Abstract
Background:
Dedicator of cytokinesis 8 (DOCK8) deficiency is the main cause of the autosomal recessive hyper IgE syndrome (HIES). We previously reported the selective loss of group 3 innate lymphoid cell (ILC) number and function in a Dock8-deficient mouse model. In this study, we sought to test whether DOCK8 is required for the function and maintenance of ILC subsets in humans.
Methods:
Peripheral blood ILC1-3 subsets of 16 DOCK8-deficient patients recruited at the pre-transplant stage, and seven patients with autosomal dominant (AD) HIES due to STAT3 mutations, were compared to those of healthy controls or post-transplant DOCK8-deficient patients (n=12) by flow cytometry and real time qPCR. Sorted total ILCs from DOCK8- or STAT3-mutant patients and healthy controls were assayed for survival, apoptosis, proliferation and activation by IL-7, IL-23, and IL-12 by cell culture, flow cytometry and phospho-flow assays.
Results:
DOCK8-deficient but not STAT3-mutant patients exhibited a profound depletion of ILC3s, and to a lesser extent ILC2s, in their peripheral blood. DOCK8-deficient ILC1-3 subsets had defective proliferation, expressed lower levels of IL-7R, responded less to IL-7, IL-12 or IL-23 cytokines and were more prone to apoptosis compared to those of healthy controls.
Conclusion:
DOCK8 regulates human ILC3 expansion and survival, and more globally ILC cytokine signaling and proliferation. DOCK8 deficiency leads to loss of ILC3 from peripheral blood. ILC3 deficiency may contribute to the susceptibility of DOCK8-deficient patients to infections.
Keywords: DOCK8, Hyper-IgE syndrome (HIES), ILC, ILC3, STAT3
INTRODUCTION
Dedicator of cytokinesis 8 (DOCK8) is a guanine nucleotide exchange factor for the Rho family member GTPase Cdc42, a prominent regulator of cytoskeleton in eukaryotic cells(1). In humans, DOCK8 deficiency results in a combined immunodeficiency, the autosomal recessive form of hyper IgE syndrome (HIES)(2,3). DOCK8-deficient patients exhibit high eosinophilia, elevated IgE levels, and have impaired T and B cell immune responses, and are prone to broad spectrum of infections, and also manifest allergic reactions(4). DOCK8 functions in both innate and adaptive immune cells. Accordingly, DOCK8 deficiency results in functional defects in dendritic cells(5), T cells(6), Treg cells(7–9), NK cells(10,11), and B cells(12) in both humans and mice. The migration of several immune cells was shown to be affected by the absence of DOCK8, which results in a defective cytoskeletal organization at the leading-edge membrane(13,14). Similarly, defective immune synapse formation, NK cytotoxic activity has been reported in DOCK8 deficiency(5,9,10). Moreover, DOCK8 was shown to interact with STAT3 and STAT5, therefore regulate cytokine signaling(9,12,15). Indeed, DOCK8-deficiency leads to impaired Th17 cell responses, as demonstrated by reduced IL-6 and IL-21 signaling; impaired Treg cell function as characterized by impaired IL-2 signaling(15).
Group 3 innate lymphoid cells (ILC3s) are a recently defined ROR-γt+ IL-23R+ innate lymphoid cell population enriched at the murine and human mucosal surfaces(16,17). ILC3s play an important role in mucosal barrier function. Importantly, ILC3s are also implicated in the protective immunity to bacterial and/or fungal infections, as well as in the pathogenesis of various chronic inflammatory diseases(17–19). With remarkable resemblance to Th17 cells, ILC3s can produce IL-17A, IL-22, IFN-γ and GM-CSF cytokines in a subset and stimulus-dependent fashion(16,20–22). In this context, IL-22 was shown to be critical for maintaining a healthy barrier by acting on epithelial cell and subsequently stimulating production of antimicrobial peptides such as Reg3γ, Reg3β and calprotectin(23–25). Recently, in Dock8pri/pri mice, we have shown that Dock8 deficiency led to a substantial reduction in ILC3 numbers and function, particularly IL-7 and IL-23 signaling was impaired(26). Consequently, those mice became susceptible to enteropathogenic Citrobacter rodentium infections. However, to date, whether DOCK8-deficient patients have any abnormalities in the number and/or function of ILC subsets, including ILC3s, have not been addressed. Such a determination is especially relevant given that ILC function may become critical in immunodeficient individuals whose adaptive immunity is already compromised.
In this study, we report, for the first time, that DOCK8-deficient patients have quantitative and qualitative defects in blood ILC3s that distinguish them from patients with the autosomal dominant form of HIES (AD-HIES) due to STAT3 loss of function mutations.
METHODS
Human Samples:
Peripheral blood samples were taken from patients, relatives or healthy donors at the respective clinics participating in this study. The three of the DOCK8 mutant patients were assessed before and after transplantation, the remaining post-transplant patients received transplantation prior to this study. The study protocol was approved by the local ethics committee of Erciyes University (#2018/388) and a written informed consent was obtained from all parents. Due to the young age of our patients, a simple oral description of the study was presented to participating children in the presence of their parent(s) and a verbal assent was requested. All methods for human studies involving human samples were performed in accordance with the relevant guidelines and regulations.
Isolation, culture and staining of cells:
Peripheral blood mononuclear cells (PBMCs) were isolated from blood via Ficoll-Paque Plus (GE17-1440-03) based on the manufacturer’s instructions and directly used for experiments without cryopreservation. The ILC polarizing conditions: ILC1:IL-2, IL-7, IL-23, IL-1β, IL-12; ILC2: IL-2, IL-7, IL-23, IL-1β, IL-4; ILC3: IL-2, IL-7, IL-23, IL-1β; 20 ng/ml each. All cytokines were purchased from BioLegend. Cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% Fetal Bovine Serum (FBS), L-glutamine, Antibiotic-Antimycotic (Anti-Anti), essential and non-essential amino acids, all purchased from Gibco. Cells were stained for the relevant antibodies after blocking 5 min with Human TruStainFcX (BioLegend) in Staining Buffer (2 % FBS in PBS) according to supplier’s dilution guidelines. Data acquisition was performed via FacsAriaIII. And the ILC subsets were sorted based on the protocol by Mjosberg et al(16). FlowJo or Diva software were used for the analysis of the flow cytometry data. Singlets were gated on FSC-H/ FSC-A chart as shown in Fig 1A. List of antibodies were given in Supplementary Methods.
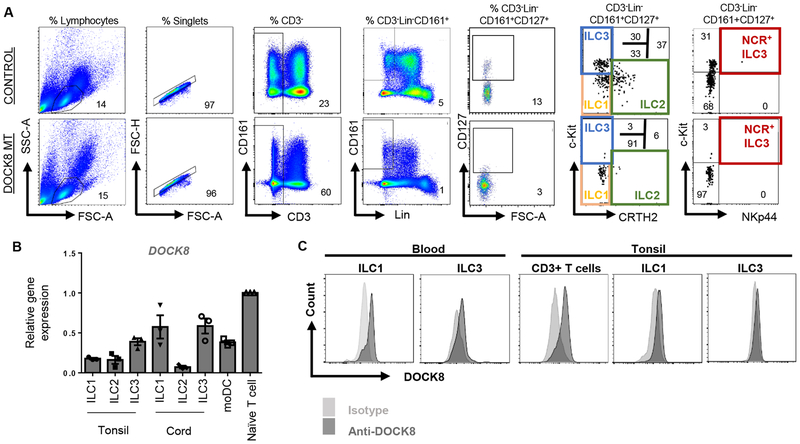
Figure 1.
Human ILCs express DOCK8. A) Gating strategy for human ILCs, representative plots for control and patient blood Blue, green, orange and red gates indicate ILC3, ILC2, ILC1 and natural cytotoxicity receptor (NCR)+ ILC3s, respectively. B) DOCK8 gene expression by sorted human ILC subsets or monocyte derived dendritic cells (moDC) or naïve T cells. DOCK8 expression was quantified as fold expression over that of T cells. C) DOCK8 intracellular staining in ILC1 and ILC3 or CD3+ T cells sorted from human peripheral blood and tonsils.
Real-time qPCR:
Sorted ILC subsets were spun and lysed with lysis buffer from RNeasy kit (Qiagen). Due to very low number of ILC isolated from DOCK8 MT patients, to increase total RNA yield, 3-5 samples were combined after lysis. We performed the same for sorted control ILCs samples. Then total RNA was extracted. cDNA was synthesized using iScript cDNA synthesis Kit (Bio-Rad). Primer sequences are shown in Table S1. LightCycler® 480 (Roche) Instrument and SYBRGreen (Bio-Rad) method were used to detect PCR products. Relative gene expression was calculated by ΔΔCT method. Expression was normalized over 18S ribosomal RNA message. mRNA levels of indicated genes for all patients were determined as fold change over the mRNA levels of controls.
Phospho-flow:
Briefly, sorted ILCs were stimulated in 100μl of complete medium with IL-7, or IL-12 or IL-23 (each 20ng/ml) for 20 min. The samples were fixed with 100μl of 4% PFA for 15 min, then washed (with staining buffer) and permeabilized with methanol for 30 min on ice. Then, stained with p-STAT5(Y694) (cat: 12-9010-42), p-STAT4(Y693) (cat: 12-9044-42) or p-STAT3(Y705) (cat: 17-9033-42) from ThermoFisher for 30 min.
Statistics:
GraphPad Prism 6 software was used for statistical analyses. Two tailed, Unpaired Student’s t test and 1-way ANOVA with Dunnett’s post-test analysis were used for significance analyses. P value <0.05 is accepted as statistically significant.
RESULTS
Human ILC subsets express DOCK8
We recruited sixteen DOCK8-deficient patients who have been diagnosed at different clinics across Turkey. Four of the patients were siblings, born to two unrelated families. The remaining twelve patients were unrelated. DOCK8 deficiency was identified by sequence analysis or copy number analysis, and where indicated, was confirmed at the protein level by immunoblotting or flow cytometry (Table S2). All of the patients presented with HIES scores above 30, and have been hospitalized due to recurrent infections (27,28). They had elevated IgE levels and eosinophilia. The patients also showed slightly reduced CD4+ T cell frequencies, consistent with DOCK8 deficiency. The features and blood work for the patients were summarized in Table S2.
ILC3s are reduced in DOCK8-deficient HIES patients
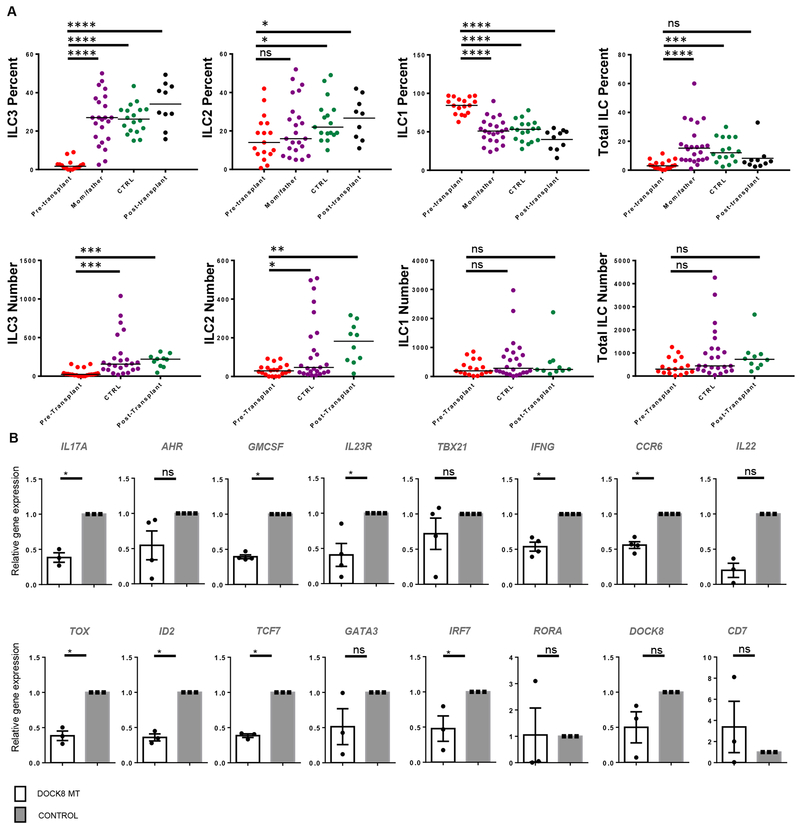
Blood samples from DOCK8-deficient patients and healthy controls were first examined for the presence of previously defined ILC subsets. In the past blood ILC3s were defined as CD3−Lineage−CD161+CD127+c-kit+CRTH2− population, and blood ILC3s lack natural cytotoxicity receptor (NCR) NKp44(16) (Figure 1A). More recently, this population has been shown to contain ILC precursors which can give rise to all ILC subsets(29). DOCK8 gene and protein expression by sorted human ILCs from tonsils and cord blood were confirmed by real-time qPCR and intracellular staining (Figure 1B, C). Similar to our previous report(26), which showed absence of ILC3s in Dock8pri/pri mice, circulating blood ILC3s were dramatically and significantly reduced in DOCK8-deficient patients both in frequency and in absolute numbers compared to the controls, or DOCK8 post-transplanted patients (Figure 2A, S1–2). ILC2 (defined as CD3−Lineage−CD161+CD127+CRTH2+) were also reduced by numbers and frequency. On the other hand, the percentage of ILC1 significantly increased due to loss of cells in the ILC3 quadrant, however, absolute number of ILC1 was comparable between controls and DOCK8-deficient patient blood. Next, we compared the sorted and pooled CD3−Lin−CD161+CD127+ population (total ILCs) obtained from control and DOCK8-deficient patients with respect to the expression of various ILC3-associated cytokines (Figure 2B). By real time qPCR, TOX, ID2, TCF7, IRF7, GMCSF, IL17A, CCR6, IFNG and IL23R mRNAs were shown to be expressed at significantly lower levels in the total ILC fraction obtained from DOCK8-deficient patients compared to control, consistent with the loss of blood ILC3s in flow data.
Figure 2.
ILC3s are reduced in DOCK8-deficient HIES patients. A) PBMCs of DOCK8-deficient patients (pre and post-transplant), mothers and fathers of patients, and control subjects were stained and gated as shown in Figure 1. CD3−Lin−CD161+CD127+ cells were gated as total ILCs and analyzed by c-kit and CRTH2 expression. Percent of ILC subsets among total ILCs, and total ILCs among CD3−Lin−CD161+ cells were shown in the top panel. Absolute number of ILC subsets or total ILCs per ml blood were shown in the bottom panel. Individual patient plots were shown in Figure S1. DOCK8-deficient patients (n=16), Healthy controls (n=14-17), Parents (n=22), post-transplant DOCK8 patients (n=10). In absolute number graphs, CTRL includes healthy controls and healthy parents. B) Reduced human blood ILC3-associated gene expression levels in DOCK8-deficient patients. CD3− Lin−CD161+CD127+ cells were sorted from peripheral blood of control and DOCK8-deficient patients (n=3-4 per group). Expression of indicated genes were assessed via real time qPCR. Results are expressed as fold change over the average of related mRNA levels in controls. Sorted and lysed cells for each group were pooled, five technical replicates run for each group. * p<0.05, ** p<0.01, *** p<0.001, ns: not significant.
Previous studies using mixed bone marrow chimera from WT and Dock8pri/pri mice have shown a requirement for DOCK8 in the generation and function of ILC3s(26). DOCK8-deficient patients require hematopoietic stem cell transplantation (HSCT)(1). To show that DOCK8 deficiency in the hematopoietic compartment is exclusively responsible for the loss of peripheral blood ILC3s, we assessed whether blood ILC3s were reconstituted following HSCT and the association between the transplant regimen and ILC3s reconstitution. The latter is also important because the recent work by Vély F. et al. reported that SCID patients with JAK3 or IL2RG mutations, who also have an ILC deficiency due to requirement of these genes for the development of the ILC lineage, did not restore their ILC numbers post-HSCT if the patients did not undergo myeloablation(30). All ten DOCK8-deficient patients that we have examined post transplantation have had complete restoration of their blood ILC3s (Figure 2A, S1C). Three of the patients have been tested prior to and after HSCT. Their ILC subsets have been separately analyzed and showed complete restoration of ILC3 and ILC2 numbers (Figure S2). All of these patients received a myeloablative regimen with Busulfan/Fludarabine/Thymoglobulin. These results argue that peripheral ILC3 loss is due to DOCK8 deficiency in the hematopoietic compartment.
DOCK8-deficient ILCs have defects in proliferation, cytokine signaling and survival
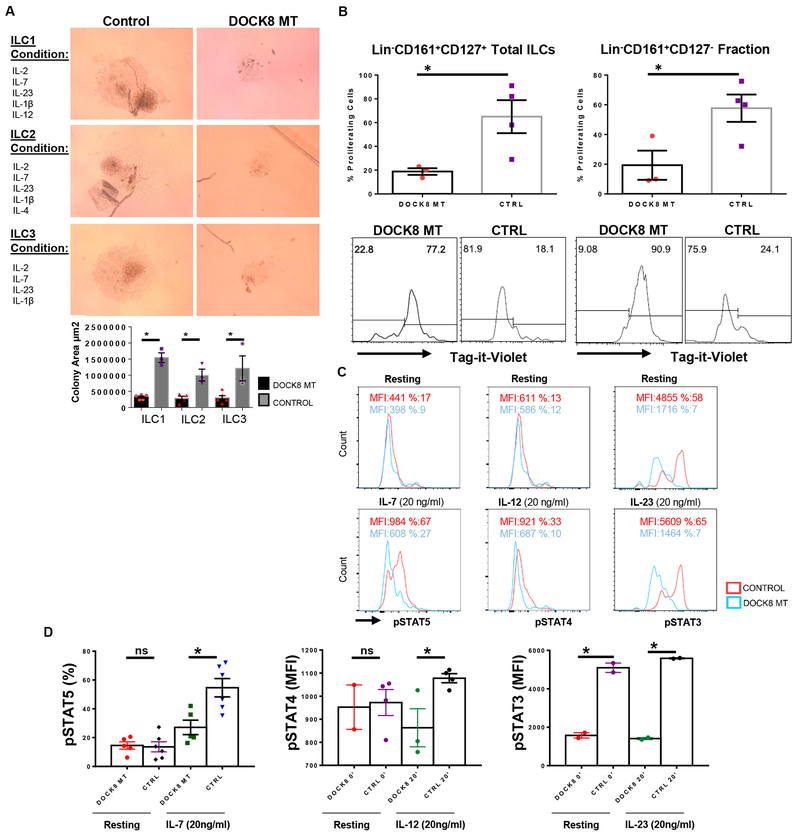
To gain insight into the mechanism of how DOCK8 deficiency causes a reduction in human ILC3s, we first compared the ex vivo proliferative capacity of sorted human ILC subsets obtained from DOCK8-deficient patients and controls. Due to the loss of ILC3 and ILC2 cells in patients, we sorted total ILCs (not subsets) from patients and controls and cultured in the presence of ILC1, ILC2 and ILC3 polarizing cytokines for 21 days based on the protocol of Lim et al.(31). DOCK8 mutant (MT) ILCs showed little proliferation under all three conditions, underlining a significant proliferative defect in response to cytokine stimulation (Figure 3A, B and S3A). Additionally, in the ILC1 condition, IFN-γ gene expression was reduced in line with impaired IL-12 signaling (data not shown).
Figure 3.
Impaired proliferation and cytokine signaling of DOCK8-deficient human ILCs. A) Equal number of sorted total ILCs (CD3−Lin−CD161+CD127+) from DOCK8-deficient patients and healthy controls were cultured for 21 days with indicated cytokine ILC subset polarizing cocktails, proliferation of ILCs were quantified by area of growth. (n= 3-5 per group). B) Sorted total ILCs (CD3−Lin−CD161+CD127+) or CD3−Lin−CD161+CD127− cells were labeled with Tag-it-violet and cultured with same ILC3 subset polarizing cytokine cocktail for 5 days. * indicates p-value <0.05. DOCK8-deficient patients (n=3), healthy controls (n=4). C) IL-7R, IL-12R and IL-23R signaling in DOCK8-deficient human ILCs are impaired. Sorted ILCs (CD3−Lin−CD161+CD127+) from control and DOCK8 patients were cultured overnight and stimulated with indicated cytokines for 20 minutes. Respective STAT phosphorylation was examined via phospho-flow. Percent or MFI indicates mean fluorescence intensity. Representative histograms belong to one patient. D) Percentages of pSTAT5 in sorted total ILCs of five (5) DOCK8 MT patients and six (6) controls upon IL-7 stimulation for 20 min (left); STAT4 phosphorylation mean fluorescent intensity (MFI) upon IL-12 stimulation for 20 min (middle), total ILCs from 2 different patients used (2 technical replicates for one patient); MFI of STAT3 phosphorylation upon IL-23 stimulation for 20 min (right), (technical replicates of one patient, the other patient’s histogram was presented in Figure S3 in the Online Repository). * indicates p-value <0.05, ns: not significant.
Next, we assessed IL-7, IL-12 and IL-23 signaling in total ILCs sorted from control and DOCK8 MT patients. Our group previously demonstrated that IL-7-dependent Stat5 and IL-23-dependent Stat3 phosphorylation are affected by the absence of Dock8 in murine ILC3s(26). In human DOCK8-deficient Th17 cells, Keles et al. showed a reduction in IL-6 or IL-21-dependent STAT3 phosphorylation(15). As expected, in line with the murine model, IL-7-dependent STAT5 phosphorylation and IL-23-dependent STAT3 phosphorylation were impaired in the total ILCs sorted from DOCK8 MT patients compared to healthy controls (Figure 3C, D and S3B). Surprisingly, IL-12-dependent STAT4 phosphorylation was also found to be defective (Figure 3C, D). Impaired signaling downstream of IL-12 suggests a defective ILC1 function in DOCK8-deficiency as observed with other STAT molecules, including STAT5 and STAT3. Defective signaling of all three aforementioned cytokine receptors may also explain the proliferative defects observed in ILC1, ILC2 and ILC3 conditions of DOCK8-deficient ILCs.
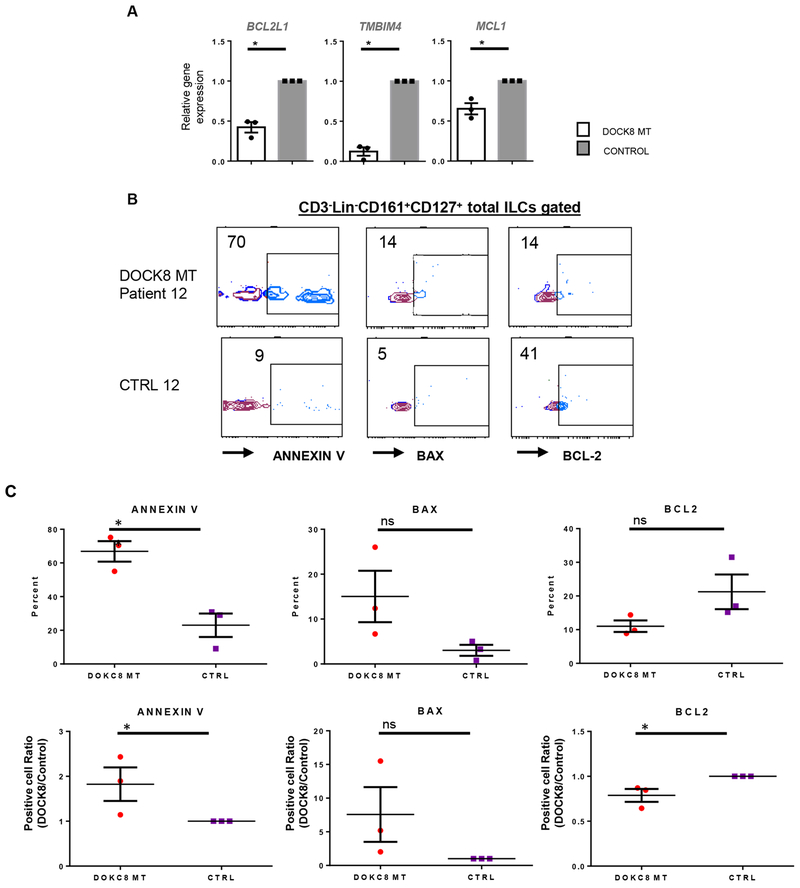
Lastly, we compared the expression of various pro- and anti-apoptotic genes in the total ILC population sorted from healthy controls and DOCK8-deficient HIES patients. Expression of the anti-apoptotic BCL2 family member genes MCL1and BCL2L1, and the transmembrane BAX inhibitor motif containing 4 gene (TMBIM4) was down-regulated in DOCK8-deficient subjects (Figure 4A). Consistent with these results, there was a significantly higher frequency of DOCK8-deficient ILCs that stained positive for the apoptotic markers ANNEXIN V and BAX as compared to control ILCs. Reciprocally, DOCK8-deficient ILCs stained lower for the pro-survival marker BCL2 compared to those of healthy controls, suggesting reduced survival in the absence of DOCK8 (Figure 4B–C). Collectively, these results argue that DOCK8-deficient ILCs have proliferative and survival defects, possibly due to impaired cytokine signaling.
Figure 4.
DOCK8-deficient human ILCs are more prone to apoptosis. A) Sorted total ILCs (CD3−Lin−CD161+CD127+) from DOCK8-deficient and sufficient donors were used to assess the expression of anti-apoptotic genes. Results are expressed as fold change over the average of related mRNA levels in controls. Sorted total ILCs from three patients or controls were pooled. B) Sorted ILCs (CD3−Lin−CD161+CD127+) from control and DOCK8 MT HIES patients were stained for ANNEXIN V, BAX or BCL2, a representative plot per patient is shown. C) Quantification of ANNEXIN V, BAX or BCL2 staining in patients and controls (three-patients per group). * p<0.05 ns: not significant.
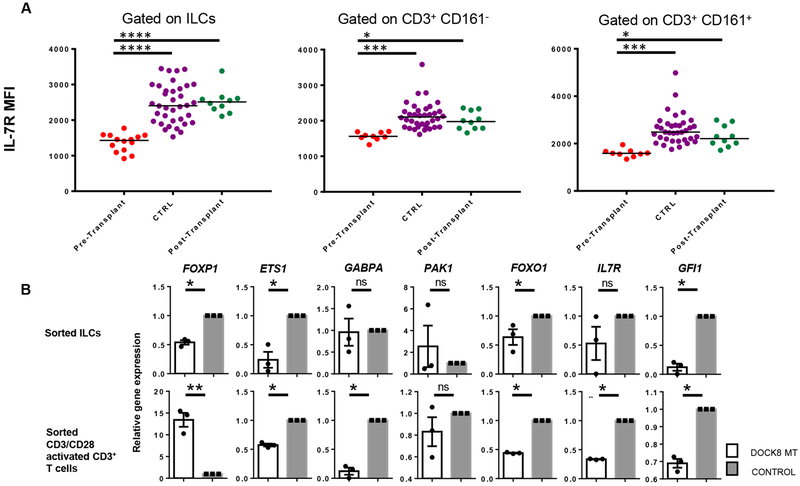
DOCK8 mutations lead to loss of surface IL-7 receptor α expression
We, then, compared IL-7Rα protein expression by the total ILCs of DOCK8 MT patients and healthy controls as this might explain the reduction in STAT5 phosphorylation upon IL-7 stimulation and survival. Previously, reduced IL-7Rα expression was reported in DOCK8-deficient T cells(7). Similar to T cells, there was a significant decrease in the mean fluorescence intensity of IL-7Rα in the peripheral blood total ILCs of DOCK8 MT patients compared with healthy controls (Figure 5A, S4), or ILCs reconstituted in patients following transplantation. Due to almost absence of ILC3s in DOCK8 MT samples, we could not test if DOCK8 deficiency also reduces surface IL-23R levels in exclusively total ILCs or specifically ILC3s. How DOCK8 may regulate IL-7Rα expression is not known. IL-7Rα expression is regulated transcriptionally and post-transcriptionally(32). In the absence of CDC42, which is activated by DOCK8, the transcriptional repressor GFI-1 was shown to bind IL-7Ra promoter and reduce T cell survival and IL-7Rα expression(33). Another repressor of IL7RA promoter is FOXP1(34). Other transcription factors that bind IL7RA promoter and positively regulate its transcription include ETS-1(35,36), FOXO1(37) and GABPA(38,39). Thus, we quantified the gene expression of these transcription factors that were shown to regulate IL7RA promoter activity including GFI1, FOXO1, FOXP1, ETS1 and GABPA or PAK1(32) in ILCs and T cells sorted from DOCK8-deficient patient and healthy controls’ blood (Figure 5B). Consistently, DOCK8-deficient ILCs had significantly lower FOXO1, FOXP1 and ETS1 gene expression compared with ILCs sorted from healthy controls. DOCK8-deficient T cells, following their activation with anti CD3/CD28, also have reduced ETS1 and FOXO1 in addition to GABPA. FOXP1 on the other hand was very highly expressed in activated T cells. Collectively, these results show that DOCK8 deficiency leads to IL-7R expression loss in human blood ILCs and that this might be mediated by reduced expression of positive regulators of IL7RA transcription FOXO1 and ETS1.
Figure 5.
Reduced IL-7Rα expression on ILC surface in DOCK8 mutant patients. A) Blood samples from DOCK8-deficient patients, controls and patients after transplantation were stained and gated as shown in Figure 1. CD3−Lin−CD161+CD127+ total ILCs or CD3+ T cells with or without CD161 expression were gated and mean fluorescent intensity (MFI) of IL-7Rα was analyzed. Individual plots were shown in Figure S4. DOCK8-deficient patients (n=16); CTRL includes both healthy controls (n=12) and healthy parents (n=22); post-transplant DOCK8 patients (n=10). B) Sorted total ILCs (CD3−Lin−CD161+CD127+) and CD3+ T cells from DOCK8-deficient patients and control donors were used to assess the expression of various transcription factors that regulate IL-7Rα expression. Results are expressed as fold change over the average of related mRNA levels in controls. * p<0.05, ** p<0.01, *** p<0.001, ns: not significant.
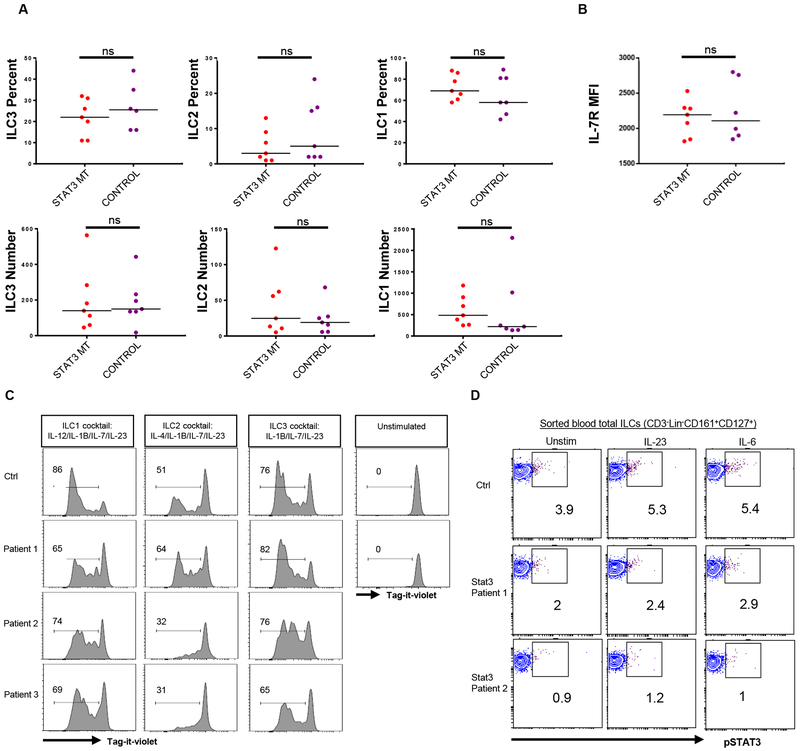
AD- HIES patients have normal ILC subsets with normal IL-7Rα expression
The autosomal dominant (AD) form of HIES is caused by loss of function mutations in STAT3(40,41). To explore whether the depletion of ILC3s observed in DOCK8 deficiency is also shared by AD-HIES, we checked ILC subsets in the peripheral blood of seven autosomal dominant HIES patients with STAT3 mutations. Absolute number and percentages of peripheral ILC subsets were comparable to that of healthy controls (Figure 6A). Additionally, we have not seen a reduction in IL-7Rα levels on the surface of ILCs (Figure 6B). IL-7-induced STAT5 phosphorylation was also comparable to controls (not shown). The cytokine cocktail we used to polarize total ILCs to ILC3 (which included IL-7, IL-2 and IL-1β) did not result in differential expansion between control and STAT3 mutant ILCs (Figure 6C). These results suggest that numeric deletion of peripheral blood ILC3 and ILC2 subsets is unique to DOCK8 deficiency but not common to STAT3 mutants although STAT3 mutant ILC3s may still be dysfunctional especially at the mucosal sites and skin, or in response to stimuli that activate exclusively STAT3 as in the case of IL-23 (Figure 6D). Nevertheless, in response to stimulation with IL2/IL-7/IL-1B/IL-23, ILCs derived from STAT3 mutant patients’ blood showed no difference with respect to proliferation compared with control ILCs and behaved healthier than DOCK8-deficient ones.
Figure 6.
Blood ILC3 numbers and IL-7Rα levels are not altered in STAT3 MT HIES patients. A) PBMCs from STAT3 mutant patients and controls were stained and gated as shown in Supplemental Figure 1. The percentage (upper panel) and absolute number (lower panel) of ILC subsets among CD3−Lin−CD161+CD127+ cells. B) Mean fluorescent intensity of IL-7Rα protein expression by ILCs obtained from controls or STAT3 MT HIES patient blood. C) Sorted total ILCs (CD3−Lin−CD161+CD127+) obtained from controls or STAT3 MT HIES patient blood were cultured in ILC1, ILC2 and ILC3 conditions after labeling with tag-it-violet. At day 7 they were examined by flow cytometry. D) Sorted total ILCs from controls or two STAT3 MT HIES patients were stimulated 20 min with media, IL-23 or IL-6 and pSTAT3 levels were measured by phospho-flow.
DISCUSSION
In this study, we demonstrate for the first time that DOCK8 deficiency results in dramatic loss of circulating ILC3s, and to lesser extend of ILC2s in human subjects. These findings extend our observations in the Dock8pri/pri mice, in which DOCK8 deficiency leads to the global loss of ILC3s, including at the mucosal sites as well. Whether DOCK8-deficiency in humans is also associated with ILC3 loss in tonsils and intestines or other organs remains to be established due to the difficulty of obtaining tissues from DOCK8-deficient patients. ILC3 subsets include lymphoid tissue inducer (LTi) cells and adult NCR+ (Nkp44+) and NCR− (NKp44−) ILC3 populations. LTi cells are critical in lymphoid tissue organogenesis(42). The presence of lymph nodes and Peyer’s patches in the Dock8pri/pri mice suggests that in humans LTi development may not be affected by DOCK8 deficiency(26). Human blood contains predominantly NKp44− ILC3 subset which was diminished in DOCK8-deficient patients. Though Dock8 deficiency reduces murine adult ILC3s regardless of NCR expression, the case for human ILC3s is less clear due to limited access to other organs or the scarcity of NCR+ subset in the blood.
Our data also provides insights into the mechanism of how DOCK8 may cause a severe reduction in human ILC3s. Singh et al. demonstrated that IL-7-dependent Stat5 and IL-23-dependent Stat3 phosphorylation in ILC3s are affected by the absence of DOCK8 in mice(26). In human Th17 cells, Keles et al. demonstrated a reduction of IL-6 and IL-21-dependent STAT3 phosphorylation in DOCK8-defective patients(15). In B cells and Treg cells STAT3 and STAT5 was shown to interact with DOCK8(9,12,15) as shown by immunoprecipitation assays. All of these pathways are instrumental in human ILC3 survival and expansion. Importantly, our data provide direct evidence for impaired IL-7, IL-12 and IL-23 signaling in DOCK8-deficient human ILCs. IL-23 mediated STAT3 signaling is critical for ILC3 expansion and function, thus its dysfunction leads to impaired IL-22, IL-17A and IFN-γ production and susceptibility to infections with extracellular pathogens. Blood ILC3s are reportedly different than mucosal ILC3s in that they express lower levels of IL-23R or RORGT, and do not produce IL-22, IL-17A(31,43). Our real time q-PCR and ELISA(43) results also confirm this by showing very low Ct values for IL23R, RORC, IL22 or undetectable protein levels for IL-22 and IL-17A, respectively. Thus, it has been difficult to demonstrate the impact of DOCK8 deficiency on the expression of these genes by blood ILC3. Recently, CD3− Lin−c-kit+CD127+ ILC3 gate was proposed to contain precursors for all ILC subsets that may seed the tissues(31). Loss of cells in the peripheral blood ILC3 gate also suggests that ILC subsets in other organs might be diminished. Importantly, reduction in IL-7R expression and impaired IL-12 signaling suggest that DOCK8 deficiency have repercussions beyond ILC3s culminating in functional or numeric deficits in ILC2 and ILC1 subsets.
IL-7 is another cytokine critical for the generation, survival, proliferation and maintenance of ILC3s in vitro and in vivo(44–49). In this study, we show that DOCK8 deficiency results in reduced IL-7Rα expression and thus signaling in human ILCs. Given that IL-7 signaling regulates BCL-2 and MCL-1 expression in T cells(32), and that DOCK8-deficient ILCs have reduced levels of IL-7R, BCL-2 and MCL-1, thus, reduced ILC survival might be as a result of impaired IL-7-mediated signaling in DOCK8-deficient ILCs. Defective proliferation and survival for DOCK8-deficient T cells, or murine ILC3s have been shown previously(6,26). Our data on human ILCs corroborates those observations. More importantly, our data provide evidence as to how DOCK8 may result in a reduction in IL-7Rα expression. Positive regulators of IL-7Rα transcription FOXO1 and ETS-1 appear to be reduced in DOCK8-deficient ILCs which may partly account for reduced IL-7Rα levels on ILCs and T cells. Whether DOCK8 deficiency promotes IL-7Rα downregulation by posttranscriptional means requires further study.
Our study also has limitations. Due to scarcity of ILCs and their subsets in the peripheral blood of DOCK8-deficient patients, we used total ILCs instead of subsets. Therefore, the initial proportion of ILC1 and ILC3 subset in total ILCs may reflect frequency of IL-12 and IL-23-phosphorylation. In contrast, since IL-7R is pan ILC marker, reduction in IL-7-dependent STAT5 phosphorylation would reflect a true signaling defect. Similarly, reduced number of ILC3, ILC2 or ILC1 among total sorted ILCs from patients may also account for the proliferation defect observed in distinct ILC polarizing conditions.
In our study, AD HIES patients with STAT3 mutations and control subjects were found to have comparable ILC3s in number. Recently, a case report revealed a reduction in a single patient(50). However, in our work, seven patients tested so far did not show a significant numeric reduction in ILC3 number or frequency, suggesting that decreased STAT3 activity, common to both DOCK8 and STAT3-deficient ILC3, does not account for their depletion(26). It should be noted however that the presence of apparently normal numbers of ILC3s in the blood of STAT3-mutant patients may not be reflective of the situation at the mucosal sites, where IL-23 is highly expressed and play a more important role in the expansion, activation and maintenance of ILC3s. In fact, IL-23 driven STAT3 phosphorylation is impaired in STAT3-deficient ILC3s. This impairment may lead to defective IL-22 production, which in turn may impact protective immune responses at the mucosal sites or skin. Nevertheless, our results suggest that DOCK8 deficiency may precipitate ILC3 deficiency by a distinct mechanism, possibly involving a combination of signaling defects including IL-7R (and its downstream STAT5 signaling module) and IL-6R/IL-23R (and their downstream STAT3 signaling modules, among others).
In addition to DOCK8, autosomal recessive form of HIES may result from mutations in PGM3 and ZNF341(2,27,41,51–57). Whether observed ILC3 defects apply to PGM3 deficiency or to the more recently reported ZNF341(56,57), which more closely phenocopies AD STAT3 deficiency, require further studies.
In summary, our work shows for the first time that DOCK8 regulates ILC3 function and maintenance in humans, and more broadly impacts the function of all three ILC subsets. We propose that the absence of ILC3s in the DOCK8-deficient patients, and the concurrent deficits in the other ILC subsets, may aggravate the defects in the adaptive immune compartment, and contribute to the susceptibility of those patients to recurrent fungal and extracellular bacterial infections.
Supplementary Material
Acknowledgement:
This work was supported partly by the Erciyes University BAP grant, TOA-2016-6130; TUBITAK grants, 215S725 and 315S315 to Ahmet Eken, and National Institutes of Health grant R01AI128976 to Talal A. Chatila.
Abbreviations
- DOCK8
Dedicator of cytokinesis 8
- HIES
Hyper-IgE syndrome
- IL
interleukin
- ILC
Innate lymphoid cells
- ILC1
group 1 ILC
- ILC2
group 2 ILC
- ILC3
group 3 ILC
- PGM3
Phosphoglucomutase 3
- STAT
signal transducer and activator of transcription
Footnotes
Disclosure of potential conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Biggs CM, Keles S, Chatila TA. DOCK8 deficiency: Insights into pathophysiology, clinical features and management. Clin Immunol 2017;181:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ et al. Combined Immunodeficiency Associated with DOCK8 Mutations. N Engl J Med 2009;361:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009;124:1289–1302.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L et al. DOCK8 Deficiency: Clinical and Immunological Phenotype and Treatment Options - a Review of 136 Patients. J Clin Immunol 2015;35:189–198. [DOI] [PubMed] [Google Scholar]
- 5.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 2012;119:4451–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randall KL, Chan SS- Y, Ma CS, Fung I, Mei Y, Yabas M et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med 2011;208:2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L et al. Dedicator of cytokinesis 8–deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol 2014;134:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AK, Eken A, Hagin D, Komal K, Bhise G, Shaji A et al. DOCK8 regulates fitness and function of regulatory T cells through modulation of IL-2 signaling. JCI Insight 2017;2. doi: 10.1172/JCI.INSIGHT.94275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen E, Kumari S, Tohme M, Ullas S, Barrera V, Tas JMJ et al. DOCK8 enforces immunological tolerance by promoting IL-2 signaling and immune synapse formation in Tregs. JCI Insight 2017;2. doi: 10.1172/jci.insight.94298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ et al. Dedicator of cytokinesis 8 interacts with talin and Wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol 2013;190:3661–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2013;131:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol 2012;13:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGhee SA, Chatila TA. DOCK8 immune deficiency as a model for primary cytoskeletal dysfunction. Dis Markers 2010;29:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Côté J-F, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007;17:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keles S, Charbonnier LM, Kabaleeswaran V, Reisli I, Genel F, Gulez N et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation. J Allergy Clin Immunol 2016;138:1384–1394.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mjösberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol 2016;138:1265–1276. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie ANJ, Spits H, Eberl G. Innate Lymphoid Cells in Inflammation and Immunity. Immunity 2014;41:366–374. [DOI] [PubMed] [Google Scholar]
- 18.Shikhagaie MM, Germar K, Bal SM, Ros XR, Spits H. Innate lymphoid cells in autoimmunity: emerging regulators in rheumatic diseases. Nat Rev Rheumatol 2017;13:164–173. [DOI] [PubMed] [Google Scholar]
- 19.Gladiator A, LeibundGut-Landmann S. Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity. PLoS Pathog 2013;9:e1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson C, Thornton EE, McKenzie B, Schaupp A- L, Huskens N, Griseri T et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. Elife 2016;5:e10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011;34:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J et al. Innate Lymphoid Cells Promote Anatomical Containment of Lymphoid-Resident Commensal Bacteria. Science (80- ) 2012;336:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cash HL, Whitham C V, Behrendt CL, Hooper L V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science (80- ) 2006;313:1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011;12:383–390. [DOI] [PubMed] [Google Scholar]
- 26.Singh AK, Eken A, Fry M, Bettelli E, Oukka M. DOCK8 regulates protective immunity by controlling the function and survival of RORγt+ ILCs. Nat Commun 2014;5:4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt KR, Gertz ME, Keles S, Schäffer AA, Sigmund EC, Glocker C et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2015;136:402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimbacher B, Schäffer AA, Holland SM, Davis J, Gallin JI, Malech HL et al. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet 1999;65:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2014;519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol 2016;17:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A et al. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell 2017;168:1086–1100.e10. [DOI] [PubMed] [Google Scholar]
- 32.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol 2012;24:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo F, Hildeman D, Tripathi P, Velu CS, Grimes HL, Zheng Y. Coordination of IL-7 receptor and T-cell receptor signaling by cell-division cycle 42 in T-cell homeostasis. Proc Natl Acad Sci 2010;107:18505–18510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol 2011;12:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenningloh R, Tai T- S, Frahm N, Hongo TC, Chicoine AT, Brander C et al. Ets-1 Maintains IL-7 Receptor Expression in Peripheral T Cells. J Immunol 2011;186:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clements JL, John SA, Garrett-Sinha LA. Impaired generation of CD8+ thymocytes in Ets-1-deficient mice. J Immunol 2006;177:905–912. [DOI] [PubMed] [Google Scholar]
- 37.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 2009;10:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue H- H, Bollenbacher J, Rovella V, Tripuraneni R, Du Y- B, Liu C- Y et al. GA binding protein regulates interleukin 7 receptor α-chain gene expression in T cells. Nat Immunol 2004;5:1036–1044. [DOI] [PubMed] [Google Scholar]
- 39.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol 2008;180:5309–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N et al. STAT3 Mutations in the Hyper-IgE Syndrome. N Engl J Med 2007;357:1608–1619. [DOI] [PubMed] [Google Scholar]
- 41.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007;448:1058–1062. [DOI] [PubMed] [Google Scholar]
- 42.Eberl G, Marmon S, Sunshine M- J, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004;5:64–73. [DOI] [PubMed] [Google Scholar]
- 43.Eken A, Yetkin MF, Vural A, Okus FZ, Erdem S, Azizoglu ZB et al. Fingolimod Alters Tissue Distribution and Cytokine Production of Human and Murine Innate Lymphoid Cells. Front Immunol 2019;10:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R et al. Cutting Edge: IL-7 Regulates the Peripheral Pool of Adult ROR + Lymphoid Tissue Inducer Cells. J Immunol 2009;183:2217–2221. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Cornelissen F, Papazian N, Reijmers RM, Llorian M, Cupedo T et al. IL-7–dependent maintenance of ILC3s is required for normal entry of lymphocytes into lymph nodes. J Exp Med 2018;215:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinette ML, Bando JK, Song W, Ulland TK, Gilfillan S, Colonna M. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat Commun 2017;8:14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chappaz S, Gartner C, Rodewald H- R, Finke D. Kit Ligand and Il7 Differentially Regulate Peyer’s Patch and Lymph Node Development. J Immunol 2010;185:3514–3519. [DOI] [PubMed] [Google Scholar]
- 48.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 2010;33:736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allan DS, Kirkham CL, Aguilar OA, Qu LC, Chen P, Fine JH et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol 2015;8:340–351. [DOI] [PubMed] [Google Scholar]
- 50.Chang Y, Kang S- Y, Kim J, Kang H- R, Kim HY. Functional Defects in Type 3 Innate Lymphoid Cells and Classical Monocytes in a Patient with Hyper-IgE Syndrome. Immune Netw 2017;17:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sassi A, Lazaroski S, Wu G, Haslam SM, Fliegauf M, Mellouli F et al. Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels. J Allergy Clin Immunol 2014;133:1410–1419, 1419.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stray-Pedersen A, Backe PH, Sorte HS, Mørkrid L, Chokshi NY, Erichsen HC et al. PGM3 Mutations Cause a Congenital Disorder of Glycosylation with Severe Immunodeficiency and Skeletal Dysplasia. Am J Hum Genet 2014;95:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Yu X, Ichikawa M, Lyons JJ, Datta S, Lamborn IT et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol 2014;133:1400–1409.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S et al. Human Tyrosine Kinase 2 Deficiency Reveals Its Requisite Roles in Multiple Cytokine Signals Involved in Innate and Acquired Immunity. Immunity 2006;25:745–755. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, Boisson B, Béziat V, Puel A, Casanova J- L. Human hyper-IgE syndrome: singular or plural? Mamm Genome 2018;29:603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Béziat V, Li J, Lin J- X, Ma CS, Li P, Bousfiha A et al. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci Immunol 2018;3:eaat4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frey-Jakobs S, Hartberger JM, Fliegauf M, Bossen C, Wehmeyer ML, Neubauer JC et al. ZNF341 controls STAT3 expression and thereby immunocompetence. Sci Immunol 2018;3:eaat4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.