Abstract
Objective:
Circulating cytokines have been associated with depression, but their detection has limitations, which may be overcome by direct detection of intracellular cytokines (ICCs) after lipopolysaccharide (LPS) stimulation in vitro. This study compared circulating versus LPS-induced inflammatory markers as correlates of subthreshold depressive symptoms.
Methods:
Secondary data analysis of a cross-sectional insomnia study in healthy community-dwelling older adults was conducted. In 117 participants (≥55 years), plasma tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), and in vitro LPS-induced monocyte production of IL-6 and TNF-α were assayed. Depressive symptoms were assessed using the clinician-rated Inventory of Depressive Symptomatology (IDS-C). Multivariate linear regression was conducted to test the associations between inflammatory markers and subthreshold depressive symptoms in the entire sample as well as in subgroups stratified into higher and lower inflammation levels.
Results:
LPS-induced TNF-α (adjusted β=0.28, p=0.04), IL-6 (0.29, p=0.03), and TNF-α+IL-6 (0.43, p=0.001) significantly positively correlated with subthreshold depressive symptoms only in higher inflammation subgroups. No circulating biomarkers positively correlated in any subgroups. In the entire sample, no biomarkers were significantly associated with subthreshold depressive symptoms.
Conclusion:
LPS-induced cytokines may be more sensitive correlates of subthreshold depressive symptoms than circulating cytokines, particularly in older adults with higher systemic inflammation.
Keywords: inflammation, depressive symptoms, lipopolysaccharide, cytokines, C-reactive protein
1. INTRODUCTION
The inflammatory mechanisms are hypothesized to play a significant role on the onset and perpetuation of depression (Raedler 2011; Raison and Miller 2011). A meta-analysis of 24 studies of unstimulated levels of cytokines showed significantly higher concentrations of plasma interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) in patients with major depression compared to control subjects (Dowlati et al. 2010). Several studies have shown elevated circulating levels of IL-6 in depressed older adults (Dentino et al. 1999; Bremmer et al. 2008). Furthermore, elevated levels of circulating c-reactive protein (CRP) and IL-6 predict depressive symptoms (Gimeno et al. 2009; Zalli et al. 2016).
Notably, upregulation of the inflammatory markers have been demonstrated only in a subgroup of depressed individuals (Raison and Miller 2011). Increasing evidence suggests the existence of an “inflammatory biotype” of depression in which patients exhibit unique biochemical and clinical features that are different from the patients with non-inflammatory biotype of depression (Jokela et al. 2015; White et al. 2017). In a randomized placebo-controlled trial of TNF antagonist infliximab for treatment-resistant depression, infliximab was clearly superior to placebo only in patients with higher baseline CRP levels but not when the entire sample was considered (Raison et al. 2013). Therefore, baseline inflammatory biomarkers may be important in distinguishing between the inflammatory and non-inflammatory biotypes of depression when investigating the association between inflammatory signals and development or maintenance of depression.
Most of the evidence linking inflammation to depression is based on the measurement of circulating plasma cytokine levels. However, direct detection of plasma cytokines has clear limitations due to the difficulty detecting cytokines when they are protein-bound as well as the presence of naturally occurring biological inhibitors interfering with immunodetection (Wadhwa and Thorpe 1998). Many cytokines are undetectable in plasma because they are produced locally and have a very short half-life. The detection of intracellular cytokine (ICC) production avoids the influence of the extracellular milieu by in vitro stimulation and permeabilization of peripheral blood cells and then direct detection of intracellular cytokines with flow cytometry (Jung et al. 1993). In addition, this methodology has the advantage of being able to identify which specific cells are producing the cytokines of interest (Rossol et al. 2011). Furthermore, there has been evidence of positive associations between severity of depression and monocyte-associated proinflammatory cytokines as measured by detection of stimulated ICC (Suarez et al. 2003; Suarez et al. 2004).
The purpose of this study was to directly compare circulating inflammatory marker levels in plasma and ICC production in stimulated monocytes as correlates of subthreshold depressive symptoms. In particular, in order to distinguish inflammatory vs. non-inflammatory biotypes of such symptoms, we examined these correlations in higher versus lower inflammation groups as defined by the levels of inflammatory biomarkers. The in vivo circulating levels of cytokines reflect the degree of active inflammation that an individual is currently undergoing whereas the in vitro stimulated ICC production evaluates the potential magnitude of the inflammatory response that an individual is likely to undergo when exposed to an inflammatory stimulus, e.g., physiological or psychological stress. We performed a secondary analysis of an existing data set which included depression symptom scores, plasma levels of inflammatory markers (i.e., cytokines and CRP), and ICC measurements acquired from insomniac older adults who were otherwise healthy (Irwin et al. 2014). To our knowledge, no other studies have directly compared these two modalities of inflammatory marker measurements in association with depressive symptoms.
2. MATERIALS AND METHODS
2.1. Participants
This study was a secondary cross-sectional analysis of the data and blood samples collected prior to starting interventions in a previously reported randomized controlled comparative efficacy trial of insomnia treatments (Irwin et al. 2014). Briefly, after obtaining UCLA institutional review board approval, subjects were recruited from the surrounding Los Angeles community through advertisements and the randomized controlled trial was conducted in the period of April 2006 through August 2011. Participants in the original study were 123 healthy community-dwelling older adults (>55 years old) who met criteria for primary insomnia as previously described (Irwin et al. 2014). Of the 123 original participants, 3 were outliers on the depression measure (Clinician-rated Inventory of Depressive Symptomatology [IDS-C)] ≥24) and 3 subjects had missing IDS-C values; therefore, the final analytical sample of this study included 117 participants (Table 1).
Table 1.
Demographic, clinical and biomarker characteristics of study participants
| Variable | Participants (N=117) |
p-value# |
|---|---|---|
| Age (55 to 85 years; mean ± SD) | 65.7 ±7.1 | 0.39 |
| Female (%) | 71.8% | 0.10 |
| Race, Non-white (%)* | 14.9% | 0.02 |
| Education (years; mean ± SD) | 15.7 ± 1.5 | 0.01 |
| Body mass index (kg/m2; mean ± SD) | 25.8 ±3.8 | 0.64 |
| Depressive symptoms (IDS-C; mean ± SD) | 8.9 ± 4.5 | NA |
| Inflammation (Plasma) | See Table 2 | |
| CRP (mg/L; median [IQR]) | 1.10 (0.60-2.50) | |
| IL-6 (pg/mL; median [IQR]) | 1.38 (1.02-2.11) | |
| TNF-α (pg/mL; median [IQR]) | 1.00 (0.80-1.35) | |
| Inflammation (ICC) | See Table 2 | |
| CD14+TNF-α+IL-6+ (%; mean ± SD) | 50.5 ± 17.7 | |
| CD14+TNF-α+ (%; mean ± SD) | 57.8 ± 18.0 | |
| CD14+IL-6+ (%; mean ± SD) | 63.4 ± 18.4 |
Note: Non-normally distributed variables were described using median and IQR.
IDS-C, Clinician-rated Inventory of Depressive Symptomatology; ICC, intracellular cytokine; CRP, C-reactive protein; IL-6, Interleukin-6; TNF-α, Tumour Necrosis Factor-alpha; IQR, interquartile range
missing 3 values, n=114
p-value derived using Pearson correlation for correlation between a continuous variable and IDS-C scores and t-test for association between a categorical variable and IDS-C scores.
2.2. Procedures
Depressive symptoms were assessed using the IDS-C as previously reported (Irwin et al. 2014). Scores of 11 or less indicate no depressive symptoms whereas scores greater than 11 but less than 24 indicate mild depressive symptoms. Systemic inflammation was measured by the plasma concentrations of CRP as previously reported (Irwin et al. 2014). Plasma concentrations of TNF-α and IL-6 were measured by high-sensitivity Quantikine ELISAs according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN), with lower limits of detection of 0.5 and 0.2 pg/mL, respectively. For ICC measurements, heparinized whole blood was stimulated with LPS (100 pg/mL) for 4 hours and % of CD14-positive monocytes producing TNF-α and IL-6 were measured by flow cytometry as previously described (Irwin et al. 2006); additional details can be found in Supplementary Figure 1. Percentages of monocytes producing IL-6, TNF-α, or both after stimulation were reported previously (Irwin, Olmstead, Breen, et al. 2015).
2.3. Statistical Analysis
For 3 samples with plasma TNF- α concentrations below the limit of detection, a value equal to half the lower limit (0.25 pg/mL) was assigned. Deviations from normality were assessed using the Skewness and Kurtosis test, and non-normally distributed variables (i.e., circulating inflammatory markers) were natural log transformed. Sample characteristics were described in terms of frequency, mean, or median, and their associations with depressive symptoms were examined using t-test or Pearson correlation. Associations between inflammatory markers and depressive symptoms were tested using multivariate linear regression analyses with standardized regression coefficients (β), which facilitate comparison across models. Selection of covariates for multivariate analyses relied on empirical evidence rather than predetermined P-value criteria; the latter approach, which selects factors for inclusion in a multivariate model only if the factors are ‘statistically significant’ in bivariate screening, is considered less optimal because a factor can be a confounder even if it is not statistically significant by itself because it changes the effect of the exposure of interest when it is included in the model, or because it is a confounder only when included with other covariates (Sun et al. 1996). The covariates were age, sex, race, education, and body-mass index (BMI).
Multivariate linear regression analyses were conducted first in the entire sample. Then, subgroup analyses were conducted, using the following stratification strategy for relatively higher and lower inflammation levels for each inflammatory plasma and ICC biomarker. The American Heart Association/ Center for Disease Control (AHA/CDC) guidelines establish a plasma or serum CRP value of 1.00 mg/L as the cut-off value between low cardiovascular risk and average cardiovascular risk (Pearson et al. 2003). Given that the CRP median in this study was 1.10 mg/L, the AHA/CDC 1.00 mg/L cut-off was used to stratify the sample into higher and lower inflammation subgroups for CRP analysis (of note, using 1.10 mg/L as the cut-off generated the identical subgroups as using 1.00 mg/L as the cut-off). As there are no established clinical guidelines for plasma levels of IL-6 and TNF-α or for ICC production of IL-6 and TNF-α corresponding to risk, subgroups of relatively higher and lower inflammation for each of these biomarkers were created using a similar method to plasma CRP stratification by median split. All analyses were performed using STATA 15 (StataCorp, College Station, TX).
3. RESULTS
3.1. Sample Characteristics
Sample characteristics of the 117 participants and their associations with IDS-C scores are shown in Table 1. Participant ages ranged from 55 to 85 years old with a mean age of 66 years. 71.8% of participants were female and 14.9% identified as non-white. Mean BMI was 25.8 kg/m2 (overweight BMI is 25.0-29.9). Participant education ranged from 12 years to 17 years with mean of 15.7 years of education. Participants had relatively low CRP levels (median 1.1mg/L) compared to the US adult population (median 2.1mg/L) (Woloshin and Schwartz 2005). The depressive symptoms at baseline were largely in the subthreshold range in this sample with 79.5% of participants with IDS-C scores of 11 or less indicating no depressive symptoms and 20% of participants with IDS-C scores in the mild range (IDS-C scores >11 and ≤23 =mild). Age, gender, and BMI were not significantly associated with baseline depressive symptoms while race and education were significantly associated with IDS-C scores (Table 1). Mean IDS-C score of non-white race was 2.8 less than that of white race. Number of years of education negatively correlated with IDS-C scores (r = −0.22).
When the correlations between various inflammatory markers themselves were examined, there was a significant positive association between plasma CRP and plasma IL-6 (unadjusted β=0.45, p<0.01; adjusted β=0.35, p<0.01) as well as between plasma CRP and plasma TNF-α (unadjusted β=0.21, p=0.06; adjusted β=0.27, p=0.01) even after adjusting for covariates (Supplementary Table 1 & 2). However, the percentage of cells positive for intracellular TNF-α, IL-6 or TNF-α+IL-6 showed no significant correlations with corresponding circulating cytokines or with plasma CRP levels (Supplementary Table 1 & 2).
3.2. Inflammatory Markers and Subthreshold Depressive Symptoms in the Higher and Lower Inflammation Subgroups
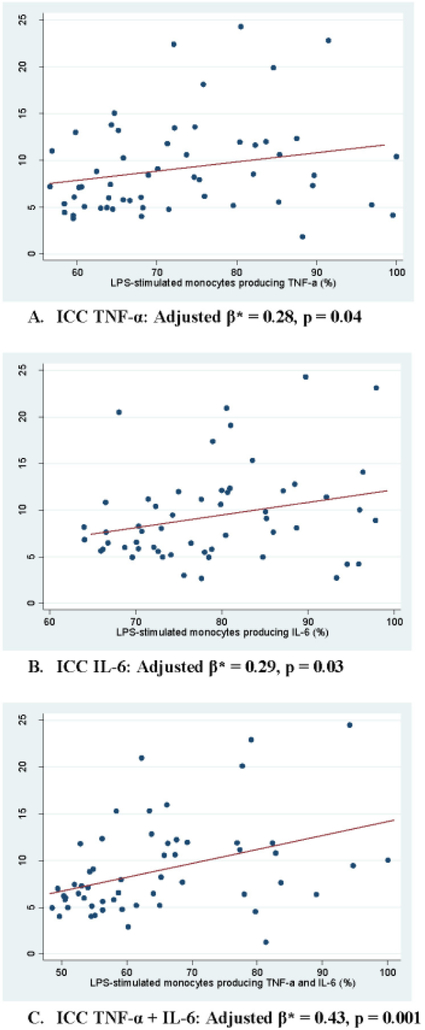
The circulating inflammatory biomarker levels in plasma (CRP, IL-6, TNF-α) and percentage of LPS-induced monocytes positive for intracellular cytokine (IL-6, TNF-α, TNF-α+IL-6) were examined for correlation with subthreshold depressive symptoms within the higher inflammation subgroups using median split for each biomarker to represent the inflammatory biotype of depression. In the higher inflammation subgroups, all LPS-induced intracellular cytokines were positively correlated with IDS-C scores (ICC TNF-α: unadjusted β=0.23, p=0.09; ICC IL-6: unadjusted β=0.26, p=0.06; ICC TNF-α+IL-6: unadjusted β=0.39, p=0.003) (Supplementary Table 3). The positive correlation was significant for all three LPS-induced inflammatory biomarkers after multivariable linear regression analyses that adjusted for sex, age, gender, education and BMI (ICC TNF-α: adjusted β=0.28, p=0.04; ICC IL-6: adjusted β=0.29, p=0.03; ICC TNF-α+IL-6: adjusted β=0.43, p=0.001) (Figure 1, Table 2). By comparison, neither plasma TNF-α nor IL-6 had any significant relationship with depressive symptoms in the higher inflammation subgroups. Plasma CRP had an unexpectedly negative correlation with IDS-C scores, which was not impacted by covariate adjustment (unadjusted β=−0.36, p=0.02; adjusted β=−0.36, p=0.02) (Table 2, Supplementary Table 3). In the lower inflammation subgroups of both LPS-induced intracellular cytokines and plasma biomarkers, no significant association was found between any of the inflammatory markers and IDS-C scores. After covariate adjustment with multivariable linear regression analyses, intracellular TNF-α demonstrated an unexpected significant negative correlation with IDS-C scores (unadjusted β=−0.26, p=0.05; adjusted β=−0.30, p=0.02) (Table 2).
Figure 1.
Correlations between depressive symptoms and the percentage of monocytes producing TNF-α (A), IL-6 (B), or both (C) after LPS stimulation in the higher inflammation subgroup
Note: β, standardized regression coefficient; CRP, C-reactive protein; IDS-C, Clinician-rated Inventory of Depressive Symptomatology; ICC, intracellular cytokine; IL-6, Interleukin-6; LPS, lipopolysaccharide; TNF-α, Tumour Necrosis Factor-alpha
*Adjusted for sex, age, race, education, and body-mass index
Table 2.
Associations between depressive symptoms (IDS-C scores) and inflammatory biomarkers
| Entire Sample | Lower Inflammation subgroup |
Higher Inflammation subgroup |
||||
|---|---|---|---|---|---|---|
| Variable | Adjusted β* | P | Adjusted β* | P | Adjusted β* | P |
| Plasma CRP (mg/L) | −0.16 | 0.14 | 0.01 | 0.97 | −0.36 | 0.02 |
| Plasma IL-6 (pg/mL) | −0.03 | 0.74 | −0.02 | 0.91 | −0.04 | 0.78 |
| Plasma TNF-α (pg/mL) | 0.03 | 0.75 | 0.10 | 0.48 | −0.06 | 0.68 |
| CD 14+TNF-α+IL-6+(%) | 0.17 | 0.07 | −0.06 | 0.66 | 0.43 | 0.001 |
| CD 14+TNF-α+(%) | 0.09 | 0.33 | −0.30 | 0.02 | 0.28 | 0.04 |
| CD14+IL-6+(%) | 0.12 | 0.21 | −0.01 | 0.94 | 0.29 | 0.03 |
Note: β, standardized regression coefficient CRP, C-reactive protein; IDS-C, Clinician-rated Inventory of Depressive Symptomatology; IL-6, Interleukin-6; TNF-α, Tumour Necrosis Factor-alpha
Adjusted for age, sex, race, education, and body-mass index
3.3. Inflammatory Markers and Subthreshold Depressive Symptoms in the Entire Sample
Within the entire sample, there was no significant association between IDS-C scores and circulating plasma CRP, IL-6 or TNF-α before or after adjusting for covariates (Table 2 & Supplementary Table 3). There were also no significant correlations between IDS-C scores and percentages of stimulated monocytes positive for intracellular TNF-α+IL-6 (unadjusted β=0.14, p=0.14), TNF-α (unadjusted β=0.05, p=0.58), or IL-6 (unadjusted β=0.12, p=0.22) before or after adjusting for covariates (Table 2 & Supplementary Table 3).
3.4. Relationship between Insomnia Severity and Inflammatory Markers
As the study subjects all met criteria for primary insomnia, potential role for the severity of insomnia mediating the relationship between the depressive symptoms and inflammatory markers were examined. In all groups examined, no significant relationships were found between sleep severity measured by the Pittsburgh Sleep Quality Index (PSQI) and circulating plasma CRP, IL-6 or TNF-α or the ICC measurements of TNF-α+IL-6, TNF-α, or IL-6 before or after adjusting for covariates (Supplementary Tables 4 & 5). Potential confounding effect of insomnia severity mediating the positive correlation between the subthreshold depressive symptoms and inflammation was addressed by removing the sleep-related items from the IDS-C scores and performing the multivariate linear regression analyses with inflammatory markers. In the higher inflammation subgroups, all LPS-induced ICCs were positively and significantly correlated with IDS-C scores without the sleep related items after covariate adjustments (ICC TNF-α: adjusted β=0.25, p=0.046; ICC IL-6: adjusted β=0.36, p=0.004; ICC TNF-α+IL-6: adjusted β=0.44, p=0.001) (Supplementary Table 7). The unexpected negative correlation between plasma CRP and IDS-C scores was unchanged after the sleep related items were removed from the IDS-C scores in the higher inflammation subgroup (adjusted β=−0.39, p=0.005) (Supplementary Table 7). In the lower inflammation subgroups, the unexpected negative association between the LPS-induced TNF-α and IDS-C scores was observed again after the sleep related items were removed (adjusted β=−0.32, p=0.01) (Supplementary Table 6). No other significant associations were found between the inflammatory markers and IDS-C scores without the sleep items (Supplementary Tables 6 & 7).
4. DISCUSSION
In this study, we conducted a secondary cross-sectional analysis of association between inflammatory markers and subthreshold depressive symptoms in otherwise healthy insomniac older adults. To our knowledge, this study is the first to directly compare circulating versus LPS-induced inflammatory markers as correlates of subthreshold depressive symptoms. We stratified the study sample into subjects with relatively higher and lower levels of individual inflammatory marker being tested in order to differentiate between inflammatory and non-inflammatory biotypes of depressive symptoms. In the higher inflammation subgroups, LPS-induced inflammatory cytokines measured by ICC TNF-α, IL-6, and especially co-production of TNF-α+IL-6 were significantly positively correlated with subthreshold depressive scores. This is consistent with numerous previous studies demonstrating only a subgroup of individuals with depression showing increased inflammation levels and also supports the rationale for stratifying the sample based on the inflammation levels when trying to capture the inflammatory biotype of depression. As expected, no positive correlation was found between the inflammatory biomarkers and depressive symptoms within the lower inflammation subgroup. Interestingly, there were no significant associations between ICC biomarkers and depressive symptoms when the entire sample was analysed together. This finding highlights the importance of distinguishing the inflammatory biotype from the non-inflammatory biotype of depression. In addition, the study subjects were relatively healthy individuals with overall very low levels of inflammation and depressive symptoms, which may have masked some signals for relationship between the inflammatory markers and depressive symptoms.
Notably, the circulating plasma IL-6 and TNF-α were not significantly correlated with subthreshold depressive symptoms even within the higher inflammation subgroup. Previous meta-analysis has established that higher levels of plasma IL-6 and TNF-α are found in a subgroup of subjects with major depressive disorder as compared to non-depressed controls (Dowlati et al. 2010). Two longitudinal studies have found that higher plasma levels of IL-6 and CRP at baseline predict future depressive symptoms (Gimeno et al. 2009; Dowlati et al. 2010; Zalli et al. 2016). However, few studies have investigated inflammatory plasma biomarkers in subjects with subthreshold depression. Bremmer et al. investigated the association between circulating biomarkers and subthreshold depression, but found no significant association (Bremmer et al. 2008). The absence of the relationship in their study is consistent with our findings of circulating biomarkers. However, we were able to detect subtle but significant relationship between LPS-induced intracellular cytokines and subthreshold depression, which suggests that the ICC assay may be a more sensitive measure of inflammation with respect to depressive symptoms than circulating plasma inflammatory markers in a relatively healthy population with subthreshold symptomatology.
Whereas the in vivo circulating levels of cytokines reflect the degree of active inflammation that an individual is currently undergoing, the in vitro LPS-stimulated ICCs represent sensitivity to triggering cellular inflammation and thus evaluate the potential magnitude of the inflammatory response that an individual may undergo when exposed to an inflammatory stimulus, e.g., physiological or psychological stress. When we assessed for any relationships between circulating and intracellular biomarkers of inflammation, we found no associations between the percentage of ICC markers and their corresponding circulating cytokines or with CRP levels (Supplementary Table 1 & 2), further supporting the possibility that they represent distinct biological processes. Thus, this “stress responsivity” component of the ICC measures may be one of the possible explanations for the current findings regarding the relative superiority of ICC measures as correlates of subthreshold depressive symptoms. Furthermore, the detection of circulating cytokines has limitations due to, e.g., interaction with biological inhibitors, short half-life, and protein binding; however, ICC method overcomes such limitations by direct detection of intracellular cytokine production in monocytes stimulated with LPS in vitro. Given these advantages of the ICC method, including the “stress responsivity” property, LPS-induced ICC measures could potentially also serve as a predictor of depressive symptoms. However, this is a cross-sectional study, and obviously a subsequent prospective cohort study is required to test this premise.
In the two studies known to use LPS-induced intracellular cytokines to measure inflammation in depression, the Beck Depression Inventory was used to assess depressive symptoms. In one study, subjects reported only minimal to mild symptoms, and in the second, subjects reported mild to moderate symptoms (Suarez et al. 2003; Suarez et al. 2004). Although our study used a different measure, IDS-C, depressive symptoms ranged from none to moderate levels, which are comparable to those in both studies by Suarez et al.. The positive association between subthreshold depressive symptoms and LPS-induced ICC TNF-α found in this study supports those reported in the Suarez et al. papers. We found no prior studies which looked at the relationship between ICC IL-6 and subthreshold depressive symptoms, even though IL-6 is an established inflammation marker in the pathophysiology of MDD (Dentino et al. 1999; Dowlati et al. 2010).
Of note, we observed two unexpected negative (i.e., inverse) correlations between inflammatory markers and subthreshold depressive symptoms—when examining the plasma CRP levels in the higher inflammation subgroup and the LPS-induced intracellular TNF-α levels in the lower inflammation subgroup. While explaining the reasons for these unexpected findings is beyond the scope of our study, it should be noted that negative correlations between inflammatory markers and depressive symptoms have been reported (Joyce et al. 1992; Kim et al. 2012; Shelton et al. 2015; Schmidt et al. 2016) and extensive heterogeneity has been observed across individual studies included in meta-analyses with differing strengths and directions of associations (Howren et al. 2009; Hiles et al. 2012; Haapakoski et al. 2015; Smith et al. 2018).
Chronic insomnia has been associated with increases in CRP and other markers of inflammation in various studies (Irwin, Olmstead, Carroll 2015). As the study subjects met the DSM criteria for primary insomnia, we have addressed the potential confounding effect of insomnia on the positive correlation between ICC inflammatory biomarkers and subthreshold depressive symptoms. First, we looked for any positive association between insomnia severity measured by PSQI and the inflammatory markers. In all groups examined, there was no significant association between PSQI and circulating or ICC inflammatory biomarkers. Additionally, we removed the sleep-related items from the IDS-C scores to control for any contribution from the sleep disturbance and found essentially identical association patterns as the initial analysis using the total IDS-C scores. Taken together, we were able to demonstrate that insomnia severity was not a confounder in the positive association between ICC inflammatory biomarkers and subthreshold depression.
The following limitations should be considered. First, the cross-sectional design of this study did not allow any inference on the directionality of the associations between inflammation and depressive symptoms. Second, a limitation to this secondary analysis is that the original study had excluded individuals with major depressive disorder (Irwin et al. 2014); stronger associations could potentially be seen if IDS-C scores had been higher. However, despite this limitation, we were able to demonstrate subtle associations between LPS-induced cytokines and subthreshold depressive symptoms in individuals with relatively higher inflammation levels. Third, the study sample was, despite the presence of insomnia, generally healthy with overall low levels of systemic inflammation, which may have masked some of the effects of inflammatory process on depression. Fourth, stratifying subjects into higher and lower inflammation subgroups further decreased the sample size. However, the observation of significant correlations between LPS-induced ICC measures and subthreshold depressive symptoms in the higher inflammation subgroup in this study seems a notable finding, perhaps indicating the potential value of ICC measures as a sensitive biomarker for depressive symptoms.
Notwithstanding the limitations, this study used a novel approach to assessing correlations between inflammation and depression. Despite the overall low levels of both inflammation and depression, a significant relationship was found between ICC inflammatory biomarkers and IDS-C scores among otherwise healthy insomniac older adults, but only after the sample was stratified based on inflammatory marker levels to delineate inflammatory versus non-inflammatory biotypes of depressive symptoms. The study needs to be replicated in a larger sample of participants including those with major depressive disorder and higher levels of inflammation to fully determine whether intracellular cytokeins, as compared to plasma cytokines, are a better measure of inflammation with respect to the association with depressive symptoms.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health (R01AG034588 and K23AG049085). The National Institutes of Health had no role in the design and conduct of the study.
Footnotes
Clinical Trials Registry: ClinicalTrials.gov NCT00280020.
STATEMENT OF INTEREST
None to declare.
REFERENCES
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. 2008. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 106(3):249–255. eng. [DOI] [PubMed] [Google Scholar]
- Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ. 1999. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 47(1):6–11. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry. 67(5):446–457. eng. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG et al. 2009. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological medicine. 39(3):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. 2015. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 49:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. 2012. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun. 26(7): 1180–1188. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 71(2): 171–186. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, Arevalo JM, Ma J, Nicassio P, Bootzin R et al. 2015. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry. 78(10):721–729. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, Yokomizo M, Lavretsky H, Carroll JE, Motivala SJ et al. 2014. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 37(9):1543–1552. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. 2015. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. 2006. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 166(16):1756–1762. [DOI] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty G, Kivimäki M. 2015. Inflammation and specific symptoms of depression. JAMA Psychiatry.1–2. [DOI] [PubMed] [Google Scholar]
- Joyce PR, Hawes CR, Mulder RT, Sellman JD, Wilson DA, Boswell DR. 1992. Elevated levels of acute phase plasma proteins in major depression. 32(11):1035–1041. [DOI] [PubMed] [Google Scholar]
- Jung T, Schauer U, Heusser C, Neumann C, Rieger C. 1993. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 159(1-2):197–207. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Kim J-M, Kim S-W, Shin I-S, Park M-H, Yoon J-H, Choi C, Yoon J-S. 2012. Associations between plasma cytokines and depressive mood in patients with breast cancer. The International Journal of Psychiatry in Medicine. 43(1):1–17. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL et al. 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 107(3):499–511. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. 2011. Inflammatory mechanisms in major depressive disorder [Review]. Curr Opin Psychiatry. 24(6):519–525. eng. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. 2011. Is depression an inflammatory disorder? [Research Support, N.I.H., Extramural Review]. Curr Psychiatry Rep. 13(6):467–475. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 70(1):31–41. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. 2011. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 31(5):379–446. [DOI] [PubMed] [Google Scholar]
- Schmidt FM, Schröder T, Kirkby KC, Sander C, Suslow T, Holdt LM, Teupser D, Hegerl U, Himmerich H. 2016. Pro-and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. 239:85–91. [DOI] [PubMed] [Google Scholar]
- Shelton MM, Schminkey DL, Groer MW. 2015. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biological research for nursing. 17(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Au B, Ollis L, Schmitz N. 2018. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp Gerontol. 102:109–132. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. 2003. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 65(3):362–368. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. 2004. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 29(9):1119–1128. [DOI] [PubMed] [Google Scholar]
- Sun GW, Shook TL, Kay GL. 1996. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 49(8):907–916. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Thorpe R. 1998. Cytokine immunoassays: recommendations for standardisation, calibration and validation. J Immunol Methods. 219(1-2):1–5. [DOI] [PubMed] [Google Scholar]
- White J, Kivimaki M, Jokela M, Batty GD. 2017. Association of inflammation with specific symptoms of depression in a general population of older people: The English Longitudinal Study of Ageing. Brain Behav Immun. 61:27–30. Eng. [DOI] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM. 2005. Distribution of C-reactive protein values in the United States. N Engl J Med. 352(15):1611–1613. [DOI] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H, Carvalho LA. 2016. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 233(9):1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.