Abstract
Visceral pain is the cardinal symptom of functional gastrointestinal (GI) disorders like the irritable bowel syndrome (IBS) and the leading cause of patients’ visit to gastroenterologists. IBS-related visceral pain usually arises from the distal colon and rectum (colorectum), an intraluminal environment that differs greatly from environment outside the body in chemical, biological, thermal and mechanical conditions. Accordingly, visceral pain is different from cutaneous pain in several key psychophysical characteristics, which likely underlies the unsatisfactory management of visceral pain by drugs developed for other types of pain. Colorectal visceral pain is usually elicited from mechanical distension/stretch, rather than from heating, cutting, pinching, or piercing that usually evoke pain from the skin. Thus, mechanotransduction, i.e., the encoding of colorectal mechanical stimuli by sensory afferents is crucial to the underlying mechanisms of GI-related visceral pain. This review will focus on colorectal mechanotransduction, the process of converting colorectal mechanical stimuli into trains of action potentials by the sensory afferents to inform the central nervous system (CNS). We will summarize neurophysiological studies on afferent encoding of colorectal mechanical stimuli, highlight recent advances in our understanding of colorectal biomechanics that plays critical roles in mechanotransduction, and review studies on mechanosensitive ion channels in colorectal afferents. This review calls for focused attention on targeting colorectal mechanotransduction as a new strategy for managing visceral pain, which can also have an added benefit of limited CNS side effects because mechanotransduction arises from peripheral organs.
Keywords: Mechanotransduction, Irritable Bowel Syndrome, visceral afferents, colorectum, mechanosensitive ion channel
INTRODUCTION
Pain as a protection mechanism is a perception in the brain in response to threats and hazards from the environment, which are usually in the form of tissue-injurious stimuli including hot, cold, acidic, inflammatory, chemical, stabbing, rubbing, and tearing/stretching stimuli etc. Our skin is directly exposed to the outside environment, and is equipped with sensory nerve endings dedicated to reliable and robust encoding of aforementioned stimuli to inform the brain (see (Gold and Gebhart 2010) for review). In contrast, solid visceral organs inside the body like the pancreas, lung, liver, and spleen usually lack prominent sensory innervations to cause pain (Cervero and Laird 1999). Extensive tissue injury and damage to those solid visceral organs can go unnoticed by patients until at the late stage of severe diseases, e.g., pancreatic cancer (Greenwald et al. 1987; Kelsen et al. 1995) and liver cirrhosis (Marotta et al. 2000). Hollow visceral organs are inside the body, but their mucosa faces an environment also ‘outside’ the body, e.g. the stomach, bladder, urethra, reproductive organs, and gastrointestinal (GI) lumen of the colon and rectum (Cervero 1994; Cervero and Laird 1999). Visceral pain is often broadly defined as pain arising from inside of the body, but is usually evoked from hollow visceral organs by excessive contraction, stretching, tension or ischemia of the organ walls (Miranda 2018). If we generally consider the hollow volume of visceral organs, e.g., the GI lumen, as a continuation of the outside environment, then both cutaneous and visceral pain share a common function, i.e., to protect tissues from ‘external’ injurious stimuli.
Despite their common protective function, visceral pain from the GI tract differs significantly from its cutaneous counterpart in several psychophysical characteristics as summarized below. First, unlike the protective role of cutaneous pain to prevent tissue injuries, GI visceral pain can be dissociated from gut injuries and inflammation. Visceral pain is the major complaint of patients with irritable bowel syndrome (IBS) whose colons lack apparent organ damage or inflammations and appear ‘normal’ as those from healthy controls (Feng et al. 2012a). In contrast, patients with inflammatory bowel disease (IBD) in relapse, i.e., with ongoing colon inflammation show normo-or even hypo-sensitivity to noxious colorectal distension (Bernstein et al. 1996; Chang et al. 2000; Annahazi et al. 2009). Second, the protective role of cutaneous pain is accompanied by an immediate motor withdrawal to move affected body part away from harmful stimuli, whereas GI visceral pain lacks a similar motor withdrawal with rapid responses (Cervero and Laird 2004); the GI reflexes like vomiting and peristalsis do not immediately follow the onset of visceral pain and are likely medium and long-term coping mechanisms. The fast motor responses require the cutaneous pain to be ‘sharp’, accurate in painful locations, and rapid in information transmission, whereas visceral pain in the absence of fast motor responses is ‘dull’, diffuse in localization often with characteristic referral, and slow in information transmission (Pasricha et al. 2006). Third, visceral pain is usually accompanied with stronger emotional and psychological components than cutaneous pain, which is likely caused by the significant overlapping between the visceral pain circuits and the autonomic nervous system that plays major roles in the body’s emotional responses (Cueva et al. 2007).
This review will focus on visceral pain that arises from the colon and rectum, especially the distal colon and rectum (colorectum) that are the focal origin of GI-related visceral pain (Ness and Gebhart 1988, 1990). Due to the unique thermal, chemical, biological and mechanical environments inside the colorectum, the perception modality of visceral pain is significantly limited compared to a vast array of cutaneous pain modalities. First, the temperature in the colorectum usually remains constant, and thus hot or cold stimuli to the colorectum generally do not evoke visceral pain (Falt et al. 2013). Second, the large intestine is host to microbiomes and immune cells (Kau et al. 2011), and is regularly exposed to chemicals like bile acids, fatty acids, peptides, and carbohydrate molecules. Hence, chemicals and acids that reliably evoke cutaneous pain can be less efficient to trigger visceral pain and vice versa. For example, inflammatory stimuli that cause severe pain in the skin do not trigger heightened perception of visceral pain in IBD patients with ongoing gut inflammation (Bernstein et al. 1996; Chang et al. 2000; Annahazi et al. 2009), whereas glycerol causes severe visceral pain when infused into the colon and rectum (Louvel et al. 1996; Bouin et al. 2001) but is not an effective skin irritant. Third, mechanical stimuli are noxious to both skin and viscera, but via different types of mechanical stress. Skin afferents are capable to encode both noxious shear and normal stress. Thus, skin can detect cutting and pinching (shear stress) as well as stabbing and tearing (mostly normal stress). In contrast, the colorectum is more sensitive to noxious normal stress evoked by colorectal distension (Ness and Gebhart 1988; Ness et al. 1990), whereas shear stress from cutting, pinching or piercing the colorectum is inadequate in evoking visceral pain (Lewis 1942; Ness and Gebhart 1990; Brierley et al. 2018).
As summarized above, many stimulus modalities that are noxious to the skin (e.g., mild hot, cold, acidic, chemical and inflammatory) are often inadequate to evoke colorectal visceral pain. Accumulating clinical and preclinical evidence has suggested colorectal mechanical stimuli as adequately noxious to evoke visceral pain (Ness and Gebhart 1990). In this review, we will focus on visceral pain evoked by colorectal mechanotransduction, the process of converting mechanical stimuli into trains of action potentials to inform the central nervous system. We will first summarize extrinsic afferent innervations of the colorectum that encode mechanical stimuli. We will then highlight recent advances in our understanding of colorectal tissue biomechanics that has profound impact on mechanotransduction of the colorectum. We will also systematically review studies on mechanosensitive ion channels on colorectal mechanotransduction, which play critical roles in depolarizing the afferent membrane to generate action potentials.
MECHANOTRANSDUCTION BY MECHANOSENSITIVE AFFERENTS AND MECHANO-NOCICEPTORS OF THE COLORECTUM
Mechanical stimuli to the colorectum are encoded by extrinsic afferents with sensory endings embedded in the colorectum. Extrinsic afferents subserve colorectal mechanotransduction to inform the CNS of noxious mechanical stimuli from the colorectum and drive conscious perceptions arising from the viscera, which are usually discomfort and pain (Gebhart 2000). The colorectum is also host to the enteric nervous system (ENS) which consists of intrinsic afferents that also encode mechanical stimuli. Information from the ENS can be potentially transmitted to the CNS via rectospinal or instestinofugal neural pathways (Furness 2006), but there is no direct evidence to support that activation of intrinsic afferents alone is sufficient to elicit conscious perception of visceral pain. Thus, it is the extrinsic afferents that likely dominate the detection of noxious mechanical stimuli in the colorectum and the transmission of tissue-injurious information to the CNS.
Extrinsic innervation of the colorectum
The colorectum is mainly innervated by extrinsic afferents in the spinal nerves, including the lumbar splanchnic nerves (LSN) and pelvic nerves (PN) with afferent somata in the thoracolumbar (TL) and lumbosacral (LS) dorsal root ganglia (DRG), respectively. Anatomic evidence has indicated that vagal afferent innervation can extend to the distal portion of the colon (Wang and Powley 2007; Herrity et al. 2014). Since activation of vagal afferents alone does not reliably evoke visceral pain (Andrews and Sanger 2002; Bulmer and Roza), we will focus on the spinal afferent innervation of the colorectum in this review.
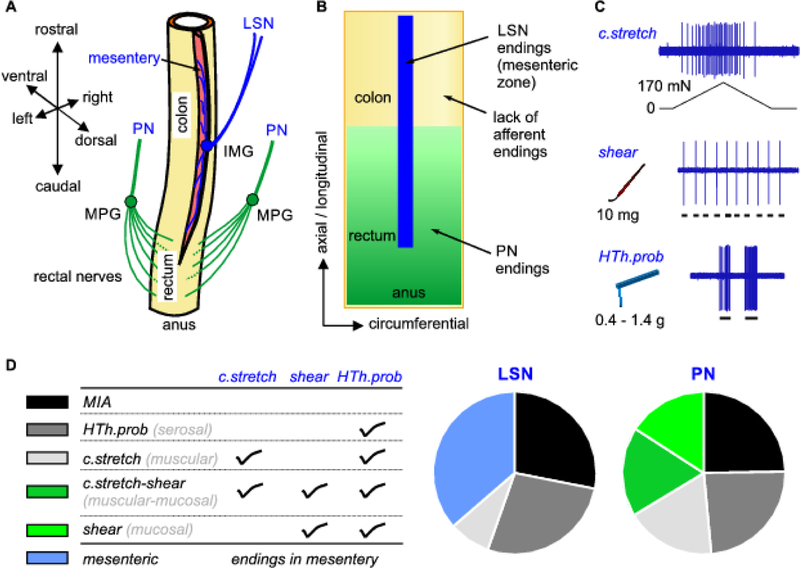
Knowledge on the neural encoding functions of colorectal spinal afferents were mostly derived from electrophysiological recordings on guinea pigs, rats and mice whose colorectums are cylindrical in the absence of a sigmoidal curvature as in human colorectum. As illustrated in Fig. 1A, afferents in the LSN pathway enter and travel longitudinally up and down the mesentery, and usually end in the narrow longitudinal strip of the colorectum next to the mesentery, i.e., the mesenteric zone as highlighted in blue in Fig. 1B. Receptive endings of LSN afferents are concentrated in the mesenteric zone and absent in other regions of the colorectum. In contrast, PN branch into fine rectal nerve fibers after passing the major pelvic ganglion and enter the rectal region at multiple locations. Within the colorectum, extrinsic PN afferents travel extensive distance in the myenteric plexus with their receptive endings spreading evenly along the circumference of the colorectum and up into the colonic region as highlighted in green in Fig. 1B (Spencer et al. 2014). The PN afferent endings are more concentrated at the distal rectal region with gradually reduced ending distributions towards the proximal colonic region. It is worth emphasizing that extrinsic spinal afferents are absent in the proximal colonic region not adjacent to the mesentery as illustrated in yellow in Fig. 1B, similar to other solid visceral organs that lack afferent innervations.
Figure 1.
Extrinsic afferents in the spinal innervation of mouse colon and rectum (colorectum). A) The lumbar splanchnic (LSN) and pelvic nerves (PN) dominate the innervation of the colonic and rectal regions, respectively. B) Afferent endings are differentially distributed in the colorectum between the LSN and PN innervations. Notice that proximal colonic regions out of the mesenteric zone have no spinal afferent innervations. C) Three mechanical stimuli are applied to the afferent receptive fields in the colorectum to evoke responses revealed by single-unit electrophysiological recordings. D) Colorectal afferents are categorized into 5 classes based on their response profiles to the three mechanical stimuli. Mesenteric afferents have endings not in the colorectum but in the mesentery. MPG: major pelvic ganglion; IMG: inferior mesenteric ganglion; c.stretch: circumferential stretch; HTh.prob: high-threshold probing;
The neural encoding function of colorectal afferents was systematically characterized by us via an unbiased electrical search strategy, i.e., identifying afferent endings by focal electrical stimulation with a concentric electrode (Feng and Gebhart 2011, 2015; Feng et al. 2012c; Feng et al. 2012b; La et al. 2012; Kiyatkin et al. 2013). Afferents ‘found’ by electrical stimulation were subsequently characterized by their responses to three mechanical stimuli to the receptive fields as illustrated in Fig. 1C:
c.stretch: circumferential colorectal stretch up to 170 mN that generates ~56 kPa circumferential normal stress.
shear: mucosal shearing by fine stroking of ~10 mg brushing force.
high-threshold probing (HTh.prob): perpendicular probing to the colorectal surface by von Frey-like monofilaments that generate 125–195 kPa normal stress (0.4–1.4 g buckling force in 0.03–0.07 mm2 tip area).
All three mechanical stimuli have physiological correlates. C.stretch recapitulates the circumferential colorectal distension up to ~50 mmHg that is beyond the noxious threshold of 20 mmHg in mice (Feng et al. 2010). Shear mimics the surface shear force on the mucosa by contents passing through the colorectal lumen. High-threshold probing (HTh.prob) generates focal mechanical stress of 125 to 195 kPa comparable to injurious colorectal distension beyond at least 80 mmHg based on calculations with Laplace’s law. Besides a small proportion of afferents that are unresponsive to any of the three mechanical stimuli (categorized as mechanically insensitive afferents [MIA]), most colorectal afferents (67% in LSN, 77% in PN) are mechanosensitive and respond to at least HTh.prob, the strongest stimuli of the three (Feng and Gebhart 2011).
Based upon their response profiles to the three mechanical stimuli as listed in Fig. 1D, we generally categorize the mechanosensitive colorectal afferents into four classes: c.stretch-shear, c.stretch, shear, and HTh.prob. In addition to responses to HTh.prob, c.stretch afferents also respond to circumferential colorectal stretch, shear afferents also respond to mucosal shearing, and c.stretch-shear afferents also respond to both stimuli. The rest mechano-sensitive afferents do not respond to either circumferential colorectal stretch or mucosal shearing and are classified as HTh.prob afferents. In addition to endings inside the colorectum, LSN afferent endings are also found in the mesentery, and are categorized as the mesenteric class. With a more stringent classification, mesenteric afferents with endings outside the colorectum are not colorectal afferents. As shown in Fig. 1D, all four colorectal afferent classes are about evenly represented in the PN innervation, while the LSN afferents consist mostly of HTh.prob and mesenteric afferents with a small proportion of c.stretch afferents. Shear and c.stretch-shear afferents that encode low-threshold mucosal shearing are absent in the LSN pathway. The mechanosensitive afferents were also studied by mechanically brushing the colorectal surface to ‘search’ the afferent endings (Brierley et al. 2004; Hughes et al. 2009), which reported similar proportions of functionally classified afferent classes as in Fig. 1D.
We need to emphasize that different terminologies are used in previous literatures that infer afferent ending locations in the colorectum based upon their functional responses, i.e., “muscular” for afferents responding to circumferential colorectal stretch, “mucosal” for afferents responding to mucosal shearing, “serosal” for those responding to high-intensity probing. The exact ending locations of those functionally classified afferents await further systematically studies that couple functional recordings with anatomic tracing at single afferent level. The name “serosal afferents” is a misnomer as anatomic studies clearly indicate the absence of extrinsic afferent endings in the serosal layer of the colorectum (Spencer et al. 2014; Zagorodnyuk et al. 2010). In this review, we have chosen to use descriptive terms that reflect the stimulus type(s) the afferents respond to, which correspond one-on-one to the previous terms as listed in parentheses in Fig. 1D.
Colorectal mechano-nociceptors
Colorectal mechano-nociceptors are afferents that detect and encode tissue-injurious mechanical stimuli to the colorectum. Nociceptors typically have high response thresholds and only encode at noxious range of stimulation. However, threshold per se is not a distinguishing characteristic of nociceptors (Gold and Gebhart 2010). Some low-threshold afferents are also categorized as nociceptors as they encode into the noxious stimulus range with increasing firing rate, i.e., intensity encoding (Perl 2007). In addition, nociceptors typically sensitize in tissue-injurious environment by reducing their response threshold and/or increasing firing rate to the same stimuli (Gold and Gebhart 2010; Gebhart and Feng 2013).
According to the above criteria, HTh.prob afferents in both LSN and PN pathways are nociceptors that encode injurious colorectal distension beyond 80 mmHg. In addition, LSN HTh.prob afferents sensitize to various chemical stimuli with enhanced firing rate in response to von-Frey mechanical probing, including capsaicin (Brierley et al. 2005a), bradykinin (Brierley et al. 2005b), inflammatory mediators (Hughes et al. 2009) and cytokines (Hughes et al. 2013). Similarly, PN HTh.prob afferents were reported to be sensitized by capsaicin (Brierley et al. 2005a) and acidic hypertonic solution (La et al. 2012). However, it remains unreported in the literature whether sensitized HTh.prob afferents have reduced response threshold to colorectal distension. Our unpublished observations indicate that serosal afferents generally do not respond to circumferential colorectal stretch (0 – 170 mN) even after exposing their endings to inflammatory mediators. Also, the intensity of 80 mmHg colorectal distension is significantly beyond the noxious threshold for mice (20mmHg) and likely causes irreversible damages to the colorectum, suggesting that HTh.prob afferents may function to inform catastrophic mechanical failure of the colorectum to the CNS. The exact role of HTh.prob afferents at distending pressure below 80 mmHg and its contribution to persistent visceral hypersensitivity await future studies.
The c.stretch and c.stretch-shear afferent classes are stretch-sensitive and encode circumferential stretch comparable to colorectal distension of 0 – 50 mmHg, covering both innocuous (< 20 mmHg) and noxious ( >20 mmHg) ranges. About 14 to 25% of the c.stretch and c.stretch-shear afferents are mechano-nociceptors with response thresholds above 20mmHg (Feng et al. 2010; Sengupta and Gebhart 1994). The vast majority of c.stretch and c.stretch-shear afferents respond to innocuous colorectal distension but also encode into the noxious range with increased firing rate (Feng et al. 2012c; Feng et al. 2010; Feng et al. 2012b; La et al. 2012; Tanaka et al. 2011; Shinoda et al. 2009). In addition, both low-threshold and high-threshold groups sensitize after ending exposure to inflammatory mediators: high-threshold afferents have reduced response threshold and enhanced firing rate to the same mechanical stretch and low-threshold afferents show significantly increased firing rate (Feng et al. 2010). More importantly, sensitization of c.stretch afferents is unanimously observed in different IBS-like mouse models with persistent visceral hypersensitivity by intracolonic treatments of zymosan (Feng et al. 2012b), TNBS (Feng et al. 2012c) and acidic hypertonic solution (AHS) (La et al. 2012), respectively. In comparison, the neural encoding of c.stretch-shear afferents is sensitized in the zymosan model (Feng et al. 2012b), attenuated in the TNBS model (Feng et al. 2012b) and unchanged in the AHS model (La et al. 2012), which likely reflects the different effects of those intracolonic treatments on colorectal mucosa. In sum, c.stretch afferents are colorectal mechano-nociceptors, and the nociceptive role of c.stretch-shear afferents may depend on the specific pathophysiological conditions in the colorectum.
The mechanically insensitive afferents (MIAs) do not encode even noxious mechanical stimuli under normal physiological conditions. However, MIAs especially in the PN pathways are capable to sensitize by acquiring responses to high-threshold mechanical probing after brief exposure of their endings to inflammatory mediators (Feng et al. 2016; Feng and Gebhart 2011). In addition, the persistent sensitization of PN MIAs is observed in IBS-like mouse models with prolonged visceral hypersensitivity (Kiyatkin et al. 2013; Feng et al. 2012c; La et al. 2012; Feng et al. 2012b). Thus, sensitized MIAs are also mechano-nociceptors that provide de novo encoding of colorectal mechanical stimuli to inform the CNS.
In summary, most mechanosensitive colorectal afferents and sensitized MIAs are mechano-nociceptors. This is consistent with the general morphologies of colorectal afferent endings, i.e., free nerve endings with prominent varicosity and lack of myelination (Spencer et al. 2014) that are hallmark features of C-fiber nociceptors in the skin (Messlinger 1996; Burgess and Perl 1973; Reynders et al. 2015). Colorectal mechanoreceptors that are probably not nociceptors include shear afferents that encode low-intensity mucosal shearing and generally do not sensitize to chemical stimuli, as well as the proportion of MIAs that do not sensitize to inflammatory stimuli but detect osmotic stimuli (Feng et al. 2016). Also, the role of c.stretch-shear afferents in visceral nociception is not consistent in all IBS-like mouse models and can be categorized as non-nociceptors by following a more stringent standard. The LSN innervation consists of mostly mechano-nociceptors of either HTh.prob afferents or MIAs capable to sensitize. In contrast, the PN innervation includes both nociceptors (HTh.prob, sensitized MIAs, and c.stretch afferents) and non-nociceptors (shear afferents, and c.stretch-shear afferents). Hence, most LSN afferents are dedicated to colorectal nociception whereas PN afferents likely participate in not only nociception but also encoding of physiological events like innocuous distension and mucosal shearing. This is also supported by the restricted distribution of afferent endings close to the mesentery in the LSN pathway versus distributed afferent endings throughout the distal colorectum in the PN pathway. Information from focal regions of the colorectum will be sufficient to detect catastrophic mechanical failure as in the LSN pathway whereas detecting physiological events like luminal shearing and distension in distal colorectum requires sensory information from all regions.
THE MULTISCALE BIOMECHANICS OF THE COLORECTUM
Colorectal mechanotransduction, i.e., encoding colorectal mechanical stimuli into trains of action potentials, is undertaken by the distal 100–200 μm of the extrinsic afferent endings embedded in the colorectal wall (Feng et al. 2015). The neurophysiology of colorectal afferents to encode bulk mechanical deformation of the colorectum has been extensively investigated and summarized in the previous section of this review. During mechanotransduction, bulk colorectal deformation causes local stress/strain distributions at the nerve endings, which drives the opening of mechanosensitive ion channels and results in neural membrane depolarization and generation of action potentials. Colorectal biomechanics, i.e., passive tissue structural and mechanical properties causally determines how bulk colorectal deformation translates to microscale stress/strain along the sensory nerve endings. Hence, the multiscale biomechanics of colorectal tissue likely has a profound impact on the process of mechanotransduction.
In contrast to the plethora of data regarding colorectal afferent neural encoding, knowledge of the colorectal biomechanics has only been reported by a handful of studies. Sokolis et al. conducted biomechanical tests to compare the mechanical strength of rat large intestine at different anatomic sites, including distal colon and rectum. Analysis of the intestine was overly simplified to assumed homogeneous material properties, and microscale mechanics was neglected (Sokolis et al. 2011; Sokolis and Sassani 2013). Carniel et al. reported mechanical testing and complementary constitutive modeling of transverse colon in pigs (Carniel et al. 2014b; Carniel et al. 2014a). This tissue is anatomically proximal to the portion of colorectum innervated by the LSN and PN pathways. Biomechanics of more proximal portions of the GI tract has been more extensively studied, including the small intestine (Storkholm et al. 1998; Zhao et al. 2002; Zeng et al. 2003; Yu et al. 2004; Lu et al. 2005; Frokjaer et al. 2006) and esophagus (Yang et al. 2004; Liao et al. 2004; Liao et al. 2003; Natali et al. 2009; Yang et al. 2006; Sommer et al. 2013). However, both the physiological function and anatomic structures of distal colorectum differ significantly from their proximal counterparts in the GI tract (Leung 2014), preventing direct translation of biomechanical findings from esophagus and small intestine to the distal colorectum. Recently, we have implemented novel experimental approaches to systematically characterize the macro-and micro-scale biomechanics of distal ~30 of mouse colorectum, i.e., focal regions innervated by the LSN and PN afferents.
Macroscale mechanical heterogeneity of the colorectum
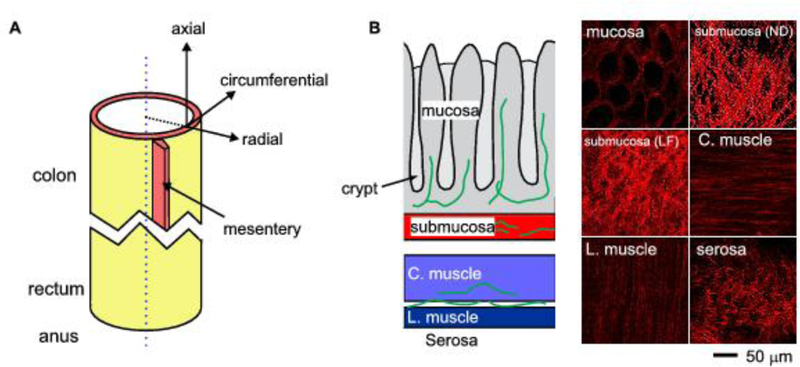
Considering the mouse distal colorectum as a cylindrical tube, we introduce a cylindrical coordinate system as shown in Fig. 2A to assess its macroscale mechanical properties along the axial, circumferential and radial directions, respectively. The heterogeneity of mechanical properties is revealed by recent biomechanical characterizations on small patches of tissues harvested from different locations in the mouse colorectum (Siri et al. 2019a) and from separate layers of colorectal wall (Siri et al. 2019b).
Figure 2.
Mechanical heterogeneity of mouse colorectum. A) The tubular colorectum is assigned with a cylindrical coordinate system. Heterogeneous macroscale mechanical properties are reported along the axial, radial and circumferential directions. B) The collagen fibers that subserve microscale soft tissue biomechanics were measured by second-harmonic generation (SHG) microscopy through the thickness of the colorectal wall. The collagen network in the submucosa is the load-bearing structure of the colorectum. C. muscle: circular muscular layer; L. muscle: longitudinal muscular layer; submucosa (LF): submucosa under load-free condition; submucosa (ND): submucosal under noxious distension.
Axial heterogeneity
We recently reported apparent difference in biomechanical properties between the colonic and rectal segments in tissue stiffness, viscoelasticity, pre-stress, and anatomic thickness (Siri et al. 2019a). Importantly, this differential biomechanics corresponds with the differential afferent neural encoding between the LSN and PN pathways that dominate the innervation of the colonic and rectal regions, respectively. The rectum is more compliant in the circumferential direction than the colon, which is consistent with the predominant presence of c.stretch and c.stretch-shear afferents in the PN innervation that encode circumferential colorectal stretch. The higher circumferential compliance of the rectum than the colon also consists with higher firing rates of rectal afferents to circumferential stretch than colonic afferents (Feng et al. 2010). The colorectum tissue is viscoelastic and dissipates more energy under deformation in the circumferential direction than in the axial, which could underlie the adaptation of afferent activities to circumferential colorectal stretch (Feng et al. 2010; Feng and Gebhart 2011). The increasing opening angle from the colon towards the rectum indicates that the rectal afferents in the muscular layers are pre-stretched in the physiological conditions, which might contribute to their higher firing rate than afferents in the colonic segments (Feng et al. 2010). Last, the rectum is significantly thicker than the colon (Feng et al. 2010), especially at the circular muscular layers, which implies that PN afferents are more affected by smooth muscle activities during normal GI functions than the LSN counterparts.
Radial heterogeneity
In addition to the differential mechanical properties along the axial direction, the colorectum biomechanics is also heterogeneous through the thickness of the wall, i.e., along the radial direction in Fig. 2A. The colorectal wall consists of multiple layers, including from outer to inner: serosal, longitudinal muscular, intermuscular, circular muscular, submucosal, muscularis mucosal, and mucosal layers. In a recent study, we separated the colorectal wall at the interstitial space between the submucosal and circular muscular layers into the outer muscular/serosal composite and inner mucosal/submucosal composite (Siri et al. 2019b). We reveal that the inner mucosal/submucosal composite has slightly higher axial stiffness than the outer composite while the outer muscular/serosal composite has higher circumferential stiffness. Hence, the wall tension resulting from colorectal distension will be undertaken by both composites with inner composite taking slightly more axial tension and outer composite more circumferential tension.
Circumferential heterogeneity
Tubular organs like the blood vessels are usually axisymmetric along the central axis, and material properties are homogeneous along the circumferential direction. However, the colorectum is not axisymmetric in the presence of mesentery along one side (Fig. 2A). Also, the distribution of LSN afferent endings is not homogeneous along the circumferential direction but concentrated at the mesenteric zone (Fig. 1A). This strongly suggests the differential biomechanical properties along the circumferential direction between the mesenteric and non-mesenteric zones, which has not been systematically studied or reported in the literature. Our preliminary unpublished observations indicate that mouse colorectum ruptures at the mesenteric zone when intraluminal pressure is beyond 200 mmHg. Further research is warranted to reveal the differential biomechanical properties along the circumferential direction of the colorectum.
In-plane heterogeneity
Considering the colorectum as a thin-walled cylinder, the axial and circumferential directions form a planar surface. The in-plane mechanical properties of the colorectum are also heterogeneous between the axial and circumferential directions. Colorectum has in-plane tissue anisotropy showing significantly higher stiffness in the axial direction than in the circumferential direction. Interestingly, this tissue anisotropy between the axial and circumferential directions is more pronounced in the rectal region than in the colonic region. This is caused by the aforementioned axial tissue heterogeneity of progressive reduction in circumferential stiffness from the proximal to distal colorectum. In the meanwhile, the axial tension stiffness shows no significant changes from proximal to distal colorectum. Collectively, this results in more significant in-plane tissue anisotropy in the rectum than in the colon. Enhanced in-plane tissue anisotropy in the rectum allows more circumferential compliance, and likely supports its physiological role of fecal storage.
Microscale mechanical heterogeneity of the colorectum
The macroscale mechanical properties of the colorectum are mainly determined by microscale structures of collagen fibers, which form the major load-bearing skeleton for many biological tissues, e.g., the skin (Reihsner and Menzel 1998), tendon cartilage (Ker 1999), and blood vessels (Hariton et al. 2007). Assembled from thread-like collagen fibrils, collagen fibers have diameters ranging from 0.5 to a few microns (Hulmes 2002). Collagen fiber morphology in small intestine was determined by a handful of studies on sectioned tissue slices using chromatic and immunobiological staining and scanning electron microscopy (Orberg et al. 1983; Orberg et al. 1982; Gabella 1983; Storkholm et al. 1998; Zeng et al. 2003; Yu et al. 2004). Recently, second-harmonic generation (SHG) microscopy has emerged as a powerful method for imaging collagen fibers with submicron’s resolution in a diverse range of tissues (Kaleem et al. 2017; Santos et al. 2019; Kumar et al. 2015; Birk et al. 2014). SHG microscopy is highly sensitive to the collagen fibril/fiber structure and can visualize collagen fibers several hundred microns deep into the tissue by using excitation light at infra-red range (800 – 1200 nm) (Chen et al. 2012). Comparing to staining from sectioned tissues of ~10 microns thick, our preliminary results indicate that SHG microscopy can visualize collagen fibers through the thickness of intact mouse colon (~200 microns thick) and most of the rectum (300 – 400 microns), which allows systematic characterization of the collagen fiber density, distribution, alignment and orientation at different layers of the colorectum (Feng et al. 2019).
Through SHG imaging, collagen fibers are found to be concentrated in the submucosa in the colorectum as shown in Fig. 2B. More significantly, collagen fibers in the submucosa are curved when the colorectum is in load-free condition and can gradually straighten up with increasing distension of the colorectum. This recruitment of collagen fibers agrees with the tension-stretch ratio relations recorded from the mucosal/submucosal composite which shows increased mechanical stiffness with deformation. Under noxious distension, the collagen fibers in the submucosa straighten up to reveal two principal families of fibers that orient approximately plus and minus 30 degrees from the axial direction, respectively (Siri et al. 2019a). The two families of fibers appear to not run in parallel planes but interweave with one another to form a reinforced network of collagen fibers. In addition, the collagen fiber network in the submucosa does not seem to vary significantly in thickness, fiber density or fiber diameter from proximal to distal colorectum (Siri et al. 2019b). This likely contributes to the consistent axial tension stiffness despite the significant increase in colorectal wall thickness from colonic to rectal regions. Consistent observations between the microscopic collagen fiber network and bulk mechanical properties from biaxial tensile tests strongly indicate that the submucosa is the load-bearing ‘skeleton’ for the colorectum and protects it from excessive distension.
The serosal layer as a connective tissue membrane is also rich in collagen but its contribution to the macroscale mechanical strength of the colorectum is limited by its thinness (Siri et al. 2019a). The circular muscular and longitudinal muscular layers are low in collagen fiber contents but are significantly thicker than the serosa (Feng et al. 2019). Thus, serosal and muscular layers are likely to have comparable contributions to the mechanical strength of the outer muscular/serosal composite, which shows comparable axial and circumferential stiffness as the inner mucosal/submucosal composite (Siri et al. 2019b). The collagen fiber orientations in the two muscular layers are well aligned with the muscle fiber directions, i.e., longitudinal and circumferential, respectively. Those two families of collagen fibers perpendicular to one another collectively lead to the reduced in-plane tissue anisotropy in the outer muscular/serosal composite as compared with more pronounced tissue anisotropy in the inner mucosal/submucosal composite (Siri et al. 2019b).
The collagen fibers in the mucosal layers appear to wrap around individual colonic crypts (Fig. 2B), which are invaginated tube-like structures in the mucosa. Collagen fibers there do not seem to form an in-plane network like their counterpart in the submucosa, and thus are unlikely to play a significant load-bearing role to resist colorectal distension (Feng et al. 2019). However, mucosal collagen fibers likely provide structural support of the crypt structures which are innervated by afferent endings (Spencer et al. 2014). Collagen fibers in the mucosa likely contribute to colorectal mechanotransduction by translating mucosal shearing into local mechanical stress/strain around the crypts.
Putative nociceptors that detect concentrations of mechanical stress in the colorectum
To effectively detect injurious colorectal distension, it is reasonable to assume that the nociceptive nerve endings are strategically located at the concentrations of mechanical stress in the colorectum. Overall, the colorectum is stiffer in the axial direction than in the circumferential direction, and the axial stiffness is higher in the inner mucosal/submucosal composite than in the outer muscular/serosal composite. Micromechanics of the colorectal wall reveals concentrated collagen fiber network in the submucosa. These pieces of evidence strongly indicate concentrations of axial mechanical stress in the submucosa during noxious colorectal distension. In turn, nociceptive endings are likely present in the submucosa to detect axial mechanical stresses, which remains to be validated by further studies. In addition, more focused studies are required to systematically characterize the encoding of axial stretch by LSN and PN colorectal afferents, which are potential nociceptors to detect axial overstretch of the colorectum.
THE ION CHANNELS IN MECHANOTRANSDUCTION OF THE COLORECTUM
The colorectal biomechanics determines the microscale mechanical stress/strain around individual afferent nerve endings in the colorectum. The key process of colorectal mechanotransduction is the generation of action potentials by the local stress/strain around the afferent endings, which requires the opening of putative mechanosensitive ion channels to depolarize the ending membrane. Thus, mechanosensitive ion channels are central to this biophysical process of converting mechanical stress/strain into electrical membrane depolarization/repolarization. Mechanosensitive ion channels in neuronal and non-neuronal systems have been extensively summarized by previous reviews, for example (Xiao and Xu 2010). Here, we will narrow our focus on channels that are directly gated by mechanical forces and studied in the GI tract. Channels that play contributing roles to colorectal mechanotransduction but are indirectly modulated by mechanical forces are excluded from this review, e.g., TRPV1, TRPA1, and P2X3.
Transient receptor potential (TRP) channel families
TRP channels are non-selective cation channels that are usually activated by endogeneous and exogeneous molecules. Among them, TRPV4 and TRPC1 are prominently gated by mechanical stimuli, and both are present in afferent neurons.
TRPV4 is expressed in 38% of gastro-oesophageal vagal neurons, 65–76% of splanchnic colonic DRG neurons, 58% of pelvic colonic DRG neurons (Brierley 2010) and also in brush-bordered epithelial cells (Cenac et al. 2008). TRPV4 is mechanosensitive as evidenced by agonist and genetic knockout studies on a colorectum-nerve preparation (Brierley et al. 2008). McGuire subsequently provides compelling evidence for the regulation of serosal units by manipulation of TRPV4 from human bowel, which is consistent with rodent literature (McGuire et al. 2018). Activation of TRPV4 by mechanical stretch is revealed using in vitro expression system of Xenopus oocytes, indicating robust response of TRPV4 channels to membrane suction (Loukin et al. 2010). Desensitization of mouse muscle fibers resulting from TRPV4 gene deletion further supports the mechanosensitivity of TRPV4 channel (Ho et al. 2012).
The gating of TRPV4 channels is modulated by protease-activated receptor 2 (PAR2) in the GI tract. Agonist-induced TRPV4 currents in dorsal root ganglion (DRG) neurons, i.e., the afferent somata are enhanced by the treatment of PAR2 agonist, while PAR2 agonist-induced sensitization is absent in TRPV4-deficient DRG neurons (Cenac et al. 2008; Grant et al. 2007; Sipe et al. 2008). Intracellular signaling pathways regarding the coupling of PAR2 with TRPV4 are more recently defined (Darby et al. 2018; Grace et al. 2014; Poole et al. 2013; Cenac et al. 2015; Sostegni et al. 2015). In human embryonic kidney cells expressing TRPV1, the activation of PAR2 leads to the synthesis of 5,6-EET, an agonist of TRPV4 (Poole et al. 2013), which is enriched in the biopsies from IBS patients (Cenac et al. 2015). In addition, tyrosine-dependent phosphorylation of TRPV4 is mediated by PAR2 through the key residue (TRPV4-Tyr-110) (Grace et al. 2014; Poole et al. 2013). Pho-kinase (Sostegni et al. 2015) and two kinases MAPK13 and WNK4 (Darby et al. 2018) are recently identified to play critical roles for PAR2-dependent sensitization of TRPV4. Thus, TRPV4 can play critical roles in both normal colorectal mechanotransduction and mechano-nociception following PAR2-mediated sensitization.
TRPC1 is a fundamental component in the body’s mechanotransduction to pressure, light touch, or tissue stretch. TRPC1 is widely present in the enteric nervous system, and mainly distributed in neurons in the myenteric plexus that are cholinergic, nitrergic and positive for calretinin, as well as in secretomotor neurons in the submucosal plexus of guinea pigs (Liu et al. 2008). TRPC1 is also expressed in the DRG and appears to play critical roles in murine afferent mechanotransduction: suppression of TRPC1 leads to reduced responses to mechanical stimuli (Staaf et al. 2009), and TRPC1 gene deletion decreases the response of saphenous nerve to innocuous stimuli and behavioral response to light touch by nearly 50% (Garrison et al. 2012). However, the exact role of TRPC1 on afferent encoding of colorectal mechanical stimuli remains unexplored in the literature.
The mechanotransduction of TRPC1 is extensively studied in other tissues. Activation of TRPC1 by pancreatic tissue pressure leads to influx of Ca2+ from extracellular space, which is the prerequisite for the migration of murine pancreatic stellate cells (Fels et al. 2016). In human bronchial epithelia cells, silencing of TRPC1 via siRNA suppresses the influx of Ca2+ initiated by stretch force. TRPC1 is crucial to pressure-mediated response in airway as asthmatic condition enhances the expression of TRPC1 (Li et al. 2019). TRPC1 also plays pivotal roles in guiding axon outgrowth of Xenopus spinal cord via TRPC1-dependent Ca2+ influx (Kerstein et al. 2013).
Degenerin / epithelial sodium channels (DEG/ENaC).
Functions for DEG/ENaC channels have been implicated in mechanotransduction as well as chemosensory transduction (see (Ben-Shahar 2011) for a detailed review). The essential mechanosensitive role of DEG/ENaC is demonstrated in C. elegans, in which two of the 15 identified genes that form the mechanosensory protein complex (mec-4 and mec-10) are from the DEG/ENaC family (Goodman and Schwarz 2003; Cueva et al. 2007; Brown et al. 2007). In Drosophila, DEG/ENaC channels are expressed in class IV mechano-nociceptive sensory neurons and play critical roles to the sensation of harsh mechanical stimuli (Tracey Jr et al. 2003). In contrast, the mechanosensitive roles of DEG/ENaC channels in mammals are less clearly demonstrated. For mechanotransduction in mammalian GI tract, ASIC3 appears to be the only channel in the DEG/ENaC family that plays critical roles in colorectal mechanotransduction. In contrast, genetic deletion of ASIC1a or 2 channels either has no effect or increased effect on colorectal mechanotransduction (Page et al. 2005).
ASIC3 is a mammalian homologue of mechanotransducer mec-4/mec-10 in C. elegans, and its mechanical gating can be similarly described by a tether model (Cheng et al. 2018). The conceptual tether model for mechanical transduction likely incorporates not only ASIC3 channels, but also extracellular matrix, membrane-associated protein, and cytoskeleton. This is supported by the finding that interaction between membrane protein stomatin/STMOL3 with ASCI2 or ASIC3 significantly modulates mechanosensitivity of mouse skin nociceptors (Moshourab et al. 2013). The expression of ASIC3 is almost exclusively in sensory afferents (Holzer 2015), in 48.7% of large-diameter DRG neurons and 39.2% of small-diameter DRG neurons of mice (Lin et al. 2016). In addition, ASIC3 expression is concentrated in mouse colorectal DRG neurons: up to 73% of colorectal DRG neurons in the LSN innervation are positive for ASIC3 (Hughes et al. 2007; Christianson et al. 2006). Moreover, increased ASIC3 is found in whole intestine and separated muscular and mucosal layers, as well as myenteric and submucosal plexuses of patients with inflammatory bowel disease (Yiangou et al. 2001). Genetic deletion of ASIC3 in mice attenuates mechanosensitivity of GI afferents in both thoracolumbar (Page et al. 2005) and lumbosacral pathways (Jones et al. 2005) as well as zymosan-induced behavioral hypersensitivity (Jones et al. 2007).
Piezo channel family
The Piezo channels were first discovered by Coste et al. in 2010 as non-selective cation channels that are gated by membrane mechanical stretch and blocked by ruthenium red and gadolinium, two known inhibitors of many mechanosensitive channels (Coste et al. 2010). Piezo channels are evolutionarily conserved in most eukaryotic organisms, suggestive of its role in mechanotransduction critical for homeostasis and survival (Xiao and Xu 2010). Piezo1 and Piezo2 are found in multiple tissues in mammals, including the colon.
Piezo1 almost has no expression in sensory afferents but is widely expressed in the enteric nervous system in the GI tract of guinea pigs, mice and humans (Mazzuoli-Weber et al. 2018). Particularly, 50%–80% somata in myenteric plexus express Piezo1, most of which co-express nitric oxide synthase and to a less extent choline acetyltransferase. In the submucosal plexus, 15%–35% somata are immune-positive to Piezo1, and they mostly co-localize with vasoactive intestinal peptide. Functional studies with voltage-sensitive dies on enteric neural somata indicate that 38–78% of mechanosensitive neurons express Piezo1. However, mechanotransduction of enteric neural somata is not affected by pharmacological application of either Piezo1 agonist or antagonist, suggesting no major contributions of Piezo1 to enteric mechanotransduction (Mazzuoli-Weber et al. 2018).
Piezo2 is, quite the opposite, enriched in DRG neurons but absent in enteric neural somata. Piezo2 is widely expressed in rodent DRG neurons including small-diameter unmyelinated ones (putative nociceptors), suggesting its role in mechano-nociception (Bagriantsev et al. 2014). This is supported by a behavioral assay on rats with noxious colorectal distension reported by Yang el al. (Yang et al. 2016), which indicates the necessary role of Piezo2 for behavioral visceral hypersensitivity in rats receiving neonatal colonic treatment of acetic acid. Besides expression in DRG, Piezo2 also exists in human and mouse enterochromaffin cells of GI epithelium which modulates the secretion of serotonin in response to mechanical forces (Wang et al. 2017; Alcaino et al. 2018). As expected, genetic deletion of Piezo2 or application of Piezo2 antagonist inhibits the release of serotonin by stretch force, which indirectly affects the mechanotransduction via serotonin-sensitive colorectal afferents. The above evidence in the literature collectively suggests that Piezo2 plays critical roles in colorectal mechanotransduction and mechano-nociception.
Two-pore domain potassium (K2p) channels
The K2p channels belong to the potassium channel superfamily and are formed by heterodimers of K2p channel subunits, which consist of four transmembrane domains, two re-entrant pore-forming loop, and intracellular amino-and carboxyl-termini. Unlike many other voltage-gated potassium channels, the open probability of K2p channels is not prominently gated by transmembrane voltage, but rather by chemical ligands and physical stimuli like mechanical membrane stretch. Different from the aforementioned mechanosensitive channels that are either selective to sodium or nonselective to cations, K2p channels are selective to potassium to cause membrane hyperpolarize when open, and likely to function as a ‘brake’ to attenuate afferent spiking (Lesage and Lazdunski 2000). Three K2p channel subtypes (TREK-1, TREK-2 and TRAAK) are widely present in extrinsic afferents innervating mouse colorectum, which are expressed by 62% and 83% of colorectal DRG neurons in the LSN and PN pathways, respectively. Specifically, TREK-1, TREK-2, and TRAAK are expressed in 42%, 36%, and 37% of LSN colonic DRG neurons and 73%, 42%, and 43% of PN DRG neurons, respectively (La and Gebhart 2011). Further, both expression and stretch-activated outward currents of the K2p channels in colorectal DRG neurons are decreased following colorectal inflammation, indicating reduced inhibition by the K2p channels that can lead to sensitized mechanotransduction in colitis-like conditions (La and Gebhart 2011). Recently, Ma et al., reported abundant expression of TREK-1 in the longitudinal and circular smooth muscles in mouse ileum and colon, while TREK-2 and TRAAK were detected exclusively in enteric neurons but not smooth muscles (Ma et al. 2018). K2p channels appear to participate in regulating colonic motilities as agonist activation of K2p channels effectively relaxes colonic smooth muscle tone (Ma et al. 2018). In sum, TREK-1, TREK-2 and TRAAK are mechanosensitive channels that may play critical roles in colorectal nociception via a disinhibition mechanism.
SUMMARY AND CONCLUSIONS
Visceral pain arising from the colorectum has distinct psychophysical characteristics as compared to pain arising from the skin, which reflects the vast different environment in the intestinal lumen versus outside the body. Stimulus modalities like heat, cold or inflammation that are noxious to the skin are not adequately noxious to the colorectum. It is mechanical stimuli, especially luminal distension that are adequately noxious and reliably evoke visceral pain from the colorectum. Mechanotransduction that encodes mechanical colorectal stimuli into trains of action potentials is undertaken by extrinsic colorectal afferents from the lumbar splanchnic and pelvic nerve innervations, which are mostly unmyelinated free nerve endings with varicosity, hallmarks of C-fiber nociceptors. The lumbar splanchnic innervation is concentrated at regions in the colorectum next to the mesenteric attachment, and consists of mostly HTh.prob afferents that encode high-threshold mechanical probing, mesenteric afferents, and mechanically-insensitive afferents. Thus, LSN afferents likely subserve nociceptive roles in detecting catastrophic mechanical failure of the colorectum. In contrast, the pelvic innervation spreads throughout the circumferential locations and is present in both colonic and rectal regions. PN afferents consist of afferents that encode high-threshold mechanical probing as well as low-intensity mucosal shearing, indicative of their dual roles in visceral nociception and encoding of normal, innocuous physiological events. Recent advances in the literature have revealed the macro-and microscale mechanical properties of the colorectum, which determine the transmission of bulk colorectal mechanical deformation into micromechanical stress/strain around individual afferent endings in the colorectum. The colorectum is significantly stiffer in the axial direction than in the circumferential direction, and the axial stress is likely undertaken by the submucosa that comprises a dense network of collagen fibers. Thus, submucosa is probably the region with concentrations of axial mechanical stress during noxious colorectal distension, which calls for further focused research on putative nociceptive endings in the submucosa and colorectal afferents that encode axial stretch. The mechanosensitive ion channels on colorectal afferent membrane are crucial for the generation of action potentials by depolarizing the membrane potential. Among the four families of mammalian mechanosensitive ion channels (TRP, DEG/ENaC, Piezo, and K2p), the following channels appear to directly contribute to mechanotransduction of colorectal afferents: TRPV4, TRPC1, ASIC3, Piezo2, TREK-1, TREK-2, and TRAAK.
Recent reports on colorectal biomechanics nicely complement our prior neurophysiological knowledge on colorectal afferent encoding and mechanosensitive ion channels, which have synergistically advanced our mechanistic understanding of colorectal mechanotransduction. Further research is warranted to reveal the neural encoding of submucosal afferents to axial stretch and the micromechanical coupling between the mechanosensitive ion channels and the micromechanical environment, which will likely generate new targets for better strategies to manage GI-related visceral pain.
Acknowledgements:
supported by grants from the U.S. National Science Foundation #1727185 and #1844762 to Dr. Feng
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors claim no conflict of interests.
REFERENCES
- Alcaino C, Knutson K, Treichel A, Yildiz G, Strege P, Linden D, Li J, Leiter A, Szurszewski J, Farrugia G, Beyder A (2018) A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA 115 (32):E7632–E7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR, Sanger GJ (2002) Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Current Opinion in Pharmacology 2 (6):650–656. doi: 10.1016/S1471-4892(02)00227-8 [DOI] [PubMed] [Google Scholar]
- Annahazi A, Gecse K, Dabek M, it-Belgnaoui A, Rosztoczy A, Roka R, Molnar T, Theodorou V, Wittmann T, Bueno L, Eutamene H (2009) Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 144 (1–2):209–217 [DOI] [PubMed] [Google Scholar]
- Bagriantsev SN, Gracheva EO, Gallagher PG (2014) Piezo proteins: regulators of mechanosensation and other cellular processes. Journal of biological Chemistry 289 (46):31673–31681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y (2011) Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Advances in genetics 76:1–26. doi: 10.1016/B978-0-12-386481-9.00001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA (1996) Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 66 (2–3):151–161 [DOI] [PubMed] [Google Scholar]
- Birk JW, Tadros M, Moezardalan K, Nadyarnykh O, Forouhar F, Anderson J, Campagnola P (2014) Second harmonic generation imaging distinguishes both high-grade dysplasia and cancer from normal colonic mucosa. Dig Dis Sci 59 (7):1529–1534. doi: 10.1007/s10620-014-3121-7 [DOI] [PubMed] [Google Scholar]
- Bouin M, Delvaux M, Blanc C, Lagier E, Delisle MB, Fioramonti J, Bueno L, Frexinos J (2001) Intrarectal injection of glycerol induces hypersensitivity to rectal distension in healthy subjects without modifying rectal compliance. EurJ Gastroenterol Hepatol 13 (5):573–580 [DOI] [PubMed] [Google Scholar]
- Brierley SM (2010) Molecular basis of mechanosensitivity. Autonomic Neuroscience 153 (1):58–68. doi: 10.1016/j.autneu.2009.07.017 [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA (2005a) Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. Journal of Physiology 567 (Pt 1):267–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Hibberd TJ, Spencer NJ (2018) Spinal Afferent Innervation of the Colon and Rectum. Frontiers in Cellular Neuroscience 12 (467). doi: 10.3389/fncel.2018.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA (2004) Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127 (1):166–178. doi: 10.1053/j.gastro.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC III, Xu L, Gebhart GF, Blackshaw LA (2005b) Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil 17 (6):854–862 [DOI] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA (2008) Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134 (7):2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Fernandez-Illescas SM, Liao Z, Goodman MB (2007) Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. The Journal of general physiology 129 (2):161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer DC, Roza C Visceral Pain In: The Oxford Handbook of the Neurobiology of Pain. [Google Scholar]
- Burgess PT, Perl E (1973) Cutaneous mechanoreceptors and nociceptors In: Somatosensory system. Springer, pp 29–78 [Google Scholar]
- Carniel E, Gramigna V, Fontanella C, Frigo A, Stefanini C, Rubini A, Natali A (2014a) Characterization of the anisotropic mechanical behaviour of colonic tissues: experimental activity and constitutive formulation. Experimental physiology 99 (5):759–771 [DOI] [PubMed] [Google Scholar]
- Carniel EL, Gramigna V, Fontanella CG, Stefanini C, Natali AN (2014b) Constitutive formulations for the mechanical investigation of colonic tissues. J Biomed Mater Res A 102 (5):1243–1254. doi: 10.1002/jbm.a.34787 [DOI] [PubMed] [Google Scholar]
- Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N (2008) Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135 (3):937–946, 946 [DOI] [PubMed] [Google Scholar]
- Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, Bertrand J, Liedtke W, Dubourdeau M, Bertrand-Michel J, Zecchi L, Stanghellini V, Bunnett NW, Barbara G, Vergnolle N (2015) Quantification and Potential Functions of Endogenous Agonists of Transient Receptor Potential Channels in Patients With Irritable Bowel Syndrome. Gastroenterology 149 (2):433–444.e437. doi: 10.1053/j.gastro.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Cervero F (1994) Sensory innervation of the viscera: peripheral basis of visceral pain. Physiological Reviews 74 (1):95–138. doi: 10.1152/physrev.1994.74.1.95 [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM (2004) Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol 61 (1):45–54. doi: 10.1002/neu.20084 [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JMA (1999) Visceral pain. The Lancet 353 (9170):2145–2148. doi: 10.1016/S0140-6736(99)01306-9 [DOI] [PubMed] [Google Scholar]
- Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K (2000) Perceptual responses in patients with inflammatory and functional bowel disease. Gut 47 (4):497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ (2012) Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nature protocols 7 (4):654–669. doi: 10.1038/nprot.2012.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Jiang B, Chen C (2018) Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing. J Biomed Sci 25 (1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM (2006) Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol 494 (2):246–259 [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science [DOI] [PMC free article] [PubMed]
- Cueva JG, Mulholland A, Goodman MB (2007) Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J Neurosci 27 (51):14089–14098. doi: 10.1523/jneurosci.4179-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby W, Grace M, Simpson K, Woodman O, McIntyre P (2018) A Functional Kinase Short Interfering Ribonucleic Acid Screen Using Protease-Activated Receptor 2-Dependent Opening of Transient Receptor Potential Vanilloid-4. Assay Drug Dev Technol 16 (1):15–26 [DOI] [PubMed] [Google Scholar]
- Falt P, Šmajstrla V, Fojtík P, Tvrdík J, Urban O (2013) Cool water vs warm water immersion for minimal sedation colonoscopy: a double-blind randomized trial. Colorectal Disease 15 (10):e612–e617. doi: 10.1111/codi.12336 [DOI] [PubMed] [Google Scholar]
- Fels B, Nielsen N, Schwab A (2016) Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J 45 (7):657–670 [DOI] [PubMed] [Google Scholar]
- Feng B, Brumovsky PR, Gebhart GF (2010) Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298 (3):G402–409. doi: 10.1152/ajpgi.00487.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Gebhart GF (2011) Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300 (1):G170–180. doi: 10.1152/ajpgi.00406.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Gebhart GF (2015) In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp (95):52310. doi: 10.3791/52310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Joyce SC, Gebhart GF (2016) Optogenetic activation of mechanically insensitive afferents in mouse colorectum reveals chemosensitivity. Am J Physiol Gastrointest Liver Physiol 310 (10):G790–798. doi: 10.1152/ajpgi.00430.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, La JH, Schwartz ES, Gebhart GF (2012a) Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302 (10):G1085–1098. doi: 10.1152/ajpgi.00542.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF (2012b) Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302 (7):G676–683. doi: 10.1152/ajpgi.00490.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF (2012c) Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303 (7):G817–824. doi: 10.1152/ajpgi.00257.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Maier F, Siri S, Pierce DM (2019) Quantifying the Collagen-Network Morphology in Mouse Distal Colon and Rectum. Paper presented at the Proceedings of the Biomedical Engineering Society 2019 Annual Fall Meeting, Philadelphia, Oct 16–19 [Google Scholar]
- Feng B, Zhu Y, La JH, Wills ZP, Gebhart GF (2015) Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol 113 (7):2618–2634. doi: 10.1152/jn.00717.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer JB, Andersen SD, Drewes AM, Gregersen H (2006) Ultrasound-determined geometric and biomechanical properties of the human duodenum. Dig Dis Sci 51 (9):1662–1669. doi: 10.1007/s10620-005-9015-y [DOI] [PubMed] [Google Scholar]
- Furness JB (2006) Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. AutonNeurosci 125 (1–2):81–85 [DOI] [PubMed] [Google Scholar]
- Gabella G (1983) The collagen fibrils in the collapsed and the chronically stretched intestinal wall. Journal of ultrastructure research 85 (2):127–138 [DOI] [PubMed] [Google Scholar]
- Garrison S, Dietrich A, Stucky C (2012) TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol 107 (3):913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF (2000) Visceral pain-peripheral sensitisation. [Review] [6 refs]. Gut 47 Suppl 4:iv54–iv55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF, Feng B (2013) Sensitization of Visceral Nociceptors In: Gebhart GF, Schmidt RF (eds) Encyclopedia of Pain. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 3464–3468. doi: 10.1007/978-3-642-28753-4_3937 [DOI] [Google Scholar]
- Gold MS, Gebhart GF (2010) Nociceptor sensitization in pain pathogenesis. Nat Med 16 (11):1248–1257. doi: 10.1038/nm.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Schwarz EM (2003) Transducing touch in Caenorhabditis elegans. Annual review of physiology 65 (1):429–452 [DOI] [PubMed] [Google Scholar]
- Grace M, Lieu T, Darby B, Abogadie F, Veldhuis N, Bunnett N, McIntyre P (2014) The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br J Pharmacol 171 (16):3881–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578 (Pt 3):715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald HP, Bonica JJ, Bergner M (1987) The prevalence of pain in four cancers. Cancer 60 (10):2563–2569. doi: [DOI] [PubMed] [Google Scholar]
- Hariton I, deBotton G, Gasser TC, Holzapfel GA (2007) Stress-driven collagen fiber remodeling in arterial walls. Biomechanics and Modeling in Mechanobiology 6 (3):163–175. doi: 10.1007/s10237-006-0049-7 [DOI] [PubMed] [Google Scholar]
- Herrity AN, Rau KK, Petruska JC, Stirling DP, Hubscher CH (2014) Identification of bladder and colon afferents in the nodose ganglia of male rats. Journal of Comparative Neurology 522 (16):3667–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB (2012) Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 6 (4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P (2015) Acid-sensing ion channels in gastrointestinal function. Neuropharmacology 94:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Brierley S, Young R, Blackshaw L (2007) Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 500 (5):863–875 [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA (2009) Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58 (10):1333–1341. doi: 10.1136/gut.2008.170811 [DOI] [PubMed] [Google Scholar]
- Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, Andrews JM, Holtmann G, Blackshaw LA, Brierley SM (2013) Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62 (10):1456–1465. doi: 10.1136/gutjnl-2011-301856 [DOI] [PubMed] [Google Scholar]
- Hulmes DJ (2002) Building collagen molecules, fibrils, and suprafibrillar structures. Journal of structural biology 137 (1–2):2–10 [DOI] [PubMed] [Google Scholar]
- Jones RC III, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF (2007) Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133 (1):184–194 [DOI] [PubMed] [Google Scholar]
- Jones RC III, Xu L, Gebhart GF (2005) The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25 (47):10981–10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleem B, Maier F, Drissi H, Pierce DM (2017) Low-energy impact of human cartilage: predictors for microcracking the network of collagen. Osteoarthritis Cartilage 25 (4):544–553. doi: 10.1016/j.joca.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI (2011) Human nutrition, the gut microbiome and the immune system. Nature 474:327. doi:10.1038/nature1021310.1038/nature10213https://www.nature.com/articles/nature10213#supplementary-informationhttps://www.nature.com/articles/nature10213#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsen DP, Portenoy RK, Thaler HT, Niedzwiecki D, Passik SD, Tao Y, Banks W, Brennan MF, Foley KM (1995) Pain and depression in patients with newly diagnosed pancreas cancer. J Clin Oncol 13 (3):748–755. doi: 10.1200/jco.1995.13.3.748 [DOI] [PubMed] [Google Scholar]
- Ker RF (1999) The design of soft collagenous load-bearing tissues. Journal of Experimental Biology 202 (23):3315–3324 [DOI] [PubMed] [Google Scholar]
- Kerstein PC, Jacques-Fricke BT, Rengifo J, Mogen BJ, Williams JC, Gottlieb PA, Sachs F, Gomez TM (2013) Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J Neurosci 33 (1):273–285. doi: 10.1523/jneurosci.2142-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin ME, Feng B, Schwartz ES, Gebhart GF (2013) Combined genetic and pharmacological inhibition of TRPV1 and P2X3 attenuates colorectal hypersensitivity and afferent sensitization. Am J Physiol Gastrointest Liver Physiol 305 (9):G638–648. doi: 10.1152/ajpgi.00180.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gronhaug KM, Romijn EI, Finnoy A, Davies CL, Drogset JO, Lilledahl MB (2015) Polarization second harmonic generation microscopy provides quantitative enhanced molecular specificity for tissue diagnostics. J Biophotonics 8 (9):730–739. doi: 10.1002/jbio.201400086 [DOI] [PubMed] [Google Scholar]
- La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF (2012) Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 303 (7):G802–809. doi: 10.1152/ajpgi.00259.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La JH, Gebhart GF (2011) Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am J Physiol GastrointestLiver Physiol 301 (1):G165–G174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M (2000) Molecular and functional properties of two-pore-domain potassium channels. American Journal of Physiology-Renal Physiology 279 (5):F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793 [DOI] [PubMed] [Google Scholar]
- Leung PS, editor (2014) The gastrointestinal system : gastrointestinal, nutritional, and hepatobiliary physiology. Dordrecht: : Springer, Netherlands [Google Scholar]
- Lewis T (1942) Pain. Macmillan, London [Google Scholar]
- Li N, He Y, Yang G, Yu Q, Li M (2019) Role of TRPC1 channels in pressure-mediated activation of airway remodeling. Respiratory Research 20 (1):91. doi: 10.1186/s12931-019-1050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Fan Y, Zeng Y, Gregersen H (2003) Stress distribution in the layered wall of the rat oesophagus. Med Eng Phys 25 (9):731–738 [DOI] [PubMed] [Google Scholar]
- Liao D, Zhao J, Fan Y, Gregersen H (2004) Two-layered quasi-3D finite element model of the oesophagus. Med Eng Phys 26 (7):535–543. doi: 10.1016/j.medengphy.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Lin S, Cheng Y, Banks R, Min M, Bewick G, Chen C (2016) Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 7:11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Qu M, Ren W, Hu H, Gao N, Wang G, Wang X, Fei G, Zuo F, Xia Y, Wood J (2008) Differential expression of canonical (classical) transient receptor potential channels in guinea pig enteric nervous system. J Comp Neurol 511 (6):847–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C (2010) Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem 285 (35):27176–27181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvel D, Delvaux M, Staumont G, Camman F, Fioramonti J, Bueno L, Frexinos J (1996) Intracolonic injection of glycerol: a model for abdominal pain in irritable bowel syndrome? Gastroenterology 110 (2):351–361 [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao J, Gregersen H (2005) Small intestinal morphometric and biomechanical changes during physiological growth in rats. J Biomech 38 (3):417–426. doi: 10.1016/j.jbiomech.2004.04.025 [DOI] [PubMed] [Google Scholar]
- Ma R, Seifi M, Papanikolaou M, Brown J, Swinny J, Lewis A (2018) TREK-1 Channel Expression in Smooth Muscle as a Target for Regulating Murine Intestinal Contractility: Therapeutic Implications for Motility Disorders. Front Physiol 9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta PJ, Graziadei IW, Ghent CN (2000) Muscle cramps: a ‘complication’ of cirrhosis. Can J Gastroenterol 14 Suppl D:21D–25D. doi: 10.1155/2000/214916 [DOI] [PubMed] [Google Scholar]
- Mazzuoli-Weber G, Kugler E, Bühler C, Kreutz F, Demir I, Ceyhan O, Zeller F, Schemann M (2018) Piezo proteins: incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res [DOI] [PubMed]
- McGuire C, Boundouki G, Hockley JRF, Reed D, Cibert-Goton V, Peiris M, Kung V, Broad J, Aziz Q, Chan C, Ahmed S, Thaha MA, Sanger GJ, Blackshaw LA, Knowles CH, Bulmer DC (2018) Ex vivo study of human visceral nociceptors. Gut 67 (1):86–96. doi: 10.1136/gutjnl-2016-311629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K (1996) Chapter 17. Functional morphology of nociceptive and other fine sensory endings (free nerve endings) in different tissues In: Kumazawa T, Kruger L, Mizumura K (eds) Progress in Brain Research, vol 113 Elsevier, pp 273–298. doi: 10.1016/S0079-6123(08)61094-8 [DOI] [PubMed] [Google Scholar]
- Miranda A (2018) 10-Abdominal Pain In: Kliegman RM, Lye PS, Bordini BJ, Toth H, Basel D (eds) Nelson Pediatric Symptom-Based Diagnosis. Elsevier, pp 161–181.e162. doi: 10.1016/B978-0-323-39956-2.00010-8 [DOI] [Google Scholar]
- Moshourab R, Wetzel C, Martinez-Salgado C, Lewin G (2013) Stomatin-domain protein interactions with acid-sensing ion channels modulate nociceptor mechanosensitivity. J Physiol (Lond) 591 (22):5555–5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali AN, Carniel EL, Gregersen H (2009) Biomechanical behaviour of oesophageal tissues: material and structural configuration, experimental data and constitutive analysis. Med Eng Phys 31 (9):1056–1062. doi: 10.1016/j.medengphy.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF (1988) Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 450 (1–2):153–169 [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF (1990) Visceral pain: a review of experimental studies. [Review] [503 refs]. Pain 41 (2):167–234 [DOI] [PubMed] [Google Scholar]
- Ness TJ, Metcalf AM, Gebhart GF (1990) A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 43 (3):377–386 [DOI] [PubMed] [Google Scholar]
- Orberg J, Baer E, Hiltner A (1983) Organization of collagen fibers in the intestine. Connective tissue research 11 (4):285–297 [DOI] [PubMed] [Google Scholar]
- Orberg J, Klein L, Hiltner A (1982) Scanning electron microscopy of collagen fibers in intestine. Connective tissue research 9 (3):187–193 [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA (2005) Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54 (10):1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha PJ, Willis WD, Gebhart GF (2006) Chronic abdominal and visceral pain: theory and practice. Taylor & Francis US, [Google Scholar]
- Perl ER (2007) Ideas about pain, a historical view. Nature reviews Neuroscience 8 (1):71–80. doi: 10.1038/nrn2042 [DOI] [PubMed] [Google Scholar]
- Poole D, Amadesi S, Veldhuis N, Abogadie F, Lieu T, Darby W, Liedtke W, Lew M, McIntyre P, Bunnett N (2013) Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem 288 (8):5790–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihsner R, Menzel EJ (1998) Two-dimensional stress-relaxation behavior of human skin as influenced by non-enzymatic glycation and the inhibitory agent aminoguanidine. Journal of Biomechanics 31 (11):985–993. doi: 10.1016/S0021-9290(98)00088-8 [DOI] [PubMed] [Google Scholar]
- Reynders A, Mantilleri A, Malapert P, Rialle S, Nidelet S, Laffray S, Beurrier C, Bourinet E, Moqrich A (2015) Transcriptional Profiling of Cutaneous MRGPRD Free Nerve Endings and C-LTMRs. Cell Reports 10 (6):1007–1019. doi: 10.1016/j.celrep.2015.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Emery N, Neu CP, Pierce DM (2019) Propagation of microcracks in collagen networks of cartilage under mechanical loads. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2019.04.017 [DOI] [PubMed]
- Sengupta JN, Gebhart GF (1994) Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71 (6):2046–2060 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF (2009) Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137 (6):2096–2104. doi: 10.1053/j.gastro.2009.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW (2008) Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol GastrointestLiver Physiol 294 (5):G1288–G1298 [DOI] [PubMed] [Google Scholar]
- Siri S, Maier F, Chen L, Santos S, Pierce DM, Feng B (2019a) Differential biomechanical properties of mouse distal colon and rectum innervated by the splanchnic and pelvic afferents. Am J Physiol Gastrointest Liver Physiol 316 (4):G473–G481. doi: 10.1152/ajpgi.00324.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri S, Maier F, Santos S, Pierce DM, Feng B (2019b) The load-bearing function of the colorectal submucosa and its relevance to visceral nociception elicited by mechanical stretch. Am J Physiol Gastrointest Liver Physiol. doi: 10.1152/ajpgi.00127.2019 [DOI] [PMC free article] [PubMed]
- Sokolis DP, Orfanidis IK, Peroulis M (2011) Biomechanical testing and material characterization for the rat large intestine: regional dependence of material parameters. Physiol Meas 32 (12):1969–1982. doi: 10.1088/0967-3334/32/12/007 [DOI] [PubMed] [Google Scholar]
- Sokolis DP, Sassani SG (2013) Microstructure-based constitutive modeling for the large intestine validated by histological observations. J Mech Behav Biomed Mater 21:149–166. doi: 10.1016/j.jmbbm.2013.02.016 [DOI] [PubMed] [Google Scholar]
- Sommer G, Schriefl A, Zeindlinger G, Katzensteiner A, Ainodhofer H, Saxena A, Holzapfel GA (2013) Multiaxial mechanical response and constitutive modeling of esophageal tissues: Impact on esophageal tissue engineering. Acta Biomater 9 (12):9379–9391. doi: 10.1016/j.actbio.2013.07.041 [DOI] [PubMed] [Google Scholar]
- Sostegni S, Diakov A, McIntyre P, Bunnett N, Korbmacher C, Haerteis S (2015) Sensitisation of TRPV4 by PAR2 is independent of intracellular calcium signalling and can be mediated by the biased agonist neutrophil elastase. Pflugers Arch 467 (4):687–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Kyloh M, Duffield M (2014) Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One 9 (11):e112466. doi: 10.1371/journal.pone.0112466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf S, Maxvall I, Lind U, Husmark J, Mattsson J, Ernfors P, Pierrou S (2009) Down regulation of TRPC1 by shRNA reduces mechanosensitivity in mouse dorsal root ganglion neurons in vitro. Neurosci Lett 457 (1):3–7 [DOI] [PubMed] [Google Scholar]
- Storkholm JH, Villadsen GE, Jensen SL, Gregersen H (1998) Mechanical properties and collagen content differ between isolated guinea pig duodenum, jejunum, and distal ileum. DigDisSci 43 (9):2034–2041 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF (2011) Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α–3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol 300 (3):G418–424. doi: 10.1152/ajpgi.00456.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD Jr, Wilson RI, Laurent G, Benzer S (2003) painless, a Drosophila gene essential for nociception. Cell 113 (2):261–273 [DOI] [PubMed] [Google Scholar]
- Wang F-B, Powley TL (2007) Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell and Tissue Research 329 (2):221–230. doi: 10.1007/s00441-007-0413-7 [DOI] [PubMed] [Google Scholar]
- Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G, Beyder A (2017) Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 595 (1):79–91. doi: 10.1113/jp272718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZS (2010) Mechanosensitive channels: in touch with Piezo. Current biology : CB 20 (21):R936–R938. doi: 10.1016/j.cub.2010.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liao D, Zhao J, Gregersen H (2004) Shear modulus of elasticity of the esophagus. Ann Biomed Eng 32 (9):1223–1230 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Yang H, Li K, Lei X, Xu C (2016) The potential role of Piezo2 in the mediation of visceral sensation. Neuroscience letters 630:158–163 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhao J, Liao D, Gregersen H (2006) Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech 39 (5):894–904. doi: 10.1016/j.jbiomech.2005.01.022 [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Smith J, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P (2001) Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur J Gastroenterol Hepatol 13 (8):891–896 [DOI] [PubMed] [Google Scholar]
- Yu J, Zeng Y, Zhao J, Liao D, Gregersen H (2004) Quantitative analysis of collagen fiber angle in the submucosa of small intestine. Comput Biol Med 34 (6):539–550. doi: 10.1016/j.compbiomed.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ, Spencer NJ (2010) Structure-function relationship of sensory endings in the gut and bladder. AutonNeurosci 153 (1–2):3–11 [DOI] [PubMed] [Google Scholar]
- Zeng YJ, Qiao AK, Yu JD, Zhao JB, Liao DH, Xu XH, Hans G (2003) Collagen fiber angle in the submucosa of small intestine and its application in Gastroenterology. World J Gastroenterol 9 (4):804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JB, Sha H, Zhuang FY, Gregersen H (2002) Morphological properties and residual strain along the small intestine in rats. World J Gastroenterol 8 (2):312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]