Abstract
Background
Aflatoxins are carcinogenic mycotoxins that contaminate many food crops. Maize and groundnuts are prone to aflatoxin contamination, and are the major sources of human exposure to aflatoxins, due to their high intake as staple foods, particularly in low‐ and middle‐income countries (LMICs). Observational studies suggest an association between dietary exposure to aflatoxins during pregnancy and early childhood and linear growth in infants and young children.
Objectives
To assess the effects on pre‐ and postnatal growth outcomes when agricultural and nutritional education interventions during the post‐harvest period that aim to reduce aflatoxin exposure are compared to usual support or no intervention. We assessed this in infants, children, and pregnant and lactating women at the household or community level in LMICs.
Search methods
In July and August 2019, we searched: CENTRAL, MEDLINE, Embase, CINAHL, Web of Science Core Collection, Africa‐Wide, LILACS, CAB Abstracts, Agricola, and two trials registers. We also checked the bibliographies of the included studies and contacted relevant mycotoxin organisations and researchers for additional studies.
Selection criteria
We included randomised controlled trials (RCTs) and cluster‐RCTs of agricultural education and nutritional education interventions of any duration, at the household or community level, aimed at reducing aflatoxin intake by infants, children, and pregnant and lactating women, in LMICs during the post‐harvest period, compared to no intervention or usual support. We excluded studies that followed participants for less than four weeks. We assessed prespecified prenatal (at birth) and postnatal growth outcomes (during infancy, childhood, and adolescence), with linear growth (as the primary outcome), infectious disease morbidity, and unintended consequences.
Data collection and analysis
Two authors independently assessed study eligibility using prespecified criteria, extracted data, and assessed risk of bias of included RCTs. We evaluated the certainty of the evidence using GRADE, and presented the main results in a 'Summary of findings' table.
Main results
We included three recent cluster‐RCTs reporting the effects of agricultural education plus post‐harvest technologies, compared to usual agricultural support or no intervention. The participants were pregnant women and their children, lactating women and their infants (< 6 months), women of childbearing age, and young children (< 59 months), from rural, subsistence maize‐farming communities in Kenya, Zimbabwe, and Tanzania.
Two trials randomised villages to the intervention and control groups, including a total of at least 979 mother‐child pairs from 60 villages. The third trial randomised 420 households, including 189 mother‐child pairs and 231 women of childbearing age. Duration of the intervention and follow‐up ranged between five and nine months. Due to risk of attrition bias, the overall risk of bias was unclear in one trial, and high in the other two trials.
None of the included studies addressed the effects of nutritional education on pre‐ and postnatal growth.
One trial reported outcomes not prespecified in our review, and we were unable to obtain unpublished growth data from the second trial, even after contacting the authors. The third trial, in lactating women and their infants in Tanzania, reported on the infants' weight‐for‐age z‐score (WAZ) after six months. This trial found that providing agricultural education aimed at changing farmers' post‐harvest practices to reduce aflatoxin exposure, by using demonstrations (e.g. handsorting, de‐hulling of maize, drying sheets, and insecticides), may improve WAZ in infants from these farmers' households, on average, by 0.57 (95% confidence interval (CI) 0.16 to 0.98; 1 study; 249 participants; very low‐certainty evidence), compared to infants from households where the farmers received routine agricultural extension services.
Another way of reporting the effect on WAZ is to compare the proportion of underweight infants (WAZ > 2 SD below the reference median value) per group. This trial found that the intervention may reduce the proportion of underweight infants in the intervention households by 6.7% (95% CI ‐12.6 to ‐1.4; 249 participants; very low‐certainty evidence) compared to control households.
No studies reported on unintended effects of agricultural and nutritional education.
Authors' conclusions
Evidence on the effects on child growth in LMICs of agricultural or nutritional education interventions that reduce aflatoxin exposure was very limited; no included study reported on linear growth. Very low‐certainty evidence suggested that agricultural education aimed at changing farmers' post‐harvest practices to reduce aflatoxin exposure by using demonstrations, may result in an increase in WAZ, when compared to usual or no education.
Plain language summary
Reducing aflatoxin intake with agricultural and nutritional education to improve growth of infants and children in low‐ and middle‐income countries
Review question
Does providing agricultural and nutritional education about how to reduce the intake of aflatoxins (from contaminated food crops) in households and communities in low and middle‐income countries (LMICs) improve the growth of infants and children compared to usual or no education?
Background
Aflatoxins are toxins produced by moulds that contaminate food crops. Maize and groundnuts are the major dietary sources of aflatoxins, as they are eaten in large amounts by many people living in LMICs. Some research from LMICs suggests that there may be a link between aflatoxin intake during pregnancy and early childhood, and growth in infants and young children.
Study Characteristics
We included three trials, conducted in pregnant and breastfeeding women (1168 mother‐child pairs), women of childbearing age (N = 231), and infants and young children (< 59 months old), from rural, subsistence maize‐farming communities in Kenya, Tanzania, and Zimbabwe. One trial in Tanzania, at unclear risk of bias overall, provided data for this review, since one trial did not report any outcomes relevant to this review, and we were unable to obtain unpublished growth data for another, even after contacting the study authors.
The trial, conducted in breastfeeding women and their babies, studied the effects of agricultural education (demonstrations to change farmers' practices after harvesting their maize crops to reduce aflatoxins (for example, by handsorting and de‐hulling the maize, using drying sheets and insecticides) on the babies' weight, standardised for age (weight‐for‐age z‐score (WAZ)), after six months (the z‐score measures the difference between these babies and the median of a population of similar babies). Farmers in the control group received routine services from agriculture extension workers.
Key results
Very low‐certainty evidence from one trial suggested that the WAZ of 128 children from farmers' households who received agricultural education may improve by a z‐score of 0.57, compared to 121 children from households where farmers only received routine services. This means that a baby girl in the intervention group, with a healthy weight, would gain about 450 to 690 grams more weight between three to nine months, compared to a baby girl in the control group. This is a meaningful difference.
Another way of measuring the effect is to compare the proportion of underweight infants (WAZ ≥ 2 standard deviations below the reference median value) per group, after the intervention. In this case, agricultural education may reduce the proportion of underweight children, on average, by 6.7% (very low‐certainty evidence), compared to routine services.
None of the included studies addressed the effects of nutritional education on length of height, or on unintended effects of agricultural or nutritional education.
Evidence about the effects on child growth of agricultural or nutritional education interventions that reduce aflatoxin exposure in LMICs was very limited. Data from one trial suggested that agricultural education, aimed at changing farmers' post‐harvest practices to reduce aflatoxin exposure, may result in the babies' increased weight‐for‐age, compared to usual or no education.
The literature was searched to August 2019.
Quality of the evidence
We have very little confidence in the results. The true effect may be substantially different.
Summary of findings
Summary of findings for the main comparison. Agricultural education with post‐harvest technologies compared to usual agricultural support for reducing aflatoxin exposure in pregnant and lactating women, infants, and children to improve childhood growth.
| Agricultural education with post‐harvest technologies compared to usual agricultural support for reducing aflatoxin exposure in pregnant and lactating women, infants, and children to improve childhood growth | ||||||
| Patient or population: pregnant and lactating women, infants, and children Setting: households or communities in low‐ and middle‐income countries Intervention: agricultural education, with post‐harvest technologies to reduce aflatoxin exposure Comparison: usual agricultural support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual agricultural support | Risk with agricultural education, with food replacement or post‐harvest technologies | |||||
| Birth length for gestational age z‐score | ‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| Birth weight for gestational age z‐score | ‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
|
Low birth weight (less than 2500 g) |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
|
Length‐ or height‐for‐age z‐score (LAZ) |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
|
Stunting (LAZ ≥ 2 SD below reference median value) |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
|
Weight‐for‐height z‐score (WHZ) |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| Unintended effects of agricultural and nutritional education interventions to reduce the aflatoxin intake of infants, children, pregnant and lactating women | ‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
|
Weight‐for‐age z‐score (WAZ) Follow‐up: mean 6 months |
The mean WAZ was ‐0.47 z‐scorea | MD 0.57 z‐score higher (0.16 higher to 0.98 higher) | ‐ | 249 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c,d | There is very uncertain evidence about the effect on WAZ when agricultural education, along with post‐harvest technologies to reduce aflatoxin exposure is compared to usual support or no intervention. |
|
Proportion of underweight children (WAZ ≥ 2 SD below reference median value) Follow‐up: mean 6 months |
The mean proportion of underweight children was 9.0%a | MD 6.7% lower (12.6% lower to 0.8% lower) | 249 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c,d | The evidence is very uncertain about the effect on the proportion of underweight children when agricultural education, along with post‐harvest technologies to reduce aflatoxin exposure, is compared to usual support or no intervention. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMean post‐intervention value in the control group reported by a single cluster‐RCT (Kamala 2018a) bDowngraded by 1 for risk of bias: high risk of attrition bias cDowngraded by 1 for indirectness: one study conducted in a single setting dDowngraded by 1 for imprecision: optimal information size not met
Background
Aflatoxins are a type of mycotoxin that affect global food security by causing the contamination of food crops, mainly in low‐ and middle‐income countries (LMICs), particularly in Africa. Human exposure to aflatoxins is considered a major public health concern due to its potential harmful effects on health (IARC 2015). Aflatoxins are colourless, odourless compounds produced during secondary metabolism by some members of the fungal genus Aspergillus (notably species in the sectionFlavi (Frisvad 2019)). Four main forms of aflatoxin are commonly found in food crops, namely aflatoxin B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2). AFB1 is the most potent and prevalent form of aflatoxin, accounting for an average of 70% of the total aflatoxin content in food. Another important type of aflatoxin, especially to infant and child health, is aflatoxin M1 (AFM1). It is a product of AFB1 hydroxylation during the metabolism of AFB1. AFM1 is a frequent contaminant of milk in lactating animals, resulting from the consumption of AFB1‐contaminated feed. AFM1 has also been reported in human breast milk (Warth 2016; Watson 2017; WHO 2018).
A variety of staple food crops, such as cereals (e.g. maize, wheat, rice), legumes, groundnuts (also called peanuts), and tree nuts, eaten in large quantities, which form the basis of a region's diet are susceptible to contamination via colonisation with aflatoxin‐producing fungi, as are other commonly eaten foods, such as oilseeds, spices, milk, meat, and dried fruit. Aflatoxin is the only mycotoxin known to contaminate crops both pre‐ and post‐harvest (potentially along the entire value chain), making it difficult to target prevention and control interventions (Ayalew 2016). Factors influencing fungal and aflatoxin contamination of crops include a region's climate, genotype of the planted crop, soil type, stress or damage to the crop due to drought before harvesting, heavy rains at and after harvesting, insect activity, low awareness of aflatoxin control, and crop production practices leading to poor timing of harvesting, inadequate drying of the crop before storage, and poor storage conditions (Ayalew 2016; Strosnider 2006).
High‐risk households for aflatoxin exposure often rely on subsistence farming and the production or consumption of a single staple food. Maize and groundnuts are the major dietary sources of human aflatoxin exposure, due to their high consumption as single staple foods by communities in LMICs. It is also likely that animal milk may be contaminated with AFM1 in some of these communities, depending on a number of factors, such as the quantities of aflatoxin‐contaminated feed ingested by animals, genetics of the animals, seasonal variation, the milking process, and environmental conditions. Contaminated milk may also subsequently contaminate milk products, such as yoghourt and cheese (Iqbal 2015). Thus, many LMIC households are plagued with adverse health consequences of aflatoxin exposure. The negative impacts on human health include an increased risk of acute toxicity, as recorded in several outbreaks of aflatoxicosis in Africa (IARC 2015; Kamala 2018b), and chronic effects, such as an increased risk of liver cancer (IARC 2015).
To protect people from the harmful effects of aflatoxins, international food safety standards stipulate maximum levels for aflatoxins in various foods (FAO and WHO 2018). These standards are operational in industrialised nations, but may have little effect in LMICs. Typically, food consumed from smallholder and subsistence farming rarely enters any sort of regulatory inspection for aflatoxin. Even if contamination levels were below the maximum levels, many people in LMICs are heavily dependant on high aflatoxin‐risk staple crops, consuming such large amounts of maize and groundnut products that their daily aflatoxin exposure would still render them vulnerable to disease (Klangwiset 2010). Effectively managing aflatoxin contamination of food and animal feed to reduce human exposure is complex, requiring integrated, multisector, scalable, and suitably resourced control programs, including awareness of aflatoxins, adequate monitoring and surveillance, pre‐harvest, peri‐harvest, and post‐harvest prevention or reduction strategies (or both), and post‐contamination aflatoxin management (Ayalew 2016). Creating widespread awareness ‘from seed to table’ about mitigating aflatoxin exposure and its effects, specifically in at‐risk communities (e.g. targeted agricultural, nutritional, and health education), is seen as critical to its management, serving as the foundation for initiating and sustaining behavioural changes and measures to control aflatoxin exposure. Control strategies include proper agronomic and crop management practices to decrease plant stress and improve plant vigour, competitive biological control using non‐aflatoxin‐producing Aspergillus flavus strains, use of resistant crop varieties, if available, proper drying of harvested crops to safe moisture levels with clean, insect‐free, and dry storage (e.g. hermetic storage solutions), and minimising aflatoxin in food fortification supply chains, with community and household‐targeted interventions (e.g. dietary diversification), food processing, and food safety and quality, market‐based approaches and public health regulations and policies (Ayalew 2016; Bandyopadhyay 2016).
Global dietary intake estimates for aflatoxin, based on estimates of typical maize and groundnut consumption, contamination levels, and body weight, indicate a much higher burden of exposure in LMICs in Sub‐Saharan Africa, China, Southeast Asia, and Latin America, compared with Western Europe and North America. The prevalence of the toxin is higher in Africa and Southeast Asia due to the conducive climate. Compounding the risk of exposure is the low level of surveillance and monitoring of these staple foods in LMICs. These countries often lack resources and analytical capacity, and as a result, there is a widening gap between the quality and quantity of prevalence data generated by laboratories in high‐income countries and LMICs (IARC 2015). Sampling procedures of foods for analytical purposes may also be problematic, since aflatoxin contamination is often not evenly distributed throughout batches of food, for example, in stored grain (WHO 2018).
Biomarkers are objective markers of aflatoxin exposure and are more accurate in assessing the degree of individual exposure than food‐based exposure assessments. There are three validated biomarkers of aflatoxin exposure: urinary biomarkers (AFM1 and aflatoxin‐N7‐guanine), reflecting exposure in the prior 24 to 48 hours; and serum levels of aflatoxin‐albumin (AF‐alb), reflecting cumulative exposure over the prior two to three months. The application of the AF‐alb biomarker has confirmed a high prevalence of aflatoxin exposure in several locations in East and West Africa (IARC 2015), as well as parts of Asia, such as Malaysia (Leong 2012). This biomarker correlates with the dietary intake estimates of aflatoxin in children who consume maize‐based diets (Routledge 2014). Other aflatoxin metabolites (for example, serum AFM1 or AFG1, urinary AFB1 or AFG1, and milk AFM1 or AFG2) are indicative of exposure, but since their levels do not correlate with aflatoxin intake, they are not considered accurate biomarkers of exposure (Smith 2018). However, it should be noted that there is no validated ranking system available to categorise individuals with different exposure levels to aflatoxin.
Description of the condition
Child undernutrition is a major public health burden in LMICs, and refers broadly to the condition where food intake is insufficient to meet a child's needs for growth, physiological function, and the ability to respond to illness. Stunting, defined as a height‐for‐age z‐score of more than two standard deviations below the child growth standard median, is the most prevalent form of child undernutrition (WHO 1986; WHO 2006). Globally, stunting affects an estimated 151 million or 22.2% of children below the age of five years. Two‐thirds of all stunted children live in LMICs. Africa is the only region in the world where the number of stunted children has increased, from 50.6 million in 2000 to 58.7 million children in 2017 (UNICEF‐WHO‐The World Bank 2018). Growth faltering typically begins in utero, followed by subsequent growth faltering after birth, especially in the first two years of life. Foetal growth restriction is an important contributor to stunted linear growth in the postnatal period. Longitudinal data over ten years from LMICs estimate that 20% of childhood stunting can be attributed to foetal growth restriction (Christian 2013).
Dietary exposure to high levels of aflatoxins during pregnancy has been widely documented in several LMICs, and may be a major contributor to foetal growth restriction, and in turn, to childhood stunting (Castelino 2014; Piekkola 2012; Shuaib 2010; Turner 2007). Cohort studies show that this exposure may contribute to adverse birth outcomes, such as low birth weight (Shuaib 2010), and stunted growth in the first year of life (Turner 2007). However, other studies found no association between exposure to aflatoxins in pregnancy and post‐natal growth outcomes (Smith 2018). Longitudinal data from West Africa on the association of aflatoxins and growth of 16‐ to 37‐month‐old children suggest that serum AF‐alb levels are associated with stunted growth (Gong 2004). However, longitudinal studies in Nepal during the first 36 months of life and in Tanzania in children 6 to 14 months of age, found no association between aflatoxin exposure and growth faltering (Mitchell 2017; Shirima 2015)
Aflatoxin exposure may affect child growth by three potential mechanisms: damage to the intestinal epithelium (enteropathy), liver toxicity, and reduced immune function. Enteropathy is associated with a reduced uptake of nutrients, and liver damage may result in less production of insulin‐like growth factors, while immune suppression may enhance the susceptibility of aflatoxin‐exposed children to infections, such as diarrhoea (IARC 2015; Watson 2017).
Numerous factors contribute to childhood growth faltering. In the postnatal period, for example, infants in many LMIC households are introduced to weaning foods (complementary foods) of low nutritional quality and high risk of microbial contamination, resulting in high rates of diarrhoea and other infectious diseases. In addition, complementary foods fed to children are often made from locally grown, low quality, and often contaminated cereals and nuts, such that these infants are exposed to a range of mycotoxins. Several studies from Africa have reported the contamination of cereal‐ and nut‐based complementary foods with aflatoxins (Kimanya 2014; Ojuri 2018; Ojuri 2019). A household surveillance study in Nigeria reported AFB1 levels in cereal‐based complementary foods of about 100 times higher than the maximum limits set by the European Union. These levels remained high throughout the year, suggesting a chronic, high level of exposure (Ojuri 2018). Although aflatoxins are found in human breast milk in high risk regions of LMICs, the level of exposure of these infants to aflatoxins in breast milk is likely to be lower than those consuming complementary foods; thus, the exclusive breastfeeding period is a window of lower exposure, and is critical to child health (Braun 2018; IARC 2015). However, the consumption of contaminated fresh cow's milk by some young children in LMICs, who mainly consume cereal‐based diets, may contribute significantly to their level of aflatoxin exposure, as milk from small‐scale dairy farms in Kenya and Brazil is frequently contaminated (Goncalves 2017; Kagera 2018).
Limited biomarker data in children suggest that the consumption of other mycotoxins that are present in staple foods, such as fumonisin in maize or deoxynivalenol (DON) in wheat, may have interactive effects on child growth. Chronic exposure to both of these mycotoxins, in addition to aflatoxins, was documented in children aged between 6 and 24 months from Tanzania (Kimanya 2014; Shirima 2015; Srey 2014). Fumonisin alone, or in combination with aflatoxins, was associated with stunting in one of these studies (Shirima 2015).
In summary, aflatoxin exposure during the first 1000 days of life (the time spanning roughly between conception and the age of two years) may be an important contributing factor to growth faltering in infants and young children, especially in LMICs. Infants and young children from households consuming diets lacking diversity, i.e. consisting mainly of a single staple food, such as maize, are likely to be most at risk of exposure to aflatoxins.
Description of the intervention
This review examined agricultural and nutritional education interventions aimed at reducing aflatoxin intake at the household or community (smallholder) level in LMIC settings. Smallholders, which are often rural farmers, are usually the biggest consumers of the crops they produce. Therefore, household and community consumption needs to be the focus of aflatoxin control education and behaviour change incentives.
Agricultural education interventions to reduce human consumption of aflatoxins are usually aimed at family members involved in agricultural production at the household or community level, creating awareness about good agricultural practices (for example, drying, sorting, storage, etc.). This education, mostly delivered through agricultural extension services, is usually accompanied by components, such as provision of technology or food replacement, to support access to and implementation of aflatoxin control strategies (for example, specific drying sheets, storage bags, or uncontaminated maize). Nutritional education interventions to reduce the consumption of aflatoxins by young children are usually aimed at women of child‐bearing age, or caregivers from LMIC communities where diets are dominated by a single staple food, but may also target community members involved in community nutrition programs, such as school feeding. This education includes promoting the use of dietary strategies for better aflatoxin control, such as increasing the variety of foods in the diet (household dietary diversity), choosing foods at low risk of aflatoxin contamination instead of those known to be at higher risk, as well as optimal food preparation practices, including traditional practices. Nutritional education may also be delivered with enabling components, such as replacement of contaminated foods with uncontaminated foods in households, the households' food supplies, or the community food supply (for example, local food shops). Numerous cereals, crops, and herbs can be impacted by aflatoxins alongside each commodity's own value chain, resulting in a multisource risk with high contamination levels. Consequently, food safety risks, such as those from aflatoxins, cannot be tackled by specific commodity or market‐based approaches alone; dietary changes and the rediscovery of traditional diverse diets is also needed (Stepman 2018). Various factors can influence observable effects of agricultural and nutritional education interventions to reduce aflatoxin exposure on child growth, such as the child’s age, baseline nutrient adequacy, dietary diversity, and different follow‐up periods used in studies.
How the intervention might work
Agricultural education
Agricultural education aimed at household or community members may promote and enable optimal agricultural practices known to lower the accumulation of aflatoxins in the staple food crops, thereby reducing aflatoxin exposure levels in infants, children, and pregnant and lactating women. This includes, for example, early harvesting; sorting techniques for freshly harvested cereals or nuts to remove those that are physically damaged or have visible moulds using methods, such as handsorting or flotation; proper drying methods for crops as soon as possible after harvesting; optimal storage of crops in dry, well‐vented structures; and administering appropriate amounts of registered insecticides to minimise insects in storage facilities. Agricultural education may also promote the use of processing practices, such as washing, crushing, wet and dry milling, and de‐hulling, as recommended by international food safety standards to reduce aflatoxin contamination of cereals (CAC 2003; Karlovsky 2016; Matumba 2015; Okeke 2018).
Nutritional education
Dietary strategies for aflatoxin control, such as greater dietary diversity, lead to lower intake of aflatoxins for infants and young children in their households. Different food choices by mothers and caregivers, for example, consuming sorghum and millet instead of maize, can also reduce the exposure of their households to aflatoxin (Bandyopadhyay 2007; Klangwiset 2010), as can the use of various food preparation practices. Extending dietary diversity to include locally fermented foods from low mycotoxin content maize, or from grains that are less prone to aflatoxin contamination (e.g. millet, sorghum and rice) could lower household exposure, especially among children for whom these fermented foods serve as primary complementary food in the local setting (Okeke 2015; Wacoo 2019). In addition to reducing aflatoxin exposure levels, these locally fermented foods may enrich the gut with beneficial probiotic bacteria and yeasts (Marco 2017).
Aflatoxins are largely unaffected by routine cooking temperatures, since they decompose at temperatures of 237 ˚C to 306 ˚C. Boiling maize grits reduces aflatoxins by only 28% (Kabak 2008). However, the amount of water used during cooking may be a critical factor in the amount of aflatoxins left for human consumption, due to the dilution effect (Ezekiel 2019a). In regions where water is scarce, the grain is more likely to be cooked in only enough water to be soaked, whereas in others, grains are washed or cooked in plenty of water (or both), some of which is then discarded (Edwards 2018). Boiling rice in excess water or with pressure cooking methods results in a reduction of aflatoxin content by 88% to 89% (Kabak 2008). Traditional methods of cooking maize meal in Central and South America (called nixtamalisation) involves the addition of alkaline compounds, such as lime, which is then washed out. It has been shown that when there is sufficient washing of the lime‐treated product before consumption, the aflatoxin levels are reduced (IARC 2015). However, the efficacy of this method has been questioned, since the chemical reaction that temporarily inactivates aflatoxins may reverse in the gastric acid of the stomach (Strosnider 2006). Other food preparation methods that reduce aflatoxins include roasting (especially in peanuts), baking, or frying (Afolabi 2014; CAC 2003).
Access of households to industrially‐processed foods, such as infant cereals, or spreads and pastes containing nuts, can contribute to aflatoxin exposure, especially for infants and young children (Ojuri 2019). Extrusion processing is used by the food industry to manufacture processed foods, such as breakfast cereals and snacks, from maize or peanut flour, and involves high temperatures (> 150 ˚C), high pressure, and severe shear forces. The destruction of aflatoxins during this process is dependent on a number of factors, such as extruder temperature, screw speed, and moisture content of the extrusion mixture. Such destruction processes usually only remove a proportion of the aflatoxins present (Kabak 2008; Karlovsky 2016).
Why it is important to do this review
Childhood undernutrition has far‐reaching effects beyond health and nutrition. Undernourished children in LMICs are at an increased risk of death from infectious diseases (Black 2013). More specifically, linear growth faltering in the period between conception and the first two years of life is associated with poorer cognitive function in early childhood; sustained effects on cognition, executive function, and school attainment throughout childhood; and ultimately, reduced economic productivity in adulthood, due to lost cognitive potential (Black 2017; Sudfeld 2015). The costs of childhood stunting have been estimated as a reduction in the current per capita income of the workforce in countries in Sub‐Saharan Africa and South Asia, of up to 10% (Galasso 2017).
Various interventions and programmes have been implemented to address the diverse causes and dynamic biological processes that cause undernutrition. The overarching approach to tackling this burden is bringing together nutrition‐specific interventions that target mothers and their children in at‐risk populations, with nutrition‐sensitive approaches that address broader underlying issues, such as food systems, socioeconomic determinants, and disease prevention (Bhutta 2013). A recent literature review on child undernutrition stated that current interventions aimed at reducing child undernutrition may be undermined by high levels of exposure to aflatoxins in the food systems of populations of vulnerable mothers and children (Watson 2017). There is a need to systematically evaluate the effect of household and community level agricultural and nutritional education interventions, aimed at reducing aflatoxin exposure in pregnant women and children, on child growth and other important health outcomes. The findings from this review could support local, regional, and national public health decision makers and other stakeholders in affected areas. The targeted, sustained application of effective agricultural practices, or dietary strategies (or both) to reduce aflatoxin exposure in communities may contribute to preventing childhood growth faltering, and promoting the long‐term well‐being of vulnerable communities in LMICs. Klangwiset and co‐workers reviewed the costs, and estimated cost‐effectiveness (in terms of reductions in aflatoxin exposure) of some of the agricultural and nutritional education intervention strategies, as described above (Klangwiset 2010). The promotion of good post‐harvest agricultural practices, as a result of education intervention strategies, is described as highly cost‐effective. Although the costs of replacing contaminated food crops have not been reported, such strategies are likely to be costly. Therefore, understanding the costs and feasibility of different aflatoxin control interventions can also help decision makers to optimally allocate resources in LMICs.
Objectives
To assess the effects on pre‐ and postnatal growth outcomes when agricultural and nutritional education interventions during the post‐harvest period that aim to reduce aflatoxin exposure are compared to usual support or no intervention. We assessed this in infants, children, and pregnant and lactating women at the household or community level in low‐ and middle‐income countries.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐randomised controlled trials (cluster‐RCTs) with at least two intervention and two control sites (EPOC 2017a). We included cluster‐RCTs, as it was likely that eligible studies would randomise different communities, instead of individual households, or people within communities.
Types of participants
Infants and children (aged < 18 years at the start of the study), and pregnant and lactating women, from low‐ and middle‐income countries (LMIC), as defined by the World Bank (World Bank 2019).
Types of interventions
We included agricultural and nutritional education interventions – with or without related post‐harvest technologies – of any duration, aimed at reducing aflatoxin exposure in infants, children, and pregnant and lactating women, at household and community levels, in LMICs during the post‐harvest period, as detailed below.
We excluded studies that randomised two different educational interventions to reduce household or community aflatoxin consumption without including an inactive control group. We excluded intervention studies that were primarily aimed at reducing the accumulation of aflatoxins in food crops during the planting phase, such as agronomic technologies (for example, genetically resistant, or modified food crops, biocontrol methods).
Eligible interventions
Agricultural education on its own (e.g. training on handsorting; proper drying methods for crops as soon as possible after harvesting; optimal storage of crops in dry, well‐vented structures; training on the use of processing practices, such as washing, crushing, wet and dry milling), with or without post‐harvest technologies (e.g. specific drying sheets, storage bags, or uncontaminated maize), or food replacement, and that was different from the education in the control group
Nutritional education on its own (e.g. promotion of dietary diversity with the inclusion of fermented traditional foods made from sorghum, millet, or rice, or food processing methods, such as nixtamalisation), with or without post‐harvest technologies (e.g. starter culture for fermentation) or food replacement (e,g, swapping contaminated maize for uncontaminated maize), and that was different from any education provided in the control group
Eligible controls
No intervention
Waiting list control, i.e. households or communities were randomly assigned to a waiting list, and received the intervention after the intervention group (Higgins 2011)
Usual support, as reported by study authors (e.g. if a community was already supported by agricultural aid workers, these workers could provide routine mycotoxin control awareness to households in the control group, whereas households in the intervention community should receive additional education and support, such as training related to the post‐harvest technology being provided)
Ineligible interventions
Supplementation of human diets with dietary enterosorbents (specific foods or compounds to reduce the bio‐availability of aflatoxin from contaminated foods), since these interventions are primarily aimed at reducing human exposure to aflatoxins during outbreaks of acute aflatoxicosis
Ineligible controls
Agricultural or nutritional education that is the same as in the intervention group (e.g. education on post‐harvest mycotoxin control plus a post‐harvest technology in the intervention versus the same education in the control group)
Interventions were broadly categorised as:
agricultural education;
nutritional education; or
both.
Types of outcome measures
We analysed the following outcomes from our included studies, where available. However, the outcomes reported by individual studies did not form part of the eligibility criteria for this review.
Primary outcomes
Prenatal growth outcomes measured in infants at birth
Birth length for gestational age z‐score
Birth weight for gestational age z‐score
Low birth weight (less than 2500 g)
Postnatal growth outcomes measured during infancy, childhood, and adolescence (up to the age of 18 years)
Length‐for‐age (LAZ) for children aged up to 24 months; or height‐for‐age z‐score (HAZ) for children older than 24 months
Stunting (defined as LAZ or HAZ more than two standard deviations below the reference median value)
Secondary outcomes
Prenatal growth outcomes measured in infants at birth
Length at birth (cm)
Weight at birth (g)
Gestational age (weeks)
Postnatal growth outcomes measured during infancy, childhood, and adolescence (up to the age of 18 years)
Weight‐for‐height z‐score (WHZ)
Wasting (defined as WLZ or WHZ more than two standard deviations (SD) below the reference median value)
Weight‐for‐age z‐score (WAZ; see Differences between protocol and review)
Proportion of underweight children (WAZ > 2 SD below the reference median value; see Differences between protocol and review)
Other secondary outcomes
Morbidity from infectious diseases in children (for example, diarrhoea, malaria, HBV and HIV infection, diagnosed by a medical doctor)
Unintended effects of agricultural and nutritional education interventions to reduce the aflatoxin intake of infants, children, pregnant and lactating women (for example, an increase in household food expenditure)
Timing of outcome assessment
We excluded any study with a length of follow‐up (i.e. time from baseline to first outcome measurement) of less than four weeks.
We had planned to group the analyses of changes in outcomes in the short‐term (one to three months), medium‐term (longer than three months to six months, and longer than six months to 12 months), and long‐term (longer than one year, longer than two years, longer than three years, etc).
Search methods for identification of studies
We attempted to identify all relevant studies, regardless of language or publication status (published, unpublished, in press, ongoing). We applied no date limitations.
Electronic searches
We searched the following databases and trial registers using the search strategies detailed in Appendix 1.
Cochrane Central Register for Controlled Trials (CENTRAL; 2019, Issue 7) in the Cochrane Library (searched 4 July 2019);
MEDLINE PubMed (1946 to 4 July 2019);
Embase Ovid (1947 to 4 July 2019);
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 5 August 2019);
Web of Science Core Collection with Indexes = SCI‐Expanded, SSCi, CPCI‐S (Clarivate Analytics; searched 4 July 2019);
Africa‐Wide EBSCOhost (searched 5 August 2019);
LILACS (Latin American and Caribbean Health Science Information database; Virtual Health Library; searched 5 July 2019);
CAB Abstracts (CABI; searched 5 August 2019);
Agricola (https://agricola.nal.usda.gov/; searched 22 July 2019);
ClinicalTrials.gov (www.clinicaltrials.gov; searched 5 July 2019);
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/; searched 5 July 2019).
It was not necessary for us to obtain any translations of search records, since all records identified were in English.
Searching other resources
We checked the reference lists of all the included studies, and contacted relevant organisations and agencies (the Partnership for Aflatoxin Control In Africa (PACA); the Consultative Group on International Agricultural Research (CGIAR) Programme on Agriculture for Nutrition and Health; the UK Department for International development (DFID); the International Food Policy Research Institute (IFPRI); the Food Safety and Codex Unit, Food and Agriculture Organization (FAO); and the Department of Food Safety and Zoonoses, World Health Organization (WHO)). We also contacted experts in the field and the authors of relevant ongoing studies to obtain any additional or unpublished data, if available.
Data collection and analysis
Selection of studies
After removing duplicate search records, using Covidence software, three authors (MV, AS, CNE) independently screened the remaining titles and abstracts of the retrieved records, to assess eligibility for inclusion (Covidence). We obtained the full‐text articles of records identified as potentially eligible; two review authors then independently screened these to determine final eligibility. We contacted the study authors of one conference abstract and obtained full‐text reports. We resolved any disagreements at any stage of the eligibility assessment process through discussion and consultation with a third review author (CN), where necessary.
Data extraction and management
Three review authors (MV, CN, CNE) and one other researcher (AB) extracted prespecified data, independently and in duplicate, from each included study, using a data extraction form that was set up and piloted in Covidence (Covidence). We extracted information on study design, funding source, study setting, types of participants, a description of the interventions examined (based on the TIDieR items (Hoffmann 2014)), and costs of the intervention, if reported. We used the PROGRESS framework (Cochrane‐Campbell Methods Group Equity checklist) to record the relevant baseline characteristics of the studies' participants (O'Neill 2014).
We extracted data for primary and secondary outcomes at all time points, using Covidence (Covidence). Where a study reported outcome data for multiple time points (i.e. more than one time point per our prespecified analysis period), we had planned to extract and use data from the longest time point (for example, where results were available at six and nine months, we would use the nine‐month data for the analysis period 'from more than 6 months to 12 months'). However, this was not necessary because data from multiple time points in the same period was not available. Furthermore, we had planned to convert outcome data to SI units where appropriate, but this was not necessary either.
We resolved disagreements during data extraction and management through discussion and consultation with other review authors (AS, CN), where necessary.
Assessment of risk of bias in included studies
We used the Cochrane Effective Practice and Organisation of Care (EPOC) Group's ’Risk of bias’ tool as a framework to assess the risk of bias of all included studies, in Covidence (Covidence; EPOC 2017b). Three review authors (MV, CN, CNE) and one other researcher (AB) performed the risk of bias assessment independently and in duplicate. Disagreements were resolved by discussion and reaching consensus.
Assessing risk of bias in randomised controlled trials (RCTs) and cluster‐RCTs
At the study level, we assessed risk of bias by considering study design and reporting characteristics relevant to our prespecified growth outcomes at birth or postnatally, where available. All growth outcomes were observer‐reported and did not involve judgements (Higgins 2019). We assessed the following nine domains (Higgins 2017).
(1) Random sequence generation
We assessed studies as:
low risk of bias if there was a random component in the sequence generation process (for example, random number table, computer random number generator);
high risk of bias if a non‐random approach was used (for example, odd or even date of birth); or
unclear risk of bias if not specified in the paper.
(2) Allocation concealment
We assessed studies as:
low risk of bias if the unit of allocation was by individual household, and there was some form of centralised randomisation scheme;
high risk of bias if investigators enrolling households could possibly foresee assignments and potentially introduce selection bias (e.g. open random allocation); or
unclear risk of bias if not specified in the paper.
Cluster‐randomised trials often randomise all clusters (i.e. communities) at once, therefore, the lack of concealment of an allocation sequence was not considered to be a major issue in this review (Higgins 2011).
(3) Baseline outcome measurements similar
We assessed studies as:
low risk of bias if baseline outcome characteristics of participants from the study and control groups were reported and were similar;
high risk of bias if important differences in outcomes between groups were present prior to intervention and were not adjusted for in the analysis; or
unclear risk of bias if there was no baseline measure of outcome (note: if assessed as high or unclear risk, but there was sufficient information to conduct an adjusted analysis, the assessment was low).
(4) Baseline characteristics similar
We assessed studies as:
low risk of bias if individual participant or household characteristics were measured prior to the intervention, and no important differences were present across study groups. RCTs were scored as low risk if imbalanced, but appropriate adjusted analysis was performed (for example, analysis of covariance);
high risk of bias if there was no report of characteristics in text or tables, or if there were differences between control and intervention groups; or
unclear risk of bias if it was unclear in the paper (for example, when characteristics were mentioned in text but no data were presented).
Since a small number of clusters are often randomised in cluster‐randomised controlled trials, there was a possibility of chance baseline imbalance between the randomised groups of either the clusters (e.g. communities) or individual households within the clusters (Higgins 2011).
(5) Incomplete outcome data
We assessed outcomes in each included study as:
low risk of bias if missing outcome measures were unlikely to bias the results (for example, the proportion of missing data for individual pregnant women or children was similar in the intervention and control groups, or the proportion of missing data was less than the effect size, i.e. unlikely to overturn the study result);
high risk of bias if missing outcome data were likely to bias the results; or
unclear risk of bias if not specified in the paper.
(6) Knowledge of the allocated interventions adequately prevented during the study
We assessed the risk of performance and detection bias associated with blinding as:
low risk if the study authors stated explicitly that the primary outcome variables were assessed blindly, or if the outcomes were objective, for example, anthropometric measurements of infants or children (low risk of performance bias).
high risk if the outcomes were not assessed blindly, for example conducting anthropometric measurements of infants or children (high risk of detection bias); or
unclear risk if not specified in the paper.
(7) Protection against contamination
We assessed studies as:
low risk of bias if allocation was by community and it was unlikely that the control group received the intervention;
high risk of bias if it was likely that the control group received the intervention; or
unclear risk of bias if community aid workers were allocated within a specific geographical area and it was possible that communication between intervention and control aid workers could have occurred.
(8) Selective outcome reporting
We assessed studies as:
low risk of bias if there was no evidence that outcomes were selectively reported (for example, the study protocol was available and all of the study’s prespecified primary and secondary outcomes of interest in the review were reported in the prespecified way; or if the study protocol was not available, but it was clear that the published report included all expected outcomes, including those that were prespecified);
high risk of bias if some important outcomes were subsequently omitted from the results (for example, if not all prespecified primary outcomes were reported; if one or more primary outcomes were reported using measurements, analysis methods, or subsets of the data that were not prespecified; or if one or more primary outcomes were not prespecified; or if the study report failed to include results for a key outcome that would be expected to have been reported for such a study); or
unclear risk of bias if outcomes were not prespecified in the study protocol or published report.
(9) Other risks of bias
We detailed other possible sources of bias (if any) for each included study and give a rating of low, high, or unclear risk of bias for this item.
For cluster‐RCTs, we also assessed the risk of bias for the following domains (Higgins 2011):
recruitment bias (for example, when individuals are recruited to the trial after the clusters have been randomised);
incorrect analysis (when clustering is not taken into account in the analysis); and
comparability with individually randomised trials.
Overall risk of bias assessment
We assessed the overall risk of bias of an included study as follows:
low risk (low risk of bias for all key domains);
high risk (high risk of bias for one or more key domains); or
unclear risk (unclear risk of bias for one or more key domains).
Included studies at high risk of bias were those with a high risk of bias in the following key domains: similarity of baseline outcome measurements (selection bias) and incomplete outcome data (attrition bias). These 'Risk of bias' summary assessments would have informed our sensitivity analysis (see Sensitivity analysis), but due to the small number of included studies and sparse data we could not perform this.
We assessed the overall risk of bias of outcomes included in the 'Summary of findings' table across included studies as:
low risk (most information is from studies at low risk of bias);
high risk (the proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results (EPOC 2017c)); or
unclear risk (most information is from studies at low or unclear risk of bias).
These 'Risk of bias' summary assessments informed our judgements regarding the quality of the evidence for each outcome, as part of the GRADE process, in our ‘Summary of findings’ tables (see Data synthesis).
Measures of treatment effect
For dichotomous outcomes (for example, prevalence of stunting), we presented results as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes (for example, LAZ), we presented the mean differences (MDs) with 95% CIs because outcomes were measured in the same way between trials. Should we have encountered different trials using different units across included studies, we would have calculated and presented the standardised mean difference (SMD) instead. In this review, we had only endpoint data, but should we encounter a combination of studies reporting endpoint data and change data from baseline in a future update of this review, we will enter both types of data in the same meta‐analysis, if the outcomes are reported using the same unit or scale.
Unit of analysis issues
Studies with more than two intervention groups:
Neither primary nor secondary outcome data were available for one of our included studies that reported numeric outcome data for more than two intervention groups. Should we encounter this in a future review update, we would, where possible, combine groups to create a single pairwise comparison, or use the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions to avoid double counting of study participants (Higgins 2011). Should the control group be shared by two or more study arms in a meta‐analysis, we will divide the control group over the number of relevant subgroup categories to avoid double counting the participants (i.e. for dichotomous data, we will divide the events and the total population, while for continuous data, we will assume the same mean and standard deviation, but will divide the total population).
Cluster‐randomised controlled trials:
The study authors of two cluster‐randomised trials included in this review adjusted for clustering in their analysis (Hoffmann 2018b; Kamala 2018a). In the other cluster‐randomised trial, it is unclear whether there was any adjustment for clustering (Dembedza 2019). This trial reported additional review outcomes, which we have described narratively (See Effects of interventions section). For future reviews, it may be necessary adjust for clustering if the study authors of cluster‐randomised trials do not appropriately account for the cluster design in their analyses of prespecified primary or secondary review outcomes. In order to do so, we will use the ICC derived from the trial (if available), or from another source (for example, using the ICC derived from other, similar trials); or we will estimate the ICC, giving reasons for our choice, and then calculate the design effect, which is 1 + (c ‐ 1) ICC, where c is the average cluster size. Estimated values are arbitrary, but we prefer to use them to adjust the effect estimates due to the implausibility that the ICC is actually zero. For continuous data, we will only adjust the sample size with the design effect, and not the means and standard deviations (SDs). For dichotomous outcomes, we will divide both the sample size and the number of people who experienced the event by the design effect. This review included one study with a parallel group design (Dembedza 2019). In our narrative description of their outcome data, we created two pairwise comparisons; for continuous outcome data we divided the number of participants in the control group, and for dichotomous outcome data, we also divided the number of events in the control group. We were unable to conduct the planned meta‐analyses of combining the estimates from included cluster‐RCTs with trials that had parallel group designs (Higgins 2011).
Dealing with missing data
We contacted study authors of all included studies for clarification of some data (e.g. baseline characteristics), or to request missing or unreported data (such as details of attrition, details of interventions received by the control groups). We assessed the extent and impact of missing data and attrition for each included study during the ’Risk of bias’ assessment.
Assessment of heterogeneity
We could not perform any meta‐analysis in this review (i.e. available data did not allow), but for any meta‐analysis in future review updates, we will examine the forest plots visually to determine whether heterogeneity of the size and direction of treatment effect is present between studies. We will use the I² statistic, Tau², and the Chi² test to quantify the level of heterogeneity among the studies in each analysis. We define substantial heterogeneity as Tau² > 0, and either I² > 50% or P < 0.10 in the Chi² test. We will note this in the text, and explore it by conducting the prespecified subgroup analyses to account for potential sources of clinical heterogeneity (see Subgroup analysis and investigation of heterogeneity). We will also consider other potential sources of clinical heterogeneity, for example, differences in the nature of the interventions delivered, or the presence of co‐contamination with other mycotoxins. We will also examine methodological sources of heterogeneity by examining studies with different levels of risk of bias in a sensitivity analysis (see Sensitivity analysis). We will use caution in the interpretation of results with high levels of unexplained heterogeneity. We will not perform a meta‐analysis if the I² statistic is higher than 90%.
Assessment of reporting biases
We requested missing outcome data from the study authors of one trial (see Selective reporting (reporting bias). Due to the small number of included studies reporting outcome data, we could not conduct a sensitivity analysis to explore the impact of missing outcome data in the overall assessment of results.
There was not a sufficient number of included studies contributing data for any particular outcome for us to examine possible publication bias; however, for future updates of the review, if more than 10 studies reporting the same outcome of interest are available, we will generate funnel plots and visually examine them for asymmetry (Review Manager 2014).
Data synthesis
We could not perform meta‐analysis in this review, but in future review updates, where data allow meta‐analyses, we will use the random‐effects model to combine data across more than one study, as we anticipate that there may be natural heterogeneity between studies, attributable to the different study settings, intervention strategies, or both. We presented the effect size per reported outcome for which we had numeric outcome data in a forest plot.
Summary of findings and assessment of the certainty of the evidence
Three study authors (MV, AS, CN) assessed the certainty of the evidence using the GRADE approach and GRADEpro GDT software (Balshem 2011; Gradepro GDT 2019; Schünemann 2015). This involved judgements in the following domains: within‐study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and publication bias, to arrive at a high, moderate, low, or very low certainty of evidence per outcome. We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid readers’ understanding, where necessary.
We presented the main results in a 'Summary of findings’ table, by summarising the following primary and secondary outcomes (where available) at medium‐term follow‐up time points (where applicable).
Birth length for gestational age z‐score;
Birth weight for gestational age z‐score;
Low birth weight (defined as less than 2500 g);
Length‐ or height‐for‐age z‐score (LAZ or HAZ);
Stunting (defined as LAZ or HAZ more than two standard deviations below the reference median value);
Weight‐for‐height z‐score (WHZ); and
Unintended effects of agricultural and nutritional education interventions to reduce the aflatoxin intake of infants, children, pregnant and lactating women; and
We added the secondary outcomes weight‐for‐age z‐score (WAZ), and the proportion of underweight infants (WAZ ≥ 2 SD below reference median value), which is another way of reporting the effect on WAZ, to our 'Summary of findings' table (Differences between protocol and review).
We interpreted the findings and certainty of the evidence using the informative statements from Santesso 2020.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was not applicable for this review, but should it be in future review updates (i.e. where we have three or more included studies in a meta‐analysis), we plan to carry out the following subgroup analyses in primary outcomes where we detected substantial heterogeneity:
Baseline age of infant and child participants: up to six months; from older than 6 months to 12 months; from older than 12 months to 24 months; > 24 months (preschool); primary school; secondary school;
Length of follow‐up; short‐term (one to three months), medium‐term (longer than three months to six months, longer than 6 months to 12 months), and long‐term (longer than one year, longer than two years, longer than three years, etc).
-
Baseline dietary diversity (defined as a qualitative measure of food consumption reflecting household access to a variety of foods; this can also be a proxy for the nutrient adequacy of the diet of individuals (FAO 2011));
for child participants (child dietary diversity score (CDDS), calculated by counting the number of food groups consumed within a reference period; maximum score of seven (WHO 2007));
for pregnant and lactating mothers (women's dietary score (WDDS), calculated by counting the number of food groups consumed within a reference period; maximum score of nine (FAO and FHI 360 2016));
on household level (household dietary diversity score (HDDS), calculated by counting the number of food groups consumed within a reference period; maximum score of 12 (Swindale 2006)).
Sensitivity analysis
Sensitivity analysis was not applicable in this review, but should it be relevant in future review updates (i.e. if we have three or more studies per meta‐analysis), for primary outcomes, we will assess the effect of:
Risk of bias: removing studies with a high risk of bias – see Assessment of risk of bias in included studies; and
Clustering effect: assessing the strength of the clustering effect in our analysis for studies that have not been adjusted for clustering. In studies where the ICC was estimated from similar studies, sensitivity analysis will be conducted by using higher and lower assumptions of the strength of the clustering effect.
Results
Description of studies
Results of the search
The study flow diagram summarises the results of the searches conducted for this review (Figure 1). We screened the titles and abstracts of 2433 de‐duplicated records, identified through searching electronic databases, searching references, or corresponding with study authors of eligible studies; we identified 29 articles for full‐text assessment. Of these, we excluded 20 full texts excluded with reasons, and identified three studies (reported in 9 full texts) to include in this review.
1.
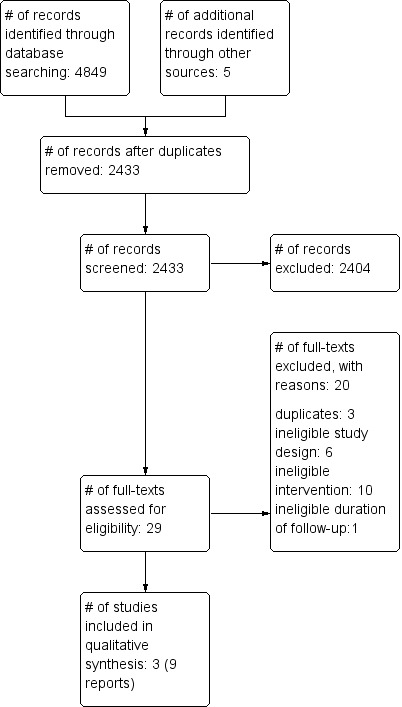
Study flow diagram
Included studies
Setting and context
Studies were undertaken in poor, rural communities where subsistence maize farming is prevalent, in the following countries:
Kenya (Hoffmann 2018b),
Tanzania (Kamala 2018a), and
Zimbabwe (Dembedza 2019).
Participants
Studies recruited farming households in villages with:
pregnant women (18 years or older) in their fifth to final month of pregnancy and their children (Hoffmann 2018b),
lactating women and their breast fed infants (less than six months old (Kamala 2018a)), and
women of childbearing age (15 to 45 years) or young children (younger than 59 months (Dembedza 2019)).
Study design, sample size, and follow‐up
All studies were cluster‐randomised controlled trials (RCT). Two trials randomised villages to the intervention and control groups, including a total of at least 979 mother‐child pairs, from a total of 60 villages (Hoffmann 2018b; Kamala 2018a). We requested details regarding randomisation from study authors for the third trial (Dembedza 2019). This cluster‐RCT randomised 420 households, including 189 mother‐child pairs and 231 women of childbearing age (Dembedza 2019). Duration of the intervention and follow‐up of these studies ranged between five and nine months. One study reported a sample size calculation, based on the expected effect size on the proportion of children exposed to fumonisins above a certain threshold, which is described in the Characteristics of included studies section (Kamala 2018a). However, the calculated study sample was underpowered to detect the expected change in weight‐for‐age z‐score (WAZ) of 0.26, reported in the literature (see Table 2).
1. Optimal information size calculations (continuous outcomes).
| Outcome | Power | Two‐sided significance level | ICC | MD | SD | Sample size (total) |
| WAZa | 80% | 95% | 0.05 | 0.26 | 1.0 | 390 (30 clusters with 13 individuals) |
Abbreviations: ICC: intracluster correlation; MD: mean difference; SD: standard deviation; WAZ: weight‐for‐age z‐score
aThis example is based on the expected effect size of nutrition education on WAZ, in food insecure populations, as reported by Bhutta 2013.
Interventions
Table 3 summarises the intervention details using the template for intervention description and replication (TIDieR) items for each included study (Hoffmann 2014). None of the included trials addressed the effects of nutritional education interventions aiming to reduce aflatoxin exposure (for example, improve dietary diversity, use aflatoxin‐reducing food preparation methods). The agricultural education interventions in two of the three included studies focused on post‐harvest technologies (for example, hermetic storage bags, maize dryer), compared to no intervention (Dembedza 2019), or usual agricultural support (Hoffmann 2018b), for five to nine months.
2. Summary of the intervention details (using TIDieR)a items for each included study.
| Why | What (materials) | What (procedures) | Who provided | How and where | When and how much | Strategies used to maintain or improve fidelity | Extent of intervention fidelity |
| Dembedza 2019 | |||||||
| Intervention group | |||||||
| Awareness and proper use of hermetic storage technology can reduce aflatoxin exposure of households with young children and women of child‐bearing age from the time of harvest throughout the storage season | (1) hermetic grain bags (Super Grain bag IV; Grain Pro Inc., Zambales, Phillipines), or (2) hermetic metal silos (Department of Agricultural Mechanisation, Harare, Zimbabwe & Action Contre La Faim) | Agricultural extension workers were first trained on how to use the technology provided, and this training was cascaded to the households. | Trained agricultural extension workers | Household level | Each household received either (1) 20 hermetic grain bags, or (2) one metal silo at the start of the 9‐month follow‐up period | Quote: "Households were strongly encouraged to store their maize in the facility allocated to them, to and diligently consume maize from these containers for the duration of the study." | NR |
| Control group | |||||||
| N/A | Ordinary polypropolene bags and grass‐thatched granaries | N/A | N/A | N/A | N/A | N/A | N/A |
| Hoffmann 2018b | |||||||
| Intervention group | |||||||
| Aflatoxin exposure in pregnant women and young children in households can be reduced by 1) providing basic information on AF prevention, along with 2) the adoption of good post‐harvest practices, 3) technology subsidies, or 4) price premiums for farmers from these households. | 1) Printed training materials given to farmers who underwent training; asked to share the information with other community members.The content included: what is aflatoxin? why should we be concerned? where is it found? what increases chances of aflatoxin formation? how can I prevent it? and how can I spread the message? (IFPRI) 2) Plastic sheeting, hermetic storage bags (available to farmers who opted to use the mobile maize dryer). An information booklet‐ written in Swahili describing recommended post‐harvest practices, how to access a mobile maize dryer, and the market incentive (if relevant) |
1) Train‐the‐trainer approach: one farmer from each village selected, in consultation with community leaders, to be trained on the causes and consequences of AF contamination in maize, and on recommended practices for its prevention 2) Farmers attended a training session re: good post‐harvest practices (drying, sorting, and storage) 3) Farmers given access to a mobile maize dryer within one of three discount categories (via public lottery): (i) no discount (350 KSh per 90kg bag), (ii) partial discount (150 KSh per bag), or (iii) full discount (no cost) 4) Farmers assigned to a post‐harvest market incentive or not |
1) Paid master trainers (specialists in food quality and post‐harvest handling training) had a facilitators guide (to help them train farmers who were selected from each village. In turn, they were expected to train other farmers in their respective villages. 2) to 4) Study staff |
1) Trained farmers facilitated training of 30 to 40 farmers in their community 2) At village meeting 3) At village meeting, farmers with expected harvests ≥ 45 kg could partake in a public lottery, through which they received vouchers indicating the discount to which they were entitled, to use the maize dryer 4) Separate village meetings for farmers in the market incentive intervention group |
1) Initial training with a group of trainee farmers within each community, followed by 2 to 3 individual follow‐up visits with each trainee farmer 3) Drying service offered immediately after harvest (transportation of farmers and their maize, measurement of grain moisture content before and after drying). Appointments scheduled by phone. Dryer transported to a central location within a village. 4) Farmers could sell up to 45 kg maize at the market price plus a price premium of approximately 50% if their maize tested below the regulatory standard for AF |
1) Farmers in each village who underwent training by a trained farmer signed a certificate, kept by a trained farmer, who received compensation 2) Provision of free plastic sheeting to farmers who attended village meetings 3) Farmers were not allowed to dry more than the prespecified amount of maize. They had to show the voucher indicating the price at which they were entitled to use the dryer, and to verify their identity |
2a) Attendance of training session: 93% ± 1% 2b) Provision of plastic sheeting: 92% ± 1% 2c) Use of plastic sheeting increased from 3% to 5% to 45% to 55% among farmers in the intervention group 2d) Drying maize after shelling, prior to storage: 76% 3) Uptake of drying service: full discount group: 89% of farmers who produced for the market vs 97% of subsistence farmers; no discount group: 15% of farmers who produced for the market vs 60% of subsistence farmers |
| Control group | |||||||
| Aflatoxin exposure in pregnant women and young children in households can be reduced by providing basic information on aflatoxin prevention | Printed training materials given to farmers who underwent training; asked to share the information with other community members.The content included: what is aflatoxin? why should we be concerned? where is it found? what increases chances of aflatoxin formation? how can I prevent it? how can I spread the message? (IFPRI) | Train‐the‐trainer approach. One farmer from each village selected, in consultation with community leaders, to be trained on the causes and consequences of AF contamination in maize, and recommended practices for its prevention | Paid master trainers (specialists in food quality and post‐harvest handling training) had a facilitators guide (to help them train farmers who were selected from each village. In turn, they were expected to train other farmers in their respective villages | Trained farmers facilitated training of 30 to 40 farmers in their community | Initial training with a group of trainee farmers within each community, followed by 2 to 3 individual follow‐up visits with each trainee farmer | Farmers in each village who underwent training by a trained farmer signed a certificate, kept by a trained farmer, who received compensation | Not assessed by study authors |
| Kamala 2018a | |||||||
| Intervention group | |||||||
| An intervention package aimed at changing farmers' post‐harvest practices to prevent or reduce aflatoxin contamination of maize, in addition to a routine service on good crop handling practices, can reduce the aflatoxin exposure of infants and young children in households | Two packs of insecticides, drying sheets | Post‐harvest intervention practices demonstrated to farmers included (1) handsorting; (2) drying surface (the use of mats, or sheets, or raised platforms; (3) adequate sun‐drying; (4) application of insecticides during storage; (5) de‐hulling of maize before milling. Routine agriculture education on good practices for handling crops. |
Agricultural extension workers and health officers | Informal meeting in health facility or school in villages | Duration of the intervention: 6 months. Agriculture extension services offered 'regularly'. | Agricultural extension workers, health officers, resident nurses, and sociologists from all participating villages guided farmers throughout study period. | (1): handsorting: 99% of participants; (2) drying on mat or raised platform: 100% of participants; (3) moisture content testing: 96% of participants; (4) insecticide use: 100% of participants; (5) de‐hulling of maize before milling: 72% of participants |
| Control group | |||||||
| Routine service on good practices for handling crops for improved well‐being and livelihoods of households offered to farmers | NR | Provided farmers with knowledge, experiences, and technologies needed to increase and sustain productivity and avoidance of crop spoilage during storage | Agriculture extension officers | At village level | Agriculture extension services offered 'regularly' | NR | NR |
aTIDieR = template for intervention description and replication. See Hoffmann 2014.
AF = aflatoxin
The study authors of one study provided us with additional information on the delivery of their intervention to households (i.e. trained agriculture extension officers who provided education to households on the use of hermetic bags or metal silos (Dembedza 2019)). Usual agricultural support in the study by Hoffmann 2018b included the training and support of one farmer from each intervention and control village, with the primary purpose of raising the awareness of all farmers within these villages on the risk of aflatoxin contamination of food crops, and basic post‐harvest agricultural practices to prevent aflatoxin contamination. The study authors provided us with the relevant training materials used in their study. In the remaining study, agricultural education and training was part of a post‐harvest package of multiple, locally available mitigation strategies, aimed at changing farmers' post‐harvest practices, to prevent or reduce aflatoxin contamination of maize, and to provide some post‐harvest technologies (for example, insecticides) to support implementation of these strategies, compared to usual agricultural support, for six months (Kamala 2018a). Contact with the study authors confirmed further intervention details (i.e. each intervention household also received drying sheets). Usual agricultural support in this trial included the regular services of agricultural extension workers to farmers on good practices for handling crops, at the village level.
Outcome measures
No included study reported any primary outcomes of interest; one reported a secondary outcome (weight‐for‐age, or WAZ (Kamala 2018a)). One study did not report any outcomes relevant to our review (Hoffmann 2018b). Although study reports by Dembedza 2019 did not report any prespecified review outcomes, the study authors indicated to us that data on postnatal growth outcomes had been collected during their study; however, this unpublished data was not available. None of the included studies reported on prenatal growth outcomes, measured at birth.
We did not prespecify individual aflatoxin exposure outcomes in our protocol; however, since the exposure under investigation is a carcinogenic toxin, we considered it important to also report changes in human exposure to aflatoxins after receiving educational interventions along with post‐harvest technologies aimed at reducing exposure. Two studies reported additional outcomes of interest: the estimated intake of aflatoxins (Kamala 2018a), and urinary concentrations of aflatoxin M1 (AFM1 (Dembedza 2019)).
Funding
Included studies were funded either by non‐profit organisations (including agricultural or food policy research institutes, non‐governmental organisations (NGO), etc) or by governmental or intergovernmental agencies, or both.
For a full description of each included study, please refer to the 'Characteristics of included studies' section.
Excluded studies
We excluded 2404 records after screening titles and abstracts, but we did not record the reasons for exclusion. During full‐text screening, we excluded 18 full texts (Figure 1). Of the excluded full texts: three were duplicates of the same article, six had an ineligible study design, ten did not address an eligible intervention (including three texts related to a published study (Hoffmann 2018a), and two texts related to an ongoing study (NCT03940547)), and one reported an ineligible duration of follow‐up. See 'Characteristics of excluded studies' section.
Ongoing studies
We did not find any potentially eligible ongoing studies.
Risk of bias in included studies
We evaluated the risk of bias of the included studies for each of the nine domains in the Cochrane Effective Practice and Organisation of Care (EPOC) Group tool (see the 'Characteristics of included studies' table). Figure 2 presents a graphical summary of the 'Risk of bias' assessments. We judged the overall risk of bias of one included study as unclear due to attrition bias (Dembedza 2019), while we judged two studies to have a high overall risk of bias due to attrition bias Hoffmann 2018b; Kamala 2018a). Only Kamala 2018a contributed outcome measures to this review's conclusions.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Random sequence generation
We judged two studies at low risk due to an adequate randomised sequence generation. One study did not provide any randomisation sequence details, and we consequently judged it at unclear risk (Dembedza 2019).
Allocation concealment
We judged all three studies to have adequate allocation concealment, since the allocation of clusters occurred at one point in time.
Baseline outcome measurements similar
Two studies did not report any relevant review outcomes, and therefore, this domain did not apply (low risk of bias). Kamala 2018a measured WAZ, but only at end of follow‐up, and thus, we do not know if there were important group differences at baseline (unclear risk of bias).
Baseline characteristics similar
One study reported baseline characteristics for the overall sample only, and therefore, we judged it at unclear risk (Dembedza 2019). We judged the remaining two studies at low risk, due to similarity of baseline characteristics between the intervention and control groups, and the adjustment of analyses for baseline characteristics.
Blinding
Blinding of participants and personnel (performance bias)
None of the studies reported on blinding of participants or study staff; therefore, we judged these studies at unclear risk.
Blinding of outcome assessment (detection bias)
One study reported that their outcome assessors were blinded (Kamala 2018a), whereas another reported objective outcome measures (urinary aflatoxin concentrations (Dembedza 2019)); therefore, we judged both at low risk.
We judged the third study at unclear risk, since it was not clear whether the outcome assessors were blinded, or how a potential lack of blinding could have affected the outcomes, which were not all objective measures.
Protection against contamination (performance bias)
We judged two studies at unclear risk, since it was possible that control villages (Kamala 2018a), or control households within a study village (Dembedza 2019), may have been exposed to the intervention. Contamination was unlikely in one study, since the intervention and control villages were far away from each other.
Incomplete outcome data
We judged the risk of attrition bias as unclear due to insufficient reporting in one study (Dembedza 2019). We judged Kamala 2018a to be at high risk of attrition bias because of significant attrition, and although not discrepant between groups, reasons were unclear and missing data were not handled appropriately. We also judged the third study at high risk due to high overall attrition (Hoffmann 2018b).
Selective reporting
The study protocol was unavailable for one study (Dembedza 2019). We judged the other two studies at low risk, as all prespecified outcomes were reported.
Other potential sources of bias
We judged two studies at high risk of other bias due to the recruitment of participants after randomisation (recruitment bias) in one (Kamala 2018a), and no adjustment for clustering in another (Dembedza 2019). Because we did not include any individually randomised trials, we could not assess the risk of bias in cluster trials in light of comparability with parallel group design trials. It should be noted that in Hoffmann 2018b, it was unclear how the 15 out of 28 originally randomised control clusters were selected for use in this trial.
Effects of interventions
See: Table 1
Here we report on all prespecified outcomes in the included studies. Post‐intervention values per group were reported by one study with available data on our prespecified outcomes. For the comparison in this review, we provide a 'Summary of findings' table containing the outcome WAZ, and proportion of underweight children (WAZ > 2 SD below reference median value; Table 1). The other prespecified outcomes for the 'Summary of findings' table were not reported by the included studies (see Data synthesis for these outcomes). Data did not allow us to do any of our preplanned subgroup or sensitivity analyses.
Agricultural education with post‐harvest technologies vs. usual agricultural support
Primary outcomes
Prenatal growth outcomes measured in infants at birth
Birth length for gestational age z‐score; birth weight for gestational age z‐score; low birth weight (less than 2500 g)
No included trials reported on any of these outcomes.
Postnatal growth outcomes measured during infancy, childhood, and adolescence (up to the age of 18 years)
Length‐ or height‐for‐age z‐score (LAZ or HAZ); stunting (defined as LAZ or HAZ > two standard deviations below the reference median value)
No included trials reported on any of these outcomes.
Secondary outcomes
Prenatal growth outcomes measured in infants at birth
Length at birth (cm); weight at birth (g); gestational age (weeks)
No included trials reported on any of these outcomes.
Postnatal growth outcomes measured during infancy, childhood, and adolescence (up to the age of 18 years)
Weight‐for‐height z‐score (WHZ); wasting (defined as WLZ or WHZ more than two standard deviations below the reference median value)
No included trials reported on any of these outcomes.
Weight‐for‐age z‐score (WAZ)
One cluster‐RCT from Tanzania in lactating women and their breast fed infants (< 6 months old) reported on the infants' WAZ at the end of the six‐month intervention; on average, the children were between 9 and 10 months old in both groups at this time (Kamala 2018a). This trial found that providing agricultural education aimed at changing farmers' post‐harvest practices to reduce aflatoxin exposure, along with the use of insecticides and drying sheets, may improve average WAZ by 0.57 z‐score (95% confidence interval (CI) 0.16 to 0.98; N = 249; Analysis 1.1; Figure 3; very low‐certainty evidence) after six months, in infants from these farmers' households, when compared to infants from households where the farmers only received routine agricultural extension services.
1.1. Analysis.
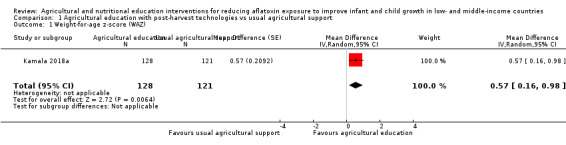
Comparison 1 Agricultural education with post‐harvest technologies vs usual agricultural support, Outcome 1 Weight‐for‐age z‐score (WAZ).
3.
Forest plot of comparison: 1. Agricultural education with post‐harvest technologies vs usual agricultural support, outcome: 1.1 Weight‐for‐age z‐score (WAZ).
Other secondary outcomes
Morbidity from infectious diseases in children (for example, diarrhoea, malaria, HBV and HIV infection diagnosed by a medical doctor); unintended effects of agricultural and nutritional education interventions to reduce the aflatoxin intake of infants, children, pregnant and lactating women (for example, an increase in household food expenditure).
No included trials reported on any of these outcomes.
Additional outcomes
Underweight (defined as WAZ of more than two standard deviations below the reference median value)
The cluster‐RCT from Tanzania in lactating women and their breast fed infants (< 6 months old) also reported on the number of infants who were underweight at the end of the six‐month intervention (Kamala 2018a). This trial found that providing agricultural education aimed at changing farmers' post‐harvest practices to reduce aflatoxin exposure, plus the use of insecticides and drying sheets, may reduce the proportion of underweight infants in these farmers' households by an average of 6.7% (95% CI ‐12.6 to ‐0.8 ; N = 249; Analysis 1.2; Figure 4; very low‐certainty evidence), when compared to infants from households where the farmers only received routine agricultural extension services.
1.2. Analysis.
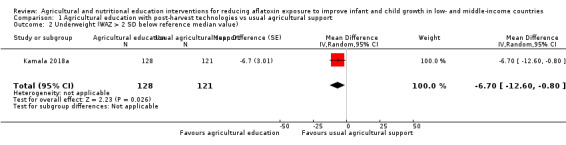
Comparison 1 Agricultural education with post‐harvest technologies vs usual agricultural support, Outcome 2 Underweight (WAZ > 2 SD below reference median value).
4.
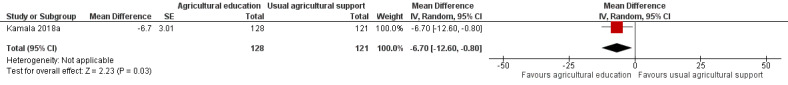
Forest plot of comparison: 1. Agricultural education with post‐harvest technologies vs usual agricultural support, outcome: 1.2 Underweight (WAZ > 2 SD below reference median value)
Estimated intake of aflatoxins
One cluster‐RCT from Tanzania estimated the intake of total aflatoxins (aflatoxin B1, B2, G1 and G2) by infants, by measuring the daily maize intake per infant, and the aflatoxin levels in the stored maize in all included households (Kamala 2018a). The study reported a mean difference in total aflatoxin intake of ‐49 ng/kg/day (95% CI ‐69 to ‐25) after six months, in infants from the group that received agricultural education aimed at changing farmers' post‐harvest practices to prevent or reduce aflatoxin exposure plus the use of insecticides and drying sheets (N = 128), when compared to the infants from households where farmers received routine agricultural extensions services (N = 121).
Urinary concentrations of aflatoxin M1 (AFM1)
One cluster‐RCT reported urinary concentrations of aflatoxin M1 (AFM1) in children (< 59 months old) from two intervention groups, compared to a control group, at three monthly intervals throughout the nine‐month intervention period (Dembedza 2019). The study reported the mean urinary AFM1 concentrations of 162 children (out of 186 children enrolled) from three groups: intervention group 1 that received agricultural education plus hermetic silos for storage (N = 50) ; intervention group 2 that received agricultural education plus hermetic bags (N = 45) ; and a control group that used ordinary polypropolene bags and grass‐thatched granaries (N = 67),
At nine months, less children in the first (RR 0.15; 95% CI 0.08 to 0.30) and second intervention groups (RR 0.60; 95% CI 0.46 to 0.79) had positive urinary AFM1 concentrations when compared to the control group, where 'positive' refers to urine containing AFM1 ≥ limit of detection of 0.0014 µg/L).
The mean AFM1 concentrations were also reported in children from intervention group 1 (GM 3.13 µg/L, N = 47, P = 0.038) and 2 (GM 22.23 µg/L, N = 37, P = 0.044) at nine months, compared to children from the control group (GM 24.34 µg/L, N = 60).
Discussion
Summary of main results
Our review assessed the effects on pre‐ and postnatal growth outcomes in infants, children, and pregnant and lactating women at the household or community level in low‐ and medium‐income countries (LMIC), when agricultural and nutritional education interventions during the post‐harvest period that aim to reduce aflatoxin exposure, were compared to usual support or no intervention, None of the included studies addressed the effects of nutritional education on pre‐ and postnatal growth. We found three eligible cluster‐randomised controlled trials (RCT; reported in nine records), one of which contributed growth outcome data to the comparison in our review, namely agricultural education with post‐harvest technologies compared to usual agricultural support (see Table 1). We addressed the review comparison with a total effective sample size of 249 children, and judged the overall risk of bias of this study as high. In this study, the mean difference in weight‐for‐age z‐score (WAZ) was 0.57 (95% confidence interval (CI) 0.16 to 0.98; N = 249; Analysis 1.1; Figure 3; very low‐certainty evidence), in favour of the children in the group where farmers received agricultural education with post‐harvest technologies (insecticides and drying sheets), compared to usual agricultural support. As an illustrative example, this mean difference in effect would translate to a baby girl with a healthy weight in the intervention group gaining about 450 grams to 690 grams more weight from age three months to nine months, compared to a baby girl in the control group, which is a meaningful difference.
Another way of reporting the effect on WAZ is to compare the proportion of underweight infants (WAZ ≥ 2 SD below the reference median value) per group after the intervention. This trial also reported that the intervention may reduce the proportion of underweight infants in intervention households by an average of 6.7% (95% CI ‐12.6 to ‐0.8; N = 249; Analysis 1.2; Figure 4; very low‐certainty evidence), when compared to those from households where the farmers only received routine agricultural extension services. No included studies reported linear growth outcomes or unintended effects of agricultural and nutritional education. Studies were funded either by non‐profit organisations or by governmental or intergovernmental agencies.
Overall completeness and applicability of evidence
This review is limited in terms of the interventions addressed, i.e. none of the studies addressed the effects of nutritional education with the aim of reducing the aflatoxin exposure, for example, improving dietary diversity, consuming more sorghum or millet instead of maize, or aflatoxin‐reducing food preparation methods (e.g. fermentation). All of the agricultural education interventions included in this review were aimed at reducing the aflatoxin contamination of maize crops. Two studies also reported daily consumption of other food crops susceptible to aflatoxin contamination, such as peanuts, or roots and tubers, by study households (Dembedza 2019), or children (Kamala 2018a).
Furthermore, the evidence from this review is also limited by lack of data for the prespecified review outcomes in the included studies. For example, one included study reported results for the anthropometrical parameter weight‐for‐age z‐score only, since length measurements were not performed (Kamala 2018a). Therefore, we were not able to make any judgement regarding the evidence for the effects of agricultural education with post‐harvest technologies, on linear growth outcomes. As stated previously, poor linear growth, or stunting, has far‐reaching implications in populations in LMICs, in terms of future loss of cognitive potential, and ultimately, reduced human capital in adulthood (Black 2017; Sudfeld 2015). It is also important to note that the infants in Kamala 2018a were only nine months old at study completion. Data from a recent longitudinal study in Gambian infants from rural communities exposed to aflatoxins, suggest that the number of underweight children increases significantly from the age of six months, up to 24 months of age. Study authors reported that the proportion of underweight infants increased between six and 24 months of age from 10% to 24.4% (Watson 2018). Lastly, the evidence from this review is also limited since none of the studies included in this review reported growth outcome measures at birth.
Although human exposure to aflatoxin contamination was not one of the prespecified outcomes for this review, we reported findings on aflatoxin exposure outcomes, namely estimated daily intake of aflatoxins in infants (Kamala 2018a), and urinary concentrations of aflatoxin M1 (AFM1) in children (Dembedza 2019).
Future reviews that synthesise measures of aflatoxin levels in harvested crops, after having implemented aflatoxin mitigation interventions or strategies, are likely to provide valuable insights pertinent to earlier activities in the pathway from agricultural production to consumption.
Finally, none of the included studies reported on the outcome of child morbidity, an important factor to consider since it has been suggested that aflatoxin‐exposed children may be at risk of developing enteropathy, as well as immune suppression, resulting in more frequent infections, such as diarrhoea (Smith 2012; Watson 2017).
In terms of costs, one study reported the estimated costs of implementing an aflatoxin awareness programme in both intervention and control communities, as well as the costs associated with the delivery of the intervention (mobile drying service, hermetic bags, plastic drying sheets (Hoffmann 2018b)). Study authors of the second trial described their intervention as a low‐cost intervention, but did not report any information related to costs incurred (e.g. provision of drying sheets, insecticides (Kamala 2018a)). The third study did not provide any information regarding the costs of their interventions (metal silos and hermetic bags (Dembedza 2019)). See Characteristics of included studies for further information.
Quality of the evidence
We considered the certainty of the evidence for two important outcomes (WAZ; proportion of underweight children (WAZ ≥ 2 SD below the reference median value) reported in one included study in this review to be very low, meaning that we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect (Kamala 2018a). We downgraded the certainty of the evidence for the following reasons:
Risk of bias: high risk of attrition bias
Imprecision: the optimal information size was not met;
Indirectness: cannot generalise the findings of a small, single trial to other LMIC settings and populations
Potential biases in the review process
It is unlikely that we missed any relevant studies, because we performed a comprehensive search of the literature, and two researchers independently selected studies for inclusion, extracted data, and assessed risk of bias of the included studies.
Because there were fewer than 10 included studies, we were unable to assess the likelihood of publication bias formally.
Agreements and disagreements with other studies or reviews
To our knowledge, no systematic review has specifically considered the effectiveness of interventions to reduce exposure of aflatoxins for improving infant and child growth in LMICs.
There is no Cochrane Review that specifically considered the effectiveness of interventions to reduce the exposure of any mycotoxin (including aflatoxins) on child health outcomes. However, there are other (non‐Cochrane) reviews that have investigated the relationship between mycotoxins and child growth outcomes. A systematic review by Tesfamariam and colleagues investigated whether dietary mycotoxin exposure influenced child growth, the immune system, morbidity, and mortality (Tesfamarium 2019). They found that the results were limited by the "reporting quality, difference in findings, heterogeneity of outcomes, mycotoxin detection methods, and the observational nature of most studies", and rated the overall quality of evidence as very low (Tesfamarium 2019). Similarly, a report by the World Health Organization (WHO) International Agency for Research on Cancer included a review to investigate the effects of aflatoxins and fumonisins on child growth in 2015 (IARC 2015). Although not fully systematic, review authors subjected studies to some quality criteria for inclusion in the review. From six included studies, the review authors concluded that aflatoxins were likely to contribute to child growth impairment, but again, the evidence was limited (IARC 2015).
Increased knowledge on mitigation strategies to reduce aflatoxin exposure in food crops resulting from agricultural education initiatives, does not necessarily translate into improved practices (Seetha 2019). Affordable, effective interventions, aimed at mitigating aflatoxin contamination of (especially staple) foods along the value chain, require priority consideration in high risk communities and the most vulnerable populations in LMICs. Stakeholder involvement is critical to drive such interventions at the household and community levels, and feasible mechanisms for frequent engagement of relevant stakeholders needs to be established (Ezekiel 2019b).
Authors' conclusions
Implications for practice.
This review provides limited implications for practice, from very low‐certainty evidence, suggesting that agricultural education may result in an increase in weight‐for‐age z‐score (WAZ) when compared to usual or no education. However, there is a need to be mindful of aflatoxin‐prone food crops and post‐harvest practices in low‐ and medium‐income countries (LMIC) when planning and implementing food security programmes and public health policies to adequately address food safety and security, particularly those affecting pregnant women and children, and smallholder farmers.
Implications for research.
This review highlighted the need for well‐designed randomised controlled trials to evaluate the effects and cost‐effectiveness of mitigation strategies, including those with agricultural and nutritional education components, to reduce the exposure of vulnerable populations, such as children and pregnant and lactating women in LMICs, to aflatoxins. Trials should ideally be conducted across several sites, whereby an intervention package is tailored to communities in several countries across regions. This will aid comparative analysis of effectiveness and applicability of interventions for diverse populations.
Intervention strategies should not focus on household consumption of certain staple foods alone, but on total food consumption at different time intervals during the study period, to capture the variation in human dietary exposure of the study population. Special attention is also required at household level in the choice and formulation of cereal and nut mixes as complementary foods for infants and children. Trials are needed that examine interventions presented as a package, and including nutritional and agricultural education, along with pre‐, peri‐, and post‐harvest agricultural interventions and technologies for a variety of food crops. Trials should be designed to include longitudinal outcome measurements that span at least one to two years, and all seasons, in order to obtain good quality effectiveness data. More trials should include the measurement of child growth outcomes, such as those specified in the methods section of this review. Growth outcomes, as well as related health outcomes, such as measures of child morbidity (e.g. infections, diarrhoea, enteropathy) are important to deduce the possible translational impact of agricultural and nutritional education interventions to reduce aflatoxin exposure.
Given the limited evidence from RCTs, and recognising the challenges of such trials that may take years to complete, it is also important to consider the value of synthesised evidence from non‐randomised studies to inform decisions in the immediate future.
Acknowledgements
We would like to thank the following individuals:
Amanda Brand (AB) for support with data extraction and 'Risk of bias' assessment;
Tonya Esterhuizen for statistical guidance and support;
Marion Kelly, Taryn Young, and Michael Routledge for their support in the identification of the review topic and guidance during the preparation of this protocol;
Jodie Doyle, Ursula Griebler, Welcome Wami, Ruth Dundas, Irma Klerings, and Miranda Cumpston from the Cochrane Public Health Group for editorial support; and
Amare Ayalew for acting as external referee on the protocol and the review; Alan Dangour for acting as external referee on the review.
Marianne Visser (MV), Anel Schoonees (AS), and Celeste Naude (CN) are partially supported by the Research, Evidence and Development Initiative (READ‐It) project (project number 300342‐104). READ‐It is funded by aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
We developed this review as part of the Aubrey Sheiham Evidence‐Based Health Care in Africa Leadership Award, which was received by MV to promote evidence synthesis capacity‐building and to support working with Chibundu N Ezekiel (CNE); and which is administered by Cochrane South Africa, based at the South African Medical Research Council.
Appendices
Appendix 1. Search strategies
CENTRAL; 2019, Issue 7 in the Cochrane Library (searched 4 July 2019)
#1 (pregnan* OR mother* OR maternal):ti,ab,kw
#2 breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk
#3 (lactating OR lactation):ti,ab,kw
#4 (newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal):ti,ab,kw
#5 (child* OR toddler* OR adolescent*):ti,ab,kw
#6 MeSH descriptor: [Pregnant Women] explode all trees
#7 MeSH descriptor: [Pregnancy] explode all trees
#8 MeSH descriptor: [Breast Feeding] explode all trees
#9 MeSH descriptor: [Adolescent] explode all trees
#10 MeSH descriptor: [Infant] explode all trees
#11 MeSH descriptor: [Child] explode all trees
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#13 aflatoxin* OR mycotoxin*
#14 MeSH descriptor: [Mycotoxins] this term only
#15 MeSH descriptor: [Aflatoxins] explode all trees
#16 #13 OR #14 OR #15
#17 #12 AND #16
#18 ((Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America")):ti,ab,kw
#19 ((Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic")):ti,ab,kw
#20 ((Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania)):ti,ab,kw
#21 ((Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico")):ti,ab,kw
#22 ((Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon or "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia)):ti,ab,kw
#23 ((developing or less* NEXT developed or "under developed" or underdeveloped or "middle income" or low* NEXT income or underserved or "under served" or deprived or poor*) NEXT (countr* or nation* or population* or world)):ti,ab,kw
#24 ((developing or less* NEXT developed or "under developed" or underdeveloped or "middle income" or low* NEXT income) NEXT (economy or economies)):ti,ab,kw
#25 (low* NEXT (gdp or gnp or "gross domestic" or "gross national")):ti,ab,kw
#26 ((low NEAR/3 middle NEAR/3 countr*)):ti,ab,kw
#27 ((lmic or lmics or "third world" or "lami country" or "lami countries")):ti,ab,kw
#28 (("transitional country" or "transitional countries")):ti,ab,kw
#29 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28
#30 #17 AND #29 in Trials
MEDLINE PubMed (searched 4 July 2019)
| #23 | Search (#17 AND #22) |
| #22 | Search (#18 OR #19 OR #20 OR #21) |
| #21 | Search (("developing country"[tw] OR "developing countries"[tw] OR "developing nation"[tw] OR "developing nations"[tw] OR "developing population"[tw] OR "developing populations"[tw] OR "developing world"[tw] OR "less developed country"[tw] OR "less developed countries"[tw] OR "less developed nation"[tw] OR "less developed nations"[tw] OR "less developed population"[tw] OR "less developed populations"[tw] OR "less developed world"[tw] OR "lesser developed country"[tw] OR "lesser developed countries"[tw] OR "lesser developed nation"[tw] OR "lesser developed nations"[tw] OR "lesser developed population"[tw] OR "lesser developed populations"[tw] OR "lesser developed world"[tw] OR "under developed country"[tw] OR "under developed countries"[tw] OR "under developed nation"[tw] OR "under developed nations"[tw] OR "under developed population"[tw] OR "under developed populations"[tw] OR "under developed world"[tw] OR "underdeveloped country"[tw] OR "underdeveloped countries"[tw] OR "underdeveloped nation"[tw] OR "underdeveloped nations"[tw] OR "underdeveloped population"[tw] OR "underdeveloped populations"[tw] OR "underdeveloped world"[tw] OR "middle income country"[tw] OR "middle income countries"[tw] OR "middle income nation"[tw] OR "middle income nations"[tw] OR "middle income population"[tw] OR "middle income populations"[tw] OR "low income country"[tw] OR "low income countries"[tw] OR "low income nation"[tw] OR "low income nations"[tw] OR "low income population"[tw] OR "low income populations"[tw] OR "lower income country"[tw] OR "lower income countries"[tw] OR "lower income nation"[tw] OR "lower income nations"[tw] OR "lower income population"[tw] OR "lower income populations"[tw] OR "underserved country"[tw] OR "underserved countries"[tw] OR "underserved nation"[tw] OR "underserved nations"[tw] OR "underserved population"[tw] OR "underserved populations"[tw] OR "underserved world"[tw] OR "under served country"[tw] OR "under served countries"[tw] OR "under served nation"[tw] OR "under served nations"[tw] OR "under served population"[tw] OR "under served populations"[tw] OR "under served world"[tw] OR "deprived country"[tw] OR "deprived countries"[tw] OR "deprived nation"[tw] OR "deprived nations"[tw] OR "deprived population"[tw] OR "deprived populations"[tw] OR "deprived world"[tw] OR "poor country"[tw] OR "poor countries"[tw] OR "poor nation"[tw] OR "poor nations"[tw] OR "poor population"[tw] OR "poor populations"[tw] OR "poor world"[tw] OR "poorer country"[tw] OR "poorer countries"[tw] OR "poorer nation"[tw] OR "poorer nations"[tw] OR "poorer population"[tw] OR "poorer populations"[tw] OR "poorer world"[tw] OR "developing economy"[tw] OR "developing economies"[tw] OR "less developed economy"[tw] OR "less developed economies"[tw] OR "lesser developed economy"[tw] OR "lesser developed economies"[tw] OR "under developed economy"[tw] OR "under developed economies"[tw] OR "underdeveloped economy"[tw] OR "underdeveloped economies"[tw] OR "middle income economy"[tw] OR "middle income economies"[tw] OR "low income economy"[tw] OR "low income economies"[tw] OR "lower income economy"[tw] OR "lower income economies"[tw] OR "low gdp"[tw] OR "low gnp"[tw] OR "low gross domestic"[tw] OR "low gross national"[tw] OR "lower gdp"[tw] OR "lower gnp"[tw] OR "lower gross domestic"[tw] OR "lower gross national"[tw] OR lmic[tw] OR lmics[tw] OR "third world"[tw] OR "lami country"[tw] OR "lami countries"[tw] OR "transitional country"[tw] OR "transitional countries"[tw])) |
| #20 | Search ((Africa[tw] OR Asia[tw] OR Caribbean[tw] OR West Indies[tw] OR South America[tw] OR Latin America[tw] OR Central America[tw] OR Afghanistan[tw] OR Albania[tw] OR Algeria[tw] OR Angola[tw] OR Antigua[tw] OR Barbuda[tw] OR Argentina[tw] OR Armenia[tw] OR Armenian[tw] OR Aruba[tw] OR Azerbaijan[tw] OR Bahrain[tw] OR Bangladesh[tw] OR Barbados[tw] OR Benin[tw] OR Byelarus[tw] OR Byelorussian[tw] OR Belarus[tw] OR Belorussian[tw] OR Belorussia[tw] OR Belize[tw] OR Bhutan[tw] OR Bolivia[tw] OR Bosnia[tw] OR Herzegovina[tw] OR Hercegovina[tw] OR Botswana[tw] OR Brasil[tw] OR Brazil[tw] OR Bulgaria[tw] OR Burkina Faso[tw] OR Burkina Fasso[tw] OR Upper Volta[tw] OR Burundi[tw] OR Urundi[tw] OR Cambodia[tw] OR Khmer Republic[tw] OR Kampuchea[tw] OR Cameroon[tw] OR Cameroons[tw] OR Cameron[tw] OR Camerons[tw] OR Cape Verde[tw] OR Central African Republic[tw] OR Chad[tw] OR Chile[tw] OR China[tw] OR Colombia[tw] OR Comoros[tw] OR Comoro Islands[tw] OR Comores[tw] OR Mayotte[tw] OR Congo[tw] OR Zaire[tw] OR Costa Rica[tw] OR Cote d'Ivoire[tw] OR Ivory Coast[tw] OR Croatia[tw] OR Cuba[tw] OR Cyprus[tw] OR Czechoslovakia[tw] OR Czech Republic[tw] OR Slovakia[tw] OR Slovak Republic[tw] OR Djibouti[tw] OR French Somaliland[tw] OR Dominica[tw] OR Dominican Republic[tw] OR East Timor[tw] OR East Timur[tw] OR Timor Leste[tw] OR Ecuador[tw] OR Egypt[tw] OR United Arab Republic[tw] OR El Salvador[tw] OR Eritrea[tw] OR Estonia[tw] OR Ethiopia[tw] OR Fiji[tw] OR Gabon[tw] OR Gabonese Republic[tw] OR Gambia[tw] OR Gaza[tw] OR Georgia Republic[tw] OR Georgian Republic[tw] OR Ghana[tw] OR Gold Coast[tw] OR Greece[tw] OR Grenada[tw] OR Guatemala[tw] OR Guinea[tw] OR Guam[tw] OR Guiana[tw] OR Guyana[tw] OR Haiti[tw] OR Honduras[tw] OR Hungary[tw] OR India[tw] OR Maldives[tw] OR Indonesia[tw] OR Iran[tw] OR Iraq[tw] OR Isle of Man[tw] OR Jamaica[tw] OR Jordan[tw] OR Kazakhstan[tw] OR Kazakh[tw] OR Kenya[tw] OR Kiribati[tw] OR Korea[tw] OR Kosovo[tw] OR Kyrgyzstan[tw] OR Kirghizia[tw] OR Kyrgyz Republic[tw] OR Kirghiz[tw] OR Kirgizstan[tw] OR "Lao PDR"[tw] OR Laos[tw] OR Latvia[tw] OR Lebanon[tw] OR Lesotho[tw] OR Basutoland[tw] OR Liberia[tw] OR Libya[tw] OR Lithuania[tw])) |
| #19 | Search ((Macedonia[tw] OR Madagascar[tw] OR Malagasy Republic[tw] OR Malaysia[tw] OR Malaya[tw] OR Malay[tw] OR Sabah[tw] OR Sarawak[tw] OR Malawi[tw] OR Nyasaland[tw] OR Mali[tw] OR Malta[tw] OR Marshall Islands[tw] OR Mauritania[tw] OR Mauritius[tw] OR Agalega Islands[tw] OR Mexico[tw] OR Micronesia[tw] OR Middle East[tw] OR Moldova[tw] OR Moldovia[tw] OR Moldovian[tw] OR Mongolia[tw] OR Montenegro[tw] OR Morocco[tw] OR Ifni[tw] OR Mozambique[tw] OR Myanmar[tw] OR Myanma[tw] OR Burma[tw] OR Namibia[tw] OR Nepal[tw] OR Netherlands Antilles[tw] OR New Caledonia[tw] OR Nicaragua[tw] OR Niger[tw] OR Nigeria[tw] OR Northern Mariana Islands[tw] OR Oman[tw] OR Muscat[tw] OR Pakistan[tw] OR Palau[tw] OR Palestine[tw] OR Panama[tw] OR Paraguay[tw] OR Peru[tw] OR Philippines[tw] OR Philipines[tw] OR Phillipines[tw] OR Phillippines[tw] OR Poland[tw] OR Portugal[tw] OR Puerto Rico[tw] OR Romania[tw] OR Rumania[tw] OR Roumania[tw] OR Russia[tw] OR Russian[tw] OR Rwanda[tw] OR Ruanda[tw] OR Saint Kitts[tw] OR St Kitts[tw] OR Nevis[tw] OR Saint Lucia[tw] OR St Lucia[tw] OR Saint Vincent[tw] OR St Vincent[tw] OR Grenadines[tw] OR Samoa[tw] OR Samoan Islands[tw] OR Navigator Island[tw] OR Navigator Islands[tw] OR Sao Tome[tw] OR Saudi Arabia[tw] OR Senegal[tw] OR Serbia[tw] OR Montenegro[tw] OR Seychelles[tw] OR Sierra Leone[tw] OR Slovenia[tw] OR Sri Lanka[tw] OR Ceylon[tw] OR Solomon Islands[tw] OR Somalia[tw] OR Sudan[tw] OR Suriname[tw] OR Surinam[tw] OR Swaziland[tw] OR Syria[tw] OR Tajikistan[tw] OR Tadzhikistan[tw] OR Tadjikistan[tw] OR Tadzhik[tw] OR Tanzania[tw] OR Thailand[tw] OR Togo[tw] OR Togolese Republic[tw] OR Tonga[tw] OR Trinidad[tw] OR Tobago[tw] OR Tunisia[tw] OR Turkey[tw] OR Turkmenistan[tw] OR Turkmen[tw] OR Uganda[tw] OR Ukraine[tw] OR Uruguay[tw] OR USSR[tw] OR Soviet Union[tw] OR Union of Soviet Socialist Republics[tw] OR Uzbekistan[tw] OR Uzbek OR Vanuatu[tw] OR New Hebrides[tw] OR Venezuela[tw] OR Vietnam[tw] OR Viet Nam[tw] OR West Bank[tw] OR Yemen[tw] OR Yugoslavia[tw] OR Zambia[tw] OR Zimbabwe[tw] OR Rhodesia[tw])) |
| #18 | Search ((Developing Countries[Mesh:noexp] OR Africa[Mesh:noexp] OR Africa, Northern[Mesh:noexp] OR Africa South of the Sahara[Mesh:noexp] OR Africa, Central[Mesh:noexp] OR Africa, Eastern[Mesh:noexp] OR Africa, Southern[Mesh:noexp] OR Africa, Western[Mesh:noexp] OR Asia[Mesh:noexp] OR Asia, Central[Mesh:noexp] OR Asia, Southeastern[Mesh:noexp] OR Asia, Western[Mesh:noexp] OR Caribbean Region[Mesh:noexp] OR West Indies[Mesh:noexp] OR South America[Mesh:noexp] OR Latin America[Mesh:noexp] OR Central America[Mesh:noexp] OR Afghanistan[Mesh:noexp] OR Albania[Mesh:noexp] OR Algeria[Mesh:noexp] OR American Samoa[Mesh:noexp] OR Angola[Mesh:noexp] OR "Antigua and Barbuda"[Mesh:noexp] OR Argentina[Mesh:noexp] OR Armenia[Mesh:noexp] OR Azerbaijan[Mesh:noexp] OR Bahrain[Mesh:noexp] OR Bangladesh[Mesh:noexp] OR Barbados[Mesh:noexp] OR Benin[Mesh:noexp] OR Byelarus[Mesh:noexp] OR Belize[Mesh:noexp] OR Bhutan[Mesh:noexp] OR Bolivia[Mesh:noexp] OR Bosnia‐Herzegovina[Mesh:noexp] OR Botswana[Mesh:noexp] OR Brazil[Mesh:noexp] OR Bulgaria[Mesh:noexp] OR Burkina Faso[Mesh:noexp] OR Burundi[Mesh:noexp] OR Cambodia[Mesh:noexp] OR Cameroon[Mesh:noexp] OR Cape Verde[Mesh:noexp] OR Central African Republic[Mesh:noexp] OR Chad[Mesh:noexp] OR Chile[Mesh:noexp] OR China[Mesh:noexp] OR Colombia[Mesh:noexp] OR Comoros[Mesh:noexp] OR Congo[Mesh:noexp] OR Costa Rica[Mesh:noexp] OR Cote d'Ivoire[Mesh:noexp] OR Croatia[Mesh:noexp] OR Cuba[Mesh:noexp] OR Cyprus[Mesh:noexp] OR Czechoslovakia[Mesh:noexp] OR Czech Republic[Mesh:noexp] OR Slovakia[Mesh:noexp] OR Djibouti[Mesh:noexp] OR "Democratic Republic of the Congo"[Mesh:noexp] OR Dominica[Mesh:noexp] OR Dominican Republic[Mesh:noexp] OR East Timor[Mesh:noexp] OR Ecuador[Mesh:noexp] OR Egypt[Mesh:noexp] OR El Salvador[Mesh:noexp] OR Eritrea[Mesh:noexp] OR Estonia[Mesh:noexp] OR Ethiopia[Mesh:noexp] OR Fiji[Mesh:noexp] OR Gabon[Mesh:noexp] OR Gambia[Mesh:noexp] OR "Georgia (Republic)"[Mesh:noexp] OR Ghana[Mesh:noexp] OR Greece[Mesh:noexp] OR Grenada[Mesh:noexp] OR Guatemala[Mesh:noexp] OR Guinea[Mesh:noexp] OR Guinea‐Bissau[Mesh:noexp] OR Guam[Mesh:noexp] OR Guyana[Mesh:noexp] OR Haiti[Mesh:noexp] OR Honduras[Mesh:noexp] OR Hungary[Mesh:noexp] OR India[Mesh:noexp] OR Indonesia[Mesh:noexp] OR Iran[Mesh:noexp] OR Iraq[Mesh:noexp] OR Jamaica[Mesh:noexp] OR Jordan[Mesh:noexp] OR Kazakhstan[Mesh:noexp] OR Kenya[Mesh:noexp] OR Korea[Mesh:noexp] OR Kosovo[Mesh:noexp] OR Kyrgyzstan[Mesh:noexp] OR Laos[Mesh:noexp] OR Latvia[Mesh:noexp] OR Lebanon[Mesh:noexp] OR Lesotho[Mesh:noexp] OR Liberia[Mesh:noexp] OR Libya[Mesh:noexp] OR Lithuania[Mesh:noexp] OR Macedonia[Mesh:noexp] OR Madagascar[Mesh:noexp] OR Malaysia[Mesh:noexp] OR Malawi[Mesh:noexp] OR Mali[Mesh:noexp] OR Malta[Mesh:noexp] OR Mauritania[Mesh:noexp] OR Mauritius[Mesh:noexp] OR Mexico[Mesh:noexp] OR Micronesia[Mesh:noexp] OR Middle East[Mesh:noexp] OR Moldova[Mesh:noexp] OR Mongolia[Mesh:noexp] OR Montenegro[Mesh:noexp] OR Morocco[Mesh:noexp] OR Mozambique[Mesh:noexp] OR Myanmar[Mesh:noexp] OR Namibia[Mesh:noexp] OR Nepal[Mesh:noexp] OR Netherlands Antilles[Mesh:noexp] OR New Caledonia[Mesh:noexp] OR Nicaragua[Mesh:noexp] OR Niger[Mesh:noexp] OR Nigeria[Mesh:noexp] OR Oman[Mesh:noexp] OR Pakistan[Mesh:noexp] OR Palau[Mesh:noexp] OR Panama[Mesh:noexp] OR Papua New Guinea[Mesh:noexp] OR Paraguay[Mesh:noexp] OR Peru[Mesh:noexp] OR Philippines[Mesh:noexp] OR Poland[Mesh:noexp] OR Portugal[Mesh:noexp] OR Puerto Rico[Mesh:noexp] OR Romania[Mesh:noexp] OR Russia[Mesh:noexp] OR "Russia (Pre‐1917)"[Mesh:noexp] OR Rwanda[Mesh:noexp] OR "Saint Kitts and Nevis"[Mesh:noexp] OR Saint Lucia[Mesh:noexp] OR "Saint Vincent and the Grenadines"[Mesh:noexp] OR Samoa[Mesh:noexp] OR Saudi Arabia[Mesh:noexp] OR Senegal[Mesh:noexp] OR Serbia[Mesh:noexp] OR Montenegro[Mesh:noexp] OR Seychelles[Mesh:noexp] OR Sierra Leone[Mesh:noexp] OR Slovenia[Mesh:noexp] OR Sri Lanka[Mesh:noexp] OR Somalia[Mesh:noexp] OR South Africa[Mesh:noexp] OR Sudan[Mesh:noexp] OR Suriname[Mesh:noexp] OR Swaziland[Mesh:noexp] OR Syria[Mesh:noexp] OR Tajikistan[Mesh:noexp] OR Tanzania[Mesh:noexp] OR Thailand[Mesh:noexp] OR Togo[Mesh:noexp] OR Tonga[Mesh:noexp] OR "Trinidad and Tobago"[Mesh:noexp] OR Tunisia[Mesh:noexp] OR Turkey[Mesh:noexp] OR Turkmenistan[Mesh:noexp] OR Uganda[Mesh:noexp] OR Ukraine[Mesh:noexp] OR Uruguay[Mesh:noexp] OR USSR[Mesh:noexp] OR Uzbekistan[Mesh:noexp] OR Vanuatu[Mesh:noexp] OR Venezuela[Mesh:noexp] OR Vietnam[Mesh:noexp] OR Yemen[Mesh:noexp] OR Yugoslavia[Mesh:noexp] OR Zambia[Mesh:noexp] OR Zimbabwe[Mesh:noexp])) |
| #17 | Search (#13 AND #16) |
| #16 | Search (#14 OR #15) |
| #15 | Search ("Mycotoxins"[Mesh:NoExp] OR "Aflatoxins"[Mesh]) |
| #14 | Search (aflatoxin* OR mycotoxin*) |
| #13 | Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) |
| #12 | Search "Child"[Mesh] |
| #11 | Search "Infant"[Mesh] |
| #10 | Search "Adolescent"[Mesh] |
| #9 | Search "Lactation"[Mesh] |
| #8 | Search "Breast Feeding"[Mesh] |
| #7 | Search "Pregnancy"[Mesh] |
| #6 | Search "Pregnant Women"[Mesh] |
| #5 | Search (child*[Title/Abstract] OR toddler*[Title/Abstract] OR adolescent*[Title/Abstract]) |
| #4 | Search (newborn*[Title/Abstract] OR neonate*[Title/Abstract] OR infant*[Title/Abstract] OR baby[Title/Abstract] OR babies[Title/Abstract] OR neonatal[Title/Abstract] OR prenatal[Title/Abstract]) |
| #3 | Search (lactating[Title/Abstract] OR lactation[Title/Abstract]) |
| #2 | Search (breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk) |
| #1 | Search (pregnan*[Title/Abstract] OR mother*[Title/Abstract] OR maternal[Title/Abstract]) |
Embase Ovid (searched 4 July 2019)
1 (pregnan* or mother* or maternal).ab. or (pregnan* or mother* or maternal).ti.
2 (breastfeed* or "breast feed" or "breast fed" or "breast feeding" or "breast milk" or breastmilk).ab. or (breastfeed* or "breast feed" or "breast fed" or "breast feeding" or "breast milk" or breastmilk).ti.
3 (lactating or lactation).ab. or (lactating or lactation).ti.
4 (newborn* or neonate* or infant* or baby or babies or neonatal or prenatal).ab. or (newborn* or neonate* or infant* or baby or babies or neonatal or prenatal).ti.
5 (child* or toddler* or adolescent*).ab. or (child* or toddler* or adolescent*).ti.
6 pregnant woman/
7 pregnancy/
8 breast feeding/
9 lactation/
10 child/
11 infant/
12 adolescent/
13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
14 (aflatoxin* or mycotoxin*).ab. or (aflatoxin* or mycotoxin*).ti.
15 aflatoxin/
16 mycotoxin/
17 14 or 15 or 16
18 13 and 17
19 developing countries.mp. or Developing Countries/
20 exp Africa/ or africa.mp.
21 asia.mp. or Asia/
22 caribbean.mp. or Caribbean/
23 west indies.mp. or Caribbean Islands/
24 south america.mp. or South America/
25 "South and Central America"/
26 (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or Burkina Faso or Burkina Fasso or Upper Volta or Burundi or Urundi or Cambodia or Khmer Republic or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or Cape Verde or Central African Republic or Chad or Chile or China or Colombia or Comoros or Comoro Islands or Comores or Mayotte or Congo or Zaire or Costa Rica or Cote d'Ivoire or Ivory Coast or Croatia or Cuba or Cyprus or Czechoslovakia or Czech Republic or Slovakia or Slovak Republic or Djibouti or French Somaliland or Dominica or Dominican Republic or East Timor or East Timur or Timor Leste or Ecuador or Egypt or United Arab Republic or El Salvador or Eritrea or Estonia or Ethiopia or Fiji or Gabon or Gabonese Republic or Gambia or Gaza or Georgia Republic or Georgian Republic or Ghana or Gold Coast or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or Isle of Man or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or Kyrgyz Republic or Kirghiz or Kirgizstan or Lao PDR or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania or Macedonia or Madagascar or Malagasy Republic or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or Marshall Islands or Mauritania or Mauritius or Agalega Islands or Mexico or Micronesia or Middle East or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or Netherlands Antilles or New Caledonia or Nicaragua or Niger or Nigeria or Northern Mariana Islands or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or Puerto Rico or Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or Saint Kitts or St Kitts or Nevis or Saint Lucia or St Lucia or Saint Vincent or St Vincent or Grenadines or Samoa or Samoan Islands or Navigator Island or Navigator Islands or Sao Tome or Saudi Arabia or Senegal or Serbia or Montenegro or Seychelles or Sierra Leone or Slovenia or Sri Lanka or Ceylon or Solomon Islands or Somalia or South Africa or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or Togolese Republic or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or Soviet Union or Union of Soviet Socialist Republics or Uzbekistan or Uzbek or Vanuatu or New Hebrides or Venezuela or Vietnam or Viet Nam or West Bank or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesi).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
27 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28 18 and 27
Africa‐Wide EBSCOhost (searched 5 August 2019)
S3 S1 AND S2
S2 ( aflatoxin* OR mycotoxin* ) OR SM Mycotoxins AND SM Aflatoxins
S1 ( pregnan* OR mother* OR maternal ) OR ( breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk ) OR ( lactating OR lactation ) OR ( newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal ) OR ( child* OR toddler* OR adolescent* ) OR SM "Pregnant women" OR SM Pregnancy OR SM "Breast feeding" OR SM Lactation OR SM Adolescent OR SM Infant OR SM Child
CINAHL EBSCOhost (searched 5 August 2019)
S14 S9 AND S13
S13 S10 OR S11 OR S12
S12 (MH "Mycotoxins+")
S11 (MM "Aflatoxins")
S10 aflatoxin* OR mycotoxin*
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
S8 (MH "Child+")
S7 (MH "Infant+")
S6 (MH "Adolescence+")
S5 (MM "Lactation")
S4 (MM "Breast Feeding")
S3 (MH "Pregnancy+")
S2 (MM "Expectant Mothers")
S1 ( pregnan* OR mother* OR maternal ) OR ( breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk ) OR ( lactating OR lactation ) OR ( newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal ) OR ( child* OR toddler* OR adolescent* )
Web of Science ‐ core collection (searched 4 July 2019)
| # 21 | #20 AND #8 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 20 | #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 19 |
TOPIC: ((("transitional country" or "transitional countries"))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 18 |
TOPIC: (((lmic or lmics or "third world" or "lami country" or "lami countries"))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 17 |
TOPIC: (((low NEAR/3 middle NEAR/3 countr*))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 16 |
TOPIC: ((low* NEXT (gdp or gnp or "gross domestic" or "gross national"))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 15 |
TOPIC: (((developing or less* NEXT developed or "under developed" or underdeveloped or "middle income" or low* NEXT income) NEXT (economy or economies))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 14 |
TOPIC: (((developing or less* NEXT developed or "under developed" or underdeveloped or "middle income" or low* NEXT income or underserved or "under served" or deprived or poor*) NEXT (countr* or nation* or population* or world))) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 13 |
TOPIC: (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon or "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 12 |
TOPIC: (Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 11 |
TOPIC: (Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 10 |
TOPIC: (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 9 |
TOPIC: (Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 8 | #7 AND #6 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 7 | #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 6 |
TOPIC: (aflatoxin* OR mycotoxin*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 5 |
TOPIC: (child* OR toddler* OR adolescent*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 4 |
TOPIC: (newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 3 |
TITLE: (lactating OR lactation) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 2 |
TOPIC: (breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
| # 1 |
TOPIC: (pregnan* OR mother* OR maternal) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S Timespan=All years |
|||||
LILACS (Latin American and Caribbean Health Science Information database) virtual health library (searched 5 July 2019)
aflatoxin OR mycotoxin OR aflatoxins OR mycotoxins [Words] and infant$ OR child$ OR toddler$ OR adolescen$ OR pregnan$ OR newborn$ OR neonat$ OR breastfeed$ OR breast feed$ OR breastmilk OR breast milk OR breastfed OR breast fed OR mother$ OR maternal OR lactation OR lactating OR prenatal OR baby OR babies [Words]
CAB Abstracts (CABI; searched 5 August 2019)
#1 pregnan* OR mother* OR maternal
#2 breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk
#3 lactating OR lactation
#4 newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal
#5 child* OR toddler* OR adolescent*
#6 (child* OR toddler* OR adolescent*) OR (newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal) OR (lactating OR lactation) OR (breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk) OR (pregnan* OR mother* OR maternal)
#7 subject:(Pregnant Women) OR subject:(Pregnancy) OR subject:("Breast feeding") OR subject:(Lactation) OR subject:(Adolescent) OR subject:(Infant) OR subject:(Child)
#8 aflatoxin* OR mycotoxin*
#9 subject:(Mycotoxins) OR subject:(Aflatoxins)
#10 (aflatoxin* OR mycotoxin*) AND ((child* OR toddler* OR adolescent*) OR (newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal) OR (lactating OR lactation) OR (breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk) OR (pregnan* OR mother* OR maternal))
#11 (Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America")
#12 (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic")
#13 (Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania)
#14 (Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia OR Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico")
#15 (Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon OR "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia)
#16 ("transitional country" or "transitional countries" OR lmic or lmics or "third world" or "lami country" or "lami countries" OR "developing country" OR "developing countries" OR "less developed country" OR "less developed countries" OR "under developed country" OR "underdeveloped country" OR "under developed countries" OR "underdeveloped countries" OR "low‐income country" OR "low‐income countries" OR "middle‐income country" OR "middle‐income countries" OR "low income country" OR "low income countries" OR "middle income country" OR "middle income countries" OR "low‐ and middle‐income country" OR "low‐ and middle‐income countries" OR "low and middle income country" OR "low and middle income countries" OR LMIC OR LMICs OR "third world country" OR "third world countries" OR underserved OR deprived OR "poor country" OR "poor countries" OR "poor nation" OR "poor nations" OR "poor population" OR "poor populations")
#17 (("transitional country" or "transitional countries" OR lmic or lmics or "third world" or "lami country" or "lami countries" OR "developing country" OR "developing countries" OR "less developed country" OR "less developed countries" OR "under developed country" OR "underdeveloped country" OR "under developed countries" OR "underdeveloped countries" OR "low‐income country" OR "low‐income countries" OR "middle‐income country" OR "middle‐income countries" OR "low income country" OR "low income countries" OR "middle income country" OR "middle income countries" OR "low‐ and middle‐income country" OR "low‐ and middle‐income countries" OR "low and middle income country" OR "low and middle income countries" OR LMIC OR LMICs OR "third world country" OR "third world countries" OR underserved OR deprived OR "poor country" OR "poor countries" OR "poor nation" OR "poor nations" OR "poor population" OR "poor populations")) OR ((Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon OR "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia)) OR ((Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia OR Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico")) OR ((Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania)) OR ((Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic")) OR ((Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America"))
#18 ((("transitional country" or "transitional countries" OR lmic or lmics or "third world" or "lami country" or "lami countries" OR "developing country" OR "developing countries" OR "less developed country" OR "less developed countries" OR "under developed country" OR "underdeveloped country" OR "under developed countries" OR "underdeveloped countries" OR "low‐income country" OR "low‐income countries" OR "middle‐income country" OR "middle‐income countries" OR "low income country" OR "low income countries" OR "middle income country" OR "middle income countries" OR "low‐ and middle‐income country" OR "low‐ and middle‐income countries" OR "low and middle income country" OR "low and middle income countries" OR LMIC OR LMICs OR "third world country" OR "third world countries" OR underserved OR deprived OR "poor country" OR "poor countries" OR "poor nation" OR "poor nations" OR "poor population" OR "poor populations")) OR ((Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or "Saint Kitts" or "St Kitts" or Nevis or "Saint Lucia" or "St Lucia" or "Saint Vincent" or "St Vincent" or Grenadines or Samoa or "Samoan Islands" or "Navigator Island" or "Navigator Islands" or "Sao Tome" or "Saudi Arabia" or Senegal or Serbia or Montenegro or Seychelles or "Sierra Leone" or Slovenia or "Sri Lanka" or Ceylon OR "Solomon Islands" or Somalia or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or "Togolese Republic" or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or "Soviet Union" or "Union of Soviet Socialist Republics" or Uzbekistan or Uzbek or Vanuatu or "New Hebrides" or Venezuela or Vietnam or "Viet Nam" or "West Bank" or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia)) OR ((Macedonia or Madagascar or "Malagasy Republic" or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or "Marshall Islands" or Mauritania or Mauritius or "Agalega Islands" or Mexico or Micronesia or "Middle East" or Moldova or Moldovia or Moldovian or Mongolia OR Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or "Netherlands Antilles" or "New Caledonia" or Nicaragua or Niger or Nigeria or "Northern Mariana Islands" or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or "Puerto Rico")) OR ((Djibouti or "French Somaliland" or Dominica or "Dominican Republic" or "East Timor" or "East Timur" or "Timor Leste" or Ecuador or Egypt or "United Arab Republic" or "El Salvador" or Eritrea or Estonia or Ethiopia or Fiji or Gabon or "Gabonese Republic" or Gambia or Gaza or Georgia or Georgian or Ghana or "Gold Coast" or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or "Isle of Man" or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or "Kyrgyz Republic" or Kirghiz or Kirgizstan or "Lao PDR" or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania)) OR ((Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or "Burkina Faso" or "Burkina Fasso" or "Upper Volta" or Burundi or Urundi or Cambodia or "Khmer Republic" or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or "Cape Verde" or "Central African Republic" or Chad or Chile or China or Colombia or Comoros or "Comoro Islands" or Comores or Mayotte or Congo or Zaire or "Costa Rica" or "Cote d'Ivoire" or "Ivory Coast" or Croatia or Cuba or Cyprus or Czechoslovakia or "Czech Republic" or Slovakia or "Slovak Republic")) OR ((Africa or Asia or Caribbean or "West Indies" or "South America" or "Latin America" or "Central America"))) AND ((aflatoxin* OR mycotoxin*) AND ((child* OR toddler* OR adolescent*) OR (newborn* OR neonate* OR infant* OR baby OR babies OR neonatal OR prenatal) OR (lactating OR lactation) OR (breastfeed* OR "breast feed" OR "breast fed" OR "breast feeding" OR "breast milk" OR breastmilk) OR (pregnan* OR mother* OR maternal)))
Agricola (https://agricola.nal.usda.gov/)
Database: NAL Article Citation Database, NAL Cataloging Database (searched 22 July 2019)
Keyword Anywhere(aflatoxin? mycotoxin?) AND Keyword Anywhere(pregnant pregnancy mother? infant? infancy child children newborn? neonate? neonatal? baby babies prenatal?)
ClinicalTrials.gov (www.clinicaltrials.gov; searched 5 July 2019)
aflatoxin OR mycotoxin OR aflatoxins OR mycotoxins
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/; searched 5 July 2019)
aflatoxin OR mycotoxin OR aflatoxins OR mycotoxins in the Title; Recruitment status: ALL
Data and analyses
Comparison 1. Agricultural education with post‐harvest technologies vs usual agricultural support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight‐for‐age z‐score (WAZ) | 1 | 249 | Mean Difference (Random, 95% CI) | 0.57 [0.16, 0.98] |
| 2 Underweight (WAZ > 2 SD below reference median value) | 1 | 249 | Mean Difference (Random, 95% CI) | ‐6.70 [‐12.60, ‐0.80] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dembedza 2019.
| Methods |
Study design: cluster‐randomised controlled trial Country: Zimbabwe Setting: smallholder farming households within villages from two maize producing districts (Mashonaland Centrla; Manicaland) Study aim: to assess the effectiveness of hermetic storage technology in reducing human aflatoxin exposure from the time of maize harvesting throughout the storage season, by measuring the levels of aflatoxin M1 present in urine from children under five years of age and women of child‐bearing age from smallholder farming households where hermetic storage technology was used to store maize grain Study dates: June 2015 to May 2016 Recruitment: all the households in each selected enumeration area (EA) were visited (1384 households) Sampling: purposive sampling of six administrative areas from each study district; random sampling of two EAs or villages from each administrative area (total of 12 EAs) Sample size justification: NR Unit of randomisation: households Total number randomised: 420 households (120 intervention 1; 120 intervention 2, and 150 control); 420 women (189 mother‐child pairs) Unit of analysis: women and children Intention‐to‐treat analysis: NR Attrition: 3 children dropped out; attrition per group unclear (number of women and children enrolled not reported per group) Relevant study limitations as reported by study authors: foods, such as vegetables and legumes, could be potential sources of exposure to aflatoxins; foods not analysed for aflatoxin content, and urinary AFM1 is not a long‐term marker of aflatoxin exposure |
|
| Participants |
Inclusion criteria: availability and permanent residence of a woman between 15 years and 45 years of age and a child under 59 months in a household, or both Exclusion criteria: NR Group differences: NR Subgroups: NR Baseline characteristics of participants Overall Mother (N = 189)
Child (N = 189)
Household (N = 420)
|
|
| Interventions |
Intervention Type: hermetic storage technology Description: households received training regarding one of two types of hermetic technologies, namely hermetic storage bags or hermetic metal silos Delivery: agricultural extension officers trained households re: the use of hermetic storage bags or silos; frequency and duration of training not specified Providers: staff of the Ministry of Agriculture, Mechanisation and Irrigation Development; the University of Zimbabwe Institute of Food, Nutrition and Family Sciences, and our research partner Action Contre’ la Faim provided technical and logistical support Duration of intervention: 9 months Duration of follow‐up: 9 months Co‐intervention: NR Cost: NR Resource requirements: NR Control Type: no intervention Description: households that continued storing their maize grain in conventional storage facilities, which included mainly ordinary polypropylene bags and grass‐thatched granaries Delivery: not applicable (n/a) Providers: n/a Duration of intervention: n/a Duration of follow‐up: 9 months Co‐intervention: NR Cost: n/a Resource requirements: n/a |
|
| Outcomes |
Outcomes:
|
|
| Notes |
Sponsorship source: International Development Research Centre, Canada; Australian Centre for International Agricultural Research, and Australian International Food Security Research Centre Grant No. 107838 Study title or name (study acronym): n/a Author’s name: MP Dembedza, LK Nyanga Email: Nyangael@yahoo.com Declarations of interest: yes; quotation: "The authors declare no conflict of interest." Trial registration: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors only stated that random allocation of interventions was performed at household level, but did not provide details re: the generation of the random sequence. |
| Allocation concealment (selection bias) | Low risk | No information about concealment, but random allocation was done at household level at one point in time, therefore, concealment of an allocation sequence should not be an issue. |
| Baseline outcome measurements similar | Low risk | Study did not report any relevant review outcomes, and therefore, this domain does not apply. |
| Baseline characteristics similar | Unclear risk | The study authors only reported baseline characteristics for the overall sample, not per group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition, missing outcome data, and reasons for missing outcome data; no mention of how missing outcome data were addressed. Reasons for variations in missing data and time points reported as "mainly due to the absence of some participants on the day of sample collection". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of personnel not reported; however, blinding of participants unlikely (intervention and control households in the same village) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors not reported, but outcome measurement (urinary aflatoxin AFM1 concentrations) not likely to be influenced by lack of blinding. |
| Protection against contamination | Unclear risk | Households in the same village were allocated to different intervention groups, presenting a risk of contamination. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, and outcomes stated in the methods section were reported in the results section; personal communication with trial authors confirmed that child growth data were collected in the trial, but these results were not included in the papers provided by authors at the time of contact. |
| Other bias | High risk | Recruitment bias (cluster‐RCT): low risk; individuals were recruited to the trial before clusters were randomised. Incorrect analysis (cluster‐RCT): high risk; no evidence that clustering was taken into account, since the unit of analysis (women and children) was different from the unit of allocation (households) in the analysis |
Hoffmann 2018b.
| Methods |
Study design: cluster‐randomised controlled trial Country: Kenya Setting: villages within two maize‐producing counties (Meru, Tharaka‐Nithi) in Eastern Kenya Study aim: to evaluate the impact of a package of post‐harvest technologies appropriate for use by smallholder farmers on contamination of maize with aflatoxin Study dates: June 2013 to October or November 2013 Recruitment: farmers from households in intervention villages were visited at their homes and invited to attend an information meeting in the village about aflatoxin prevention and access to post‐harvest technologies. Farmers from households in control villages were approached by a trained farmer in their village to attend a training session on aflatoxin prevention. Sampling: random sampling of villages Sample size justification: no Unit of randomisation: villages Total number randomised: 30 villages (15 intervention; 15 control); 679 households (350 intervention; 329 control) Unit of analysis: households; standard errors were bootstrapped to account for the effect of clustering at the village level Intention‐to‐treat analysis: Yes. Quote: “Analysis of the intervention’s impact was conducted using multivariate linear regression based on an intent‐to‐treat approach…” Missing control variables were imputed for use in multivariate linear regression." Attrition: 139 households (82 intervention; 47 control) for intent to pay for treatment technology and reported post‐harvest losses, but 508 households (277 intervention; 231 control) for aflatoxin contamination of household stored maize Relevant study limitations as reported by study authors: farmers who still had maize in their households three months after harvest were relatively more well‐off, on average, than those who did not. Small sample sizes limited conclusions on the relative contributions of training and technology. Aflatoxin contamination varies considerably by year; research spanning several seasons would have been ideal. |
|
| Participants |
Inclusion criteria: households with women who were at least 18 years of age and in their fifth to final month of pregnancy, as estimated by the woman. Infants delivered from the pregnancy for which their mothers were enrolled; if an infant died or moved outside the study area, a child aged between 12 months and 35 months, residing in the household, was taken as a replacement child. Exclusion criteria: NR Group differences: more control households had heard of aflatoxins, compared to intervention households (P = 0.01) Subgroups: subsistence farmers vs market producers Baseline characteristics of participants Intervention group (N = 329) Mother
Child
Household
Control group (N = 350) Mother
Child
Household
|
|
| Interventions |
Intervention: Type: package of post‐harvest technologies plus standard of care for aflatoxin prevention Description: aflatoxin prevention training: standard of care aflatoxin prevention training using the 'training the trainers' approach. Intervention: farmers attended village meeting during which they heard recommended post‐harvest practices for aflatoxin control and the use of a mobile maize dryer. Post‐harvest agricultural intervention: households with an expected harvest of > 45 kg randomly assigned to one of the following groups in terms of access to a mobile maize dryer and hermetic storage bags: 1. Full discount (free access); 2. Partial discount (150 KSh per 90 kg bag), or 3. No discount (350 KSh per 90 kg bag). Intervention households were also randomly assigned (1:1) to a market incentive or no market incentive. Separate meetings conducted in each village with farmers assigned to the market incentive payment vs those not offered payment. Aflatoxin prevention training by 'training the trainers' approach: at least one farmer from each of the study villages was selected, in consultation with community leaders, to receive training re: the causes and consequences of aflatoxin contamination in maize and recommended practices for aflatoxin prevention. Delivery: intervention: additional training conducted at village meetings with eligible farmers. Quotation: "more detailed than the training of trainers offered to the villages". A booklet given to each household describing recommended post‐harvest practices and how to access a mobile maize dryer. Post‐harvest agricultural technology: each household received free plastic sheeting. Appointments via telephone for the use of the dryer within a village. A study dryer transported to a central location within the village. The drying service included transportation of farmers and their maize from their homestead to the dryer location, measurement of initial grain moisture content, use of the flatbed dryer, and post‐drying moisture testing. Aflatoxin prevention training: elected representative farmers trained and asked to pass the information on to other farmers in their village Providers: study staff (intervention); master trainers (aflatoxin prevention training) Duration of intervention: five months Duration of follow‐up: five months Co‐intervention: NR Cost: standard of care for aflatoxin prevention: cost of master trainers (who facilitated the training of one farmer from each village): approximately USD 1.70 per farmer (two meetings with 25 farmers each per day, transport rental was USD 60 and trainers' wages were USD 25). Compensation for trained farmers (who facilitated training of farmers in their own community) not reported by study authors. Intervention: provision of drying sheets: USD 5 per farmer; training: USD 1.70 per farmer (included vehicle rental cost of USD 60 per day and trainer wage of USD 25 for two meetings of 25 farmers per day); mobile drying service: 2.93 Ksh per kg (fully subsidised); 0.73 Ksh per kg (partially subsidised); hermetic storage bags: 220 KSh per bag. Exchange rate 101.2 KSh = USD 1 (March 2018) Resource requirements: personnel: trainers (specialists in food quality and post‐harvest handling); equipment: plastic sheeting (500 gauge plastic); mobile maize dryer (EasyDry500; www.acdivoca.org/easydry), hermetically seal able storage bags (Purdue Improved Cowpea Storage (PICS) bags) Control Type: standard of care for aflatoxin prevention Description: aflatoxin prevention training by 'training the trainers' approach: at least one farmer from each of the study villages was selected, in consultation with community leaders, to receive training re: the causes and consequences of aflatoxin contamination in maize and recommended practices for aflatoxin prevention Delivery: elected representative farmers trained and asked to pass the information on to other farmers in their village Providers: master trainers Duration of intervention: five months Duration of follow‐up: five months Co‐intervention: NR Cost: standard of care for aflatoxin prevention: cost of master trainers (who facilitated the training of one farmer from each village): approximately USD 1.70 per farmer (two meetings with 25 farmers each per day, transport rental was USD 60 and trainers' wages were USD 25. Compensation of trained farmers (who facilitated training of farmers in their own community) not reported by study authors. Resource requirements: personnel: trainers (specialists in food quality and post‐harvest handling) |
|
| Outcomes |
Outcomes:
|
|
| Notes |
Sponsorship source: Ministry for Foreign Affairs of Finland through the FoodAfrica Programme, UK aid from the British people, the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH) led by the International Food Policy Research Institute, and the Bill and Melinda Gates Foundation Grand Challenge Explorations Award Study title or name (study acronym): Mitigating aflatoxin exposure to improve child growth in Eastern Kenya (MAICE) Author’s name: Vivian Hoffmann; Alexia Pretari Email: v.hoffmann@cgiar.org; alexia.pretari@gmail.com; Declarations of interest: yes. Quotation: "Declarations of interest: None." Trial registration: AEARCTR‐0000105 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Clusters (villages) were simultaneously assigned to intervention or control, therefore, allocation could not be foreseen in advance of enrolment. |
| Baseline outcome measurements similar | Low risk | Study did not report any relevant review outcomes, and therefore, this domain does not apply. |
| Baseline characteristics similar | Low risk | More households in control group had heard of aflatoxin prior to the study, compared to intervention households (P = 0.01). However, study authors controlled for baseline characteristics in their analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High total attrition. Attrition was a bit higher in the intervention group (23.4%), compared to the control group (17.3%). Specifically, for the measurement of aflatoxin in stored home‐produced maize, data were collected from only 171 households (no. per group not reported; attrition of 74.8%). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether the assessors who conducted the follow‐up survey were blinded. While maize contamination assays comparing intervention and control were objective, self‐reported post‐harvest practices were not. |
| Protection against contamination | Low risk | Intervention and control villages were approximately 4 km apart. Study staff held meetings with eligible households in each cluster. Access to mobile maize dryer in intervention clusters restricted to households that could produce a voucher. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available at www.socialscienceregistry.org/trials/105, and all prespecified outcomes were reported. |
| Other bias | Unclear risk | Recruitment bias (cluster‐RCT): randomisation appears to have followed recruitment as the train‐the‐trainer approach was conducted before allocation to trial arms of interest for this study: low risk. Incorrect analysis (cluster‐RCT): low risk; standard errors were bootstrapped to correct for clustering at the village level. Other bias: unclear risk. It is unclear how the study authors selected the 15 control clusters in this study from the 25 control clusters that were generated by the randomisation sequence. |
Kamala 2018a.
| Methods |
Study design: cluster‐randomised controlled trial Country: Tanzania Setting: smallholder farming households producing maize, primarily for home consumption, from villages in the Northern highland, Eastern lowland, and South‐Western highland Study aim: to evaluate the effectiveness of a post‐harvest intervention package as a mitigation strategy for reducing aflatoxin and fumonisin contamination in maize, and subsequently, dietary exposure of infants Study dates: May to November 2013 (Hanang and Kilosa districts); February to August 2014 (Rungwe district) Recruitment: eligible infants aged 0 to 6 months were identified from their registration number and date of birth, and their households were recruited one month before the harvesting period Sampling: purposive sampling of three maize‐producing districts; followed by random sampling of 30 villages, and stratified sampling of 10 households from each village Sample size justification: yes; using a difference of 50% in the proportion of children exposed to fumonisin B1 above the provisional maximum tolerable daily intake (PMTDI) of 2 ug/kg bw/day; alpha of 0.05 (two‐tailed test), variation in cluster (km) at 0.25, and power at 80%. 30 clusters with 10 households per cluster required Unit of randomisation: villages Total number randomised: 30 villages (15 intervention; 15 control); 300 households (150 intervention households; 10 households per village and 150 control households; 10 households per village) Unit of analysis: households; study authors adjusted for the effect of clustering using multilevel mixed‐effect models Intention‐to‐treat analysis: quotation: "Analysis of the primary outcomes was by intention‐to‐treat, and all missing households were analysed as part of the community in which they were enrolled." Attrition: no loss of clusters. WAZ outcome: 51 children (22 intervention, 29 control); total aflatoxin and fumonisin content of maize outcome: 39 households (14 intervention, 25 control) Relevant study limitations as reported by study authors: linear growth of children not being measured due to fieldwork limitations; lack of baseline data on various outcomes; differential dropout; lack of morbidity information for the children using maize as the only dietary source of aflatoxins and fumonisins, and the use of insecticides and consequent exposure |
|
| Participants |
Inclusion criteria: households with a breastfed infant aged 0 to 6 months, born to parents who were local residents at the time of recruitment; maize producing households with capacity of storing maize for at least six months after harvest Exclusion criteria: households with an infant having a known congenital malformation or chronic condition that could affect weight Group differences: NR Subgroups: NR Baseline characteristics of participants Intervention group (N = 150) Mother
Child
Household
Control group (N = 150) Mother
Child
Household
|
|
| Interventions |
Intervention Type: post‐harvest intervention package and agricultural extension services Description: routine agricultural extension on good practice for handling crops plus training for identifying and sorting infected or damaged maize kernels, demonstrations on drying maize on raised platforms or sheets, provision of sheets for drying, education on adequate sun drying and how to test for dryness, advice to de‐hull maize before milling, training on the use of insecticides with maize kernels and the provision of insecticides Delivery: farmers gathered at nearby health facility or school in the particular village for informal meetings; intervention and agricultural extension provided from harvest until six months post‐harvest, but frequency and duration not specified Providers: Duration of intervention: six months Duration of follow‐up: six months Co‐intervention: NR Cost: NR Resource requirements: sheets for drying provided to participants, bottles and salt for testing maize dryness, and insecticides Control Type: routine agricultural extension services Description: routine agricultural extension on good practice around the handling of farmers' crop Delivery: agricultural extension was provided from harvest until six months post‐harvest. Frequency and duration of intervention was not specified, but the study authors referred to a regular service for farmers at the village level. Providers: agricultural extension (AE) workers Duration of intervention: six months Duration of follow‐up: six months Co‐intervention: NR Cost: NR Resource requirements: NR |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Sponsorship source: Flemish Interuniversity Council‐Institutional University cooperation (VLIR‐UOS); grant number ZEIN2011PR388 Study title or name (study acronym): Intervention in minimizing aflatoxins and fumonisins exposure to children through food and breastfeeding in Tanzania Author’s name: Analice Kamala; Martin E. Kimanya Email: analice.kamala@tfda.go.tz; martin.kimanya@nm‐aist.ac.tz; Mekimanya@yahoo.co.uk Declarations of interest: no Trial registration: NCT02438774 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | All clusters assigned simultaneously by one investigator (not involved in field work) according to randomisation sequence |
| Baseline outcome measurements similar | Unclear risk | We do not know if there were important group differences in the review outcome, WAZ, at baseline, as these measurements were not done. |
| Baseline characteristics similar | Low risk | Baseline characteristics were reported as comparable for all assessed variables. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No loss of clusters. Overall attrition for the WAZ outcome was 17% (intervention 14.6% (22/150); control 19.3% (29/150)). Among the reasons cited was the category 'unwilling to participate' (intervention: 9/150; control: 13/150), which was not specific enough to assess whether this attrition impacted the reported effect. Furthermore, reasons were not provided for 12 trial participants with missing WAZ outcome data (6 participants per group). Missing data for the secondary outcome, WAZ, were not handled appropriately. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether personnel or participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The data collection team was separate from the intervention support team, and they were blinded to the village allocation. |
| Protection against contamination | Unclear risk | It is possible that agricultural extension officers, based within a specific geographical area, communicated with each other. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes indicated in NCT02438774 were reported, with the exception of aflatoxin and fumonisin levels in breast milk in the main citation. However, the study authors reported these outcomes in earlier conference proceedings. |
| Other bias | High risk | Recruitment bias (cluster‐RCT): high risk; recruitment followed randomisation. Incorrect analysis (cluster‐RCT): low risk; the study authors used multilevel mixed‐effects models, with fixed‐effect for study group, and random‐effects for the village. |
NR = not reported; n/a = not applicable; RCT = randomised controlled trial; SE = standard error; SD = standard deviation; KSh = Kenyan Shillings; WAZ = weight‐for‐age z‐score;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Grace 2015 | Ineligible study design |
| Hoffmann 2018a | Ineligible intervention. Study authors confirmed via email correspondence (see Hoffmann 2018a) that a general agricultural education programme was implemented in both intervention and control villages, primarily aimed at farmers to raise their awareness of basic prevention measures to prevent contamination of food crops with aflatoxins. Study authors described this programme as the 'standard of care' for communities at high risk for exposure to aflatoxins, and thus, the education was the same in both groups. In addition, the intervention villages had their contaminated maize replaced with uncontaminated maize. |
| IFPRI 2017 | Ineligible intervention: intervention aimed at reducing the accumulation of aflatoxins in food crops during pre‐harvesting period (biological control with non‐toxic strains of A. flavus ‐ Aflasafe) |
| IITA 2016 | Ineligible intervention: intervention aimed at reducing the accumulation of aflatoxins in food crops during pre‐harvesting period (biological control with non‐toxic strains of A. flavus ‐ Aflasafe) |
| James 2007 | Ineligible study design |
| Kuchenbecker 2017 | Ineligible intervention: intervention described by study authors as a nutritional education programme to increase dietary diversity of infants and young children, but did not include any specific educational component aimed at reducing their intake of aflatoxins |
| Mamiro 2004 | Ineligible intervention: intervention did not include any educational component; described by study authors as the administration of a processed complementary food to infants |
| NCT03940547 | Ineligible intervention: study protocol stated that both Intervention and control groups would receive infant and young child feeding (IYCF) education (no specific educational component aimed at reducing their intake of aflatoxins). Children in the intervention group would receive a very low‐aflatoxin blended porridge flour. |
| Nyanga 2017 | Ineligible study design |
| Seetha 2018 | Ineligible duration of follow‐up |
| SHINE 2015 | Ineligible intervention: intervention described by study authors as an Infant and young child feeding (IYCF) intervention, but did not include any specific educational component aimed at reducing their intake of aflatoxins |
| Smith 2015 | Ineligible study design |
| Turner 2005 | Ineligible study design |
| Xu 2017 | Ineligible study design |
Differences between protocol and review
The background was updated to reflect current information and context, including the extent of aflatoxin contamination of food crops along the value chain, the associated complexity of aflatoxin management strategies, the foundational importance of creating awareness about aflatoxin exposure, and its effects for interventions at household or community levels, as well as descriptions of potential educational interventions,
In the methods section, we expanded our descriptions of eligible and ineligible agricultural and nutritional education interventions, as well as eligible and ineligible controls, using examples in order to better clarify the boundaries between what was included and excluded in the review.
We amended the original categories of interventions to: agricultural education, nutritional education, or both, as it became clear that the original categories (agricultural education or nutritional education with or without food replacement) often overlap i.e. agricultural education may also be combined with food replacement.
The secondary outcome weight‐for‐age z‐score (WAZ), and the additional outcome, proportion of underweight children (WAZ ≥ 2 SD below the reference median value), were added to the 'Summary of findings' table, although we did not originally prespecify these for inclusion. We feel that these outcomes provide relevant child growth data in line with the review question.
We extracted data on two individual aflatoxin exposure outcomes (only reported in two of the three included studies) that were not prespecified and reported these data in the 'Effects of interventions' section. These include the estimated daily intake of aflatoxins, and urinary levels of aflatoxins. Since the exposure under investigation is a carcinogenic toxin, we considered this information important to report on these additional outcomes.
Contributions of authors
All review authors contributed to the development of the protocol. Two authors (AS, Nicola Randall (NR)) developed the search strategy. Three review authors (MV, AS, CNE) screened potential studies. Two review authors (MV, CN), with the assistance of others (AB), extracted data and conducted 'Risk of bias' assessment. Three authors (MV, AS, CN) drafted the results and discussion sections of the review, with input from two authors (CNE, NR). All authors were involved in editing the final review for submission.
Sources of support
Internal sources
Stellenbosch University, South Africa.
External sources
-
Department for International Development, UK.
Project number 300342‐104
-
Aubrey Sheiham Evidence‐Based Health Care in Africa Leadership Award, UK.
Financial support for meetings, travels and capacity building
Declarations of interest
Marianne E. Visser ‐ none known
Anel Schoonees ‐ none known
Chibundu N. Ezekiel ‐ none known
Nicola Randall ‐ none known
Celeste E. Naude ‐ none known
New
References
References to studies included in this review
Dembedza 2019 {published and unpublished data}
- Dembedza MP, Chidewe C, Benhura MA, Mvumi BM, Manema LR, Nyanga LK. Effectiveness of hermetic maize grain storage technology in limiting aflatoxin exposure in women and children from smallholder farming areas. World Mycotoxin Journal 2019;12(3):233‐43. [10.3920/WMJ2019.2435] [Google Scholar]
- Dembedza MP, Nyanga LK, Manema L, Benhura MA, Chidewe C. Hermetic technology for reduction of aflatoxin exposure in women and children under five years: a case study of Shamva and Makoni Districts, Zimbabwe. Toxins 2017;9(9):66. [Google Scholar]
- Murashiki TC, Chidewe C, Benhura MA, Manema LR, Mvumi BM, Nyanga LK. Effectiveness of hermetic technologies in limiting aflatoxin B1 and fumonisin B1 contamination of stored maize grain under smallholder conditions in Zimbabwe. World Mycotoxin Journal 2018;11(3):459‐69. [DOI: 10.3920/WMJ2017.2288] [DOI] [Google Scholar]
- Nyanga LK [pers comm]. Cochrane systematic review: request for information [personal communication]. Email to: ME Visser 22 August 2019.
Hoffmann 2018b {published and unpublished data}
- Hoffmann V. Improving food safety on the farm: experimental evidence from Kenya on incentives and subsidies for technology adoption. American Journal of Agricultural Economics (in press).
- Hoffmann V [pers comm]. IFPRI Discussion paper 01746: request for baseline results [personal communication]. Email to: ME Visser 18 October 2019.
- Hoffmann V, Jones K. Improving food safety on the farm: Experimental evidence from Kenya on agricultural incentives and subsidies as public health investments. Washington, DC.: International Food Policy Research Institute (IFPRI); 2018. IFPRI Discussion Paper 1746.
- Pretari A, Hoffmann V, Tian L. Post‐harvest practices for aflatoxin control: evidence from Kenya. Journal of Stored Products Research 2019;82:31‐9. [DOI: 10.1016/j.jspr.2019.03.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamala 2018a {published and unpublished data}
- Kamala A [pers comm]. Re. NCT02438774: request for additional information [personal communication]. Email to: ME Visser 31 October 2019.
- Kamala A, Kimanya M, Meulenaer B, Kolsteren P Jacxsens L, Haesaert G, et al. Post‐harvest interventions decrease aflatoxin and fumonisin contamination in maize and subsequent dietary exposure in Tanzanian infants: a cluster randomised‐controlled trial. World Mycotoxin Journal 2018;11(3):447‐58. [DOI: 10.3920/WMJ2017.2234] [DOI] [Google Scholar]
- Kamala A, Kimanya M, Magoha H, Meulenaer B, Kolsteren P, Jacxsens L, et al. Post‐harvest interventions decrease infants' dietary exposure to aflatoxins and fumonisins in Tanzania: a cluster randomized controlled trial. Toxins 2017;9(9):33‐4. [Google Scholar]
- NCT02438774. Intervention in minimizing aflatoxins and fumonisins exposure to children through food and breastfeeding in Tanzania. clinicaltrials.gov/show/nct02438774 (first received 8 May 2015).
References to studies excluded from this review
Grace 2015 {published data only}
- Grace D, Mahuku G, Hoffmann V, Atherstone C, Upadhyaya HD, Bandyopadhyay R. International agricultural research to reduce food risks: case studies on aflatoxins. Food Security 2015;7:569‐82. [DOI: 10.1007/s12571-015-0469-2] [DOI] [Google Scholar]
Hoffmann 2018a {published data only}
- Hoffmann V [pers comm]. IFPRI Discussion paper 01746 [personal communication]. Email to: ME Visser 29 October 2019.
- Hoffmann V, Jones K, Leroy J. Correction: The impact of reducing dietary aflatoxin exposure on child linear growth: a cluster randomized controlled trial in Kenya. BMJ Global Health 2019;4(e000983.corr1):1. [DOI: 10.1136/bmjgh-2018-000983corr1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann V, Jones K, Leroy J. Mitigating aflatoxin exposure to improve child growth in Eastern Kenya: a study protocol for a randomized controlled trial. Trials 2015;16:552. [DOI: 10.1186/s13063-015-1064-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann V, Jones K, Leroy J. The impact of reducing dietary aflatoxin on child linear growth: a cluster randomised controlled trial in Kenya. BMJ Global Health 2018;3(e00098):1‐8. [DOI: 10.1136/bmjgh-2018-000983] [DOI] [PMC free article] [PubMed] [Google Scholar]
IFPRI 2017 {published data only}
- International Food Policy Research Institute. Annual Report 2016: CGIAR Research Program on Agriculture for Nutrition and Health. Washington (DC): CGIAR Research Program on Agriculture for Nutrition and Health; 2017‐11 2017.
IITA 2016 {published data only}
- IITA. Africa research in sustainable intensification for the next generation: sustainable intensification of key farming systems in the Sudan and Guinea savanna of West Africa. Ibadan, Nigeria: International Institute of Tropical Agriculture (IITA); 2016. Technical Report, 1 October 2015 to 31 March 2016.
James 2007 {published data only}
- James B, Adda C, Cardwell K, Annang D, Hell K, Korie S, et al. Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Additives and Contaminants 2007;24(11):1283‐91. [DOI: 10.1080/02652030701416558] [DOI] [PubMed] [Google Scholar]
Kuchenbecker 2017 {published data only}
- Kuchenbecker J, Reinbott A, Mtimuni B, Krawinkel MB, Jordan I. Nutrition education improves dietary diversity of children 6‐23 months at community‐level: results from a cluster randomized controlled trial in Malawi. PloS One 2017;12(4):19. [DOI: 10.1371/journal.pone.0175216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mamiro 2004 {published data only}
- Mamiro PS, Kolsteren PW, Camp JH, Roberfroid DA, Tatala S, Opsomer AS. Processed complementary food does not improve growth or hemoglobin status of rural Tanzanian infants from 6–12 months of age in Kilosa District, Tanzania. American Society for Nutritional Sciences 2004;134:1084‐90. [DOI] [PubMed] [Google Scholar]
NCT03940547 {published data only}
- NCT03940547. Mycotoxin Mitigation Trial (MMT). clinicaltrials.gov/show/NCT03940547 (first received 7 May 2019).
- University of Buffalo. Trial to establish causal linkage between mycotoxin exposure and child stunting. www.buffalo.edu/globalhealthequity/global‐projects/the‐global‐child/trial‐to‐establish‐causal‐linkage‐between‐mycotoxin‐exposure‐and‐child‐stunting.html (accessed 9 September 2019).
Nyanga 2017 {published data only}
- Nyanga LK, Ngaru M, Kachambwa M, Madya F, Chahwanda S, Manema LR, et al. Maize grain aflatoxin contamination reduction technologies. Opportunities for gender transformation in Shamva and Makoni districts, Zimbabwe. In: Bock B, Duncan J, Buchheim E, Bultman S, Groot M, Walhout E, et al. editor(s). Yearbook of Women's History. Gendered Food Practices from Seed to Waste. 36. Hilversum, Netherlands: Verloren, 2017:79‐95. [Google Scholar]
Seetha 2018 {published data only}
- Seetha A, Tsusaka TW, Munthali TW, Musukwa M, Mwangwela A, Kalumikiza Z, et al. How immediate and significant is the outcome of training on diversified diets, hygiene and food safety? An effort to mitigate child undernutrition in rural Malawi. Public Health Nutrition 2018;21(6):1156‐66. [DOI: 10.1017/S1368980017003652] [DOI] [PMC free article] [PubMed] [Google Scholar]
SHINE 2015 {published data only}
- The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial: rationale, design, and methods. Clinical Infectious Diseases 2015;61(Suppl 7):S685–702. [DOI: 10.1093/cid/civ844] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015 {published data only}
- Smith LE, Prendergast AJ, Turner PC, Mbuya MNN, Mutasa K, Kembo G, et al. The potential role of mycotoxins as a contributor to stunting in the SHINE trial. Clinical Infectious Diseases 2015;61(Suppl 7):S733‐37. [: 10.1093/cid/civ849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2005 {published data only}
- Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, Hall AJ, et al. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community‐based intervention study. Lancet 2005;365:1950‐6. [DOI: 10.1016/S0140-6736(05)66661-5] [DOI] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu Y, Doel A, Watson S, Routledge MN, Elliott CT, Moore SE, et al. Study of an educational hand sorting intervention for reducing aflatoxin B‐1 in groundnuts in rural Gambia. Journal of Food Protection 2017;80(1):44‐9. [DOI: 10.4315/0362-028X.JFP-16-152] [DOI] [PubMed] [Google Scholar]
Additional references
Afolabi 2014
- Afolabi CG, Ezekiel CN, Kehinde IA, Olaolu AW, Ogunsanya OM. Contamination of groundnut in South‐Western Nigeria by aflatoxigenic fungi and aflatoxins in relation to processing. Journal of Phytopathology 2015;163:279‐86. [DOI: 10.1111/jph.12317] [DOI] [Google Scholar]
Ayalew 2016
- Ayalew A, Hoffmann V, Lindahl J, Ezekiel CN. The role of mycotoxin contamination in nutrition: the aflatoxin story. In: Covic N, Hendriks SL editor(s). 2015 ReSAKSS Annual Trends and Outlook Report. Washington (DC): International Food Policy Research Institute (IFPRI), 2016:98‐114. [Google Scholar]
Balshem 2011
- Balshem H, Helfanda M, Schünemann HJ, Oxman AD, Kunze R, Brozek J, et al. GRADE guidelines: rating the quality of evidence: introduction. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bandyopadhyay 2007
- Bandyopadhyay R, Kumar M, Leslie JF. Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Additives and Contaminants 2007;24(10):1109‐14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bandyopadhyay 2016
- Bandyopadhyay R, Ortega‐Beltran A, Akande A, Mutegi C, Atehnkeng J, Kaptoge L, et al. Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin Journal 2016;9(5):771‐89. [DOI: 10.3920/WMJ2016.2130] [DOI] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence‐based interventions for improvement of maternal and child nutrition; what can be done and at what cost ?. Lancet 2013;382(9890):452‐77. [DOI: 10.1016/S0140-6736(13)60996-4] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382:427‐51. [DOI: 10.1016/S0140-6736(13)60937-X] [DOI] [PubMed] [Google Scholar]
Black 2017
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C L, et al. Advancing early childhood development: from science to scale. 1. Early childhood development coming of age: science through the life course. Lancet 2017;389(10064):77‐90. [10.1016/S0140‐6736(16)31389‐7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2018
- Braun D, Ezekiel CN, Abia WA, Wisgrill L, Degen GH, Turner PC, et al. Monitoring early life mycotoxin exposures via LC‐MS/MS breast milk analysis. Analytical Chemistry 2018;90:14569‐77. [DOI: 10.1021/acs.analchem.8b04576] [DOI] [PubMed] [Google Scholar]
CAC 2003
- Codex Alimentarius Commission (CAC). Code of practice for the prevention and reduction of mycotoxin contamination in cereals. CAC‐RCP 51‐2003. Rome, Italy: FAO/WHO, Adopted 2003. Revised 2014. [Google Scholar]
Castelino 2014
- Castelino JM, Dominguez‐Salas P, Routledge MN, Prentice AM, Moore SE, Hennig BJ, et al. Seasonal and gestation‐stage associated differences in aflatoxin exposure in pregnant Gambian women. Tropical Medicine & International Health 2014;19(3):348‐54. [DOI: 10.1111/tmi.12250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christian 2013
- Christian P, Lee SE, Angel MD, Adair LS, Arifeen SE, Ashorn P, et al. Risk of childhood undernutrition related to small‐for‐gestational age and preterm birth in low‐ and middle‐income countries. International Journal of Epidemiology 2013;42:1340‐55. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 24 January 2019. Melbourne, Australia: Veritas Health Innovation, 2019.
Edwards 2018
- Edwards S [pers comm]. Use of water in reducing aflatoxins during food preparation [personal communication]. Email to: S Edwards 1 November 2018.
EPOC 2017a
- Cochrane Effective Practice, Organisation of Care (EPOC). What study designs can be considered for inclusion in an EPOC review and what should they be called? EPOC resources for review authors. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 29 0ctober 2018).
EPOC 2017b
- Cochrane Effective Practice and Organisation of Care Group (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. epoccochrane.org/resources/epoc‐resources‐review‐authors (accessed 2 November 2018).
EPOC 2017c
- Cochrane Effective Practice, Organisation of Care (EPOC). Summary assessments of the risk of bias. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources‐for‐authors2017/summary_assessments_of_the_risk_of_bias.pdf (accessed 5 May 2019).
Ezekiel 2019a
- Ezekiel CN, Sulyok M, Ogara IM, Abia WA, Warth B, Šarkanj B, et al. Mycotoxins in uncooked and plate‐ready household food from rural northern Nigeria. Food and Chemical Toxicology 2019;128:171‐9. [DOI: 10.1016/j.fct.2019.04.002] [DOI] [PubMed] [Google Scholar]
Ezekiel 2019b
- Ezekiel CN, Ortega‐Beltran A, Bandyopadhyay R. The need for integrated approaches to address food safety risk: the case of mycotoxins in Africa. The future of food safety: Transforming knowledge into action for people, economies and the environment. First FAO/WHO/AU International Food safety conference; 2019 February 12‐13; Addis Ababa, Ethiopia. 2019.
FAO 2011
- Food, Agriculture Organization (FAO). Guidelines for Measuring Household and Individual Dietary Diversity. Rome: Food and Agriculture Organization of the United Nations, 2011. [Google Scholar]
FAO and FHI 360 2016
- Food, Agriculture Organization (FAO), FHI 360. Minimum Dietary Diversity for Women: A Guide for Measurement. Rome: Food and Agriculture Organization of the United Nations, 2016. [Google Scholar]
FAO and WHO 2018
- Food and Agriculture Organization of the United Nations and World Health Organization. Codex Alimentarius International Food Standards. www.codexalimentarius.org (accessed 21 November 2018).
Frisvad 2019
- Frisvad JC, Hubka V, Ezekiel CN, Hong S‐BA, Novakova A, Chen AJ, et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Studies in Mycology 2019;93:1‐63. [DOI: 10.1016/j.simyco.2018.06.001.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galasso 2017
- Galasso E, Wagstaff A. The economic costs of stunting and how to reduce them. World Bank Group. Policy Research Note 2017, issue March:1‐40.
Goncalves 2017
- Goncalves L, Dalla Rosa A, Gonzales SL, Feltes MMC, Badiale‐Furlong E, Dors GA. Incidence of aflatoxin M1 in fresh milk from small farms. Food Science and Technology 2017;37:11‐5. [DOI: 10.1590/1678-457X.06317] [DOI] [Google Scholar]
Gong 2004
- Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, et al. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environmental Health Perspectives 2004;112(13):1334‐8. [DOI: 10.1289/ehp.6954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gradepro GDT 2019 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Statistical Methods group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for Systematic reviews of interventions. version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JPT, Savoviç J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. Draft version (29 January 2019) for inclusion in: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for Systematic reviews of interventions. version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook. London: Cochrane.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [10.1136/bmj.g 1687] [DOI] [PubMed] [Google Scholar]
IARC 2015
- Wild CP, Miller JD, Groopman JD, editor(s). Mycotoxin control in Low‐ and Middle‐Income Countries. IARC Working Group Reports, No. 9. Lyon (FR): International Agency for Research on Cancer, 2015. [ISBN 978‐92‐832‐2510‐2.] [PubMed] [Google Scholar]
IFPRI
- Agricultural Cooperative development International and Volunteers in Overseas Coopreative assistance (ACDI/VOCA). Aflatoxin awareness Facilitator's guide. International Food Policy Research Institute (IFPRI).
Iqbal 2015
- Iqbal SZ, Jinap S, Pirouz AA, Ahmad Faizal AR. Aflatoxin M1 in milk and dairy products, occurrence and recent challenges: a review. Trends in Food Science & Technology 2015;46(1):110‐9. [DOI: 10.1016/j.tifs.2015.08.005] [DOI] [Google Scholar]
Kabak 2008
- Kabak B. The fate of mycotoxins during thermal food processing. Journal of the Science of Food and Agriculture 2009;89:549‐54. [DOI: 10.1002/jsfa.3491] [DOI] [Google Scholar]
Kagera 2018
- Kagera I, Kahenyac P, Mutuab F, Anyangoe G, Kyalloa F, Grace D, et al. Status of aflatoxin contamination in cow milk produced in smallholder dairy farms in urban and peri‐urban areas of Nairobi County: a case study of Kasarani sub county, Kenya. Infection Ecology & Epidemiology 2018;9(1):1547095. [DOI: 10.1080/20008686.2018.1547095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamala 2018b
- Kamala A, Shirima C, Jani B, Bakari M, Sillo H, Rusibanayila N, et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin Journal 2018;11(3):311‐20. [DOI: 10.3920/WMJ2018.2344] [DOI] [Google Scholar]
Karlovsky 2016
- Karlovsky P, Suman M, Berthiller F, Meester J, Eisenbrand G, Perrin I, et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Research 2016;32(4):179‐205. [DOI: 10.1007/s12550-016-0257-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimanya 2014
- Kimanya ME, Shirima CP, Magoha H, Shewiyo DH, Meulenaer B, Kolsteren P, et al. Co‐exposures of aflatoxins with deoxynivalenol and fumonisins from maize based complementary foods in Rombo, Northern Tanzania. Food Control 2014;41:76‐81. [DOI: 10.1016/j.foodcont.2013.12.034] [DOI] [Google Scholar]
Klangwiset 2010
- Khlangwiset P, Wu F. Costs and efficacy of public health interventions to reduce aflatoxin‐induced human disease. Food Additives & Contaminants. Part A. 2010;27(7):998‐1014. [DOI: 10.1080/19440041003677475] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leong 2012
- Leong YH, Rosma A, Latiff AA, Izzah AN. Associations of serum aflatoxin B1‐lysine adduct level with sociodemographic factors and aflatoxins intake from nuts and related nut products in Malaysia. International Journal of Hygiene and Environmental Health 2012;215:368‐72. [DOI] [PubMed] [Google Scholar]
Marco 2017
- Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Folign B, et al. Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology 2017;44:94‐102. [DOI: 10.1016/j.copbio.2016.11.010] [DOI] [PubMed] [Google Scholar]
Matumba 2015
- Matumba L, Poucke C, Ediage EN, Jacobs B, Saeger S. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin‐contaminated white maize. Food Additives & Contaminants. Part A 2015;32(6):960‐9. [DOI: 10.1080/19440049.2015.1029535] [DOI] [PubMed] [Google Scholar]
Mitchell 2017
- Mitchell NJ, Hsu HH, Chandy RK, Shrestha B, Bodhidatta L, Tu YK, et al. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: An extension of the MAL‐ED study. PLoS ONE 2017;12(2):e0172124. [DOI: 10.1371/journal.pone.0172124] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67:56‐64. [http://dx.doi.org/10.1016/j.jclinepi.2013.08.005] [DOI] [PubMed] [Google Scholar]
Ojuri 2018
- Ojuri OT, Ezekiel CN, Sulyokb M, Ezeokolic OT, Oyedelea OA, Kolawole I, et al. Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in Nigeria. Food and Chemical Toxicology 2018;121:37‐50. [DOI: 10.1016/j.fct.2018.08.025] [DOI] [PubMed] [Google Scholar]
Ojuri 2019
- Ojuri OT, Ezekiel CN, Eskola MK, Sarkanj B, Babalola AD, Sulyok M, et al. Mycotoxin exposures in infants and young children consuming household‐ and industrially‐processed complementary foods in Nigeria and risk management advice. Food Control 2019;98:312‐22. [DOI: 10.1016/j.foodcont.2018.11.049] [DOI] [Google Scholar]
Okeke 2015
- Okeke CA, Ezekiel CN, Nwangburuka CC, Sulyok M, Ezeamagu CO, Adeleke RA, et al. Bacterial diversity and mycotoxin reduction during maize fermentation for Ogi production. Frontiers in Microbiology 2015;6(1402):1‐12. [DOI: 10.3389/fmicb.2015.01402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okeke 2018
- Okeke CA, Ezekiel CN, Sulyok M, Ogunremi OR, Ezeamagu CO, Sarkan B, et al. Traditional processing impacts mycotoxin levels and nutritional value of ogi – a maize‐based complementary food. Food Control 2018;86:224‐33. [DOI: 10.1016/j.foodcont.2017.11.021] [DOI] [Google Scholar]
Piekkola 2012
- Piekkola S, Turner PC, Abdel‐Hamid M, Ezzat S, El‐Daly M, ElKafrawy S, et al. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Additives & Contaminants: Part A 2012;29(6):962‐71. [DOI: 10.1080/19440049.2012.658442] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Routledge 2014
- Routledge MN, Kimanya ME, Shirima CP, Wild CP, Gong YY. Quantitative correlation of aflatoxin biomarker with dietary intake of aflatoxin in Tanzanian children. Biomarkers 2014;19:430‐5. [DOI: 10.3109/1354750X.2014.924998] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126‐135. [DOI: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2015
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations.(updated October 2013). Available from guidelinedevelopment.org/handbook.
Seetha 2019
- Kachulu L, Maruwo J, Machinjiri N, Botha R, Masumba J, et al. Knowledge, attitude and practices of Malawian farmers on pre‐ and postharvest crop management to mitigate aflatoxin contamination in groundnut, maize and sorghum ‐ implication for behavioural change. Toxins 2019;11(716):1‐14. [DOI: 10.3390/toxins11120716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirima 2015
- Shirima CP, Kimanya ME, Routledge MN, Srey C, Kinabo JL, Humpf HU, et al. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ Health Perspect 2015;123(2):173‐8. [DOI: 10.1289/ehp.1408097; PMID: 25325363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuaib 2010
- Shuaib F, Jolly P, Ehiri J, Yatich N, Jiang Y, Funkhouser E, et al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Tropical Medicine & International Health 2010;15:160‐7. [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith LE, Stoltzfus RJ, Prendergast A. Food chain mycotoxin exposure, gut health, and impaired growth: A conceptual framework.. Adv Nutr 2012;3:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018
- Smith LE, Prendergast AJ, Turner PC, Humphrey JH, Stoltzfus RJ. Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. American Journal of Tropical Medicine and Hygiene 2017;96(4):770‐6. [DOI: 10.4269/ajtmh.16-0730] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srey 2014
- Srey C, Kimanya ME, Routledge MN, Shirima CP, Gong YY. Deoxynivalenol exposure assessment in young children in Tanzania. Molecular Nutrition & Food Research 2014;58:1574‐80. [DOI] [PubMed] [Google Scholar]
Stepman 2018
- Stepman F. Scaling‐Up the Impact of Aflatoxin Research in Africa. The Role of Social Sciences. Toxins 2018;10:136. [DOI: 10.3390/toxins10040136] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strosnider 2006
- Strosnider H, Azziz‐Baumgartner E, Banziger M, Bhat RV, Breiman R, Brune M‐N, et al. Workgroup report: public health strategies for reducing aflatoxin exposure in developing countries. Environmental Health Perspecttives 2006;114(12):1898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudfeld 2015
- Sudfeld CR, McCoy DC, Danaei G, Fink G, Ezzati M, Andrews KG, et al. Linear growth and child development in low‐ and middle‐income countries: a meta‐analysis. Pediatrics 2015;135(5):e1266. [DOI: 10.1542/peds.2014-3111] [DOI] [PubMed] [Google Scholar]
Swindale 2006
- Swindale A, Bilinsky P. Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide. Second Edition. Washington, DC: Food and Nutrition Project (FANTA); Academy for Educational Development, 2006. [Google Scholar]
Tesfamarium 2019
- Tesfamariam K, Boevre M, Kolsteren P, Belachew T, Mesfin A, Saeger S, Lachat C. 2019. Dietary mycotoxins exposure, child growth. immune system, morbidity, mortality: a systematic literature review. Dietary mycotoxins exposure and child growth, immune system, morbidity, and mortality: a systematic literature review. Critical Reviews in Food Science and Nutrition 2019;Nov 6:1‐21. [DOI: 10.1080/10408398.2019.1685455] [DOI] [PubMed] [Google Scholar]
Turner 2007
- Turner PC, Collinson AC, Cheung YB, Gong YY, Hall AJ, Prentice AM, et al. Aflatoxin exposure in utero causes growth faltering in Gambian infants. International Journal of Epidemiology 2007;36(5):1119‐25. [DOI] [PubMed] [Google Scholar]
UNICEF‐WHO‐The World Bank 2018
- Levels and trends in child malnutrition. UNICEF‐WHO‐The World Bank: Joint Child Malnutrition Estimates – 2018 edition. data.unicef.org/resources/levels‐and‐trends‐in‐child‐malnutrition‐2018 (accessed 13 November 2018).
Wacoo 2019
- Wacoo AP, Mukisa IM, Meeme R, Byakika S, Wendiro D, Sybesma W, et al. Probiotic enrichment and reduction of aflatoxins in a traditional African maize‐based fermented food. Nutrients 2019;11(265):1‐15. [DOI: 10.3390/nu11020265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warth 2016
- Warth B, Braun D, Ezekiel CN, Turner PC, Degen GH, Marko D. Biomonitoring of mycotoxins in human breastmilk: Current state and future perspectives. Chemical research in toxicology 2016;29:1087‐1097. [DOI: 10.1021/acs.chemrestox.6b00125] [DOI] [PubMed] [Google Scholar]
Watson 2017
- Watson S, Gong YY, Routledge M. Interventions targeting child undernutrition in developing countries may be undermined by dietary exposure to aflatoxin. Critical Reviews in Food Science and Nutrition 2017;57(9):1963‐75. [DOI: 10.1080/10408398.2015.1040869] [DOI] [PubMed] [Google Scholar]
Watson 2018
- Watson S, Moore SE, Darboe MK, Chen G, Tu Y, Huang Y, et al. Impaired growth in rural Gambian infants exposed to aflatoxin: a prospective cohort study. BMC Public Health 2018;18(1247):1‐9. [DOI: 10.1186/s12889-018-6164-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1986
- World Health Organization Working Group. Use and interpretation of anthropometric indicators of nutritional status.. Bulletin of the World Health Organization 1986;64:929‐941. [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. WHO Child Growth Standards: length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: methods and development.. Geneva: World Health Organization Press, 2006. [Google Scholar]
WHO 2007
- World Health Organization (WHO). Indicators for assessing infant and young child feeding practices. Part I: Definitions. Geneva: World Health Organization, 2007. [Google Scholar]
WHO 2018
- World Health Organization (WHO). Aflatoxins. Food Safety Digest 2018:1‐5.
World Bank 2019
- World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups (accessed 31 January 2019).


