Abstract
Heterochromatin regulation is critical for genomic stability. Different H3K9 methylation states have been discovered, with distinct roles in heterochromatin formation and silencing. However, how the transition from H3K9me2 to H3K9me3 is controlled is still unclear. Here, we investigate the role of the conserved bromodomain AAA-ATPase, Abo1, involved in maintaining global nucleosome organisation in fission yeast. We identified several key factors involved in heterochromatin silencing that interact genetically with Abo1: histone deacetylase Clr3, H3K9 methyltransferase Clr4, and HP1 homolog Swi6. Cells lacking Abo1 cultivated at 30 °C exhibit an imbalance of H3K9me2 and H3K9me3 in heterochromatin. In abo1∆ cells, the centromeric constitutive heterochromatin has increased H3K9me2 but decreased H3K9me3 levels compared to wild-type. In contrast, facultative heterochromatin regions exhibit reduced H3K9me2 and H3K9me3 levels in abo1∆. Genome-wide analysis showed that abo1∆ cells have silencing defects in both the centromeres and subtelomeres, but not in a subset of heterochromatin islands in our condition. Thus, our work uncovers a role of Abo1 in stabilising directly or indirectly Clr4 recruitment to allow the H3K9me2 to H3K9me3 transition in heterochromatin.
Subject terms: Centromeres, Centromeres, Telomeres, Telomeres
Introduction
In eukaryotic cells, the regions of the chromatin that contain active genes are termed euchromatin, and these regions condense in mitosis to allow for chromosome segregation and decondense in interphase to allow for gene transcription1. The chromatin regions that remain condensed throughout the cell cycle are defined as heterochromatin regions and are transcriptionally repressed2,3. In general, heterochromatin can be defined molecularly by the modification of histone H3, specifically the absence of methylation on lysine 4 (H3K4me) and presence of methylation on lysine 9 (H3K9me)4–6. A conserved group of heterochromatin proteins, the heterochromatin protein 1 (HP1) family, specifically recognise heterochromatin regions by binding to H3K9me. Both di- and tri-methylation (H3K9me2/3) enable the binding of HP1 proteins to silence heterochromatin7,8. Heterochromatin regions can be further classified into constitutive and facultative heterochromatin9,10. Fission yeast, Schizosaccharomyces pombe, is an excellent model organism for chromatin studies because its heterochromatin organisation is similar to human cells11,12. Examples of constitutive heterochromatin regions in S. pombe are the pericentromeric regions that contain repetitive DNA sequences13. These regions are regulated by RNAi-dependent silencing machinery that involves protein Dcr1 and Ago114,15. In contrast, the silencing of facultative heterochromatin, such as subtelomeric regions and heterochromatin islands, is mediated by RNAi-independent machinery that can be dynamically modulated to express genes required for the cell cycle or adaptation to the cell environment16,17. A subset of facultative heterochromatin islands, known as “determinant of selective removal” (DSR) islands, contain meiotic genes18. These islands are silenced through a specific RNA elimination process, together with Clr4, when the cells are not in meiosis19.
Distinct roles have been observed for H3K9me2 and H3K9me3 in heterochromatin silencing. In the nematode Caenorhabditis elegans, H3K9 is methylated sequentially and H3K9me2 and H3K9me3 are present in distinct localised chromatin domains20,21. Jih et al. recently reported that the transition from H3K9me2 to H3K9me3 can be blocked by introducing point mutations in the SET and chromodomain of S. pombe Clr4 (I418P, F449Y, and W31G)22. These three Clr4 mutants exhibit increased H3K9me2 and reduced H3K9me3 levels in pericentromeric regions, which allows the transcription of dg-dh repeats22. However, the two Clr4 SET domain mutants (I418P and F449Y) block the transition to H3K9me3 in the pericentromeric region by inhibiting the methylation capacity of the enzyme, whereas the chromodomain mutant Clr4 (W31G) reduces the transition to H3K9me3 by blocking recognition and binding to methylated H3K922,23.
In S. pombe, H3K9me2 regions allow transcription, whereas H3K9me3 regions are transcriptionally silent22. The Clr4 enzyme is comprised of the chromodomain and SET domains and binds to methylated H3K924. The HP1 homologue Swi6 also binds H3K9me and acts together with the histone deacetylase Clr3 to prevent histone turnover in heterochromatin regions, repressing transcription25. Clr4 is responsible for both H3K9me2 and H3K9me3 in fission yeast, but the determinants and auxiliary proteins leading to the transition from H3K9me2 to H3K9me3 remain unknown.
The bromodomain is an extensive evolutionarily conserved protein domain that recognises acetylated lysine residues and plays multiple roles in transcriptional activation26. The human bromodomain-containing proteins, ATAD2 and ATAD2B, belong to the AAA (ATPases Associated with diverse cellular Activities) ATPase family. Proteins belonging to this family hydrolyse ATP to mediate conformational changes in substrate proteins, resulting in diverse cellular processes27,28. ATAD2 plays a key role in cell proliferation and is upregulated as a potential oncogene in cervical, colorectal, breast, and lung cancers29–32. In the budding yeast Saccharomyces cerevisiae, the ATAD2 homologue Yta7 binds to histones and participates in the heterochromatin boundary function in the HMR region, where it counteracts the spread of silencing into the neighbouring euchromatin33,34. This ScYta7 boundary function is possibly related to the regulation of nucleosome density through eviction of H3-H4 tetramers from the chromatin35.
Two AAA-ATPase bromodomain family members have been identified in S. pombe, Abo1 and Abo236. A recent study showed in vitro that Abo1 structure works as a hexamer using Cryo-EM analysis and is able to load H3-H4 onto DNA in a ATP-hydrolysis dependent manner37. In vivo, cells lacking Abo1 exhibit a global reduction in nucleosome levels, nucleosome redistribution, silencing defects at centromeric heterochromatin regions, and a chromosome segregation phenotype. Abo1 plays a role in centromere formation, as Abo1 is localised at pericentromeric heterochromatin and abo1∆ mutant cells present with silencing and defects in chromosome segregation 38Intriguingly, pericentromeric heterochromatin in abo1∆ has a slight increase in H3K9me2 enrichment, with clear defects in silencing38. However, the mechanism underlying Abo1 function is still unknown.
Here, our synthetic genetic screening of Abo1-interacting genes in S. pombe revealed several heterochromatin factors, including the histone deacetylase Clr3, H3K9 methyltransferase Clr4, and HP1 homologue Swi6. We found that cells lacking abo1 exhibit a strong imbalance in H3K9me2 and H3K9me3 levels within different heterochromatin regions, resulting in transcriptional deregulation. We show that Abo1 is involved in the heterochromatin assembly process by stabilising Clr4 binding and mediating the transition between H3K9me2 and H3K9me3.
Results
Abo1 interacts genetically with heterochromatin factors Clr3, Clr4, and Swi6
To explore the function of Abo1 in chromatin structure and function, we conducted a focused genetic screen to identify partners of Abo1. We performed a Synthetic Genetic Array (SGA) assay39 in which S. pombe strains harbouring deletions of genes involved in chromatin regulation were crossed with a strain carrying an abo1 deletion. Among 711 investigated genes, the strongest negative genetic interactions were recorded with genes encoding proteins involved in heterochromatin assembly: histone deacetylase Clr3, H3K9 methyltransferase Clr4, and chromodomain HP1 homologue Swi6 (Supplementary Table S1).
Other bromodomain family members in human cells are implicated in the modulation of gene expression in heterochromatin during temperature stress40. Therefore, we also analysed the cell viability of wild-type and abo1 single/double-mutant cells during temperature stress. At the standard growth temperature (30 °C), the population of dead cells was increased in the abo1∆ culture compared to wild-type. A synergistic increase in cell death was observed for the double mutants of abo1∆ combined with clr4∆ and swi6∆ (proportion of dead cells for triplicate culture in WT: 0.76 ± 0.41%; abo1∆: 2.28 ± 0.36%; clr4∆: 1.04 ± 0.05%; abo1∆clr4∆: 5.85 ± 0.69%; swi6∆: 0.67 ± 0.07%; abo1∆swi6∆: 8.65 ± 1.06%). No additive effects were detected with abo1∆ and clr3∆ (clr3∆: 0.43% ± 0.13; abo1∆clr3∆: 2.88% ± 0.24; Fig. 1). Growth experiments under cold stress (25 °C) and heat stress (37 °C) for 4 hours revealed that abo1∆ mutant cells poorly adapt to temperature changes compared to wild-type cells. The double mutants abo1∆clr3∆, abo1∆clr4∆, and abo1∆swi6∆ had a synergistic increase in dead cells under temperature stress compared to the normal growth temperature. The negative genetic interactions of abo1 and clr3/clr4/swi6 suggest that Abo1 is involved in some aspect of heterochromatin assembly.
Figure 1.
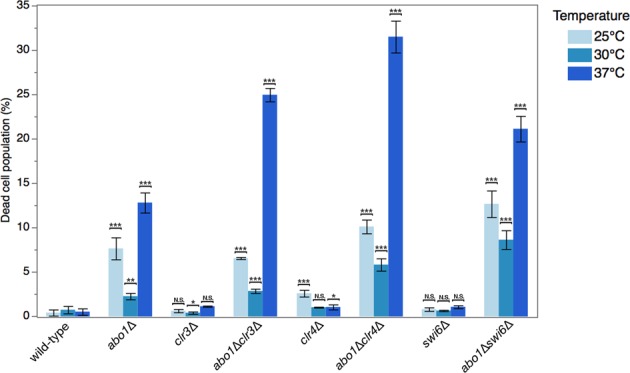
Abo1 interacts genetically with heterochromatin factors Clr3, Clr4, and Swi6. A histogram showing the results of flow cytometry analyses of the population of dead cells of single clr3∆, clr4∆, and swi6∆ mutants and their corresponding double mutants combined with abo1 deletion at normal culture temperature (30 °C) or under cold stress (25 °C) and heat stress (37 °C) conditions for 4 hours. The following strains were used: Hu2185, Hu2318, Hu3022, Hu3026, Hu3023, Hu3027, Hu3024, and Hu3028. The percentage of dead cells at different temperatures for the different deleted mutants. Error bars indicate standard error of the mean (n = 3). *p < 0.1, **p < 0.05, ***p < 0.01, two-tailed unpaired t-test. N.S., not significant (i.e., p > 0.1).
Deletion of abo1 alters H3K9me2 and H3K9me3 levels and causes silencing defects in subtelomeric and pericentromeric heterochromatin regions
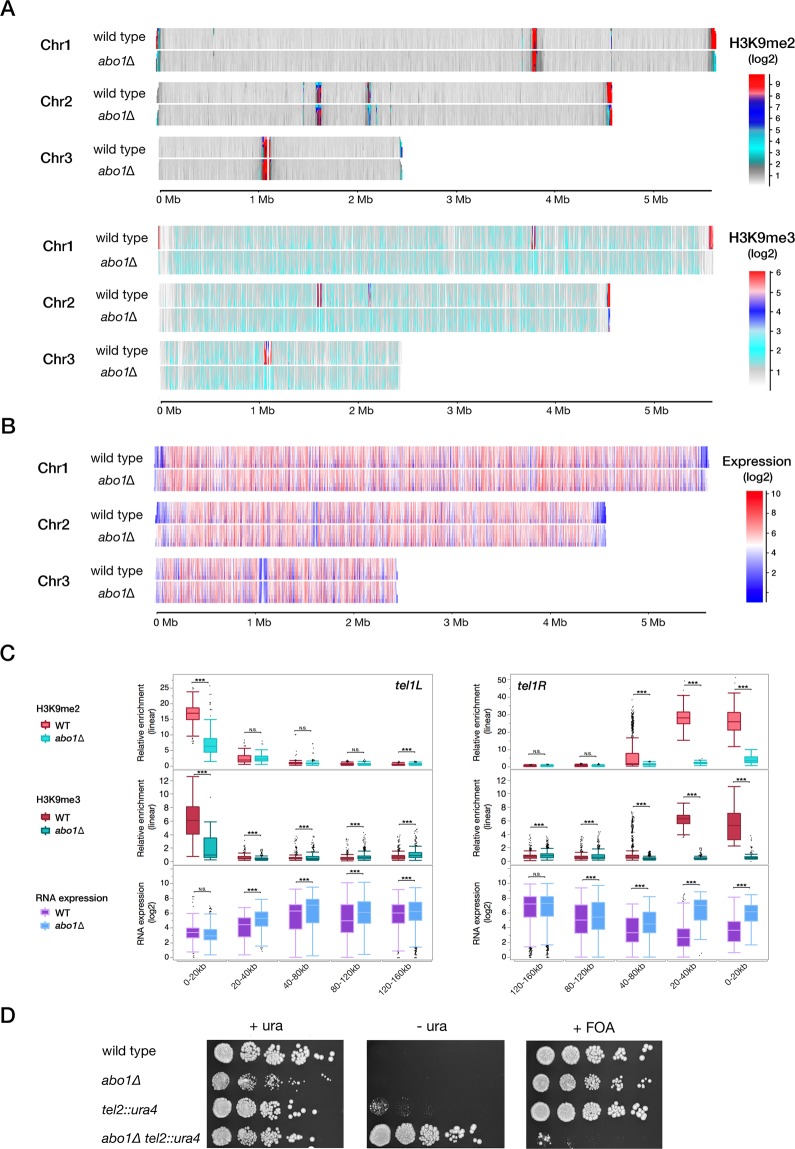
Based on previous observations of abo1∆ silencing defects38, and to further investigate the genome-wide role of Abo1 in heterochromatin formation and its effect on transcription, we performed ChIP-seq of H3K9me2/3 marks (Fig. 2A) and RNA profiling (Fig. 2B). The most striking effects occurred in the subtelomeric regions (Fig. 2A-C). For example, at subtelomeres in chromosomes I and II, the H3K9me2 and H3K9me3 levels respectively decreased at least 4-fold and 3-fold in abo1∆ compared to wild-type cells. De-repression of RNA levels was also observed in the same regions (Supplementary Fig. S1). This effect was especially pronounced in the regions 20 to 40 kb from the chromosome ends (Fig. 2C; Supplementary Fig. S1). These regions are strongly enriched in H3K9me2/3 and Swi6 in wild-type cells41. In the left subtelomeric region of chromosome II, the H3K9me2 and H3K9me3 levels were not affected. At chromosome III, the H3K9me2 levels were increased only within 20 kb from the right end, and RNA expression in that area was generally not affected by deletion of Abo1 (Supplementary Fig. S1). Subtelomeric regions of chromosome III are differentially regulated from other subtelomeres, as they contain the rDNA loci.
Figure 2.
Deletion of abo1 changes H3K9me2 and H3K9me3 enrichment and causes silencing defects in pericentromeric and subtelomeric heterochromatin regions. (A) Domainogram of H3K9me2 enrichment (up-panel) and H3K9me3 (down-panel) on three chromosomes in wild-type and abo1∆ cells grown at 30 °C. The enrichment is represented by log2 values as indicated by the colours, ranging from weak (grey) to strong (red). (B) Domainogram of RNA expression at 30 °C in wild-type and abo1∆ cells. The Hu2185 and Hu2318 strains were used. The expression is represented by log2 values indicated by the colours, ranging from weak expression (blue) to high expression (red). (C) Box plots of the left and right subtelomere regions of chromosome I, showing H3K9me2 enrichment (upper panel), H3K9me3 enrichment (middle panel) and gene expression levels (lower panel) in wild-type cells and abo1∆ cells grown at 30 °C. The plots represent log2 values. (D) Pictures of spotting assays using wild-type and abo1∆ cells with the ura4 gene reporter located in the Tel2L region. Cells were grown for 4 days at 30 °C in PMG + uracil, PMG -uracil, and PMG + 5-FOA as indicated. The following strains were used: Hu2185, Hu2318, FY1862, and Hu3030. All ChIP –seq experiments were performed in at least two independent bio-replicates.Error bars indicate standard error of the mean. *p < 0.1, **p < 0.05, ***p < 0.01, two-tailed unpaired t-test. N.S., not significant (i.e., p > 0.1).
We further examined the silencing in subtelomeres of abo1-deleted cells using the ura4+ reporter gene inserted in the left subtelomere of chromosome II (tel2L::ura4). Silencing of tel2L::ura4 was examined by the growth of colonies in media without uracil and media containing FOA. In abo1-deleted cells, silencing of tel2L::ura4 was clearly reduced, confirming that Abo1 is required for gene silencing in subtelomeric regions (Fig. 2D).
Our genome-wide mapping of H3K9me2 corroborates the discrete change in abo1∆ cells at the pericentromeric regions presented in the supplementary data by Gal et al.38.as in the otr1R region in abo1∆ cells (Supplementary Fig. S2). A significant reduction of H3K9me3 marks was observed in pericentromeric otr regions of all three chromosomes contrasting with H3K9me2 alteration in the same regions, as in otr1R region in abo1∆ cells (Supplementary Fig. S2). In addition, RNA profiling analysis showed that the otr1R region was 1.4-fold de-repressed in abo1Δ cells compared to wild-type (Supplementary Fig. S3C). As the abo1∆ mutant was temperature-sensitive, we examined whether Abo1 is linked to changes in chromatin expression. However, comparisons of genome-wide expression in abo1-deleted cells did not reveal notable differences in the silencing of heterochromatic regions at different temperatures (Supplementary Fig. S3A-C). Thus, the silencing defects observed in abo1∆ cells appear to be mostly independent of growth temperature.
Abo1 deletion results in a similar phenotype as Clr4 point mutation
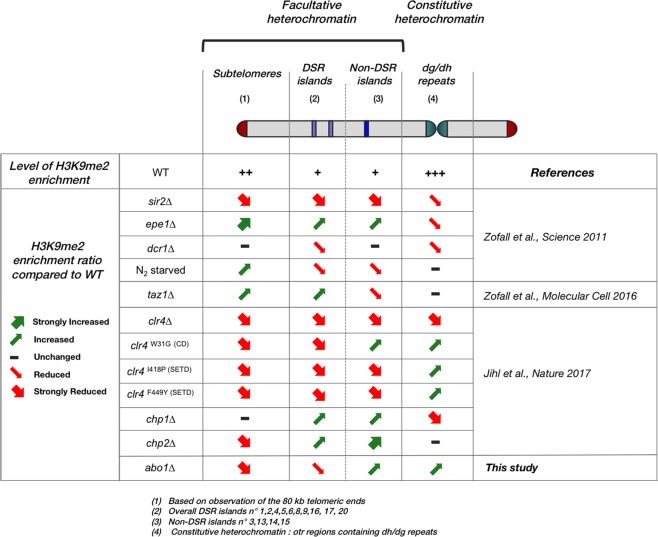
To search for the heterochromatin assembly mechanism involving Abo1, we extracted published H3K9me2 ChIP-seq data from several different mutant strains19,22,42. In addition to the Clr4 mutant strains (clr4∆, clr4I418P, clr4F449Y, clr4W31G), other mutations affecting heterochromatin-associated factors, such as the deacetylate Sir2, putative histone H3 demethylase Epe1, siRNA-producing enzyme Dcr1, telomere binding protein Taz1, and HP1 family members Chp1 and Chp2, were included. The effects of these mutations on H3K9me2 levels in the different heterochromatin regions were compared to the H3K9me2 levels in cells lacking Abo1 (Fig. 3; Supplementary Figs. S4-S8). These comparisons revealed a similarity between the clr4W31G strain and abo1∆ cells. For example, in the otr1R region, H3K9me2 levels were increased but H3K9me3 levels were reduced compared to wild-type in both abo1∆ and clr4W31G mutant cells (Supplementary Fig. S2, middle panel; Supplementary Fig. S6, top panel)43, which suggested that abo1 deletion may also disrupt the transition of H3K9me2 to H3K9me3 as clr4W31G mutation.
Figure 3.
Cells with abo1 deletion present a similar phenotype as the clr4 point mutation. The tables summarize changes in H3K9me2 levels in fission yeast cells with the indicated mutant backgrounds compared to wild-type. Small arrows represent a partial or no reduction/increase in H3K9me2 levels in the designated chromatin regions, whereas large arrows indicate a stronger reduction in most of the target compared to the wild-type. Observations were made on the effects based on the mean value of the corresponding region compared to the wild type of extracted published or present data or showed by the authors themselves. H3K9me2 levels of telomeric heterochromatin and islands are shown in Supplementary Data S1, S2, S4, S5, S6, S7, S8. The data were extracted from the publications indicated in the right column.
Abo1 is required for the transition of H3K9me2 and H3K9me3 in pericentric and telomeric heterochromatin
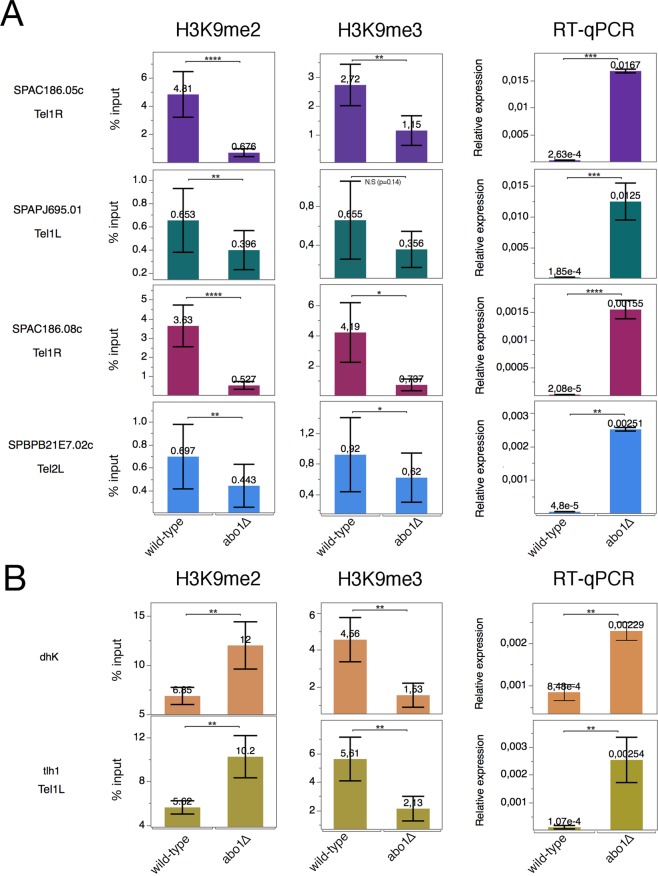
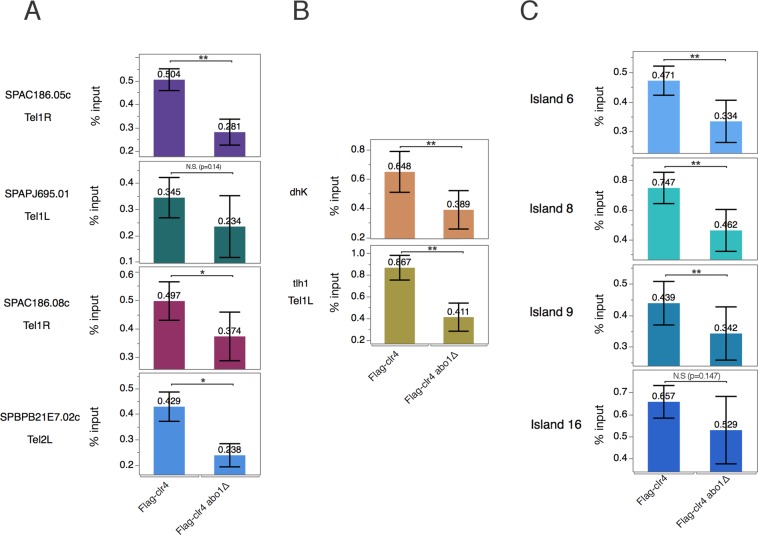
To confirm our observation on the role of Abo1 in histone methylation, we validated H3K9me2/me3 enrichment by ChIP-qPCR and expression by RT-qPCR of several genes located at subtelomeres, in the pericentromeric dh repeats, and at tlh1, a gene located near the end of chromosome I that contains dg-dh-like repeats (Fig. 4). The four genes located in the subtelomeric regions Tel1R and Tel1L exhibited robust methylation levels in wild-type and a clear reduction of both H3K9me2 and H3K9me3 in abo1∆ cells (Fig. 4A). All four genes that were tested had 100-fold increased expression in abo1∆ cells (Fig. 4A, right panel). In contrast, at pericentromeric repeats (dhK) and the tlh1 locus, abo1∆ cells had increased H3K9me2 enrichment and reduced H3K9me3 compared to wild-type (Fig. 4B). These changes were accompanied by silencing defects (Fig. 4B, right panel). These observations corroborate the role of Abo1 in mediating the transition from H3K9me2 to H3K9me3, specifically in heterochromatin at telomeres and in centromeric repeat regions. However, subtelomeric genes require Abo1 for both H3K9me2 and H3K9me3 (Fig. 4A), possibly reflecting different Clr4 recruitment mechanisms compared to telomeric (tlh1) and centromeric (dhK) repeat regions (see Discussion).
Figure 4.
Differential requirement for Abo1 in H3K9me2 and H3K9me3, and silencing of gene expression in subtelomeric and pericentromeric regions. Bar diagrams showing the ChIP and RT-qPCR results. The Hu2185 and Hu2318 strains were used. (A) Analysis of four genes located in subtelomeric regions: H3K9me2 (left panels), H3K9me3 ChIP-qPCR (middle panels), and RT-qPCR (right panels). (B) Analysis of dh repeats located in pericentromeric regions and the Tel1L (tlh1) region: H3K9me2 ChIP-qPCR (left panels) and RT-qPCR (right panels). All qPCR experiments were performed in at least three independent experiments. Error bars indicate standard error of the mean. *p < 0.1, **p < 0.05, ***p < 0.001, two-tailed unpaired t-test. N.S., not significant (i.e., p > 0.1).
Abo1 is required to maintain H3K9me2 and H3K9me3 levels in DSR islands without any effect on gene expression
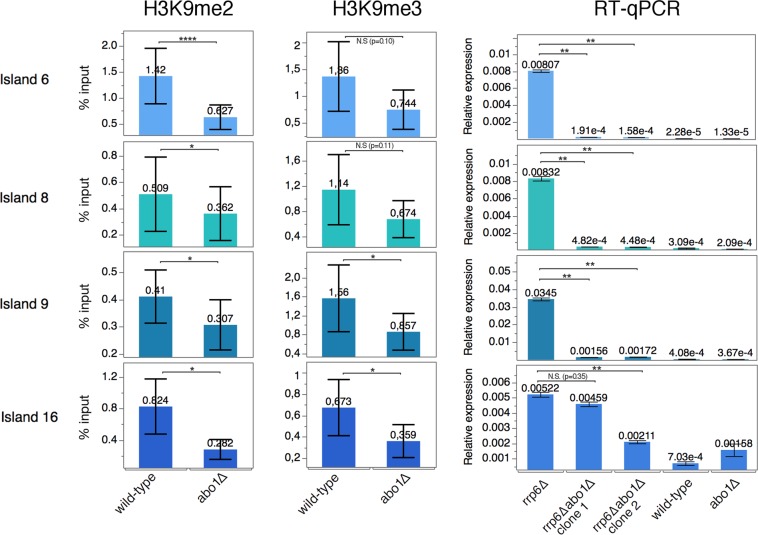
Next, we analysed the role of Abo1 in DSR islands by comparing our abo1∆ ChIP-seq results to publicly available data19,42. The average H3K9me2 levels in abo1∆ and clr4W31G cells exhibited a decreased level of H3K9me2 in 9 of 10 DSR islands, whereas the levels of H3K9me2 were increased in 3 of 4 non-DSR islands (Fig. 3, Supplementary Fig. S8A). The reduced H3K9me2 levels were confirmed by ChIP-qPCR at several DSR islands (Fig. 5, left panel). H3K9me3 levels were also significantly affected by abo1 deletion (Fig. 5, middle panel). Next, we analysed the expression of these islands in rrp6∆abo1∆ cells. The rrp6+ gene was deleted to abolish RNA degradation of the DSR genes19. Surprisingly, despite the loss of the H3K9me2/me3 heterochromatin markers, deletion of abo1 led to reduced expression of DSR islands in rrp6∆abo1∆ compared to rrp6∆ cells (Fig. 5, right panel). These results show that Abo1 is required for the establishment of heterochromatin and contributes to the transition of H3K9me2 to me3 at DSR islands. Furthermore, Abo1 somehow negatively affects the transcription or stability of DSR island mRNA (see Discussion).
Figure 5.
Loss of Abo1 does not affect silencing in DSR islands despite decreased H3K9me2 and H3K9me3 levels. Bar diagrams showing the ChIP and RT-qPCR results for four different heterochromatin DSR islands: Island6 (ssm4), Island8 (mcp5), Island9 (mei4), and Island16 (pvg4). H3K9me2 ChIP-qPCR (left panels), H3K9me3 ChIP-qPCR (middle panels), and RT-qPCR (right panels). The genotypes are indicated (wild-type, abo1∆, and rrp6∆). The following strains were used: Hu2185, Hu2318, Hu2464, and Hu3031 (clone1 and clone2). ChIP-qPCR experiments were performed in at least three independent experiments. RT-qPCR of abo1∆rrp6∆ was performed in two independent colonies. Error bars indicate standard error of the mean. *p < 0.1, **p < 0.05, ***p < 0.001, two-tailed unpaired t-test. N.S., not significant (i.e., p > 0.1).
Clr4 and H3K9 methylation are needed for heterochromatin association of Abo1
Attempts were also made to address whether Abo1 directly interacts with Clr4 by performing Abo1-TAP purification using mass spectrometry. However, no trace of Clr4 was found, suggesting an indirect interaction between Clr4 and Abo1 (Supplementary Fig. S11). We also analyzed at the data from Iglesias et al.44 who recently determined the Swi6-associated heterochromatin proteome using a method of native chromatin domains coupled with quantitative mass spectrometry (nChIP-MS). In S. pombe, Abo1 was found in association with Swi6 that specifically bind domains containing H3K9me25 with a similar enrichment level (23 fold, p-value 1.15 10−2) (Supplementary Fig. S12B) compared to heterochromatin core proteins. Similar results were found for the closely related species S. japonicus that diverged from S. pombe approximately 30 million years ago (420 fold, p-value 1.19.10−2). Interestingly, when Swi6 nChIP-MS was performed in the clr4 catalytically dead (clr4∆CD) and H3K9R substitution mutants, Abo1 was not found (Supplementary Fig. S12B). We also checked in their data if Abo1 Swi6 heterochromatin association could depend on others heterochromatin components required for silencing such as Chp2, Dcr1, Ago1, Sir2, Epe1. None of these factors affects the association between Abo1 with FLAG-Swi6 (Supplementary Fig. S12C). Thus, we conclude from this analysis that Abo1 is associated with Swi6 chromatin and this is dependent of H3K9me and Clr4.
Abo1 is required to stabilise Clr4 recruitment at heterochromatin
Using ChIP-qPCR, we compared Clr4 enrichment at heterochromatin regions between the Clr4 flag-tagged and Clr4 flag-tagged abo1∆ strains. At the four genes tested in the subtelomeric regions, we observed a marked decrease in Clr4 occupancy in abo1 deletion (Fig. 6A) consistent with the decrease of H3K9me3 observed in the same loci in abo1∆ cells (Fig. 5A). At the centromeric (dhK) and telomeric repeats (tlh1), Clr4 occupancy was also significantly reduced in abo1∆ cells (Fig. 6B). A similar decrease in Clr4 was observed at DSR islands (Fig. 6C). Generally, these results support the role of Abo1 to stabilise Clr4 recruitment to allow the H3K9me2-H3K9me3 transition at different heterochromatin regions.
Figure 6.
Deletion of Abo1 leads to reduced enrichment of Clr4 in all heterochromatin regions. Bar diagrams showing the Flag ChIP-qPCR results. The SPJ390 and Hu3040 strains were used. (A) Analysis of four genes located in subtelomeric regions. The locations of genes are indicated. (B) Analysis of dh repeats located in pericentromeric regions and the Tel1L (tlh1) region. (C) Analysis of four different heterochromatin DSR islands: Island6 (ssm4), Island8 (mcp5), Island9 (mei4), and Island16 (pvg4). All qPCR experiments were performed in at least three independent experiments. Error bars indicate standard error of the mean error. *p < 0.1, **p < 0.05, ***p < 0.001, two-tailed unpaired t-test. N.S., not significant (i.e., p > 0.1).
Discussion
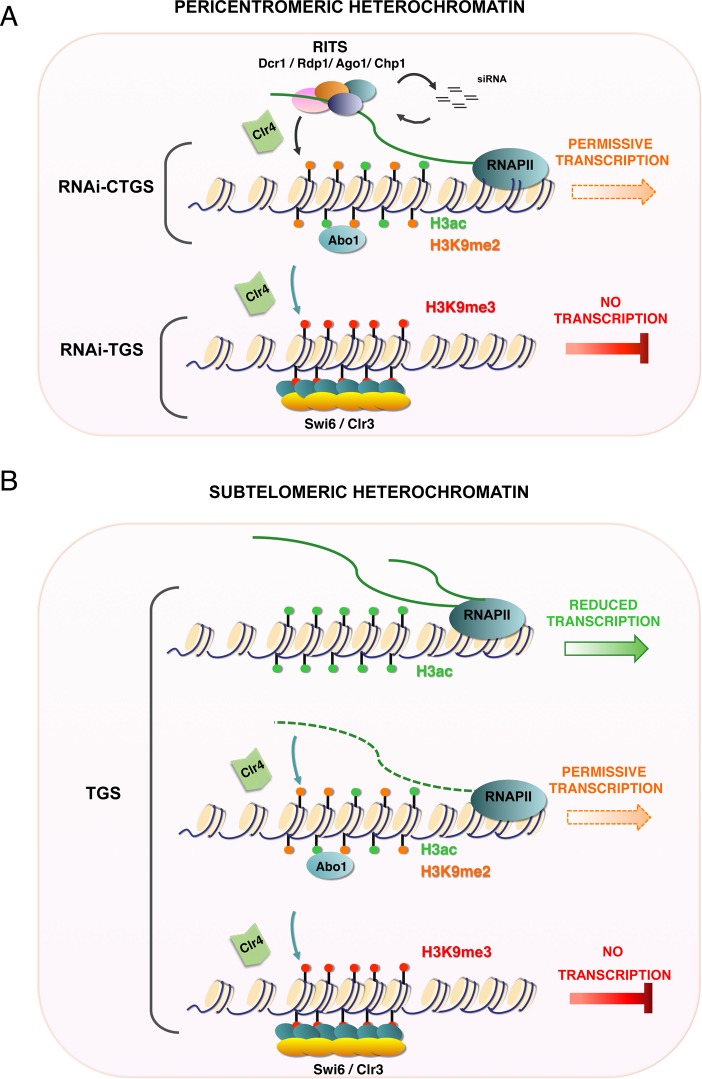
The present study showed that Abo1 is required for the proper regulation of chromatin silencing and maintenance of the H3K9me2/3 levels at both facultative and constitutive heterochromatin regions. Until recently, it has generally been thought that the H3K9me2 marker in S. pombe heterochromatin inversely correlates with expression45. In 2017, a study in S. pombe revealed that H3K9me2 and H3K9me3 have distinct roles in gene silencing, with H3K9me2 being permissive for transcription22. Our work identifies Abo1 as a new factor involved in the H3K9 methylation process. In pericentromeric heterochromatin, silencing occurs in two steps: RNAi co-transcriptional transcriptional gene silencing (RNAi-CTGS) followed by transcriptional gene silencing (RNAi-TGS)22. In the RNAi-CTGS step, the dg-dh repeats can still be transcribed, and siRNAs are generated by Dicer and Ago1, consequently activating the RITS complex (Fig. 7A)46–48. Next, the siRNA-activated RITS complex helps recruit the methyltransferase Clr4 to perform di-methylation of H3K949,50. At this stage, H3K9me2 and euchromatic H3 acetylation (H3ac) are present, allowing for a low level of transcription. In the RNAi-TGS step, H3K9 is tri-methylated, Swi6 binds, and the Clr3 deacetylase enzyme removes acetylation of H3 to stop transcription (Fig. 7A). We hypothesise that Abo1 appears in the first step when H3K9me2 has been added by Clr4. Our finding that Abo1 association with heterochromatin depends on Clr4 and H3K9 methylation is consistent with this idea (Supplementary Fig. S12). Furthermore the reduction of Clr4 recruitment that we observe at dhK and tlh1 in abo1∆ strains suggests that Clr4 is stabilised by Abo1 (Fig. 6B).
Figure 7.
Model of the proposed role of Abo1 in heterochromatin in S. pombe. Schematic models of Abo1 function in constitutive heterochromatin (A) and facultative heterochromatin regions (B).
In contrast, at genes in subtelomeric regions (Fig. 7B), di-methylation on H3K9 does not require the siRNA-RITS complex. Here, Abo1 may help to recruit Clr4 promoting both H3K9me2 and H3K9me3, leading to TGS. The data in Fig. 6A, showing that Clr4 binding to subtelomeres depends on Abo1, supports this notion. At both pericentric and telomeric regions, the physical interaction observed between Abo1 and Swi651 may contribute to the assembly of heterochromatin.
Interestingly, the reduced expression and H3K9me2 levels at the DSR islands (Fig. 5) suggest that Abo1 plays a different role with Clr4 in this facultative heterochromatin. A recent study showed that, despite its presence at DSR genes, Clr4 is not involved in regulating mRNA levels at normal growth temperature, whereas Set1-mediated deacetylation by Clr3 and other HDACs plays a key role52. However, loss of Clr4 in cells cultivated at 18 °C alters meiotic gene silencing but also the assembly of new facultative heterochromatin islands indicating a critical role for Clr4 at lower temperature53. Since Abo1 deletion have been show to affect the global gene expression and nucleosome occupancy in vivo38, we cannot rule out the possibility that Abo1 acts across the genome affecting indirectly the gene expression. Whether Abo1 modulates Set1 and HDAC activity at facultative heterochromatin islands or mediates their silencing through the Clr4 recruitment at low temperature remains to be determined.
One possibility is that the observed effect on methylation patterns in the tested strains could be linked to nucleosome dynamics. We first looked at nucleosome positioning using data from Gal et al.38 in the same regions analysed in our study. However, no obvious changes were observed at either the positioning or nucleosome levels in abo1∆ compared to wild-type cells at the subtelomeric, pericentromeric, and non-DSR island regions (Supplementary Fig. S9). Absence of change was also observed when the H3 occupancy was tested for the same regions using ChIP-qPCR abo1∆, clr4W31G, and abo1∆ clr4W31G strains (Supplementary Fig. S10). Together, both of these results suggest that nucleosome dynamics defects are not the main cause of H3K9me transition defects in abo1∆ cells.
In C. elegans, the heterochromatin includes telomeric and subtelomeric regions, as well as centromeres and repetitive DNA transposons 54,55. Similar to fission yeast, these heterochromatin regions are enriched in H3K9 methylation54, and an HP1-like protein, HPL-2, binds to regions correlating with H3K9me2/3 enrichment55,56. The analysis of H3K9me2 and H3K9me3 patterns has revealed that these markers can be enriched in different chromatin regions associated with distinct functions in C. elegans20. H3K9 methylation occurs step-wise by different enzymes: MET2 for mono-/di-methylation and SET25 for tri-methylation. The latter step brings heterochromatin to the nuclear periphery and leads to TGS21. In S. pombe, whether tri-methylation is coupled to peripheral localisation in the nucleus remains to be tested. Given our findings for Abo1, it would be interesting to test whether an ATAD2 homologue in C. elegans modulates the activity of MET2 or SET25. A candidate for this function could be the ATAD2 homologue LEX-1, as it has been reported to modulate gene expression at repetitive DNA sequences57.
The exact function of the human Abo1 homologue ATAD2 in both cancer and non-cancer cells remains unclear. However, ATAD2 has been proposed to be involved in the regulation of many key factors, such as E2F, Myc, and EZH2, as well as downstream crosstalk with P53/P21 pathways58–61. In embryonic stem cells, knockdown of ATAD2 leads to an overall increase in nucleosome density and reduced histone turnover62. In breast cancer, ATAD2 has higher affinity for heterochromatin than euchromatin during S phase63. Although it is conceivable that the mechanistic role of AAA family proteins is conserved in eukaryotes, future studies need to address the putative role of human Abo1 homologues in heterochromatin assembly.
Materials and Methods
Schizosaccharomyces pombe strains and growth conditions
Culture, genetic manipulation, and fission yeast strains are described in Supplementary Table S2. Standard YES medium was used for cell culture. Standard fission yeast genetic techniques and media were used according to Moreno et al.64. Yeast cells were grown to mid-logarithmic phase. Cells were then counted and diluted to the concentration of 1.25 × 106 cells/ml. Five-fold serial dilution was performed. A total of 5 μl of each dilution was spotted on complete PMG media plates containing 1 g/L FOA (5-fluorouracil-6-carboxylic acid monohydrate) and PMG media lacking uracil. The plates were incubated at 30 °C.
FACS analysis
S. pombe cells were grown to log phase in triplicate for fluorescence-activated cell sorting (FACS). Cells were then washed with 1x PBS and subsequently resuspended in 50 mM sodium citrate (pH 7) with 50 μg/ml propidium iodide (PI) for 30 min in the dark at room temperature (Sigma Aldrich). Cells were analysed immediately using a flow cytometer (Cytoflex, Beckman Coulter). Cells were sorted by forward (FSC) and side (SSC) light scattering. After gating for 20,000 single cells using FSC and SSC, dead cells stained with PI were counted through the FL2- channel.
RNA extraction and RNA microarray
Wild-type (Hu2185) and abo1∆ strains (Hu2318) were cultured to mid-log exponential phase and continuously induced at 25 °C, 30 °C, or 37 °C for 4 hours. The cells were then harvested by centrifugation at 3000 rpm and lysed by incubation with TES buffer (10 mM Tris-HCl, pH 7.5, 10 mM EDTA, and 0.5% SDS). RNA was extracted using the hot acid-phenol method. Biological duplicates of RNA samples were sent for hybridisation to Gene Chip S. pombe Tilling 1.0FR Arrays (Affymetrix, Santa Clara, CA, USA) at the Affymetrix core facility (BEA) of Karolinska Institutet. For RNA microarray analysis, the signal raw data files were normalised using Affymetrix Tiling Analysis Software (TAS). The normalised microarray data were mapped to the S. pombe genome (Sanger 2007). Microarray results were visualised using SeqMonk software (https://www.bioinformatics.babraham.ac.uk/projects/seqmonk/). The microarray data can be accessed at GEO accession GSE 125910.
RT- qPCR
For each cell culture, 1.0 μg of the extracted RNA was treated with DNase I (18068015, Thermo Fisher) and isolated for reverse transcription by SuperScript™ III First-Strand Synthesis Master Mix (11752050, Invitrogen). RT-qPCR was then applied by using the SYBR™ Green PCR Master Mix (436870, Thermo Fisher). The reactions were performed using an Applied Biosystems® 7500 Real-Time PCR System. The primer sequences are listed in Supplementary Table S3.
Chromatin immunoprecipitation
Chromatin samples from wild-type (Hu2185), abo1∆ cells (Hu2318), Flag-clr4 cells (SPJ390), and abo1∆ Flag-clr4 cells (Hu3040) were isolated in duplicate and subjected to ChIP according to Durand-Dubief et al.65. A total of 50 μg (for ChIP-seq) or 4 μg (for ChIP-qPCR) of H3K9me2 antibody (ab1220, Abcam), 4 μg (for ChIP-seq) or 1 μg (for ChIP-qPCR) of H3K9me3 biotinylated antibody (C1550003, Diagenode), 4 μg of Flag M2 antibody (F1804, Sigma), and 4 μg of H3 antibody (ab1791, Abcam) were added to 30 μl of sheared chromatin (for ChIP-qPCR) or 100 μl of sheared chromatin (for H3K9me2 ChIP-seq) or 200 μl of sheared chromatin (for H3K9me3 ChIP-seq) for each experiment. Protein A Dynabeads (10001D, Invitrogen) were used for H3K9me2 ChIP, and m280 streptavidin Dynabeads (11205D, Invitrogen) for H3K9me3 ChIP with an additional antibody pre-incubation step. ProteinA/G Magnetic beads (88802, Thermo Scientific) were used for Flag ChIP and H3 ChIP. ChIP DNA was sequenced and the sequencing data processed by the core facility (BEA) of Karolinska Institutet. Processed data were quantified using a read count per million reads normalisation, and then relative enrichment compared to the DNA input using SeqMonk software (https://www.bioinformatics.babraham.ac.uk/projects/seqmonk/). ChIP-DNA was also quantified for quantitative PCR using Applied Biosystems® 7500 Real-Time PCR Systems. SYBR™ Green PCR Master Mix (436870, Thermo Fisher). Primers are listed in Expanded View Table EV2. ChIP-seq data can be accessed at GEO accession GSE125911.
Synthetic genetics array analysis
A total of 760 haploid S. pombe strains harbouring deletions of genes involved in chromatin processes were selected from the Bioneer deletion library V.566 using Gene Ontology terms “chromatin binding”, “DNA binding”,”chromosome binding”, “chromosome”, and “transcription”. These strains have gene∆::KanR deletions and carry the auxotrophic markers ade6-M216 (or ade6-M210) ura4-D18 and leu1-32. The 760 strains were crossed with the smt0 abo1∆::hygR strain (Hu3021), spores transferred in YES media, and successively selected three times on YES with G418 (200 μg/ml) and YES with hygromycin (200 μg/ml). The cross resulted in 716 double mutants carrying a deletion for a gene involved in the chromatin process combined with the abo1 gene deletion using the RoToR robot (Singer Instruments). For analysis, single and double mutants were grown for 2 days using semi-solid YES media at 30 °C with the 384-plate format in triplicate. Plates were scanned and the colony sizes measured for four replicates and normalised using SGAtools67. Next, the colony fitness score was obtained using the normalised colony sizes of the single and double mutants. Negative scores indicate that the double mutant induces an increased fitness defect than expected; conversely, positive scores correspond to greater fitness compared to the control. The scoring distribution of these strains was analysed and a cut-off score of <−0.45 applied.
Protein purification and mass spectrometry
Six litres of Abo1-TAP (Hu2368) and wild-type cells were cultured to log-phase. Pelleted cells were then ground using the grinding machine (6875 Freezer/Mill® High Capacity Cryogenic Grinder) with liquid nitrogen. The frozen yeast powders were resuspended in lysis buffer (150 mM NaCl, 1% NP-40, 50 mM Tris-HCl). Cell lysates were ultra-centrifuged and the supernatant removed. The supernatant was flowed through pre-washed IgG Sepharose 6 Fast Flow beads (17-0969-01, GE Healthcare). Using TEV protease (AcTEV Protease, 12575-015, NOVEX), TAP-tagged Abo1 protein was eluted (TAP affinity purification protocol according to (http://cshprotocols.cshlp.org/content/2006/1/pdb.prot4153.full)68,69. Eluted samples were quantified and sent for mass spectrometry at the chemical core facility in Karolinska Institutet70.
Supplementary information
Acknowledgements
This work was supported by grants from the Swedish Cancer Society (CF) and the Swedish Research Council (VR) to KE and AL. We thank Patrick Varga-Weisz for the strains. We thank the BEA facility for their help processing the Affymetrix arrays and the ChIP high-throughput sequencing, and the MedH Flow Cytometry core facility at KI. Open access funding provided by Karolinska Institute.
Author contributions
M.D.D., W.D., K.E. and P.P. planned and designed the experiments. W.D., M.D.D., Y.Z., E.O. and J.P.S. performed the experiments and analysed the data. M.D.D., W.D., K.E., J.P.S. and A.L. wrote the manuscript with input from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63209-y.
References
- 1.Babu A, Verma RS. Chromosome structure: euchromatin and heterochromatin. Int. Rev. cytology. 1987;108:1–60. doi: 10.1016/S0074-7696(08)61435-7. [DOI] [PubMed] [Google Scholar]
- 2.Alberts, B. J. A. et al. Chromosomal DNA and Its Packaging in the Chromatin Fiber. Molecular Biology of the Cell. 4th edition (2002).
- 3.Elgin SC. Heterochromatin and gene regulation in Drosophila. Curr. Opin. Genet. Dev. 1996;6:193–202. doi: 10.1016/S0959-437X(96)80050-5. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 2002;12:198–209. doi: 10.1016/S0959-437X(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 5.Santos-Rosa H, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 6.Fang TC, et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 2012;209:661–669. doi: 10.1084/jem.20112343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskeland R, Eberharter A, Imhof A. HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol. Cell Biol. 2007;27:453–465. doi: 10.1128/Mcb.01576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachner M, O’Carroll N, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 9.Peters AHFM, et al. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat. Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 10.Grewal SIS, Jia ST. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 11.Fukagawa T, Earnshaw WC. The centromere: chromatin foundation for the kinetochore machinery. Dev. Cell. 2014;30:496–508. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez DE, et al. Telomere structure and telomerase in health and disease (Review) Int. J. Oncol. 2012;41:1561–1569. doi: 10.3892/ijo.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Jia ST, Jia S. New Insights into the Regulation of Heterochromatin. Trends genetics: TIG. 2016;32:284–294. doi: 10.1016/j.tig.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpe TA, et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 15.Djupedal I, et al. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes. Dev. 2005;19:2301–2306. doi: 10.1101/gad.344205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ai WD, Bertram PG, Tsang CK, Chan TF, Zheng XFS. Regulation of subtelomeric silencing during stress response. Mol. Cell. 2002;10:1295–1305. doi: 10.1016/S1097-2765(02)00695-0. [DOI] [PubMed] [Google Scholar]
- 17.Trojer P, Reinberg D. Facultative heterochromatin: Is there a distinctive molecular signature? Mol. Cell. 2007;28:1–13. doi: 10.1016/j.molcel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Harigaya Y, et al. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature. 2006;442:45–50. doi: 10.1038/nature04881. [DOI] [PubMed] [Google Scholar]
- 19.Zofall M, et al. RNA Elimination Machinery Targeting Meiotic mRNAs Promotes Facultative Heterochromatin Formation. Science. 2012;335:96–100. doi: 10.1126/science.1211651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessler JB, Andersen EC, Villeneuve AM. Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line. PLoS Genet. 2010;6:e1000830. doi: 10.1371/journal.pgen.1000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towbin BD, et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–947. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Jih G, et al. Unique roles for histone H3K9me states in RNAi and heritable silencing of transcription. Nature. 2017;547:463–467. doi: 10.1038/nature23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K, Mosch K, Fischle W, Grewal SI. Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat. Struct. Mol. Biol. 2008;15:381–388. doi: 10.1038/nsmb.1406. [DOI] [PubMed] [Google Scholar]
- 24.Ivanova AV, Bonaduce MJ, Ivanov SV, Klar AJ. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat. Genet. 1998;19:192–195. doi: 10.1038/566. [DOI] [PubMed] [Google Scholar]
- 25.Aygun O, Mehta S, Grewal SI. HDAC-mediated suppression of histone turnover promotes epigenetic stability of heterochromatin. Nat. Struct. Mol. Biol. 2013;20:547–554. doi: 10.1038/nsmb.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng L, Zhou MM. Bromodomain: an acetyl-lysine binding domain. Febs Lett. 2002;513:124–128. doi: 10.1016/S0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 27.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat. reviews. Mol. Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 28.Fouret R, et al. A comparative and integrative approach identifies ATPase family, AAA domain containing 2 as a likely driver of cell proliferation in lung adenocarcinoma. Clin. cancer research: an. Off. J. Am. Assoc. Cancer Res. 2012;18:5606–5616. doi: 10.1158/1078-0432.CCR-12-0505. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, et al. Oncogene ATAD2 promotes cell proliferation, invasion and migration in cervical cancer. Oncol. Rep. 2015;33:2337–2344. doi: 10.3892/or.2015.3867. [DOI] [PubMed] [Google Scholar]
- 30.Shang P, Meng F, Liu Y, Chen X. Overexpression of ANCCA/ATAD2 in endometrial carcinoma and its correlation with tumor progression and poor prognosis. Tumour biology: J. Int. Soc. Oncodev. Biol. Med. 2015;36:4479–4485. doi: 10.1007/s13277-015-3089-8. [DOI] [PubMed] [Google Scholar]
- 31.Kalashnikova EV, et al. ANCCA/ATAD2 overexpression identifies breast cancer patients with poor prognosis, acting to drive proliferation and survival of triple-negative cells through control of B-Myb and EZH2. Cancer Res. 2010;70:9402–9412. doi: 10.1158/0008-5472.CAN-10-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caron C, et al. Functional characterization of ATAD2 as a new cancer/testis factor and a predictor of poor prognosis in breast and lung cancers. Oncogene. 2010;29:5171–5181. doi: 10.1038/onc.2010.259. [DOI] [PubMed] [Google Scholar]
- 33.Gradolatto A, et al. Saccharomyces cerevisiae Yta7 regulates histone gene expression. Genetics. 2008;179:291–304. doi: 10.1534/genetics.107.086520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gradolatto A, et al. A Noncanonical Bromodomain in the AAA ATPase Protein Yta7 Directs Chromosomal Positioning and Barrier Chromatin Activity. Mol. Cell Biol. 2009;29:4604–4611. doi: 10.1128/Mcb.00160-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi LM, Ellahi A, Rine J. Direct regulation of nucleosome density by the conserved AAA-ATPase Yta7. P Natl Acad. Sci. USA. 2011;108:E1302–E1311. doi: 10.1073/pnas.1116819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpy A, et al. Absolute proteome and phosphoproteome dynamics during the cell cycle of Schizosaccharomyces pombe (Fission Yeast) Mol. Cell Proteom. 2014;13:1925–1936. doi: 10.1074/mcp.M113.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho C, et al. Structural basis of nucleosome assembly by the Abo1 AAA+ ATPase histone chaperone. Nat. Commun. 2019;10:5764. doi: 10.1038/s41467-019-13743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gal C, et al. Abo1, a conserved bromodomain AAA-ATPase, maintains global nucleosome occupancy and organisation. Embo Rep. 2016;17:79–93. doi: 10.15252/embr.201540476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong AH, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 40.Col E, et al. Bromodomain factors of BET family are new essential actors of pericentric heterochromatin transcriptional activation in response to heat shock. Sci. Rep. 2017;7:5418. doi: 10.1038/s41598-017-05343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cam HP, et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 2005;37:809–819. doi: 10.1038/ng1602. [DOI] [PubMed] [Google Scholar]
- 42.Zofall M, Smith DR, Mizuguchi T, Dhakshnamoorthy J, Grewal SIS. Taz1-Shelterin Promotes Facultative Heterochromatin Assembly at Chromosome-Internal Sites Containing Late Replication Origins. Mol. Cell. 2016;62:862–874. doi: 10.1016/j.molcel.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jih G, et al. Unique roles for histone H3K9me states in RNAi and heritable silencing of transcription. Nature. 2017;547:463–+. doi: 10.1038/nature23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglesias N, et al. Native Chromatin Proteomics Reveals a Role for Specific Nucleoporins in Heterochromatin Organization and Maintenance. Mol. Cell. 2020;77:51–66 e58. doi: 10.1016/j.molcel.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 46.Buhler M, Verdel A, Moazed D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell. 2006;125:873–886. doi: 10.1016/j.cell.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Carmichael JB, Provost P, Ekwall K, Hobman TC. ago1 and dcr1, two core components of the RNA interference pathway, functionally diverge from rdp1 in regulating cell cycle events in Schizosaccharomyces pombe. Mol. Biol. Cell. 2004;15:1425–1435. doi: 10.1091/mbc.e03-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekwall K. The RITS complex-A direct link between small RNA and heterochromatin. Mol. Cell. 2004;13:304–305. doi: 10.1016/S1097-2765(04)00057-7. [DOI] [PubMed] [Google Scholar]
- 49.Locke, S. & Martienssen, R. In Epigenomics-Uk (eds. Anne, C. Ferguson-Smith, John M. Greally & Robert A. Martienssen) 149–162 (Springer Netherlands, 2009).
- 50.Ragunathan K, Jih G, Moazed D. Epigenetics. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science. 2015;348:1258699. doi: 10.1126/science.1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motamedi MR, et al. HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol. Cell. 2008;32:778–790. doi: 10.1016/j.molcel.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watts BR, et al. Histone deacetylation promotes transcriptional silencing at facultative heterochromatin. Nucleic Acids Res. 2018;46:5426–5440. doi: 10.1093/nar/gky232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallagher PS, et al. Iron homeostasis regulates facultative heterochromatin assembly in adaptive genome control. Nat. Struct. Mol. Biol. 2018;25:372–383. doi: 10.1038/s41594-018-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grewal SI, Elgin SC. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 2002;12:178–187. doi: 10.1016/S0959-437X(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 55.Garrigues JM, Sidoli S, Garcia BA, Strome S. Defining heterochromatin in C. elegans through genome-wide analysis of the heterochromatin protein 1 homolog HPL-2. Genome Res. 2015;25:76–88. doi: 10.1101/gr.180489.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couteau F, Guerry F, Muller F, Palladino F. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. Embo Rep. 2002;3:235–241. doi: 10.1093/embo-reports/kvf051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng RJ, Armstrong KR, Wang X, Chamberlin HM. The bromodomain protein LEX-1 acts with TAM-1 to modulate gene expression in C. elegans. Mol. Genet. genomics: MGG. 2007;278:507–518. doi: 10.1007/s00438-007-0265-6. [DOI] [PubMed] [Google Scholar]
- 58.Ciro M, et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 2009;69:8491–8498. doi: 10.1158/0008-5472.CAN-09-2131. [DOI] [PubMed] [Google Scholar]
- 59.Duan Z, et al. Developmental and androgenic regulation of chromatin regulators EZH2 and ANCCA/ATAD2 in the prostate Via MLL histone methylase complex. Prostate. 2013;73:455–466. doi: 10.1002/pros.22587. [DOI] [PubMed] [Google Scholar]
- 60.Lu WJ, Chua MS, So SK. Suppression of ATAD2 inhibits hepatocellular carcinoma progression through activation of p53- and p38-mediated apoptotic signaling. Oncotarget. 2015;6:41722–41735. doi: 10.18632/oncotarget.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altintas DM, et al. Direct cooperation between androgen receptor and E2F1 reveals a common regulation mechanism for androgen-responsive genes in prostate cells. Mol. Endocrinol. 2012;26:1531–1541. doi: 10.1210/me.2012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morozumi Y, et al. Atad2 is a generalist facilitator of chromatin dynamics in embryonic stem cells. J. Mol. Cell Biol. 2016;8:349–362. doi: 10.1093/jmcb/mjv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koo SJ, et al. ATAD2 is an epigenetic reader of newly synthesized histone marks during DNA replication. Oncotarget. 2016;7:70323–70335. doi: 10.18632/oncotarget.11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods enzymology. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 65.Durand-Dubief M., E. K. In DNA Microarrays for Biomedical Research Vol. 529 (Human Press, 2009).
- 66.Asrani SK, Kim WR. Predicting response to pegylated interferon/ribavirin-based therapy in genotype 1 chronic hepatitis C patients: results of 3 independent genome-wide association studies. Gastroenterology. 2010;138:1622–1624. doi: 10.1053/j.gastro.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 67.Wagih O, et al. SGAtools: one-stop analysis and visualization of array-based genetic interaction screens. Nucleic acids Res. 2013;41:W591–596. doi: 10.1093/nar/gkt400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tasto JJ, Carnahan RH, McDonald WH, Gould KL. Vectors and gene targeting modules for tandem affinity purification in Schizosaccharomyces pombe. Yeast. 2001;18:657–662. doi: 10.1002/yea.713. [DOI] [PubMed] [Google Scholar]
- 69.Puig O, et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 70.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.