankin, 1995). The purpose of these studies was to determine if transplanted retina tissue could extend axons towards the superior colliculus—one of the main RGC targets in the rodent. Hankin and Lund’s rationale was that if retina tissue transplanted into an ectopic location can extend axons to the superior colliculus, then perhaps RGCs hold intrinsic properties to help guide their axons to proper brain targets regardless of the environment the retina tissue is placed in. Indeed, when placed in several ectopic locations in the brain, E13 mouse RGCs grew axons towards the superior colliculi in P1 rat brains. It is important to note that the transplanted RGCs were newly born and in midst of finding their appropriate brain targets when they were removed from the mouse embryos. It is possible that, as suggested by Hertz et al (Hertz et al., 2014), RGCs isolated from early stages of development are more plastic and amenable to transplant.
Similarly, whole-thickness retinal sheets can be rolled up into a cannula and injected into the subretinal space of host retinas. The main purpose of these studies was to restore photoreceptor function (Ghosh et al., 1999b; Ghosh et al., 1999a; Ghosh and Arner, 2002) but RGCs were also transplanted in the process. Unfortunately, in cat and pig transplant recipients, little integration with host retinal tissue was observed, suggesting that the retinal sheet may not be an effective avenue for developing RGC transplant methods.
Several encouraging RGC transplant studies have been performed by Jeffery Goldberg’s group (Hertz et al., 2014; Venugopalan et al., 2016; Tang et al., 2018). Rather than using retinal sheets for their transplants, these studies purified postnatal mouse RGCs and transplanted them either onto cultured adult rodent retinas or into host eyes in vivo by intravitreal injection. Several common themes arose from these studies. First, RGCs isolated from younger (embryonic or early postnatal) ages resulted in better survival after transplantation. For example, Hertz et al. found that between 40% and 50% of RGCs from E18 to P9 survived 24 hours after ex vivo transplantation, however the survival rate for adult-derived RGCs dropped to below 20%. Second, the success rate of RGC transplants is very modest but, remarkably, some RGCs transplanted intravitreally engrafted with the host retina, acquired the normal morphology of endogenous RGCs, responded to light and even established synaptic contacts with the lateral geniculate nucleus and the superior colliculus.
Chao and collaborators (Chao et al., 2017) injected 1,000,000 hESC-derived retinal cells into the subretinal space of squirrel monkeys (Saimiri sciureus). The cells were injected in the temporal macula and, interestingly, some axonal projections emanating from the transplant extended along the host nerve fiber layer towards the ONH. The ability of these RGCs to project into the optic nerve suggests that hESC-derived donor cells may also have the ability to integrate into host retinal and central nervous system circuitry.
In combination, these studies suggest that RGC transplantation is possible, albeit not very efficient. These results have established the groundwork for a major direction the field has taken in recent years: identifying the major barriers to RGC transplantation and identifying methods to overcome them. For example, delivering RGCs into a host vitreal space is not difficult, but once RGCs have been transplanted, they must overcome physical barriers preventing them from surviving and integrating with the host retina. Both the inner limiting membrane (ILM) and the neural fiber layer (NFL) are likely to thwart the engraftment potential of any transplanted cell. The ILM consists of astrocytes and Müller glia endfeet, and covered by a basal membrane of thick extracellular matrix proteins, while the NFL contains the axons of the ganglion neurons coursing on the vitreal surface of the retina from the periphery to the optic nerve head.
Many RGC transplant studies have used healthy adult animals as transplant recipients, and in any healthy adult animal, the NFL/ILM are fully intact and densely packed. Therefore, in rodent RGC transplant models, it may be useful to analyze methods to disrupt the NFL/ILM in order to allow transplanted RGCs to physically reach the GCL. Ultimately, it may be more appropriate to transplant RGCs into a retina that has a disrupted NFL or has undergone some RGC degeneration since humans who may receive RGC transplants in the future surely will not have a healthy retinal environment.
Johnson et al. (Johnson et al., 2010) investigated the effect of disrupting the ILM and modulating Müller glia activity on the ability of mesenchymal stem cells to integrate into the GCL of rat retinas. Two methods for disrupting the ILM of rat retinas were tested: mechanically peeling off the ILM, and digestion with collagenase. In retinal explants both methods improved mesenchymal stem cell engraftment. However, neither would be useful for clinical purposes, since mechanical peeling of the ILM is too disruptive to the existing intact retina and in vivo collagenase treatment caused extensive retinal hemorrhaging. These experiments suggest that likely the ILM is a physical barrier to transplant engraftment, and that ILM disruption may improve RGC transplant engraftment. However, gentler, perhaps more transient, methods of ILM disruption must be discovered before ILM disruption is a feasible technique for improving RGC engraftment into a host retina.
Alpha-Aminoadipic acid (AAA) is a glutamate analogue that, in the retina, is specifically gliotoxic. AAA has also been shown to impair Müller glia cell function. Therefore Johnson et al. hypothesized that treating retinas with AAA, and thereby disrupting Müller glia activity, mesenchymal stem cell engraftment would improve. Indeed, the authors observed a significant increase in the number of transplanted cells being able to enter the retina rather than sit on the vitreal side of the ILM. These results suggest that modulation of Müller glia activity can improve transplanted cell engraftment in the retina, which may allow for harsher ILM disruption methods to be avoided in order to improve cell engraftment in the GCL.
Material transfer: lessons from photoreceptor transplants
Before the widespread use of genetically-encoded fluorescent reporters, cells transplanted into host retinas were labeled by several means, including transplantation of pigmented cells into albino animals, tracer injections, LacZ, etc. (De Robertis, 2009). Once genetically-encoded reporters for a growing number of cell types became available, they were an obvious choice for researchers to use to label donor cells for transplantation approaches because they can be visualized in live tissue, are constantly renewed during the life of the cell, and were thought to be specific thanks to promoter-specific transgene expression. Interestingly, recent work in the field of photoreceptor transplants has demonstrated that genetically encoded cell markers may not be as ideal of a donor cell labeling tool as once believed. Work from several groups has revealed that mouse photoreceptors transplanted into mouse retinas transfer cellular material, including fluorescent proteins, to photoreceptor cells of the host retina (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016; Decembrini et al., 2017; Ortin-Martinez et al., 2017; Waldron et al., 2018). Therefore, fluorescently-labeled rods in the host ONL that were previously assumed to be engrafted donor cells are merely host cells that have up-taken proteins from the donor cell mass. While it remains unknown if material transfer can occur in other cells, material transfer in the GCL must be considered a possibility, and many of the existing studies should be re-evaluated.
Moving forward, several considerations must be made when performing RGC transplants to ensure material transfer is not causing false positives in transplant success results. First, proteins or RNA produced in donor cells can be transferred from donor cells in the subretinal space (a space produced during photoreceptor transplants between the photoreceptor layer and RPE) to endogenous photoreceptors in host retinas (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016; Decembrini et al., 2017; Ortin-Martinez et al., 2017; Waldron et al., 2018). Pearson et al. injected recombinant EGFP into the subretinal space of adult mice and analyzed the retinas for EGFP positive photoreceptors (Pearson et al., 2016). Unlike when GFP positive photoreceptors are transplanted into wild-type host retinas, injection of recombinant EGFP protein resulted in very few EGFP positive photoreceptors in the host retina. This result suggests that host photoreceptors do not normally uptake large quantities of free protein from the environment, rather that transplanted photoreceptors actively exchange molecules with host photoreceptors, or at least confer some specificity for the material to be transferred. It is also possible that the transfer is not mediated by proteins but by RNA from donor to host cells. Similarly, it is unclear whether the exchange of material is driven by exosomes/small vesicles or by direct cell contacts; however, it is now obvious that proteins not expressed by wild type photoreceptors—GFP and CRE for example—can somehow be transferred from donor cells into endogenous photoreceptors. Further experimentation will be required to understand the basic mechanism of donor-to-host material transfer in photoreceptor transplants. How a similar mechanism may affect transplants in the vitreal side of the eye remains unexplored.
Secondly, material transfer is not the result of nuclear fusion between donor and host photoreceptors. Several groups transplanted male -derived mouse photoreceptors into female hosts, and analyzed host retinas for Y chromosome presence (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016). These studies did not observe cells with a Y chromosome within the ONL. Two conclusions can be drawn from this result: nuclear fusion does not occur between donor and host photoreceptors, and that donor cell integration in these studies is a rare occurrence. Additionally, Ortin-Martinez et al. used EdU, a thymidine analogue, to pre-label donor photoreceptors to assay donor cell integration into host retinas. The authors observed no EdU positive nuclei in the photoreceptors of host retinas (Ortin-Martinez et al., 2017).
Finally, material transfer studies have shown that the host environment is important in determining the success of photoreceptor transplants. Waldron et al. investigated the role of the host environment on the success of photoreceptor transplants by transplanting photoreceptors into two different mouse models of retinal degeneration (Waldron et al., 2018). In the first model, Nrl−/−; Prph2rd2/rd2 mice contained no rods and slowly degenerated over time. In this mouse, some transplanted photoreceptors were observed in the outer nuclear layer of host retinas, and were conclusively not the result of material transfer (despite that some transplanted photoreceptors in this study, as expected, underwent material transfer with host photoreceptors). However, in a second model of retinal degeneration, Nrl−/−; rpe65R91W/R91W, in which a point mutation in the RPE65 gene leads to a more robust outer limiting membrane, neither material transfer nor donor cell integration were observed. Thus, Waldron et al. concluded that a more robust outer limiting membrane can prevent both material transfer and donor cell integration. It appears the host environment plays a role in promoting or inhibiting donor photoreceptor integration, and likely will be an important factor to consider in RGC transplants as well.
The discovery of material transfer in photoreceptor transplant studies is a reminder that experimental results must be examined with multiple points of view, considering all possible explanations for an experimental result. However, the fact that material transfer occurs is not a death nail for retinal transplants—it is possible that material transfer itself may be a useful therapeutic strategy to deliver factors to the retina. While it may be easy to view material transfer as a setback for the field, at its core knowledge of its existence has the potential to allow researchers to learn more about basic mechanisms neurons use to communicate with each other, and will further refine future retinal transplant studies in a positive manner.
Alternative strategies: Müller glia-mediated endogenous repair
In teleost fish, Müller glia cells can robustly regenerate different retinal cell types after damage (Hitchcock et al., 2004). However, the mammalian Müller glia lack these regenerative abilities as Müller cells do not spontaneously re-enter the cell cycle after damage. Recently, several studies have focused on using Müller glia as an endogenous source of cells for regeneration. For instance, Karl and colleagues showed that a cocktail of growth factors allowed Müller glia to re-enter the cell cycle after NMDA damage (Karl et al., 2008). However, in this paradigm, most of the newly born cells became amacrine cells and not RGCs. Interestingly, the transcription factor Ascll has been shown to be able to reprogram Muller glia into neuronal fates (Pollak et al., 2013; Ueki et al., 2015), and when combined with epigenetic manipulations, adult Müller glia can regenerate inner retinal neurons that closely resemble bipolar cells (Jorstad et al., 2017). More recently, Yao et al. (Yao et al., 2018) showed that Müller glia can regenerate rod photoreceptors when stimulated with B-catenin and then reprogrammed with a combination of factors (Otx2, Crx, Nrl) known to be essential for rod photoreceptor fate. While regeneration of RGCs through endogenous mechanisms has not been achieved yet, the identification of the right factors to reprogram Müller glia into RGC fates could open novel avenues for the treatment of glaucoma and other optic neuropathies.
Concluding remarks
Organoid and 2D protocols for generating RGCs from human and mouse SCs have been proposed as a promising solution to a lack of donor tissue for RGC replacement. However, optimizing these protocols for producing RGCs in large quantities that can be stored for an extended period of time has been proven challenging. Moving forward, it will be important to establish rigorous quality control standards for SC-derived RGCs for use in a human clinic. For example, what parameters can the scientific community and appropriate governmental bodies agree define an RGC? Sanes et al. presented a useful discussion in their review of neuronal classification which focused on how RGC subtypes are classified (Sanes and Masland, 2015). How pure is pure enough in terms of producing ‘pure’ RGC cultures for transplants? Will SC-derived RGC cultures need to be more than just pure RGCs, and perhaps have specific ratios of different subtypes of RGCs? Additionally, in the context of glaucoma RGCs will probably need to be transplanted in combination with treatments to lower IOP as the increased pressure could also affect the newly transplanted cells. Answering these outstanding questions will be critical to advancing the field towards a feasible RGC transplant therapy and investigating these problems will certainly uncover others.
Even considering all these challenges, it is becoming apparent that stem cell-based therapies hold enormous potential for the treatment of retinal ganglion cell and other neural degenerations. With the unceasing progress in the development of new protocols to generate bona-fide RGCs, and the recent possibilities of genetic editing offered by CRISPR, we hope that in the future, reversing vision loss by RGC transplantation can be a reality.
Acknowledgements
We thank Drs. Corinne Fairchild and Sergi Simó for critical reading of the manuscript, and Drs. Edward Pugh, Nadean Brown and Tom Glaser for many helpful discussions. This work was supported by the National Eye Institute [R01EY026942 to ALT]. The T32 Vision Science Training grant [4T32EY015387–14 to MPI] to A.M.M. We also benefited from the use of the NEI Core Facilities, which is supported by the National Eye Institute [P30 EY012576].
References
- Adler R, Canto-Soler MV. 2007. Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev Biol 305:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldredge A, Fuhrmann S. 2016. Loss of Axin2 Causes Ocular Defects During Mouse Eye Development. Invest Ophthalmol Vis Sci 57:5253–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. 2012. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31:152–181. [DOI] [PubMed] [Google Scholar]
- Alsio JM, Tarchini B, Cayouette M, Livesey FJ. 2013. Ikaros promotes early-born neuronal fates in the cerebral cortex. Proc Natl Acad Sci U S A 110:E716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio JG, Hopp H, Choi A, Mandayam Comar J, Liao VC, Harutyunyan N, Lee TC. 2017. Temporal expression of CD184(CXCR4) and CD171(L1CAM) identifies distinct early developmental stages of human retinal ganglion cells in embryonic stem cell derived retina. Exp Eye Res 154:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. 1988. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1:791–803. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Wallace VA. 2012. Cell fate determination in the vertebrate retina. Trends Neurosci 35:565–573. [DOI] [PubMed] [Google Scholar]
- Berson DM. 2008. Retinal ganglion cell types and their central projections Albright TD, Masland R (Eds.), The Senses: A Comprehensive Reference (Vision 1) Vol.1:491–520. [Google Scholar]
- Boucherie C, Mukherjee S, Henckaerts E, Thrasher AJ, Sowden JC, Ali RR. 2013. Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells 31:408–414. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Pekny M, Cooper ML, Benowitz L, Lasker IIoA, Glaucomatous Neurodegeneration P. 2017. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp Eye Res 157:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski EE, Simonett JM, Clark EM, Wright LS, Howden SE, Wallace KA, Petelinsek AM, Pinilla I, Phillips MJ, Meyer JS, Schneider BL, Thomson JA, Gamm DM. 2014. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Hum Mol Genet 23:6332–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V, La Morgia C, Ross-Cisneros FN, Sadun AA. 2017. Optic neuropathies: the tip of the neurodegeneration iceberg. Hum Mol Genet 26:R139–R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C 2014. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci 15:615–627. [DOI] [PubMed] [Google Scholar]
- Chao JR, Lamba DA, Klesert TR, Torre A, Hoshino A, Taylor RJ, Jayabalu A, Engel AL, Khuu TH,Wang RK, Neitz M, Neitz J, Reh TA. 2017. Transplantation of Human Embryonic Stem Cell- Derived Retinal Cells into the Subretinal Space of a Non-Human Primate. Transl Vis Sci Technol 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. 2001. Early eye development in vertebrates. Annu Rev Cell Dev Biol 17:255–296. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. 1990. Topography of ganglion cells in human retina. J Comp Neurol 300:5–25. [DOI] [PubMed] [Google Scholar]
- Daniszewski M, Senabouth A, Nguyen QH, Crombie DE, Lukowski SW, Kulkarni T, Sluch VM, Jabbari JS, Chamling X, Zack DJ, Pebay A, Powell JE, Hewitt AW. 2018. Single cell RNA sequencing of stem cell-derived retinal ganglion cells. Sci Data 5:180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. 2009. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev 126:925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y. 2014. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports 2:853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S, Martin C, Sennlaub F, Chemtob S, Biel M, Samardzija M, Moulin A, Behar-Cohen F, Arsenijevic Y. 2017. Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation. Mol Ther 25:634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Huberman AD. 2014. Retinal ganglion cell maps in the brain: implications for visual processing. Curr Opin Neurobiol 24:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A. 2018. Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors. Stem Cell Reports 10:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Sasai Y. 2011. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc 7:69–79. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–56. [DOI] [PubMed] [Google Scholar]
- Eldred KCH SE; Hussey KA; Brennerman B; Zhang P; Chamling X; Sluch VM; Welsbie DS; Hattar S; Taylor J; Wahlin K; Zack DJ; Johnston RJ 2018. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J, Jolicoeur C, Ramamurthy V, Cayouette M. 2008. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron 60:26–39. [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. 2007. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol 308:1–14. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S 2010. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol 93:61–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi SA, Reh TA. 2010. Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J Neurosci 30:4048–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh F, Arner K. 2002. Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina 22:478–486. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Bruun A, Ehinger B. 1999a. Graft-host connections in long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci 40:126–132. [PubMed] [Google Scholar]
- Ghosh F, Bruun A, Ehinger B. 1999b. Immunohistochemical markers in full-thickness embryonic rabbit retinal transplants. Ophthalmic Res 31:5–15. [DOI] [PubMed] [Google Scholar]
- Gill KP, Hung SS, Sharov A, Lo CY, Needham K, Lidgerwood GE, Jackson S, Crombie DE, Nayagam BA, Cook AL, Hewitt AW, Pebay A, Wong RC. 2016. Enriched retinal ganglion cells derived from human embryonic stem cells. Sci Rep 6:30552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. 2002a. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33:689–702. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. 2002b. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296:1860–1864. [DOI] [PubMed] [Google Scholar]
- Hankin MH, Lund RD. 1990. Directed early axonal outgrowth from retinal transplants into host rat brains. J Neurobiol 21:1202–1218. [DOI] [PubMed] [Google Scholar]
- Harris RE, Coulombe MG, Feller MB. 2002. Dissociated retinal neurons form periodically active synaptic circuits. J Neurophysiol 88:188–195. [DOI] [PubMed] [Google Scholar]
- Hertz J, Qu B, Hu Y, Patel RD, Valenzuela DA, Goldberg JL. 2014. Survival and integration ofdeveloping and progenitor-derived retinal ganglion cells following transplantation. Cell Transplant 23:855–872. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. 2004. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res 23:183–194. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Kretz A, Cellerino A. 2003. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res 22:483–543. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. 1998. The major cell populations of the mouse retina. J Neurosci 18:8936–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR. 2010. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci 51:960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. 2017. Glaucoma. Lancet 390:2183–2193. [DOI] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. 2017. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 548:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. 2008. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A 105:19508–19513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama M, Kurokawa MS, Ueda Y, Ueno H, Kumagai Y, Chiba S, Takada E, Ueno S, Tadokoro M, Suzuki N. 2010. Transfection with pax6 gene of mouse embryonic stem cells and subsequent cell cloning induced retinal neuron progenitors, including retinal ganglion cell-like cells, in vitro. Ophthalmic Res 43:79–91. [DOI] [PubMed] [Google Scholar]
- Kelley KA, Friedrich VL Jr., Sonshine A, Hu Y, Lax, Li J, Drinkwater D, Dressler H, Herrup K. 1994. Expression of Thy-1/lacZ fusion genes in the CNS of transgenic mice. Brain Res Mol Brain Res 24:261–274. [DOI] [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. 1997. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol 115:1031–1035. [DOI] [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. 2000. Number ofganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 41:741–748. [PubMed] [Google Scholar]
- Kobayashi W, Onishi A, Tu HY, Takihara Y, Matsumura M, Tsujimoto K, Inatani M, Nakazawa T, Takahashi M. 2018. Culture Systems of Dissociated Mouse and Human Pluripotent Stem Cell-Derived Retinal Ganglion Cells Purified by Two-Step Immunopanning. Invest Ophthalmol Vis Sci 59:776–787. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Doe CQ. 2013. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci 14:823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Linberg KA, Fisher SK. 1992. Neurons of the human retina: a Golgi study. J Comp Neurol 318:147–187. [DOI] [PubMed] [Google Scholar]
- La Torre A, Georgi S, Reh TA. 2013. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci U S A 110:E2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre A, Hoshino A, Cavanaugh C, Ware CB, Reh TA. 2015. The GIPC1-Akt1 Pathway Is Required for the Specification of the Eye Field in Mouse Embryonic Stem Cells. Stem Cells 33:2674–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre A, Lamba DA, Jayabalu A, Reh TA. 2012. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol Biol 884:229–246. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. 2006. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A 103:12769–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer KB, Ohlemacher SK, Phillips MJ, Fligor CM, Jiang P, Gamm DM, Meyer JS. 2018. Retinal Ganglion Cell Diversity and Subtype Specification from Human Pluripotent Stem Cells. Stem Cell Reports 10:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA. 1997. Mechanisms of optic neuropathy. Curr Opin Ophthalmol 8:9–15. [DOI] [PubMed] [Google Scholar]
- Lewis SL, Khoo PL, De Young RA, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson RV, Robb L, Tam PP. 2008. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development 135:1791–1801. [DOI] [PubMed] [Google Scholar]
- Li H, Tierney C, Wen L, Wu JY, Rao Y. 1997. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development 124:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Hankin MH. 1995. Pathfinding by retinal ganglion cell axons: transplantation studies in genetically and surgically blind mice. J Comp Neurol 356:481–489. [DOI] [PubMed] [Google Scholar]
- Mann F, Holt CE. 2001. Control of retinal growth and axon divergence at the chiasm: lessons from Xenopus. Bioessays 23:319–326. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. 1994. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A 91:7286–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. 1999. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development 126:5713–5724. [DOI] [PubMed] [Google Scholar]
- Medeiros FA, Lisboa R, Weinreb RN, Liebmann JM, Girkin C, Zangwill LM. 2013. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 120:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. 2009. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A 106:16698–16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. 2002. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 3:271–280. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Gumuscu B, Hartman BH, Reh TA. 2006. Notch activity is downregulated just prior to retinal ganglion cell differentiation. Dev Neurosci 28:128–141. [DOI] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. 2012. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 35:153–179. [DOI] [PubMed] [Google Scholar]
- Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, Meyer JS. 2016. Stepwise Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells Enables Analysis of Glaucomatous Neurodegeneration. Stem Cells 34:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A, Tsai EL, Nickerson PE, Bergeret M, Lu Y, Smiley S, Comanita L, Wallace VA.2017. A Reinterpretation of Cell Transplantation: GFP Transfer From Donor to Host Photoreceptors. Stem Cells 35:932–939. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. 2008. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26:215–224. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. 2011. Induction of human neuronal cells by defined transcription factors. Nature 476:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran S, Balasubramanian S, Babai N, Qiu F, Eudy JD, Thoreson WB, Ahmad I. 2010. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells 28:695–703. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, Sampson RD, Georgiadis A, Waldron PV, Duran Y, Naeem A, Kloc M, Cristante E, Kruczek K, Warre-Cornish K, Sowden JC, Smith AJ, Ali RR. 2016. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun 7:13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Wessely O, Li SY, De Robertis EM. 2001. Neural and head induction by insulin-like growth factor signals. Dev Cell 1:655–665. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM. 2014. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells 32:1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker A, Brand M. 2005. Fgf signals from a novel signaling center determine axial patterning of the prospective neural retina. Development 132:4951–4962. [DOI] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. 1997. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 124:805–816. [DOI] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA. 2013. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development 140:2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini T, Barber L, Burton D. 2002. Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucoma. Ophthalmology 109:1367–1371. [DOI] [PubMed] [Google Scholar]
- Riazifar H, Jia Y, Chen J, Lynch G, Huang T. 2014. Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem Cells Transl Med 3:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg AN, Liu Z, Kopan R, Brown NL. 2009. Rbpj cell autonomous regulation of retinal ganglion cell and cone photoreceptor fates in the mouse retina. J Neurosci 29:12865–12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. 1973. The vertebrate retina; principles of structure and function. San Francisco,: Freeman; x, 1044 p. pp. [Google Scholar]
- Rucker JC, Biousse V, Newman NJ. 2004. Ischemic optic neuropathies. Curr Opin Neurol 17:27–35. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Gan L, Yang XJ. 2009. Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina. J Neurosci 29:6932–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta H, Takahashi H, Shintani T, Etani K, Aoshima A, Noda M. 2006. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci 26:10868–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. 2015. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38:221–246. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M. 2016. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun 7:13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong RO. 2001. Development of retinal ganglion cell structure and function. Prog Retin Eye Res 20:139–174. [DOI] [PubMed] [Google Scholar]
- Singh MS, Balmer J, Barnard AR, Aslam SA, Moralli D, Green CM, Barnea-Cramer A, Duncan I, MacLaren RE. 2016. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat Commun 7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit AJ. 2014. Intraocular pressure variations: causes and clinical significance. Can J Ophthalmol 49:484–488. [DOI] [PubMed] [Google Scholar]
- Sluch VM, Davis CH, Ranganathan V, Kerr JM, Krick K, Martin R, Berlinicke CA, Marsh-Armstrong N, Diamond JS, Mao HQ, Zack DJ. 2015. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep 5:16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. [DOI] [PubMed] [Google Scholar]
- Takata N, Abbey D, Fiore L, Acosta S, Feng R, Gil HJ, Lavado A, Geng X, Interiano A, Neale G, Eiraku M, Sasai Y, Oliver G. 2017. An Eye Organoid Approach Identifies Six3 Suppression of R- spondin 2 as a Critical Step in Mouse Neuroretina Differentiation. Cell Rep 21:1534–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. 2015. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep 5:8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Jing L, Willard VP, Wu CL, Guilak F, Chen J, Setton LA. 2018. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res Ther 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotia P, Chopra DA, Dravid SM, Van Hook MJ, Qiu F, Morrison J, Rizzino A, Ahmad I. 2017. Generation of Functional Human Retinal Ganglion Cells with Target Specificity from Pluripotent Stem Cells by Chemically Defined Recapitulation of Developmental Mechanism. Stem Cells 35:572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Marshall VS. 1998. Primate embryonic stem cells. Curr Top Dev Biol 38:133–165. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Mason DF, Miller DH. 2014. Optic neuritis. Lancet Neurol 13:83–99. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. 2013. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl Med 2:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. 2011a. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One 6:e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, Jacobson SG, Sheffield VC, Stone EM. 2011b. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci U S A 108:E569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA. 2015. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A 112:13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL. 2016. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun 7:10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara MN, Flores-Bellver M, Aparicio-Domingo S, McNally M, Wahlin KJ, Saxena MT, Mumm JS, Canto-Soler MV. 2017. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development 144:3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkner M, Zschatzsch M, Rostovskaya M, Overall RW, Busskamp V, Anastassiadis K, Karl MO. 2016. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports 6:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, Duran Y, Blackford SJI, Kloc M, Goh D, Zabala Aldunate E, Sampson RD, Bainbridge JWB, Smith AJ, Gonzalez-Cordero A, Sowden JC, Ali RR, Pearson RA. 2018. Transplanted Donor- or Stem Cell-Derived Cone Photoreceptors Can Both Integrate and Undergo Material Transfer in an Environment-Dependent Manner. Stem Cell Reports 10:406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, Kageyama R, Wallace VA. 2009. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol 184:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dakubo GD, Thurig S, Mazerolle CJ, Wallace VA. 2005. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development 132:5103–5113. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA. 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B. 2018. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 560:484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T, Tanaka T, Matsuzaka E, Tamalu F, Watanabe SI, Nishina S, Azuma N. 2017. Effects of neuroactive agents on axonal growth and pathfinding of retinal ganglion cells generated from human stem cells. Sci Rep 7:16757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Gupta VK, Li JC, Klistorner A, Graham SL. 2013. Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci 24:301–321. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920. [DOI] [PubMed] [Google Scholar]
- Zhao X, Liu J, Ahmad I. 2002. Differentiation of embryonic stem cells into retinal neurons. Biochem Biophys Res Commun 297:177–184. [DOI] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. 2014. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 5:4047. Zuber ME. 2010. Eye field specification in Xenopus laevis. Curr Top Dev Biol 93:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. 2003. Specification of the vertebrate eye by a network of eye field transcription factors. Development 130:5155–5167. [DOI] [PubMed] [Google Scholar]


