Abstract
Background
Obesity disproportionately affects more women than men. The loss of ovarian function during the menopause transition coincides with weight gain, increases in abdominal adiposity, and impaired metabolic health. Racial differences in obesity prevalence that results from the menopause transition are not well understood
Objectives
To assess longitudinal changes in body composition and cardiometabolic risk among Black and White women during the menopausal transition.
Study Design
In a secondary analysis of a prospective, observational cohort study—the Healthy Transitions study—161 women aged 43 years and older with body mass index between 20 and 40 kg/m2 and who had not yet transitioned through menopause were enrolled at Pennington Biomedical Research Center. Women were seen annually for body composition by dual-energy X-ray absorptiometry; abdominal adipose tissue distribution by computed tomography; sex steroid hormones; and cardiometabolic risk factors including fasting glucose, insulin, and lipids. Surrogate measures of insulin sensitivity were also calculated.
Results
Ninety-four women (25 Black, 69 White) transitioned through menopause and were included within the analyses. At menopause onset, Black women weighed more (77.8±3.0 vs. 70.8±1.8 kg), and had higher systolic (125±16 vs. 118±14 mmHg) and diastolic (80±8 vs. 74±7 mmHg) blood pressure compared to White women (all p≤0.05). No other differences in body composition, sex steroid hormones, or cardiometabolic risk factors were observed at menopause onset. Before menopause, White women gained significant weight (+3 kg), total body adiposity (+6% percent body fat, +9% fat mass, +12% trunk fat mass), and abdominal adipose tissue (+19% subcutaneous fat, +15% visceral fat, +19% total adipose tissue) which coincided with significant decreases in estradiol, sex hormone-binding globulin, and estrone sulfate, as well as increases in follicle-stimulating hormone, total cholesterol, and low-density lipoprotein cholesterol. Conversely, Black women had more abdominal adipose tissue before menopause, which was maintained across the menopause transition. Black women also had significant decreases in estrone sulfate and total testosterone, as well as increases in follicle-stimulating hormone before menopause. In the postmenopausal years, abdominal subcutaneous adipose tissue, total adipose tissue, follicle-stimulating hormone, total cholesterol, and low-density and high-density lipoprotein cholesterol increased only in White women.
Conclusions
White women gained more abdominal adiposity during the menopause transition compared to Black women, which may be due in part to differences in the pattern of sex steroid hormone changes between women of different racial backgrounds. The gains in abdominal adiposity in White women were observed in tandem with increased cardiometabolic risk factors. Future studies should consider comprehensive lifestyle approaches to target these increased gains in abdominal adiposity (i.e., nutrition and physical activity coaching), while also taking into account the potential interactions of race, body adiposity, sex steroid hormones, and their influence on cardiometabolic risk.
Keywords: abdominal fat, adipose tissue, hormones, menopause, race, visceral fat
INTRODUCTION
Obesity increases significantly in women after 40 years of age in the United States, with the prevalence reaching 45% between 40 and 59 years, and 43% over the age of 60.1,2 Rapid increases in body weight (adiposity) occurs in the years leading up to menopause—known as the menopause transition (or perimenopause)—when sex steroid hormones, energy balance, and body fat distribution are in flux.3 There is substantial evidence that the menopause transition is associated with a redistribution of subcutaneous fat from the gluteal/femoral depot to the abdominal depot3–7 which is commensurate with the loss of ovarian function and decreases in physical activity, energy expenditure, and fat oxidation.3 Undoubtedly, these changes predispose the estimated 2 million women that reach menopause annually in the United States to increased cardiometabolic risk including insulin resistance, type 2 diabetes, and cardiovascular disease.8–10 These observations have even led some experts to suggest that a BMI greater than 24.9 kg/m2 (rather than 30 kg/m2) may be more appropriate to denote obesity in midlife women.11,12
This growing body of research, however, lacks an understanding of the potential racial disparity in obesity prevalence across the menopause transition. In a cross-sectional analysis of women prior to menopause,13 we observed that sleeping energy expenditure, as well as consumption of protein, fiber, and other dietary nutrients, were lower in Black compared to White women. Prior to menopause, Black women also had significantly more abdominal subcutaneous adipose tissue and slightly less visceral adipose tissue compared to White women.14 Longitudinal data from the Study of Women’s Health Across the Nation (SWAN) revealed that Black and White women actually experience similar changes in body composition during the menopause transition.4 We confirmed these findings in a previously published analyses of 51 women taking part in the Healthy Transitions study and who transitioned through menopause.3 Previous studies, however, have not investigated whether changes in body composition affect cardiometabolic risk differently among Black and White women during the menopause transition.
A better understanding of how race may affect changes in abdominal adiposity is important so that clinicians can target care to help women minimize the cardiometabolic burdens observed with menopause. We hypothesize that Black women would have similar increases in abdominal adiposity (particularly abdominal subcutaneous adipose tissue and visceral adipose tissue) compared to White women, but that Black women will experience disproportionate increases in cardiometabolic risk factors (e.g., glucose, insulin, lipids, and insulin resistance) which would ultimately increase risk for developing type 2 diabetes.
MATERIALS & METHODS
Study Design
Healthy Transitions was a 4-year prospective, observational cohort study that investigated the effect of the menopause transition on obesity, energy expenditure, and insulin sensitivity [clinicaltrials.gov identifier: NCT00412269] at the Pennington Biomedical Research Center (PBRC) in Baton Rouge, Louisiana from 1998 to 2002. The study was approved by the PBRC Institutional Review Board, and participants provided written informed consent before participation. Women who had not yet transitioned through menopause were enrolled and completed annual visits during the years before menopause (premenopause and/or perimenopause) as well as during the years after menopause (postmenopause). The study was extended for an additional 3 years to continue annual visits for those women who did not transition through menopause from 2003 to 2006. Study outcomes included changes in body weight and body composition by dual-energy X-ray absorptiometry (DXA) and computed tomography (CT); sex steroid hormones; and cardiometabolic risk factors including fasting glucose, insulin, and lipids.
A subset of 80 women (40 Black, 40 White) were targeted for additional outcome measures of 24-hour energy expenditure (by respiratory chamber) at baseline, 4 years, and 6 years post-enrollment, as well as insulin sensitivity (by frequently sampled intravenous glucose tolerance test) at baseline, 4 years, and 8 years post-enrollment. Due to the small sample size and unequal race distribution of women who transitioned through menopause who had both premenopause and postmenopause measures of 24-hour energy expenditure (n=25; 4 Black, 21 White), we were unable to sufficiently explore how changes in energy expenditure differed by race. While frequently sampled intravenous glucose tolerance test data were also only available in 19 women (2 Black, 17 White), we applied commonly used surrogate measures of insulin sensitivity to our cohort.
Study Participants
Women were recruited by print and radio advertisement, as well as targeted mailings and word of mouth from the Baton Rouge, Louisiana area. Women were required to be healthy, 43 years or older, with body mass index (BMI) between 20 and 40 kg/m2, and premenopausal (i.e., have had at least 5 menstrual cycles in the last 6 months and have confirmed follicle-stimulating hormone (FSH) <30 mIU/mL15). Women were ineligible if they were taking regular medication (including oral contraceptives or other hormones), were not having regular menstrual cycles, or had clinically abnormal results on laboratory tests or physical examination including diagnosed hypertension, hypercholesterolemia, diabetes, or cardiovascular disease. Women self-reported their race and ethnic background. Potential confounders of changes in body adiposity across the menopause transition included age, as well as highest level of education and smoking status (self-reported).
A total of 161 premenopausal women (55 Black, 102 White, 2 Asian/Asian-Indian, and 2 biracial) enrolled in the study.3 Of the 157 women who were self-reported as Black or White, 94 (or 60%; 25 Black, 69 White) transitioned through menopause at some point during the study and, therefore, were included within the analyses. Of the 63 women who did not transition and were excluded from the analyses, 28 (12 Black, 16 White) dropped from the study and 35 (17 Black, 18 White) did not transition (see Supplemental Figure 1). Women were classified retrospectively as having transitioned through menopause (indicated as Year 0, or ‘menopause onset’) at the annual visit where both an absence of menstrual cycles for 1-year was reported with an FSH>30 mIU/mL. None of the women self-reported being non-Hispanic or Latino.
Study Procedures
Body composition and abdominal adipose tissue distribution
Weight was measured in the morning after an overnight fast (Scale-Tronix 5200; Scale-Tronix Inc.) while the subject wore a surgical gown, which was subtracted from the total weight. Body composition was measured by DXA (QDR 2000; Hologic, Waltham, MA) in the morning following an overnight fast. Within-subject precision of the DXA, which is considered the gold standard for quantifying body adiposity in adults,16 was excellent (coefficient of variation for percent body fat was 1.5% [SD=0.51]). Estimates of percent body fat, fat mass, and fat-free mass were measured in ~10–15 minutes while subject wore a surgical gown. Abdominal adipose tissue distribution, including abdominal subcutaneous adipose tissue (SAT), deep subcutaneous adipose tissue (deep SAT),17 superficial subcutaneous adipose tissue (superficial SAT), visceral adipose tissue (VAT), and abdominal total adipose tissue (TAT) was measured by a single 10-mm slice at the level of the interspace between the L4-L5 vertebrae by computed tomography (GE High Speed Advantage; GE Medical Systems, Milwaukee, WI) as previously described.18
Sex steroid hormones
Hormone measurements were conducted on serum following an overnight fast. Estradiol, FSH, and sex hormone-binding globulin (SHBG) were measured using immunoassay with chemiluminescent detection (Siemens; Immulite 2000). Estrone sulfate was measured using radioimmunoassay (Siemens; Diagnostic Systems Laboratories) and total testosterone was measured using radioimmunoassay (Siemens; Diagnostic Products Corporation). All sex steroid hormones were measured once during a single cycle.
Cardiometabolic risk factors
Systolic and diastolic blood pressure were measured in triplicate at rest. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured in an overnight fasted blood sample using a Beckman Synchron CX5 autoanalyzer. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation, assuming triglycerides were within normal limits. Glucose (Beckman Synchron CX7, Beckman; Brea, CA, USA) and insulin (Abbott IMx analyzer, Abbott Laboratories; Abbott Park, IL, USA) were measured, and the homeostatic model assessment-insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated as previously described.19,20 Higher HOMA-IR and lower QUICKI are indicative of increased insulin resistance. Presence (or absence) of the metabolic syndrome was also determined and classified dichotomously.21
Statistical Analysis
The primary outcome of the original cohort study was VAT, while key secondary outcomes included abdominal SAT, fat mass, and fat-free mass. We conservatively estimated that 160 women (80 Black, 80 White) would need to be enrolled in our longitudinal cohort study to ensure detectable differences in VAT in women of different racial backgrounds (Black vs. White), as well as differences between women who and did not transition through menopause, would be feasible. With this sample size, along with a conservative assumption that possibly only one-third of the 160 women (or 53 women) would transition through menopause, we calculated >95% power to detect differences in both main effects (race and menopause status) and ~60% power to detect their interactions (race*menopause status) for a difference in VAT of 25 cm2.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) with a significance level set at α=0.05. All characteristics at menopause onset (Year 0) are expressed as raw (unadjusted) means with 95% confidence intervals. Between-race comparisons were made using a two-sample t-test. A linear mixed effect repeated measure model, with a compound symmetric covariance matrix, was used to estimate the percent change from menopause onset (Year 0) across all years. Fixed effects in the model included race, time (as a categorical variable), and race*time interaction, while only a subject random effect was used. Both between-race and within-race comparisons were calculated using two-sample t-tests constructed from estimates of least squares means ± standard error of the mean (SEM) from the linear model. Similarly, repeated measures models were used to estimate the associations between body composition measures and hormones levels between Black and White women leading up to menopause using slope estimates and t-tests. Age was not adjusted for within the linear models due to the lack of difference at menopause onset (Year 0) between Black and White women. Highest level of education and smoking status was also not adjusted for within the linear models due to the lack of difference at screening between Black and White women.
RESULTS
Characteristics at menopause onset
Ninety-four women (25 Black, 69 White) transitioned through menopause. Participant characteristics at menopause onset (Year 0) are displayed in Table 1. At menopause onset, Black women weighed more and had higher systolic and diastolic blood pressure compared to White women (all p≤0.05). No other differences in body fatness, sex steroid hormones, or cardiometabolic risk factors were observed between Black and White women. Importantly, the 94 women who transitioned through menopause (as well as within each racial group) were not different from those that did not transition through menopause in terms of percent body fat and adipose tissue distributions (abdominal SAT, VAT, and TAT) at baseline.
Table 1.
Characteristics of Women by Race at Menopause Onset (Year 0)1
| Everyone (n=94) | Black (n=25) | White (n=69) | Difference | p-value | |
|---|---|---|---|---|---|
| Age, years | 53 (52, 53) | 52 (51, 53) | 53 (52, 53) | −1 (−2, 0) | 0.23 |
| Weight, kg | 72.7 (69.6, 75.8) | 77.8 (71.9, 83.8) | 70.8 (67.2, 74.4) | 7.0 (0.1, 13.9) | 0.05 |
| BMI, kg/m2 | 27.0 (25.8, 28.2) | 28.8 (26.5, 31.0) | 26.3 (24.9, 27.7) | 2.4 (−0.2, 5.1) | 0.07 |
| Waist Circumference, cm | 83.7 (80.8, 86.6) | 86.6 (81.0, 92.2) | 82.6 (79.3, 86.0) | 4.0 (−2.6, 10.5) | 0.23 |
| Waist-to-Hip Ratio | 0.80 (0.79, 0.82) | 0.81 (0.77, 0.84) | 0.80 (0.78, 0.82) | 0.01 (−0.03, 0.04) | 0.79 |
| Sex Steroid Hormones | |||||
| Estradiol, pg/mL | 68.6 (46.5, 90.6) | 76.3 (29.8, 122.9) | 66.3 (41.0, 91.6) | 10.0 (−42.9, 63.0) | 0.71 |
| Estrone Sulfate, ng/mL | 3.1 (2.0, 4.2) | 3.7 (1.6, 5.8) | 2.9 (1.6, 4.2) | 0.8 (−1.7, 3.2) | 0.54 |
| FSH, mIU/mL | 63.9 (55.9, 71.9) | 75.0 (59.6, 90.4) | 59.9 (50.6, 69.2) | 15.1 (−2.9, 33.1) | 0.10 |
| SHBG, nmol/L | 63.6 (54.6, 72.7) | 61.4 (43.7, 79.2) | 64.4 (53.8, 75.1) | −3.0 (−23.7, 17.7) | 0.77 |
| Total Testosterone, ng/dL | 25.8 (24.5, 27.1) | 24.8 (22.2, 27.3) | 26.2 (24.6, 27.7) | −1.4 (−4.4, 1.5) | 0.34 |
| Cardiometabolic Risk Factors | |||||
| Systolic Blood Pressure, mmHg | 120 (117, 123) | 125 (119, 130) | 118 (115, 122) | 7 (0, 13) | 0.04 |
| Diastolic Blood Pressure, mmHg | 76 (74, 78) | 80 (78, 83) | 74 (72, 76) | 6 (3, 10) | <0.001 |
| Heart Rate, bpm | 56 (53, 60) | 57 (51, 63) | 56 (53, 60) | 1 (−7, 8) | 0.90 |
| Total Cholesterol, mg/dL | 211 (204, 219) | 221 (207, 236) | 208 (199, 216) | 13 (−3, 30) | 0.11 |
| HDL Cholesterol, mg/dL | 66 (63, 69) | 67 (60, 73) | 66 (63, 70) | 0 (−7, 7) | 0.94 |
| LDL Cholesterol, mg/dL | 123 (116, 130) | 133 (121, 146) | 119 (112, 127) | 14 (−0, 29) | 0.06 |
| Triglycerides, mg/dL | 110 (95, 125) | 107 (78, 136) | 111 (94, 129) | −4 (−38, 30) | 0.80 |
| Glucose, mg/dL | 95 (93, 96) | 94 (91, 97) | 95 (93, 97) | −1 (−5, 3) | 0.70 |
| Insulin, mU/mL | 8.8 (7.7, 9.9) | 10.0 (7.9, 12.1) | 8.4 (7.1, 9.6) | 1.7 (−0.8, 4.1) | 0.19 |
| HOMA-IR | 2.1 (1.8, 2.4) | 2.4 (1.9, 3.0) | 2.0 (1.7, 2.3) | 0.4 (−0.2, 1.1) | 0.21 |
| QUICKI | 0.35 (0.35, 0.36) | 0.35 (0.33, 0.36) | 0.36 (0.35, 0.37) | −0.01 (−0.03, 0.00) | 0.14 |
| Body Composition by DXA | |||||
| Percent Body Fat, % | 41.0 (39.2, 42.9) | 42.3 (38.7, 45.9) | 40.6 (38.4, 42.7) | 1.7 (−2.5, 5.9) | 0.43 |
| Fat Mass, kg | 30.8 (28.1, 33.5) | 34.1 (28.9, 39.3) | 29.7 (26.6, 32.8) | 4.4 (−1.7, 10.5) | 0.15 |
| Fat-Free Mass, kg | 41.7 (40.7, 42.8) | 43.3 (41.2, 45.3) | 41.2 (40, 42.4) | 2.0 (−0.3, 4.4) | 0.09 |
| Trunk Fat Mass, kg | 13.3 (11.6, 14.9) | 14.4 (11.2, 17.6) | 12.8 (10.9, 14.7) | 1.6 (−2.1,5.3) | 0.40 |
| Body Composition by CT | |||||
| Abdominal SAT, cm2 | 350 (322, 378) | 377 (320, 433) | 341 (309, 374) | 36 (−30, 101) | 0.28 |
| VAT, cm2 | 100 (86, 114) | 96 (68, 125) | 101 (85, 118) | −5 (−38, 28) | 0.77 |
| Abdominal TAT, cm2 | 449 (411, 487) | 470 (393, 546) | 443 (399, 486) | 27 (−61, 115) | 0.54 |
| Deep SAT, cm2 | 181 (166, 197) | 192 (160, 223) | 178 (160, 196) | 14 (−23, 51) | 0.45 |
| Superficial SAT, cm2 | 163 (144, 182) | 162 (124, 200) | 163 (141, 185) | −1 (−45, 43) | 0.97 |
Abbreviations: BMI, body mass index; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment-insulin resistance; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check index; SAT, subcutaneous adipose tissue; SHBG, sex hormone-binding globulin; TAT, total adipose tissue; VAT, visceral adipose tissue.
Values are expressed as raw means (95% confidence intervals) at menopause onset (Year 0).
Changes in body composition and abdominal adipose tissue distribution
Before menopause
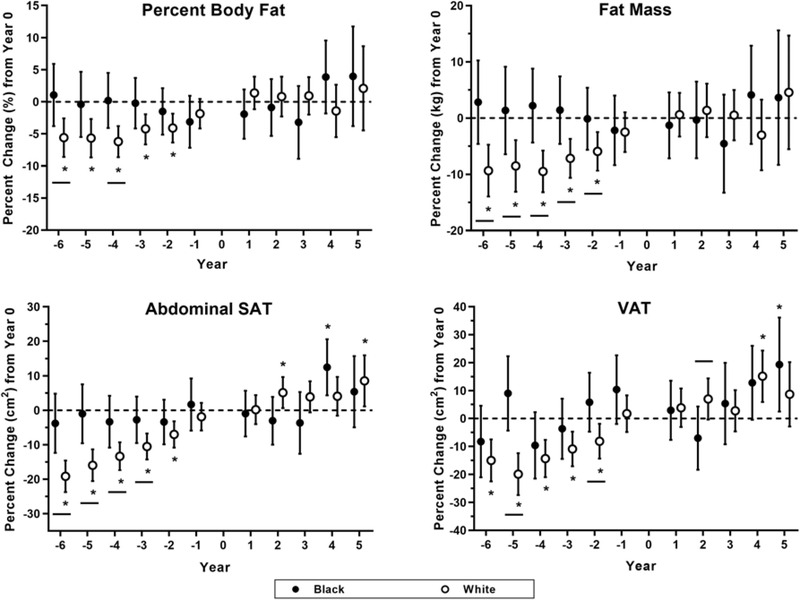
In the 2 to 6 years before menopause onset (Figure 1), White women gained significant weight (+3 kg) and significantly more total body adiposity (+6% percent body fat, +9% fat mass, +12% trunk fat mass) and abdominal adipose tissue (+19% abdominal SAT, +19% abdominal TAT, +15% VAT). Conversely, Black women maintained body composition and abdominal adiposity across menopause. Similar trends in waist circumference, deep SAT, and superficial SAT were observed in both Black and White women (see Supplemental Table 1). Fat-free mass was maintained in both Black and White women.
Figure 1. Percent Change in Body Composition & Adipose Tissue Distribution.
Percent change in percent body fat (%), fat mass (kg), abdominal subcutaneous adipose tissue (SAT; cm2), and visceral adipose tissue (VAT; cm2) are displayed across the menopause transition in years. Menopause onset is denoted as Year 0, with negative years implying years before menopause onset and positive years implying years after menopause onset. Data are displayed as means with 95% confidence intervals for each race. Closed circles (•) indicate data from Black women, and open circles (○) indicate data from White women.
Postmenopause
Both Black and White women tended to maintain total body adiposity and abdominal adipose tissue achieved at the time of menopause onset into their postmenopausal years. However, abdominal TAT did increase among White women in years 2, 4, and 5, as well as abdominal SAT in years 2 and 5, and VAT in year 4 after menopause.
Changes in sex steroid hormones
Before menopause
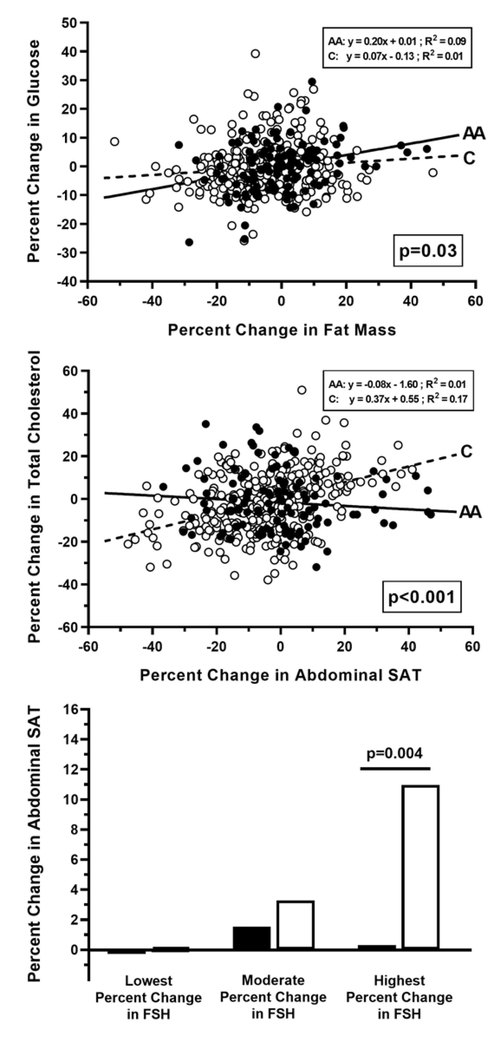
In the 1 to 6 years before menopause, White women had significant decreases in estradiol and SHBG, while Black women had significant decreases in total testosterone but no changes in estradiol or SHBG concentrations (see Supplemental Table 2). Importantly, for every 1% decrease in total testosterone, VAT mass decreased 0.05% in White women and increased 0.05% in Black women (between-race p=0.03). Both Black and White women had significant decreases in estrone sulfate and increases in FSH. We separated both Black and White women into tertiles based on FSH levels to determine if racial differences occur within each tertile (lowest, moderate, and highest percent changes in FSH). White women with the largest percent increases in FSH had the greatest percent increases in abdominal SAT. Specifically, the largest FSH tertile had significant abdominal SAT differences between Black and White women (p=0.004). Conversely, there was no relationship between the percent changes in FSH tertiles and abdominal SAT among Black women (Figure 2).
Figure 2. Percent Change in Body Composition & Adipose Tissue Distribution.
Percent change in body composition (fat mass and abdominal subcutaneous adipose tissue (SAT)) are plotted against changes in cardiometabolic risk and sex steroid status including glucose, total cholesterol, and follicle-stimulating hormone (FSH). Individual data are provides for each race. Closed circles (•) or black bars indicate individual data from Black women, and open circles (○) or white bars indicate data from White women. Solid lines (—) and dashed lines (- - -) are indicative of overall trends for Black and White women, respectively. Respective equations, R-squared value, and/or p-value are provided for (or between) each race.
Postmenopause
Concentrations of estradiol, estrone sulfate, SHBG, and total testosterone were maintained postmenopause in both races. Conversely, FSH continued to increase among White women, whereas FSH concentrations increased among Black women only in years 2 and 5 postmenopause.
Changes in cardiometabolic risk factors
Before menopause
In the 2 to 6 years before menopause, a significant increase in total cholesterol and a significant decrease in insulin sensitivity (measured by QUICKI) was observed among White women (see Supplemental Table 3). White woman also had a significant increase in LDL cholesterol in years 4 and 6 only. All other cardiometabolic risk factors, including HOMA-IR and prevalence of the metabolic syndrome, remained relatively unchanged before menopause among White women. Conversely, no consistent changes in cardiometabolic risk factors were observed among Black women before menopause.
Pronounced differences in the association between the changes in body composition and changes in cardiometabolic risk leading up to menopause were observed. Specifically, for every 1% increase in abdominal SAT: (1) total cholesterol increased 0.36% in White women and decreased 0.04% in Black women (between-race p<0.0001) (Figure 2); and (2) LDL cholesterol increased 0.44% in White women and 0.02% in Black women (between-race p<0.01). Additionally, for every 1% increase in VAT mass: (1) total cholesterol increased 0.21% in White women and decreased 0.06% in Black women (between-race p<0.0001); and (2) LDL cholesterol increased 0.27% in White women and decreased 0.04% in Black women (between-race p=0.0002). And finally, for every 1% increase in fat mass, glucose increased 0.04% in White women compared to 0.20% in Black women (between-race p=0.03) (Figure 2).
Postmenopause
In the 2 to 5 years postmenopause, White women had significant increases in HDL cholesterol, as well as increases in total cholesterol and LDL cholesterol (both in the 2 to 3 years postmenopause). No consistent changes in other cardiometabolic risk factors or prevalence of the metabolic syndrome were observed among White women. Conversely, no changes in cardiometabolic risk factors were observed among Black women postmenopause.
COMMENT
Principal Findings
White women gained significantly more weight in the years leading up to menopause, which was primarily due to significant increases in abdominal adiposity. Commensurate with these increases in abdominal adiposity, White women had more dynamic changes in sex steroid hormones and increases in cardiometabolic risk, including increased total cholesterol and LDL cholesterol. In contrast, Black women had more abdominal adiposity prior to menopause yet maintained these adiposity levels and overall cardiometabolic health profile across the menopause transition.
Results
Our current findings are contrary to our original hypothesis that Black women would have disproportionate increases in cardiometabolic risk compared to White women despite similarly proposed increases in abdominal adiposity. This hypothesis was supported by the combined analysis of three longitudinal cohorts (ARIC, CARDIA, and Framingham Heart Study) that found for a given level of weight gain among middle-age adults ages 45 to 60 years, diabetes incidence is higher in Black compared to White individuals.22 Black individuals also have less visceral adiposity and intrahepatic lipid,23–25 higher HDL cholesterol,26 lower triglycerides23,26 and prevalence of hepatic steatosis,27 yet are paradoxically more insulin resistant compared to White individuals.28–30 These findings demonstrate that Black women may be more susceptible to increased cardiometabolic risk in response to weight gain because of menopause.31,32 Recent findings further support the notion that Black women may have greater insulin response and lower insulin clearance in response to glucose administration and, therefore, may suffer more detrimental effects of weight gain during the menopause transition.33,34Our findings in this completed sample (n=94) are also in contrast with our preliminary analysis (n=51) where we reported that Black and White women had similar changes in body adiposity and sex steroid hormones across menopause.3 To our knowledge, no longitudinal studies have examined whether Black and White women differ in response to gains in abdominal adiposity across the menopause transition using such robust measures of abdominal adiposity.
There are several possible explanations for why White women gained more weight and abdominal adiposity—and caught up to Black women. First, Black women had borderline obesity prior to menopause and lacked the hallmark increases in FSH and decreases in estradiol and SHBG throughout the menopause transition which may have contributed to the absence of gains in abdominal adiposity. These observations were not the same among White women. Whether changes in body adiposity precludes changes in sex steroid hormones (rather than vice versa) remains unclear. In SWAN, obesity status was both an important predictor of sex steroid hormone levels as well as the degree in which these sex steroids change across the menopause transition.35,36 Specifically, women with obesity—especially Black women—often maintain estradiol concentrations and have smaller increases in FSH.35 These observations are important since more mechanistic studies in both animals and humans have shown that blocking FSH37 and gonadal suppression with estradiol add-on38 limits any gain in adiposity. While further investigation is warranted, the gains in abdominal adiposity observed among White women in our study are likely due in part to the interaction between both obesity status and sex steroid hormones. Furthermore, we cannot understate the complex interactions that exist between whole-body metabolism, sex steroid hormones, genetics, and environmental factors, as well as our inability to evaluate all of these factors within the present analyses, in the worsening of adiposity and cardiometabolic health in midlife women.
One interesting finding was that Black women had a significant decrease in testosterone in the years prior to menopause, which was not observed among White women. It has been previously reported that testosterone concentrations are positively associated with body size39,40 with some reporting that Black women have lower testosterone levels than White women after adjusting for body size.41 In the present study, Black women had greater levels of adiposity and higher (yet declining) testosterone levels during the menopause transition. Thus, the maintenance of estradiol combined with decreasing testosterone among Black women may have contributed to the absence of increased adiposity.
Clinical Implications
Our study supports increased attention towards potential race-specific dynamics in how obesity status before menopause may affect sex steroid hormones and thus subsequent changes in abdominal adiposity. While the present study did not find any increases in cardiometabolic risk or abdominal adiposity among Black women, our observations do not indicate that Black women are less likely to develop type 2 diabetes and metabolic syndrome as they age. Indeed, gains in abdominal adiposity may progress at a slower rate and into the postmenopausal years creating a lag in the development of cardiometabolic disease.39 In addition, we observed that Black women had greater increases in fasting glucose level for each small increase in total fat mass, suggesting potentially greater metabolic vulnerability to excess weight gain. Furthermore, we feel there is a potential opportunity for physicians to consider more comprehensive risk profiling of women during the menopause transition as part of their routine clinic visits. Specific assessments may include standardized waist circumference measurements, as well as a more complex lipid panel and fasting glucose level so that changes in cardiometabolic risk can be evaluated over time.
Research Implications
More studies are needed to disentangle which women are more susceptible to weight gain and increased cardiometabolic risk during the menopause transition. While interventions targeting body weight change during perimenopause are scarce,42,43 future studies should consider more comprehensive lifestyle approaches to target these increased in abdominal adiposity, including nutrition and physical activity coaching.44 Future treatment approaches for midlife women should also consider the interactions of race, body adiposity, sex steroid hormones, and their influence on cardiometabolic risk.
Strengths and Limitations
The strengths of our study include the relatively large sample size of women with robust longitudinal measurements of body composition and regional adiposity. First, existing longitudinal cohort studies in menopause often use simple outcome measures like BMI and waist circumference4,36,39 or body composition by bioelectrical impedance analysis (BIA)4 to assess abdominal adiposity. While important epidemiologic studies benefit from the simplicity of these tools to estimate body adiposity (i.e., accessible, ease of transport), BMI, waist circumference, and skin calipers simply do not accurately quantify body composition to the precision that is required.45 The accuracy and reliability of BIA for quantifying body adiposity is also limited, in part because of the issue of fluctuating hydration status and also because many BIA instruments perform poorly on individuals with larger body sizes.45 Having more robust measures of regional (upper versus lower) adiposity is important. Indeed, upper-body (abdominal) adiposity is associated with more obesity-related disorders,8–10,46–48 while lower-body (femoral) adiposity may be more metabolically protective.48–52 To our knowledge, only a few longitudinal studies have used more robust measures such as DXA or CT to quantify changes in body adiposity across the menopause transition5,6 in addition to our longitudinal cohort.3 Neither of those studies,5,6 however, examined racial disparities across menopause. Indeed, longitudinal studies that undertake comprehensive metabolic phenotyping in large sample sizes across the menopause transition are difficult to execute due to both poor retention rates and the inherent variability in the length of the menopause transition.
Our study has several limitations. First, we were unable to evaluate the influence of menopause stage or length of perimenopause on our study outcomes due to the absence of cycle variability data. We were also unable to quantify predictors of diabetes incidence since only two of the women that transitioned through menopause actually developed type 2 diabetes. Instead, we quantified presence or absence of the metabolic syndrome (>3 conditions indicative of presence) as a surrogate marker of metabolic health. While our large sample size is indeed a strength of the study, we still lacked a truly equal race distribution of women who transitioned through menopause, which likely accounts for some of the larger standard deviations/errors observed among Black women. Furthermore, we did not time our measurement of sex steroid hormones due to logistical concerns and the complexities that a larger sample size would entail. We also did not routinely measure changes in thyroid hormones—particularly thyroid stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4)—which are responsible for regulating metabolism. Additionally, our follow-up period after menopause onset may not be sufficient in length to capture all potential increases in cardiometabolic risk that arise in the postmenopausal years. Some longitudinal studies have reported that Black women may have a slower trajectory to develop the metabolic syndrome and type 2 diabetes despite higher levels of body adiposity at baseline.39 Differences in physical activity and dietary intake (and diet quality) may also have contributed to the observed changes in body adiposity and cardiometabolic risk.53–56 Despite our preliminary findings that physical activity and dietary intake decreases in the years before menopause,3 we were unable to evaluate racial differences in physical activity and dietary changes due to the discontinued collection of these data during the 3-year extension of the study.
Conclusions
To our knowledge, our study is the first to examine whether women of different racial backgrounds differ in response to gains in abdominal adiposity across the menopause transition using robust measures of abdominal adiposity. We found that White women gained more abdominal adiposity during the menopause transition compared to Black women, which may be due in part to differences in the pattern of sex steroid hormone changes between women of different racial backgrounds. Future studies should evaluate which women are more susceptible to weight gain and increased cardiometabolic risk during the menopause transition.
Supplementary Material
AJOG AT A GLANCE.
A. Why was this study conducted?
To assess longitudinal changes in body composition and cardiometabolic risk among Black and White women during the menopause transition.
B. What are the key findings?
White women gained more abdominal adiposity during the menopause transition compared to Black women, which may be due in part to differences in the pattern of sex steroid hormone changes between women of different racial backgrounds.
C. What does this study add to what is already known?
This study demonstrates that Black women may not gain as much abdominal adiposity across the menopause transition due to both higher abdominal adiposity and smaller fluctuations in sex steroid hormones in the years leading up to menopause.
ACKNOWLEDGEMENTS
Other Key Contribution: We acknowledge the support from Kelsey N. Olson in helping quality control check our data.
Source of Funding: The initial Healthy Transitions study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK50736 (to J.C.L.). An extension study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK50736 (to S.R.S.). This research was also supported by the NIDDK sponsored Ruth L. Kirschstein National Research Service T32 Research Training Grant T32-DK064584 (to K.L.M.), as well as by the National Institute of General Medical Sciences of the National Institutes of Health (U54-GM104940), which funds the Louisiana Clinical and Translational Science Center (LA CaTS).
Footnotes
Conflict of Interest Statement: The authors declare there are no competing or conflicts of interest associated with the proposed manuscript.
Clinicaltrials.gov Identifier: Healthy Transitions study ( NCT00412269) – https://clinicaltrials.gov/show/NCT00412269
CONDENSATION: During the menopause transition, White women had greater gains in abdominal adiposity and increased cardiometabolic risk compared to Black women.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovejoy JC, Champagne CM, De Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sowers Mf, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulnour J, Doucet E, Brochu M, Lavoie JM, Strychar I, Rabasa-Lhoret R, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: A Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19(7):760–7. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: A 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric. 2004;7(4):375–89. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: The study of women’s health across the nation (SWAN) fat patterning study. Obesity. 2010;18(3):604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–8. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–83. [DOI] [PubMed] [Google Scholar]
- 11.Banack HR, Wactawski-Wende J, Hovey KM, Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause. 2018;25(3):307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rm Blew, Sardinha LB, Milliken LA, Teixeira PJ, Going SB, Ferreira DL, et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res. 2002; 10(8):799–808. [DOI] [PubMed] [Google Scholar]
- 13.Lovejoy JC, Champagne CM, Smith SR, De Jonge L, Xie H. Ethnic differences in dietary intakes, physical activity, and energy expenditure in middle-aged, premenopausal women: The Healthy Transitions Study. Am J Clin Nutr. 2001;74(1):90–5. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The healthy transitions study. Obes Res. 2001;9(1):10–6. [DOI] [PubMed] [Google Scholar]
- 15.Burger HG, Dudley Ec, Hopper JL, Shelley JM, Green A, Smith A, et al. The endocrinology of the menopausal transition: A cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995;80(12):3537–45. [DOI] [PubMed] [Google Scholar]
- 16.Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, et al. A better index of body adiposity. Obesity. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SR, Lovejoy jC, Greenway F, Ryan D, De Jonge L, De La Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001; [DOI] [PubMed] [Google Scholar]
- 18.Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y. Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas. 2002;42(4):301–6. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and p-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 20.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 22.Wei GS, Coady SA, Reis JP, Carnethon MR, Coresh J, D’agostino RB, et al. Duration and degree of weight gain and incident diabetes in younger versus middle-Aged black and white Adults: ARIC, CARDIA, and the framingham heart study. Diabetes Care. 2015;38(11):2042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovejoy Jc, De La Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: Effects of race. Metabolism. 1996;45(9):1119–24. [DOI] [PubMed] [Google Scholar]
- 24.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61(4):765–71. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African-Americans and whites. Obes Res. 2005;13(1):66–74. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Sacks FM, Furtado JD, Ricks M, Courville AB, Sumner AE. Racial differences between African-American and white women in insulin resistance and visceral adiposity are associated with differences in apoCIII containing apoAI and apoB lipoproteins. Nutr Metab. 2014;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–95. [DOI] [PubMed] [Google Scholar]
- 28.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11(8):755–62. [DOI] [PubMed] [Google Scholar]
- 30.Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in p-cell function. Diabetes Care. 2000;23(9):1353–8. [DOI] [PubMed] [Google Scholar]
- 31.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17(1):39–58. [DOI] [PubMed] [Google Scholar]
- 32.Centers For Disease and Control P. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2017. US Department of Health and Human Services; 2017. [Google Scholar]
- 33.Chung ST, Galvan-De La Cruz M, Aldana PC, Mabundo LS, DuBose CW, Onuzuruike AU, et al. Postprandial insulin response and clearance among black and white women: the Federal Women’s Study. J Clin Endocrinol Metab [Internet]. 2019;104(1):181–92. Available from: https://academic.oup.com/jcem/artide7104/1/18l/5106940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cree-Green M Postglucose hyperinsulinemia in black women is not what we thought. J Clin Endocrinol Metab [Internet]. 2019;104(2):266–8. Available from: https://academic.oup.com/jcem/article/104/2/266/5144618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tepper PG, Randolph JF, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildman RP, Tepper PG, Crawford S, Finkelstein JS, Sutton-Tyrrell K, Thurston RC, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the study of women’s health across the nation. J Clin Endocrinol Metab. 2012;97(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, Demambro VE, Dhawan S, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shea KL, Schwartz RS, Wolfe P, Stavros A, Wierman ME, Kittelson JM, et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton-Tyrrell K, Zhao X, Santoro N, Lasley B, Sowers M, Johnston J, et al. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171(11):1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manson JM, Sammel MD, Freeman eW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertil Steril. 2001;75(2):297–304. [DOI] [PubMed] [Google Scholar]
- 41.Randolph JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–22. [DOI] [PubMed] [Google Scholar]
- 42.Garcfa-Unciti M, Izquierdo M, Idoate F, Gorostiaga E, Grijalba A, Ortega-Delgado F, et al. Weight-loss diet alone or combined with progressive resistance training induces changes in association between the cardiometabolic risk profile and abdominal fat depots. Ann Nutr Metab. 2012;61(4):296–304. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C-C, Hsu C-Y, Liu J-F. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women. Menopause [Internet]. 2018;25(7):1 Available from: http://insights.ovid.com/crossref?an=00042192-900000000-97597 [DOI] [PubMed] [Google Scholar]
- 44.Marlatt KL, Beyl RA, Redman LM. A qualitative assessment of health behaviors and experiences during menopause: A cross-sectional, observational study. Maturitas [Internet]. 2018;116(July):36–42. Available from: 10.1016/j.maturitas.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piers LS, Soares MJ, Frandsen SL, O’dea K. Indirect estimates of body composition are useful for groups but unreliable in individuals. Int J Obes. 2000; [DOI] [PubMed] [Google Scholar]
- 46.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996; [DOI] [PubMed] [Google Scholar]
- 48.Amati F, Pennant M, Azuma K, Dube JJ, Toledo FGS, Rossi AP, et al. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity. 2012; [DOI] [PubMed] [Google Scholar]
- 49.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. International Journal of Obesity. 2010. [DOI] [PubMed] [Google Scholar]
- 50.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CDA, Yudkin JS, et al. Trunk Fat and Leg Fat Have Independent and Opposite Associations with Fasting and Postload Glucose Levels: The Hoorn Study. Diabetes Care. 2004; [DOI] [PubMed] [Google Scholar]
- 51.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CDA, Kostense PJ, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: The Hoorn Study. Am J Clin Nutr. 2003; [DOI] [PubMed] [Google Scholar]
- 52.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Metab. 2015; [DOI] [PubMed] [Google Scholar]
- 53.Ward E, Gold EB, Johnson WO, Ding F, Chang P-Y, Song P, et al. Patterns of Cardiometabolic Health as Midlife Women Transition to Menopause: A Prospective Multi-Ethnic Study. J Clin Endocrinol Metab. 2018;104(5):1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohrt WM. Menopause and weight gain. Menopause [Internet]. 2009;64(6):28–9. Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/menopause-and-weight-gain [PubMed] [Google Scholar]
- 55.Binder EF, Birge SJ, Kohrt WM. Effects of endurance exercise and hormone replacement therapy on serum lipids in older women. J Am Geriatr Soc. 1996;44(3):231–6. [DOI] [PubMed] [Google Scholar]
- 56.Grindler NM, Santoro NF. Menopause and exercise. Menopause. 2015;22(12): 1351–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.