Abstract
Flower color can be applied to landscaping and identification of the purity of seeds in hybrid production. However, the molecular basis of white flower trait remains largely unknown in Brassica rapa. In this study, an F2 population was constructed from the cross between 15S1040 (white flower) and 92S105 (yellow flower) for fine mapping of white flower genes in B. rapa. Genetic analysis indicated that white flower trait is controlled by two recessive loci, Brwf1 and Brwf2. Using InDel and SNP markers, Brwf1 was mapped to a 49.6-kb region on chromosome A01 containing 9 annotated genes, and among them, Bra013602 encodes a plastid-lipid associated protein (PAP); Brwf2 was located in a 59.3-kb interval on chromosome A09 harboring 12 annotated genes, in which Bra031539 was annotated as a carotenoid isomerase gene (CRTISO). The amino acid sequences of BrPAP and BrCRTISO were compared between two yellow-flowered and three white-flowered lines and critical amino acid mutations of BrPAP and BrCRTISO were identified between yellow-flowered and white-flowered lines. Therefore, Bra013602 and Bra031539 were predicted as potential candidates for white flower trait. Our results provide a foundation for further identification of Brwf and increase understanding of the molecular mechanisms underlying white flower formation in Chinese cabbage.
Subject terms: Plant breeding, Plant genetics
Introduction
In nature, flower color was used to attract insect for pollination in plants1. There are three chemically distinct pigments, carotenoids, flavonoids, and betalains, responsible for flower color, and among them, carotenoids accumulating in petals can generate yellow, orange, and red flower colors2,3. The most common carotenoids in petals are xanthophylls, which show high specificity in composition and quantity among plant species or varieties4.
Carotenoid accumulation was modulated by its biosynthesis, degradation, and sequestration5–8. The mutation of key genes involved in the above three processes could result in the conversion of flower and fruit colors. For example, a single-nucleotide mutation in β-carotene hydroxylase 2 (CHYB2) caused orange fruit phenotype in pepper9. In Chrysanthemum morifolium, Brassica napus, and B. oleracea, the loss-of-function mutation of carotenoid cleavage dioxygenase 4 (CCD4) led to change in flower color from white to yellow7,10–14. The mutation of pale yellow petal (PYP1) that was involved in xanthophyll ester production was responsible for pale yellow petal phenotype in tomato8.
The Brassica genus includes important oil crops and vegetables15 with yellow flower color as the most common form, while there are other colors, such as pale yellow, white, orange, and tangerine16–22. Compared with the studies of other traits in Brassica genus, such as yield23–28, fertility29–32, disease resistance33–35, the genetic studies of flower colors have been conducted earlier16,17,36. However, only the molecular mechanisms of white flower formation were understood11–14,37,38. Recently, a few genes controlling flower colors have been reported. For example, a carotenoid isomerase gene (CRTISO) related to pale yellow flower in B. rapa22 and a carotenoid cleavage dioxygenase 4 gene (CCD4) associated with white flower in B. napus11 and B. oleracea12–14 were cloned, respectively. In B. napus and B. oleracea, a CACTA-like transposable element insertion caused disruption of CCD411,13,14 and CCD4 from white-flowered line could rescue the petal color of yellow-flowered line11,12. In addition, Zhang et al.37,38 reported that the BjuA008406 and BjuB027334 genes, which might be involved in carotenoid esterification, were predicted as the potential candidates for white flower in B. juncea. However, none of white flower genes has been cloned yet, and the molecular mechanism of white flower formation remains poorly understood in B. rapa.
In this study, the inheritance pattern of white flower trait was analyzed using an F2 segregating population developed from the crossing of white flower line 15S1040 and yellow flower line 92S105. Molecular markers designed based on the genome re-sequencing data of 15S1040 and 92S105 were used to map white flower genes, and then the prediction of the candidate genes was performed; the coding sequences of two candidate genes (BrPAP and BrCRTISO) were compared between three white-flowered and two yellow-flowered lines; the expression levels of two candidate genes were tested in different tissues. Our findings provide insights in molecular mechanisms controlling flower color variation in B. rapa.
Results
Genetic analysis of the white flower trait in B. rapa
The flower colors of F1 plants derived from the cross between white parent 15S1040 and yellow parent 92S105 were all yellow (Fig. 1a–c). Among 1282 F2 individuals, 718 individuals were yellow flower, 257 individuals were milky yellow flower, 227 individuals were pale yellow flower, and 80 individuals were white flower (Fig. 1d–g). The F2 segregation ratio was fitted into an expected ratio of 9:3:3:1 (χ2 = 1.908, df = 3, P > 0.05) using χ2 test (Table 1). These results indicated that yellow flower trait was dominant over white flower and the white flower trait was controlled by two recessive genes, Brwf1 and Brwf2, therefore the genotypes of four flower color plants may be yellow flower (BrWF1BrWF1BrWF2BrWF2, BrWF1BrWF1BrWF2Brwf2, BrWF1Brwf1BrWF2BrWF2, or BrWF1Brwf1BrWF2Brwf2), milky yellow flower (Brwf1Brwf1BrWF2BrWF2 or Brwf1Brwf1BrWF2Brwf2), pale yellow flower (BrWF1BrWF1Brwf2Brwf2 or BrWF1Brwf1Brwf2Brwf2) and white flower (Brwf1Brwf1Brwf2Brwf2), respectively.
Figure 1.
Flower colors of the two parents and their F1 (c) and F2 (d–g) generations. (a) 15S1040, (b) 92S105; (c) F1 individual; (d) Yellow flower F2 individual, (e) Milky yellow flower F2 individual, (f) Pale yellow flower F2 individual, (g) White flower F2 individual.
Table 1.
The segregation of flower colors in the F1 and F2 population.
| Cross combination | Generation | Total plants | Yellow flower plants | Milky yellow flower plants | Pale yellow flower plants | White flower plants | Mendelian expectation | χ2 valuea(df = 3, P > 0.05) |
|---|---|---|---|---|---|---|---|---|
| 15S1040×92S105 | F1 | 20 | 20 | |||||
| F2 | 1282 | 718 | 257 | 227 | 80 | 9:3:3:1 | 1.908 |
a χ2 > χ2(0.05, 3) = 7.815 is considered significant.
Carotenoid accumulation and ultrastructural analysis of chromoplasts in yellow and white petals
Carotenoid composition and content in yellow and white petals at the flowering stage were analyzed using high performance liquid chromatography (HPLC). The results showed that the major carotenoids in yellow and white petals were both violaxanthin and lutein, however, the total carotenoid contents of yellow and white petals were 211.69 ± 21.70 μg/g and 10.49 ± 1.21 μg/g (Fig. 2a), respectively, which may result in the difference in color between yellow and white petals.
Figure 2.

Carotenoid composition and content and chromoplast ultrastructure in anthesis petals of 92S105 and 15S1040. (a) Carotenoid composition and content of 92S105 and 15S1040. Error bars indicate the standard deviation (SD), and asterisks represent significant difference (t-test, P < 0.05) between 92S105 and 15S1040. (b) Chromoplast morphology in the petal of 92S105. (c) Chromoplast morphology in the petal of 15S1040. PG: plastoglobule, ST: starch, bar= 0.5 μm (b,c).
To study whether there were differences in chromoplast structures between yellow and white petals, the ultrastructural analysis of chromoplasts in the two parents was performed using transmission electron microscopy (TEM). The results indicated that yellow-flowered individuals had normal chromoplasts with numerous fully developed plastoglobules (PGs), however, white-flowered individuals showed abnormal chromoplasts with few PGs (Fig. 2b,c).
Preliminary mapping of the Brwf genes
To determine the locations of genes controlling white flower trait, 81 insertion/deletion (InDel) markers distributed on 10 chromosomes were developed based on the re-sequencing data of the two parents, and 34 InDel markers (W1-W11, W101-W288) exhibited polymorphism between 15S1040 and 92S105 (Supplementary Table S1). These polymorphic markers were used for bulk segregant analysis (BSA) of flower color trait. As a result, six markers (W101, W105, W107, W112, W114, and W116) on chromosome A01 and three markers (W1, W5, and W11) on chromosome A09 were linked with Brwf genes. Among them, W105 and W112 markers, W5 and W11 markers were randomly chosen to assay 30 milky yellow-flowered and 30 pale yellow-flowered plants from F2 population. The results showed that W105 and W112 markers were linked with the Brwf1 gene controlling milky yellow flower and W5 and W11 markers were linked with the Brwf2 gene controlling pale yellow flower, which indicated that Brwf1 and Brwf2 were located on chromosomes A01 and A09, respectively.
For preliminary mapping of the Brwf1 and Brwf2 genes, newly designed 36 InDel markers on chromosome A01 and 35 InDel markers on chromosome A09 were screened between the two parental lines, and 14 (W310-W339) and 12 (W23-W60,W67) markers showed polymorphism, respectively (Supplementary Table S1). These polymorphic markers were used for BSA of flower color trait and 14 markers on chromosome A01 and 5 markers on chromosome A09 were linked with the Brwf genes. To preliminarily map the Brwf1 and Brwf2 genes separately, A and B groups that were segregated in BrWF1/Brwf1 and BrWF2/Brwf2 loci, respectively, were selected from F2 population and A group included 108 yellow-flowered and 36 milky yellow-flowered individuals and B group included 108 yellow-flowered and 36 pale yellow-flowered individuals. Then obtained 19 linkage markers from chromosomes A01 and A09 were used to detect A and B groups, respectively. In A group, the Brwf1 gene, co-segregating with W323 marker, was localized to a region between W322 and W331 markers on chromosome A01, and the genetic and physical distances were 0.74 cM and 186.1 kb, respectively (Fig. 3a). In B group, the Brwf2 gene was mapped to a 0.71 cM interval flanked by W5 and W67 markers with the corresponding physical distance of 216.3 kb on chromosome A09, and one marker W11 co-segregated with Brwf2 (Fig. 3b).
Figure 3.
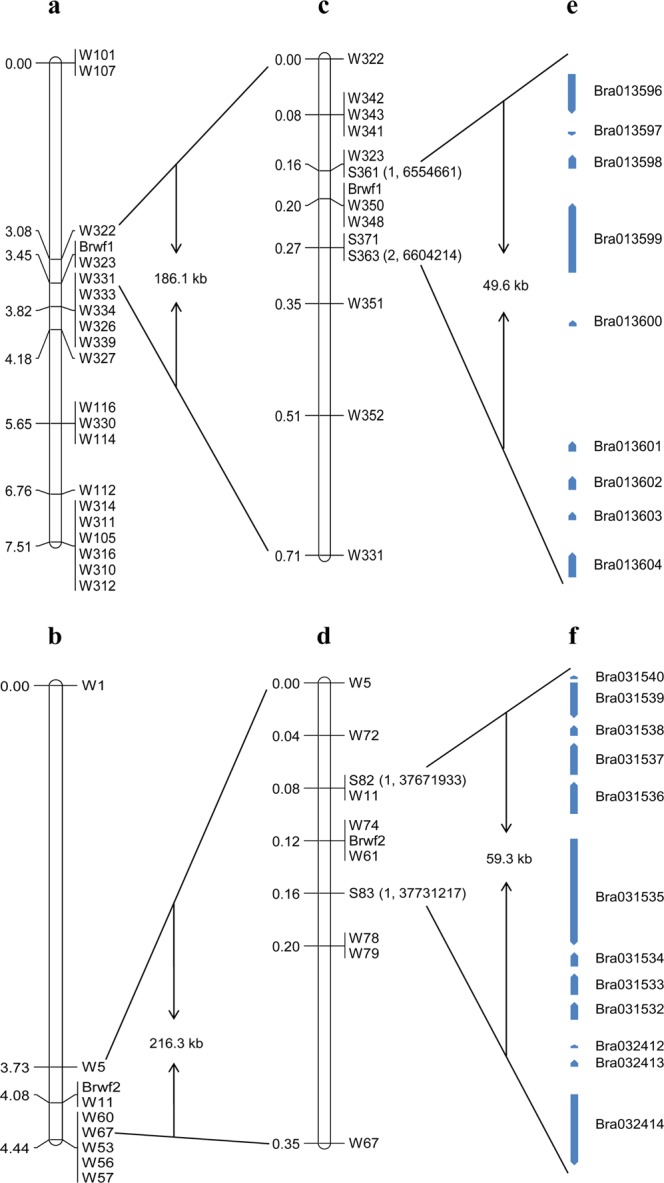
Fine mapping of Brwf genes in Chinese cabbage. Preliminary genetic map of Brwf1 (a) and Brwf2 (b) based on 144 F2 individuals. Fine linkage map of Brwf1 (c) and Brwf2 (d) based on 1282 F2 individuals. Annotated genes within the mapping intervals of Brwf1 (e) and Brwf2 (f). The bracket contains two numbers; the former represents recombinants between an individual marker and the white flower gene; the latter represents the physical position of SNP markers according to BRAD B. rapa reference genome.
Fine mapping of the Brwf genes
For fine mapping of the Brwf1 gene, the two markers W322 and W331 were used to detect recombination events in all F2 plants, and a total of 18 recombinants including 5 recombination events with W322 marker and 13 recombination events with W331 marker were obtained. Using the re-sequencing data of the two parents, 10 new InDel markers were developed from the preliminary mapping region and seven of them (W341-W352) exhibited polymorphism in the two parents (Supplementary Table S1). These polymorphic markers were unceasingly used to screen all the 18 recombinants. The results indicated that the Brwf1 gene was delimited to a shortened interval between W323 and W351 markers with one recombinant and four recombinants, respectively (Fig. 4a–c and Supplementary Fig. S1). To further narrow down the mapping interval, two single-nucleotide polymorphism (SNP) markers (S361 and S371) were developed and used to test recombination events. As a result, one recombination event with S361 marker and two recombination events with S371 marker were found, and then a developed SNP marker S363 on the side of S371 was also used to detect two recombination events (Supplementary Table S1 and Fig. S2). The two SNP markers S361 and S363 further narrowed the Brwf1 gene to an interval of 0.11 cM with the corresponding physical distance of 49.6 kb, Finally, two markers, W348 and W350, co-segregating with Brwf1 were obtained (Fig. 3c).
Figure 4.
Genotyping of screened recombinants for fine mapping of Brwf1 (a-c) and Brwf2 (d-f) using closely linked markers. (a) W323, (b) W348, (c) W351; (d) W11, (e) W61, (f) W78. M: DL50 marker; P1: 92S105; P2: 15S1040; F1: F1 individual; R: screened recombinants from the F2 population using markers W322 and W331, W5 and W67; H: heterozygous genotype plant; B: recessive homozygous genotype plant.
To fine map the Brwf2 gene, a total of nine recombinants were identified using W5 and W67 markers, which included three recombination events occurring between W5 marker and Brwf2 and six recombination events occurring between W67 marker and Brwf2. Among 15 InDel markers developed from the preliminary mapping interval, five markers (W61, W72-W79) were polymorphic between 15S1040 and 92S105 (Supplementary Table S1). The nine recombinants were screened by five new polymorphic markers. As a result, the Brwf2 gene was restricted to a region between W11 and W78 markers and there were one recombinant with W11 marker and two recombinants with W78 marker (Fig. 4d–f and Supplementary Fig. S1). For further narrow down the mapping region, two more SNP markers (S82 and S83) were developed for detecting recombination events (Supplementary Table S1). The result showed that one recombination event was found between each of the two SNP markers and Brwf2 (Supplementary Fig. S2) respectively, so the Brwf2 gene was delimited to a 0.08 cM region flanked by S82 and S83 markers, and the corresponding physical distance was 59.3 kb. Finally, two markers, W61 and W74, co-segregating with Brwf2 were obtained (Fig. 3d).
Identification and sequence analysis of the candidate genes
According to the B. rapa reference genome in BRAD (Brassica database, http://brassicadb.org/brad), 9 and 12 genes were annotated within the two final mapping intervals of Brwf1 and Brwf2 genes, respectively (Fig. 3e,f). Among 9 annotated genes in the Brwf1 interval on chromosome A01, Bra013602 encodes a plastid-lipid associated protein (PAP) that was previously reported to regulate carotenoid accumulation39,40 (Table 2). Out of 12 annotated genes in the Brwf2 interval on chromosome A09, Bra031539 was predicted to encode a carotenoid isomerase (CRTISO) that was involved in carotenoid biosynthesis41–44 (Table 2). Therefore, Bra013602 and Bra031539 were predicted as the two candidates for Brwf1 and Brwf2 genes, respectively.
Table 2.
Annotated genes within the mapping intervals of Brwf1 and Brwf2 on chromosomes A01 and A09.
| Chr. | B. rapa | Gene positiona | Gene functiona | Arabidopsis thaliana homolog |
|---|---|---|---|---|
| A01 | Bra013596 | 6555476…6558338 | Protein kinase | AT4G23280 |
| Bra013597 | 6559518…6559832 | F-box family protein | AT4G22170 | |
| Bra013598 | 6561855…6562856 | Unknown protein | AT4G22190 | |
| Bra013599 | 6567555…6572888 | AKT2/3: cyclic nucleotide binding/inward rectifier potassium channel/protein binding | AT4G22200 | |
| Bra013600 | 6577601…6578014 | PRA1.H: prenylated PAB acceptor 1.H | AT4G27540 | |
| Bra013601 | 6597194…6597946 | ISU1: structural molecule | AT4G22220 | |
| Bra013602 | 6598836…6599884 | PAP: plastid-lipid associated protein | AT4G22240 | |
| Bra013603 | 6600885…6601496 | Zinc finger (C3HC4-type RING finger) family protein | AT4G22250 | |
| Bra013604 | 6602225…6604058 | Alternative oxidase | AT4G22260 | |
| A09 | Bra031532 | 37708145…37710011 | 2-oxoglutarate-dependent dioxygenase | AT1G06650 |
| Bra031533 | 37705347…37707116 | 2-oxoglutarate-dependent dioxygenase | AT1G06650 | |
| Bra031534 | 37703357…37704748 | PSBP-1: Photo system II subunitp-1 | AT1G06680 | |
| Bra031535 | 37693586…37703026 | Ribosome biogenesis | AT1G06720 | |
| Bra031536 | 37686812…37689589 | Unknown protein | AT1G06750 | |
| Bra031537 | 37681637…37684412 | GAUT6: Galacturonosyltransferase 6 | AT1G06780 | |
| Bra031538 | 37680010…37681151 | RNA polymerase Rpb7 N-terminal domain-containing protein | AT1G06790 | |
| Bra031539 | 37676627…37679733 | Carotenoid isomerase | AT1G06820 | |
| Bra031540 | 37674379…37674678 | Glutaredoxin family protein | AT1G06830 | |
| Bra032412 | 37717578…37717784 | 2-oxoglutarate-dependent dioxygenase | AT1G06640 | |
| Bra032413 | 37718500…37719120 | 2-oxoglutarate-dependent dioxygenase | AT1G06650 | |
| Bra032414 | 37722562…37728738 | Unknown protein | AT1G06590 |
aGene position and annotation based on BRAD B. rapa reference genome data (chromosome v1.5).
The specific primers WY503 was designed for cloning and sequencing of the cDNA sequences of BrPAP (Supplementary Table S1). The gene sequence comparison showed that there were 15 SNPs in the coding region of BrPAP between 92S105 and 15S1040 (Supplementary Fig. S3a), which resulted in four amino acid residue mutations (Supplementary Fig. S4a). Based on previous studies42,44, two designed primers, WY571 and WY572, were used to clone the cDNA sequences of BrCRTISO in 92S105 and 15S1040, respectively (Supplementary Table S1). The sequence alignment indicated that there were many SNPs, one small deletion, and one large insertion in the coding region of BrCRTISO in 15S1040. This large insertion had 943 bp that was located at the 3′ end of BrCRTISO (Supplementary Fig. S3b). After the amino acid sequence alignment, 17 amino acid residue changes and the deletion of two amino acid residues were found in BrCRTISO of 15S1040, however, at the 3′ end, the large insertion resulted in mutations of 15 amino acid residues, one amino acid residue insertion, and three amino acid residue deletions in BrCRTISO of 15S1040 (Supplementary Fig. S4b).
To identify the key mutations of the two candidate genes between white-flowered and yellow-flowered lines, the genomic sequences of two candidate genes from one yellow-flowered line (09Q5) and two white-flowered lines (15S1001 and 17S690) were cloned using designed specific primers, which included WY503 for BrPAP of yellow-flowered and white-flowered lines, and WY561, WY562, WY563 for the BrCRTISO of yellow-flowered line and WY561, WY562, WY566 for the BrCRTISO of white-flowered lines according to previous studies42,44 (Supplementary Table S1). The deduced amino acid sequences of BrPAP and BrCRTISO from three yellow-/white-flowered lines were compared with that from the two parental lines. The results indicated that the deduced amino acid sequence of BrPAP in 09Q5 was same as that in 92S105, while there were seven amino acid residue mutations among 15S1040, 15S1001, and 17S690, but only one mutant amino acid residue (Leu → Pro) was found between two yellow-flowered and three white-flowered lines and it was located in the conserved domain of BrPAP (Fig. 5a; Supplementary Fig. S4a); the deduced amino acid sequence of BrCRTISO in 09Q5 had 17 amino acid residue mutations and one deletion of two amino acid residues compared with 92S105, while the sequences from 15S1001 and 17S690 were identical to that from 15S1040, however, two amino acid residue mutations (Ile → Val, Leu → Phe) and many amino acid residue changes at the end of sequences were consistent with the flower color and the two amino acid residues were located in the conserved domain of BrCRTISO (Fig. 5b; Supplementary Fig. S4b).
Figure 5.
Gene structures and amino acid sequence analyses of BrPAP and BrCRTISO. (a) The coding region of BrPAP includes two exons and one intron. The nonsynonymous SNP mutation (T → C) in exon 2 results in the amino acid residue conversion (Leu → Pro) between yellow-flowered and white-flowered lines. (b) The coding region of BrCRTISO contains 13 exons and 12 introns. The nonsynonymous SNP mutation (A → G) in exon 2 and (C → T) in exon 6 and a large insertion in exon 13 cause the conversion of Ile to Val and Leu to Phe, and many amino acid residue changes, respectively, between yellow-flowered and white-flowered lines. The above amino acid residue mutations are consistent with flower color phenotypes. Black backgrounds indicate mutant amino acid residues. 92S105 and 09Q5 are yellow-flowered lines; 15S1040, 15S1001, and 17S690 are white-flowered lines.
Expression analysis of the candidate genes and carotenoid metabolic genes
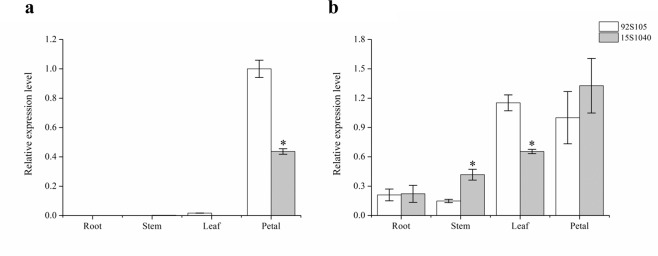
Expression pattern analysis of BrPAP and BrCRTISO was conducted using Quantitative real-time PCR (qPCR) in different tissues (roots, stems, cauline leaves, and petals) from the two parental lines. BrPAP expressed mainly in petals and could hardly be detected in other tissues with expression level of BrPAP in petals of 92S105 being twofold higher than that in 15S1040 (Fig. 6a); BrCRTISO had relatively higher expression levels in cauline leaves and petals than in roots and stems, however, BrCRTISO did not exhibit significant difference in expression between the petals of the two parental lines (Fig. 6b). Moreover, the expression levels of genes related to carotenoid metabolism in petals were detected. The results indicated that CRTISO and Lycopene ε-cyclase (LCYE) had no significant differences in expression between the petals of the two parental lines, but expression of other seven genes showed down-regulated in petals of 15S1040 compared with 92S105 (Supplementary Fig. S5).
Figure 6.
Expression pattern analysis of BrPAP (a) and BrCRTISO (b) in different tissues of 92S105 and 15S1040. Error bars represent the SD, and asterisks indicate significant difference (t-test, P < 0.05) between 92S105 and 15S1040. The expression of the two genes in petals of 92S105 was considered as the standard of ‘relative’ expression.
Discussion
In Brassica species, genetic analysis of flower color traits has been carried out early16,17,36. The previous investigations showed that white flower trait was dominant over yellow flower and controlled by a single gene in B. napus11,45 and B. oleracea13,14,18,46,47. However, several studies have reported that white flower trait was a recessive trait controlled by two major genes20,37,38,48. In this study, genetic analysis of white flower trait in B. rapa was conducted with F2 population derived from a cross between white-flowered line 15S1040 and yellow-flowered line 92S105. Our results showed that white flower trait was controlled by two separate loci and the white flower trait is recessive to yellow flower, consistent with previous reports20,37,38,48.
Multiple studies have reported recently on gene mapping of white flower trait in Brassica species. In B. napus, a white flower gene was mapped to a 0.39 cM region on chromosome C0311. Ashutosh et al.46 and Han et al.12,47 also mapped a white flower gene on chromosome C03 using populations derived from the crosses between broccoli and Chinese kale, cabbage and Chinese kale, respectively. In Chinese kale, a white flower gene was also delimited to chromosome C0313,14. The above results indicated that a single gene controlling white flower trait might be the same gene in B. napus and B. oleracea. In B. juncea, two recessive genes that controlled white flower trait were restricted to chromosomes A02 and B04 and the genetic distances were 0.13 cM and 0.25 cM, respectively37,38. In this study, we found that white flower trait in B. rapa was also controlled by two genes (Brwf1 and Brwf2), which were mapped to intervals of 0.11 cM and 0.08 cM on chromosomes A01 and A09, respectively.
PAP, also called fibrillin, found in the pepper fruit chromoplasts and its homologous protein in chromoplasts of cucumber flower, was named as chromoplast-specific carotenoid-associated protein (CHRC)49. In chromoplast, fibrillin and CHRC were positively associated with carotenoid accumulation39,40. The suppression of the expression of CHRC gene in tomato flowers resulted in decreased carotenoids39, which indicated that CHRC plays a role in mediating carotenoid storage in chromoplasts of flowers. Over-expression of the pepper fibrillin gene in tomato increased the levels of carotenoids in fruit40. In this study, the Bra013602 gene encoding PAP was located in the final mapping region of Brwf1, which deduced amino acid sequence has four amino acid residue mutations between the two parents and one of the mutations (Leu → Pro) occurred in the conserved domain of BrPAP between yellow-flowered lines (92S105 and 09Q5) and white-flowered lines (15S1040, 15S1001, and 17S690), which might affect the function of BrPAP in white-flowered lines. In addition, fibrillin was involved in plastoglobule formation based on previous investigations40,50. Over-expression of the fibrillin gene from pepper in tobacco resulted in the increased number of PGs in plastids of leaves and petals50. In this study, ultrastructural analysis of chromoplasts in the two parents revealed that the number of PGs in yellow petal chromoplasts was more than that in white petal chromoplasts. Expression pattern analysis of BrPAP indicated that the expression level of BrPAP in petals was much higher than that in other tissues. These results indicated that BrPAP was the most possible candidate for white flower trait.
It was known that the role of CRTISO is the control of the conversion of prolycopene to lycopene. The functional disruption of BrCRTISO gene resulted in the orange head leaf formation in Chinese cabbage41–44. However, Lee et al.22 reported that 19 amino acid residue changes and deletion of two amino acid residues were found in the amino acid sequence of BrCRTISO from pale-yellow flower cultivar compared with that in yellow flower cultivar. In this study, Bra031539 encoding BrCRTISO was located in the final delimited genomic region of Brwf2. The amino acid sequence analysis of BrCRTISO indicated that two amino acid residue mutations (Ile → Val, Leu → Phe) that were located in the conserved domain of BrCRTISO and many amino acid residue changes at the end of sequences were found between two yellow-flowered lines (92S105 and 09Q5) and three white-flowered lines (15S1040, 15S1001, and 17S690). In addition, although Zhang et al.42 reported that the amino acid residue mutation (Leu → Phe) of BrCRTISO could not affect the protein function in leaves, this mutation which was found in petals might affect BrCRTISO function in this study. Taken together, two amino acid residue mutations (Ile → Val, Leu → Phe) and many amino acid residue changes in the C-terminal end of BrCRTISO might affect its function, which suggested that BrCRTISO was the most promising candidate for white flower trait.
In B. napus11 and B. juncea37, the major carotenoid in yellow and white petals was violaxanthin, but the total carotenoid contents in yellow petals were forty-twofold and eightfold higher than that in white petals, respectively. In the present study, carotenoid analysis of yellow and white petals showed that violaxanthin and lutein were mainly accumulated in yellow and white petals of Chinese cabbage, however, the total carotenoid content was twenty times higher in yellow petals than in white petals, which were consistent with the previous studies11,37. Moreover, because light could partially replace CRTISO activity22,44, which combined with the phenotypic observation of F2 plants and the results of amino acid sequence comparison of BrPAP and BrCRTISO, we hypothesized that the mutations of BrPAP and BrCRTISO and light might jointly affect the prolycopene accumulation and resulted in barely detecting it in 15S1040. In B. napus, a single dominant gene, BnaCCD4, controls the white flower trait and associated with carotenoid degradation11. In B. juncea, the white flower trait was jointly controlled by two recessive genes, Bjpc1 and Bjpc2 which encode esterase/lipase/thioesterase family protein and phytyl ester synthase 2, respectively, and were involved in carotenoid esterification37,38. In this study, the potential candidate genes for the white flower trait in Chinese cabbage were BrPAP and BrCRTISO that were associated with carotenoid storage and biosynthesis, respectively. The results of TEM analysis and amino acid sequence alignment of BrPAP indicated that the mutation of BrPAP resulted in decrease of carotenoid accumulation by blocking PG formation. The mutant types of BrCRTISO in the present study were incompletely consistent with the previous investigations42,44, which indicated that the function of BrCRTISO in 15S1040 might not be complete disruption. In addition, expression analysis of genes associated with carotenoid metabolism showed that the majority of carotenoid biosynthesis pathway genes were down-regulated expression in petals of 15S1040 compared with 92S105. Hence, the mutation of BrCRTISO might decrease the flux of carotenoid biosynthesis pathway. Taken together, we inferred that both mutations of BrPAP and BrCRTISO maybe lead to the white flower formation by decreasing total carotenoid content in 15S1040 (Fig. 7).
Figure 7.

Proposed molecular mechanism diagram of white flower formation in Chinese cabbage. PSY: phytoene synthase, PDS: phytoene desaturase, Z-ISO: ζ-carotene isomerase, ZDS: ζ-Carotene desaturase, CRTISO: carotenoid isomerase, LCYE: Lycopene ε-cyclase, LCYB: Lycopene β-cyclase, CHYB: β-carotene hydroxylase, CYP97: cytochrome P450-type monooxygenase 97, ZEP: zeaxanthin epoxidase, PAP: plastid-lipid associated protein. Enzymes with green represent the genes that encode these enzymes were down-regulated expression in white flower. Gray frames represent mutated genes.
Methods
Plant materials
The five Chinese cabbage lines, the white-flowered 15S1040, 15S1001, 17S690, and the yellow-flowered 92S105 and 09Q5, were used in this study. 15S1040 and 92S105 (Fig. 1a,b) were selected as parents for constructing F2 population. To study the inheritance pattern of white flower trait and fine map the Brwf genes, a cross between the two parental lines, 15S1040 and 92S105, was used to produce the hybrid F1, then one F1 plant was self-pollinated to generate the F2 population with 1282 individuals. Other white-flowered and yellow-flowered lines were used for the identification of the candidate genes. All materials were bred and provided by the Chinese cabbage research group at the Northwest A&F University, Yangling, China.
All plants used in the present study were grown and naturally vernalized at the experimental field of the Northwest A&F University in 2018. During the flowering stage, the observations of at least ten flowers per plant were performed twice to evaluate the flower color of each individual with an 8-day interval.
Carotenoid extraction and analysis
Carotenoid were extracted from fresh petals at the flowering stage and detected following the methods of Cao et al.51. Carotenoid analysis was performed using LC-2010AHT HPLC (Shimadzu, Kyoto, Japan) with C30 column (YMC, Kyoto, Japan). Carotenoids were identified by the typical retention time of the standard compounds, including violaxanthin (Sigma-Aldrich, Saint Louis, America), lutein (Solarbio, Beijing, China), α-carotene and β-carotene (Wako, Osaka, Japan). The identification of prolycopene was performed based on reported the typical retention time and relative order of carotenoid compound peaks22,43,51. Carotenoid content was quantified according to Morris’ method52. The total carotenoid content was the sum of all the detected carotenoid compound contents. Three biological replicates were used for all analyses and the calculation of means and standard deviations were conducted. The significant difference between 92S105 and 15S1040 was analyzed by t-test.
Transmission electron microscopy analysis
Petals from 92S105 and 15S1040 flowers at the flowering stage were cut into 0.3 × 0.6 cm sections, fixed with 2.5% glutaraldehyde. The preparation of observation samples of petals and TEM analysis were performed according to Yi et al.53 described methods.
DNA and RNA extraction, first-strand cDNA synthesis, and gel electrophoresis
Total genomic DNA was isolated from fresh leaves using the cetyl trimethylammonium bromide (CTAB) method described by Porebski et al.54. Using the MiniBEST Plant RNA Extraction Kit (TaKaRa, Dalian, China), total RNA was extracted from petals of open flowers, roots, stems, and cauline leaves from the two parental lines, and first-strand cDNA, which was used for quantitative real-time PCR (qPCR), was synthesized by PRIMESCRIPT 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). To clone the cDNA sequences of the candidate genes, first-strand cDNA synthesis was performed with PRIMESCRIPT II 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China).
Two (yellow and white flowers) and four (yellow, milky yellow, pale yellow, and white flowers) kinds of F2 individuals were used for BSA and fine mapping, respectively. Two DNA pools, yellow-flowered pool and white-flowered pool, were created by mixing equal amounts of DNA from 8 individuals with yellow flower and 8 individuals with white flower, respectively, which were randomly selected from F2 population. The PCR reaction and separation of its products were performed as described by Zhang et al.31.
Development of InDel and SNP markers
To develop InDel and SNP markers, the two parental lines, 15S1040 and 92S105, were re-sequenced with HiSeq X Ten (Gene Denovo, Guangzhou, China) at 30- and 91-fold sequencing depths. The re-sequencing data of 15S1040 and 92S105 were mapped to the B. rapa reference genome in BRAD, the genomic variants were found using Genome Analysis Toolkit (GATK), and the annotation of the physical location of each genomic variant was carried out. The insertions/deletions> 3 bp and single-nucleotide polymorphism loci were used to develop InDel and SNP markers, respectively, with the Primer Premier 5.0 (http://www.premierbiosoft.com/primerdesign/) software based on the corresponding flanking sequences in the B. rapa reference genome. The primers used in the present study were synthesized by Sangon Biotech Co., Ltd (Shanghai, China).
Identification of recombination events
To obtain the DNA fragments that contained SNP loci in the recombinants, the specific primers were designed according to the reference genome of B. rapa. The purification of PCR products and sequencing were conducted using our previous method55. The nucleotide sequences were analyzed using the DNASTAR Lasergene 7.1 (http://www.dnastar.com) and Chromas 2.4.1 (http://technelysium.com.au/wp/chromas/) softwares.
Fine mapping of the Brwf genes and identification of the candidate genes
The polymorphic molecular markers were utilized to assay genotype of plants in the F2 populations. The linkage analyses were conducted using the genotypic data of the polymorphic markers and phenotypic data of each individual in F2 segregating population. The linkage map was then constructed using the JoinMap 4.0 (https://www.kyazma.nl/index.php/JoinMap/) software based on a LOD threshold score of 6.0. The candidate genes in the final delimited region were analyzed based on the annotation data of the B. rapa reference genome in BRAD.
Cloning and sequence analysis of the candidate genes
To clone the DNA and cDNA sequences of the putative candidate genes, the primers were designed according to the B. rapa reference genome. The cloning of putative candidate genes and sequencing were performed according to our previous method55. The complete coding sequences of two candidate genes from two yellow-flowered and three white-flowered lines were submitted to GenBank, the accession numbers: BrPAP: MN338556 (92S105), MN338557 (09Q5), MN338558 (15S1040), MN338559 (15S1001), and MN338560 (17S690); BrCRTISO: MN338561 (92S105), MN338562 (09Q5), MN338563 (15S1040), MN338564 (15S1001), and MN338565 (17S690).
Expression analysis of the candidate genes and carotenoid metabolic genes
qPCR was used to investigate the expression pattern of the candidate genes in different tissues and the expression levels of carotenoid metabolic genes in petals of the two parental lines, and carotenoid metabolic genes included phytoene synthase (PSY), phytoene desaturase (PDS), ζ-Carotene desaturase (ZDS), carotenoid isomerase (CRTISO), Lycopene ε-cyclase (LCYE), Lycopene β-cyclase (LCYB), β-carotene hydroxylase (CHYB), zeaxanthin epoxidase (ZEP), and carotenoid cleavage dioxygenases 4 (CCD4). The specific primers were designed for qPCR using the Primer Premier 5.0 software (Supplementary Table S2), and Chinese cabbage elongation-factor 1α (EF1α) gene was selected as the internal reference56. The qPCR tests were performed following the method described by Ren et al.57. All gene expression analyses were repeated three times with independent samples. The calculation of relative expression level was performed using the 2−ΔΔCT method58. The significant difference analysis of expression data between 92S105 and 15S1040 was performed using t-test.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFD0101802, 2016YFD0101701). We are thankful to Baohua Li for his linguistic assistance during the revision of this paper.
Author contributions
L.Z. and N.Z. conceived and designed the experiments. N.Z. performed phenotypic observation, HPLC and TEM analysis, the genetic linkage map construction, and cloning of the candidate genes in the mapped region, and wrote this paper. L.C. participated in the genetic linkage map construction and cloning of the candidate genes. S.M., R.W., and M.T. participated in phenotypic observation, DNA and RNA extraction, and cDNA synthesis. Q.H. helped the sequence analysis. L.Z. provided the B. rapa materials, revised this paper, and supervised the research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63165-7.
References
- 1.Kevan PG, Baker HG. Insects as flower visitors and pollinators. Ann. Rev. Entomol. 1983;28:407–453. doi: 10.1146/annurev.en.28.010183.002203. [DOI] [Google Scholar]
- 2.DellaPenna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- 3.Grotewold E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 4.Ohmiya A. Diversity of carotenoid composition in flower petals. Jpn. Agr. Res. Q. 2011;45:163–171. doi: 10.6090/jarq.45.163. [DOI] [Google Scholar]
- 5.Galpaz N, Ronen G, Khalfa Z, Zamir D, Hirschberg J. A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell. 2006;18:1947–1960. doi: 10.1105/tpc.105.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y, Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008;19:190–197. doi: 10.1016/j.copbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka S, Aida R, Yamamizo C, Shibata M, Ohmiya A. The carotenoid cleavage dioxygenase 4 (CmCCD4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica. 2012;184:377–387. doi: 10.1007/s10681-011-0602-z. [DOI] [Google Scholar]
- 8.Ariizumi T, et al. Identification of the carotenoid modifying gene PALE YELLOW PETAL 1 as an essential factor in xanthophyll esterification and yellow flower pigmentation in tomato (Solanum lycopersicum) Plant J. 2014;79:453–465. doi: 10.1111/tpj.12570. [DOI] [PubMed] [Google Scholar]
- 9.Borovsky Y, et al. Induced mutation in β-CAROTENE HYDROXYLASE results in accumulation of β-carotene and conversion of red to orange color in pepper fruit. Theor. Appl. Genet. 2013;126:557–565. doi: 10.1007/s00122-012-2001-9. [DOI] [PubMed] [Google Scholar]
- 10.Ohmiya A, et al. Mechanism behind petal color mutation induced by heavy-ion-beam irradiation of recalcitrant chrysanthemum cultivar. J. Jpn. Soc. Hort. Sci. 2012;81:269–274. doi: 10.2503/jjshs1.81.269. [DOI] [Google Scholar]
- 11.Zhang B, et al. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015;206:1513–1526. doi: 10.1111/nph.13335. [DOI] [PubMed] [Google Scholar]
- 12.Han FQ, et al. Map-based cloning and characterization of BoCCD4, a gene responsible for white/yellow petal color in B. oleracea. BMC Genomics. 2019;20:242–252. doi: 10.1186/s12864-019-5596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu XM, et al. Fine mapping and candidate gene analysis of the yellow petal gene ckpc in Chinese kale (Brassica oleracea L. var. alboglabra Bailey) by whole-genome resequencing. Mol. Breeding. 2019;39:96–106. doi: 10.1007/s11032-019-1011-6. [DOI] [Google Scholar]
- 14.Zhang B, et al. Insertion of a CACTA-like transposable element disrupts the function of the BoCCD4 gene in yellow-petal Chinese kale. Mol. Breeding. 2019;39:130–137. doi: 10.1007/s11032-019-1008-1. [DOI] [Google Scholar]
- 15.Branca, F. & Cartea, E. Wild crop relatives: genomic and breeding resources. 2, 17–36 (Springer-Verlag, 2011).
- 16.Mohammad A, Sikka SM, Aziz MA. Inheritance of seed colour in some oleiferous Brassicae. Indian J. Genet. Plant Breed. 1942;2:112–127. [Google Scholar]
- 17.Cours, B. J. Genetic studies in Brassica campestris L. MSc thesis, University of Wisconsin (1977).
- 18.Kianian SF, Quiros CF. Trait inheritance, fertility, and genomic relationships of some n=9 Brassica species. Genet. Res. Crop Evol. 1992;39:165–175. [Google Scholar]
- 19.Li M, Chen WJ, Yi DL. Studies on the inheritance of CMS restorer R18 with red color flower in rapeseed (Brassica napus L.) Sci. Agric. Sin. 1999;32:27–30. [Google Scholar]
- 20.Singh KH, Chauhan JS. Genetics of flower colour in Indian mustard (Brassica juncea L. Czern & Coss) Indian J. Genet. 2011;71:377–378. [Google Scholar]
- 21.Feng H, Li YF, Liu ZY, Liu J. Mapping of or, a gene conferring orange color on the inner leaf of the Chinese cabbage (Brassica rapa L. ssp. pekinensis) Mol. Breeding. 2012;29:235–244. doi: 10.1007/s11032-010-9542-x. [DOI] [Google Scholar]
- 22.Lee S, et al. Association of molecular markers derived from the BrCRTISO1 gene with prolycopene-enriched orange-colored leaves in Brassica rapa. Theor. Appl. Genet. 2014;127:179–191. doi: 10.1007/s00122-013-2209-3. [DOI] [PubMed] [Google Scholar]
- 23.Fan CC, et al. Mapping of quantitative trait loci and development of allele-specific markers for seed weight in Brassica napus. Theor. Appl. Genet. 2010;121:1289–1301. doi: 10.1007/s00122-010-1388-4. [DOI] [PubMed] [Google Scholar]
- 24.Cai GQ, et al. Genetic dissection of plant architecture and yield-related traits in Brassica napus. Sci. Rep. 2016;6:21625. doi: 10.1038/srep21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YK, et al. Fine mapping of a dominant gene conferring chlorophyll-deficiency in Brassica napus. Sci. Rep. 2016;6:31419. doi: 10.1038/srep31419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, et al. Identification of BnaYUCCA6 as a candidate gene for branch angle in Brassica napus by QTL-seq. Sci. Rep. 2016;6:38493. doi: 10.1038/srep38493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He YJ, et al. GWAS, QTL mapping and gene expression analyses in Brassica napus reveal genetic control of branching morphogenesis. Sci. Rep. 2017;7:15971. doi: 10.1038/s41598-017-15976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen WH, et al. Fine mapping of a silique length- and seed weight-related gene in Brassica napus. Theor. Appl. Genet. 2019;132:2985–2996. doi: 10.1007/s00122-019-03400-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z, Dun X, Xia S, Tu J, Fu T. The BnMs3 regulatory pathway is required for tapetal function and pollen development in Brassica napus. J. Exp. Bot. 2012;63:2041–2058. doi: 10.1093/jxb/err405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, et al. A mitochondria-targeted PPR protein restores pol cytoplasmic male sterility by reducing orf224 transcript levels in oilseed rape. Mol. Plant. 2016;9:1082–1084. doi: 10.1016/j.molp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang HM, et al. Allelism analysis of BrRfp locus in different restorer lines and map-based cloning of a fertility restorer gene, BrRfp1, for pol CMS in Chinese cabbage (Brassica rapa L.) Theor. Appl. Genet. 2016;130:539–547. doi: 10.1007/s00122-016-2833-9. [DOI] [PubMed] [Google Scholar]
- 32.Wei C, et al. Construction of restorer lines and molecular mapping for restorer gene of hau cytoplasmic male sterility in Brassica napus. Theor. Appl. Genet. 2019;132:2525–2539. doi: 10.1007/s00122-019-03368-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YY, et al. Overexpression of three glucosinolate biosynthesis genes in Brassica napus identifies enhanced resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS One. 2015;10:e0140491. doi: 10.1371/journal.pone.0140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, et al. Genome-wide association study identifies new loci for resistance to Sclerotinia Stem Rot in Brassica napus. Front. Plant Sci. 2016;20:1418. doi: 10.3389/fpls.2016.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu FY, et al. Fine mapping of Brassica napus blackleg resistance gene Rlm1 through bulked segregant RNA sequencing. Sci. Rep. 2019;9:14600. doi: 10.1038/s41598-019-51191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson OH. A dominant white flower color in Brassica oleracea L. Am Nat. 1929;63:561–565. doi: 10.1086/280291. [DOI] [Google Scholar]
- 37.Zhang XX, et al. Fine-mapping and candidate gene analysis of the Brassica juncea white-flowered mutant Bjpc2 using the whole-genome resequencing. Mol. Genet. Genomics. 2017;293:359–370. doi: 10.1007/s00438-017-1390-5. [DOI] [PubMed] [Google Scholar]
- 38.Zhang XX, et al. Inheritance and gene mapping of the white flower trait in Brassica juncea. Mol. Breeding. 2018;38:20–29. doi: 10.1007/s11032-017-0771-0. [DOI] [Google Scholar]
- 39.Leitner-Dagan Y, et al. Expression and functional analyses of the plastid lipid-associated protein CHRC suggest its role in chromoplastogenesis and stress. Plant Physiol. 2006;142:233–244. doi: 10.1104/pp.106.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simkin AJ, et al. Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochemistry. 2007;68:1545–1556. doi: 10.1016/j.phytochem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JX, et al. Fine mapping and identification of candidate Br-or gene controlling orange head of Chinese cabbage (Brassica rapa L. ssp. pekinensis) Mol. Breeding. 2013;32:799–805. doi: 10.1007/s11032-013-9907-z. [DOI] [Google Scholar]
- 42.Zhang JX, et al. Molecular characterization and transcriptome analysis of orange head Chinese cabbage (Brassica rapa L. ssp. pekinensis) Planta. 2015;241:1381–1394. doi: 10.1007/s00425-015-2262-z. [DOI] [PubMed] [Google Scholar]
- 43.Li PR, et al. Carotenoid identification and molecular analysis of carotenoid isomerase-encoding BrCRTISO, the candidate gene for inner leaf orange coloration in Chinese cabbage. Mol. Breeding. 2015;35:72–83. doi: 10.1007/s11032-015-0190-z. [DOI] [Google Scholar]
- 44.Su TB, et al. Loss of function of the carotenoid isomerase gene BrCRTISO confers orange color to the inner leaves of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Plant Mol. Biol. Rep. 2015;33:648–659. doi: 10.1007/s11105-014-0779-0. [DOI] [Google Scholar]
- 45.Quazi MH. Interspecific hybrids between Brassica napus L. and B. oleracea L. developed by embryo culture. Theor. Appl. Genet. 1988;75:309–318. doi: 10.1007/BF00303970. [DOI] [Google Scholar]
- 46.Ashutosh SB, Shinada T, Kifuji Y, Kitashiba H, Nishio T. Molecular mapping of a male fertility restorer locus of Brassica oleracea using expressed sequence tag-based single nucleotide polymorphism markers and analysis of a syntenic region in Arabidopsis thaliana for identification of genes encoding pentatricopeptide repeat proteins. Mol. Breeding. 2012;30:1781–1792. doi: 10.1007/s11032-012-9761-4. [DOI] [Google Scholar]
- 47.Han FQ, et al. Inheritance and InDel markers closely linked to petal color gene (cpc-1) in Brassica oleracea. Mol. Breeding. 2015;35:160–167. doi: 10.1007/s11032-015-0354-x. [DOI] [Google Scholar]
- 48.Rawat DS, Anand IJ. Inheritance of flower colour in mustard mutant. Indian J. Agric. Sci. 1986;56:206–208. [Google Scholar]
- 49.Vishnevetsky M, Ovadis M, Vainstein A. Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci. 1999;4:232–235. doi: 10.1016/S1360-1385(99)01414-4. [DOI] [PubMed] [Google Scholar]
- 50.Rey P, et al. Over-expression of a pepper plastid lipid-associated protein in tobacco leads to changes in plastid ultrastructure and plant development upon stress. Plant J. 2000;21:483–494. doi: 10.1046/j.1365-313x.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- 51.Cao HB, et al. Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J. Exp. Bot. 2012;63:4403–4417. doi: 10.1093/jxb/ers115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris WL, et al. Carotenogenesis during tuber development and storage in potato. J. Exp. Bot. 2004;55:975–982. doi: 10.1093/jxb/erh121. [DOI] [PubMed] [Google Scholar]
- 53.Yi B, et al. Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. Plant J. 2010;63:925–938. doi: 10.1111/j.1365-313X.2010.04289.x. [DOI] [PubMed] [Google Scholar]
- 54.Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997;15:8–15. doi: 10.1007/BF02772108. [DOI] [Google Scholar]
- 55.Zhang N, et al. Genetic analysis and gene mapping of the orange flower trait in Chinese cabbage (Brassica rapa L.) Mol. Breeding. 2019;39:76–86. doi: 10.1007/s11032-019-0984-5. [DOI] [Google Scholar]
- 56.Qi, J. N. et al. Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Rep.28, 597–604 (2010).
- 57.Ren YJ, et al. Characteristics of color development in seeds of brown- and yellow-seeded heading Chinese cabbage and molecular analysis of Brsc, the candidate gene controlling seed coat color. Front. Plant Sci. 2017;8:1410. doi: 10.3389/fpls.2017.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.