Abstract
The therapeutic effect of reactive oxygen species (ROS)-involved cancer therapies is significantly limited by shortage of oxy-substrates, such as hypoxia in photodynamic therapy (PDT) and insufficient hydrogen peroxide (H2O2) in chemodynamic therapy (CDT). Here, we report a H2O2/O2 self-supplying nanoagent, (MSNs@CaO2-ICG)@LA, which consists of manganese silicate (MSN)-supported calcium peroxide (CaO2) and indocyanine green (ICG) with further surface modification of phase-change material lauric acid (LA). Under laser irradiation, ICG simultaneously generates singlet oxygen and emits heat to melt the LA. The exposed CaO2 reacts with water to produce O2 and H2O2 for hypoxia-relieved ICG-mediated PDT and H2O2-supplying MSN-based CDT, acting as an open source strategy for ROS production. Additionally, the MSNs-induced glutathione depletion protects ROS from scavenging, termed reduce expenditure. This open source and reduce expenditure strategy is effective in inhibiting tumor growth both in vitro and in vivo, and significantly improves ROS generation efficiency from multi-level for ROS-involved cancer therapies.
Subject terms: Cancer microenvironment, Cancer therapy, Nanoscale materials
The supply of oxygen species for dynamic therapy is a major limitation. Here, the authors report on a complex of calcium peroxide with photo/chemo dynamic agents, where NIR heating causes the release of calcium peroxide to generate oxygen and hydrogen peroxide for photodynamic and chemodynamic therapy.
Introduction
Reactive oxygen species (ROS), mainly including active superoxide anions (O2−), hydroxyl radicals (•OH), and singlet oxygen (1O2), act as significant signaling and regulatory molecules at physiologic levels, conversely, will damage cells once the concentration elevate at an abnormal level1. Elevated ROS level is one of the characteristics of tumor microenvironment (TME), and along with high ROS elimination rates exist in cancer cells to maintain a steady equilibrium state, called self-adaptation mechanisms. Thus, cancer cells are more sensitive to further enhanced oxidative stress beyond the cellular tolerability threshold2. On this basis, ROS-mediated therapies, such as photodynamic therapy (PDT)3–6 and chemodynamic therapy (CDT)7–11, are developed to disrupt the cellular self-adaptation mechanisms and induce cell death based on ROS-generating agents12.
The PDT utilities light-activated photosensitizers to convert oxygen (O2) to ROS13, whereas CDT takes advantage of an in situ Fenton or Fenton-like reaction between hydrogen peroxide (H2O2) and catalysts to generate cytotoxic hydroxyl radical (•OH)14,15. Recently, the PDT/CDT combination therapy has been continuously explored to amplify the tumor oxidative stress and achieve better anticancer therapeutic effect than monotherapy16–20. However, the TME feature of hypoxia, depletable amount of H2O2 and the glutathione (GSH) depletion effect on ROS still limit ROS efficiency15,21,22. Two different feasible strategies have been proposed to relieve hypoxia in PDT and supplement the cellular amount of H2O2 in CDT, respectively, amplifying endogenous O2/H2O2 generation11,23–26 or directly delivering exogenous O2/H2O2 into cells27–29. To date, there have indeed been some nanosystems for synergistic PDT/CDT, but most of them only overcome part of the limitations. For example, Liu et. al constructed sorafenib@Fe3+-tannic acid nanoparticles with GSH depletion property for PDT/CDT19. Copper ferrite nanospheres18, copper/manganese silicate nanospheres16, and ROS-activatable liposomes30 have been reported for hypoxia-relieved and GSH-depleting synergistic PDT/CDT. At present, simultaneous hypoxia relief, H2O2 supplement, and GSH-depletion nanosystems have been little reported, which is highly desirable in PDT/CDT combination therapy. CaO2, a safe solid inorganic peroxide, can decompose to simultaneously release O2 and H2O2 in contacting with water31 and have been widely applied in remediation of environmental contamination32. Therefore, the introduction of CaO2 into ROS-involved therapies seem to hold great promise for enhanced ROS generation.
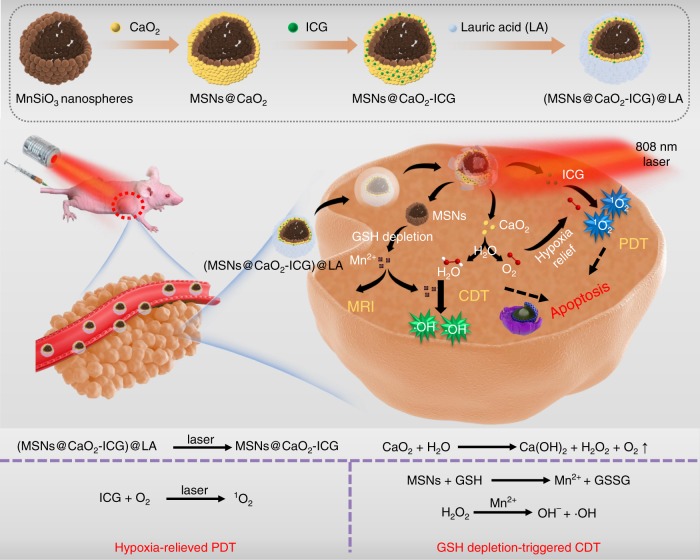
Herein, a H2O2/O2 self-supplying thermoresponsive nanosystem, (MSNs@CaO2-ICG)@LA, consisting of manganese silicate (MSNs) supported calcium peroxide (CaO2) nanoparticles (NPs) and indocyanine green (ICG) with further surface coating of a phase-change material lauric acid (LA, melting point: 44~46 °C), is reported for photodynamic/chemodynamic synergistic cancer therapy (Fig. 1). In this nanosystem, the CaO2 is protected from water by LA until the outer layer LA is melted owing to the photothermal effect of ICG under the irradiation of a near-infrared (NIR) 808 nm laser. The exposed CaO2 reacts with water to rapidly generate H2O2 and O2, and accompanies exposure of inner MSNs. The released O2 can relieve hypoxia for enhanced ICG-mediated PDT. The interaction between MSNs and GSH lead to release of Fenton-like agent Mn2+ for H2O2-supplementing CDT and magnetic resonance imaging (MRI)-guided therapy. This GSH depletion further enhance the ROS generation efficiency. Thus we report a smart system (MSNs@CaO2-ICG)@LA can simultaneously overcome the main limitations including hypoxia, depletable amount of H2O2 and GSH elimination effect on ROS for synergistic PDT/CDT, and this open source and reduce expenditure ROS-produced way obtain excellent tumor inhibition effect both in vitro and in vivo, provide a universal idea of therapeutic nanoagents design for synergistic PDT/CDT.
Fig. 1. Open source and reduce expenditure ROS generation strategy.
The scheme of fabrication process and therapeutic mechanism of thermo-responsive (MSNs@CaO2-ICG)@LA NPs for synergistic CDT/PDT with H2O2/O2 self-supply and GSH depletion.
Results
Preparation and characterization of (MSNs@CaO2-ICG)@LA
The MSNs with an average diameter of 120 nm were synthesized by a hydrothermal method (Supplementary Figure 1a). The CaO2 NPs with an average diameter of 20 nm were prepared through a hydrolysis-precipitation process (Supplementary Figure 1b)33,34. Then, the CaO2 NPs were assembled onto the surface of MSNs to form MSNs@CaO2 NPs in methanol through electrostatic adsorption, confirmed by the transmission electron microscope (TEM) images (Fig. 2a) and X-ray diffraction (XRD) pattern with the characteristic peaks belong to MSNs and CaO2, respectively (Fig. 2b). The successful assembly of MSNs@CaO2 was also validated by the X-ray photo-electron spectroscopy (XPS) analysis (Supplementary Figure 2a), and the high resolution XPS revealed that the O 2 s existed primarily in the form of silicate and peroxo groups (Supplementary Figure 2b), and the Mn 2p3/2 mainly consisted of 34.65% Mn2+ (641 eV), 55.74% Mn3+ (642 eV), and 9.61% Mn4+ (644 eV) (Fig. 2c)11,16. The high content of Mn3+ in MSNs made it possible to react with endogenous GSH for further biodegradation16,35, and the TEM analysis confirmed the gradual biodegradation of MSNs in the presence of GSH (Supplementary Figure 3). Thus, MSNs could degrade to release Mn2+ for CDT by depleting ROS scavenger GSH, which was beneficial to amplify the therapeutic effect36.
Fig. 2. Physiochemical characterizations.
a TEM images of MSNs@CaO2 NPs. b XRD spectra of MSNs, CaO2 NPs, and MSNs@CaO2. c High-resolution Mn 2p XPS spectra of MSNs@CaO2. d UV-vis spectra of ICG, MSNs@CaO2, and MSNs@CaO2-ICG. e TEM images of (MSNs@CaO2-ICG)@LA. f DSC curves of LA and (MSNs@CaO2-ICG)@LA.
ICG is a NIR tricarbocyanine dye approved by the US Food and Drug Administration for clinical use and show great potential both in PDT and PTT37,38. We further incorporated ICG into the nanosystem to utilize its photothermal property and 1O2 generation capacity. The strong absorption peak centered at 800 nm in UV–vis spectra of MSNs@CaO2-ICG validated the integration of ICG molecules into the nanosystem, and the loading content of ICG was estimated to be 7.87 wt % (Fig. 2d). The phase-change material LA with good biocompatibility and biodegradability39,40 was added as coating to obtain (MSNs@CaO2-ICG)@LA with thermo-responsive property. The TEM image of (MSNs@CaO2-ICG)@LA showed the uniform size (Fig. 2e) and the change of surface zeta potential (Supplementary Figure 4) confirmed the preparation process. The differential scanning calorimetry (DSC) curves of (MSNs@CaO2-ICG)@LA exhibited a similar melting point to pure LA, validating the successful coating of LA on MSNs@CaO2-ICG (Fig. 2f). The resulting (MSNs@CaO2-ICG)@LA also showed good stability in water, PBS (10 mm, pH 7.4) and Dulbecco's modified eagle medium (DMEM) solution demonstrated by the hydrodynamic particle size and surface zeta potential analysis after 24 h (Supplementary Figure 5).
In vitro H2O2 and O2 generation and thermo-responsive property
Cumulative amount of H2O2 released from CaO2 was measured by Cu(II)-neocuproine spectrophotometric method41. Pure CaO2 immediately reacted with water to generate H2O2 up to 75 μm at 25 °C, whereas the cumulative H2O2 amount of CaO2@LA remained at the same level lower than 30 μm within 7 h at 25 °C (Fig. 3a), suggesting the LA coating can protect CaO2 from reacting with water beforehand. In addition, the H2O2 releasing was increased when the pH descended, suggesting the acidic environment in tumors was good for the H2O2 generation (Supplementary Figure 6). When the temperature increase to 46 °C, the CaO2@LA recovered the rapid H2O2 generation ability similar to pure CaO2 owing to the melting of LA coating. The CaO2@LA solution at 25 °C displayed no significant change in dissolved O2 level within 15 min monitored by a portable dissolved oxygen meter in real time (Fig. 3b). However, when heated to 46 °C, the O2 concentration showed a rapid increase in the first 3 min faster than pure CaO2 at 25 °C, then fell slowly. These results fully demonstrated the H2O2 and O2 simultaneous generation capacities of CaO2 NPs in the reaction with water, and preliminarily verified the thermalresponsive property of LA.
Fig. 3. ROS generation and in vitro MRI.
a H2O2 cumulative release profile ([CaO2]=10 μg mL−1) and b O2 concentration measurement in PBS (10 mm, pH = 7.4) ([CaO2]=100 μg mL−1). c Time-dependent degradation of DPBF irradiated by laser for 10 min. ([MSNs] = 50 μg mL−1, [ICG] = 8 μg mL−1. Laser: 808 nm, 0.64 W cm−2). d EPR analysis of •OH production in each group with different treatment. DMPO was used as the spin-trapping agent. The sample in each group was (MSNs@CaO2-ICG)@LA aqueous solution ([MSNs] = 50 μg mL−1). e In vitro MRI of different solution and f the corresponding r1 value ([MSNs] = 50 μg mL−1. Laser: 808 nm, 0.64 W cm−2, 10 min).
Enhanced ROS generation measurements of (MSNs@CaO2-ICG)@LA
The (MSNs@CaO2-ICG)@LA exhibited an ICG concentration-dependent photothermal effect under an 808 nm irradiation (Supplementary Figure 7). Upon irradiation, the temperature increase induced melting of LA (Supplementary Figure 8), and the ICG was gradually released from (MSNs@CaO2-ICG)@LA (Supplementary Figure 9), demonstrating the successful design of this thermoresponsive nanosystem. The 1O2 generation of (MSNs@CaO2-ICG)@LA in vitro was monitored using 1,3-diphenylisobenzofuran (DPBF) as chemical probe. The DPBF content of ICG, MSNs or CaO2 group displayed slight decline compared with the control group under the irradiation of 808 nm laser (Fig. 3c and Supplementary Figure 10a). The temperature rise and NIR laser irradiation alone showed negligible effect on the degradation of DPBF (Supplementary Figure 10b). In contrast, the DPBF treated with MSNs@CaO2-ICG and (MSNs@CaO2-ICG)@LA showed a sharp decrease within 10 min owing to O2 self-supplying PDT effect of ICG. The •OH generation ability was evaluated by methylene blue (MB) degradation. The redox reaction between MSNs and GSH induced Mn2+ release and GSH depletion, and the Mn2+ reacted with H2O2 to produce active •OH through Fenton-like reaction (Supplementary Figure 11a). It was worthy to mention that the MB degradation increased with the increase of GSH concentration from 0 to 1.0 mm, but decreased when further increased GSH concentration as excessive GSH would scavenge •OH conversely (Supplementary Figure 11b). The H2O2 concentration-dependent Fenton-like effect provided the great possibility of enhanced •OH generation by H2O2 self-supply from CaO2 (Supplementary Figure 11c). Similar results to the MB degradation experiments were also obtained by electron paramagnetic resonance (EPR) analysis of •OH production of (MSNs@CaO2-ICG)@LA as shown in Fig. 3d.
The released Mn2+ from MSNs could also be utilized as MRI contrast agent. As shown in Fig. 3e, the T1 signal intensity of MSNs (group I) had negligible change and the released Mn2+ were at very low concentration, whereas MSNs treated with 10 mm GSH (group II) exhibited enhanced brightness derived from paramagnetic Mn2+ centers because MSN was reduced by GSH and the increasing free Mn ions (Supplementary Table 1) were easier to proceed chemical exchange with protons than isolated Mn centers in MSNs for enhanced T1 signal42. Thus, it was rational that (MSNs@CaO2-ICG)@LA displayed enhanced brightness only when co-treated with GSH and NIR laser irradiation (group IV). Remarkably, the longitudinal relaxivity coefficient (r1) of group IV and correspongding released Mn concentration were larger than that of group II (Fig. 3f). This was attributed to the enhanced release of Mn2+ from MSNs accelerated by photothermal effect of ICG (Supplementary Table 1).
Intracellular uptake of (MSNs@CaO2-ICG)@LA
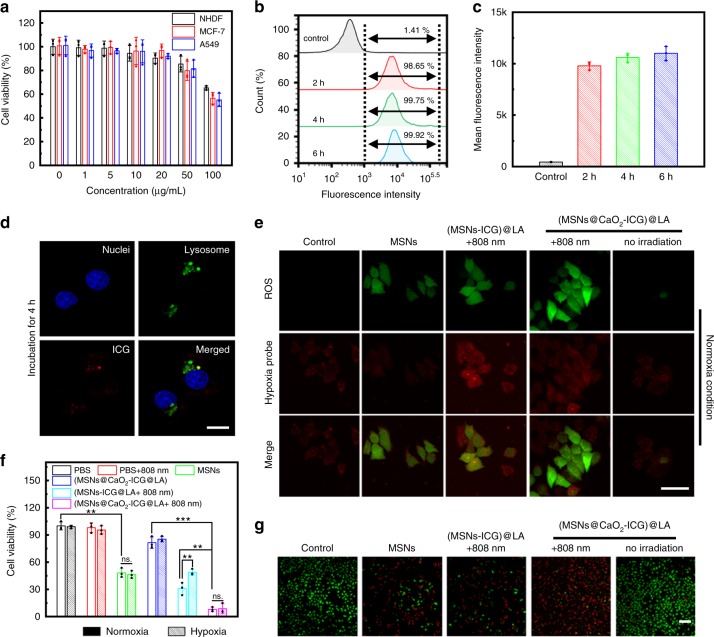
Before evaluating the feasibility of (MSNs@CaO2-ICG)@LA for in vivo antitumor therapy, the cytotoxicity and cell uptake of (MSNs@CaO2-ICG)@LA were first investigated. As shown in Fig. 4a, the (MSNs@CaO2-ICG)@LA showed little cytotoxicity toward MCF-7, A549, and NHDF cells when the concentration was from 0 to 50 μg mL−1 after incubation for 12 h, indicating good biocompatibility. The flow cytometry was conducted to measure the fluorescence intensity of ICG in MCF-7 cells treated with (MSNs@CaO2-ICG)@LA at different incubation time point (Fig. 4b), and the corresponding analysis of mean fluorescence intensity was shown in Fig. 4c. Comparing with the blank control group, the cells treated with (MSNs@CaO2-ICG)@LA demonstrated high uptake rate as the incubation time extended, and incubation for 4 h was sufficient. Figure 4d demonstrated that the location of (MSNs@CaO2-ICG)@LA in MCF-7 cells overlapped with lysosome after incubation for 4 h, suggesting the endolysosomal pathway.
Fig. 4. Intracellular uptake and ROS generation of (MSNs@CaO2-ICG)@LA.
a Cell viability of MCF-7, A549, and NHDF cells treated with different concentrations of (MSNs@CaO2-ICG)@LA by CCK-8 assay. Data are presented as mean ± SD (n = 3). b The rate of MCF-7 cells uptaking (MSNs@CaO2-ICG)@LA ([MSNs] = 25 μg mL−1), and c corresponding mean fluorescence intensity by flow cytometry after different incubation time. Data are presented as mean ± SD (n = 3). d Fluorescence images showing cellular uptake of (MSNs@CaO2-ICG)@LA ([MSNs] = 25 μg mL−1) in MCF-7 cells after incubation for 4 h. Scale bar: 20 μm. e Fluorescence images showing ROS and hypoxia level in MCF-7 cells with different treatment under normoxia condition. ([MSNs] = 25 μg mL−1. Laser: 0.64 W cm−2, 10 min). Scale bar: 50 μm. f Cell viability of MCF-7 cells with different treatments under hypoxia or normoxia condition ([MSNs] = 25 μg mL−1. Laser: 0.64 W cm−2, 10 min). The mean value was calculated by the two-tailed t test (mean ± SD, n = 3). **P < 0.01 and ***P < 0.001, compared with the indicated group. g Fluorescence images of Calcein-AM- and propidium iodide (PI)-costained MCF-7 cells with different treatments under normoxia condition ([MSNs] = 25 μg mL−1. Laser: 0.64 W cm−2, 10 min). Scale bar: 200 μm.
Intracellular enhanced ROS production
To investigate the enhanced ROS production of (MSNs@CaO2-ICG)@LA in living cells, we first explored the intracellular O2 self-supplying property of CaO2. As shown in Supplementary Figure 12, after hypoxia treatment, the red fluorescence intensity related to hypoxia of cells nearly remained unchanged when incubated with CaO2@LA, while that of CaO2-treated group was significantly weaken, confirming the intracellular hypoxia relief by CaO2. The CaO2-mediated O2 self-supply provided the possibility to enhance the ROS production and PDT efficiency. As expected, the intracellular fluorescence imaging by ROS probe (DCFH-DA, green) and hypoxia probe demonstrated that the (MSNs@CaO2-ICG)@LA-treated cells with irradiation displayed the strongest green fluorescence and slight enhanced red fluorescence compared with other groups both under normoxia or hypoxia conditions (Fig. 4e and Supplementary Figure 13), indicating tremendous ROS generation enhanced by O2 and H2O2 self-supply from CaO2. In order to further investigate the enhanced CDT/PDT therapeutic effect, we used MCF-7 cancer cells as model cell to examined the anticancer effect of (MSNs@CaO2-ICG)@LA in normoxia as well as hypoxia environment as contrast. Upon a 808 nm laser irradiation, the (MSNs@CaO2-ICG)@LA treatment showed greatly enhanced cytotoxicity than both MSNs and (MSNs-ICG)@LA in normoxia environment (Fig. 4f), in consistent with the calcein-AM and propidium iodide (PI) co-staining results that most of cancer cells were killed when treated with (MSNs@CaO2-ICG)@LA plus irradiation (Fig. 4g). Notably, the hypoxia condition had obvious influence on the cell killing ability of (MSNs-ICG)@LA-treated group, whereas that of (MSNs@CaO2-ICG)@LA-treated group almost the same both in normoxia and in hypoxia conditions owing to the O2 self-supplying property of CaO2 (Fig. 4f).
In vivo tumor treatment efficiency of (MSNs@CaO2-ICG)@LA
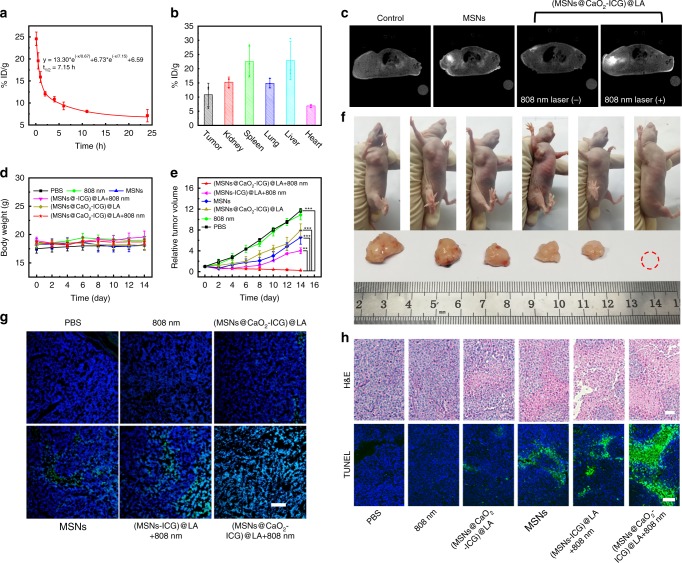
The antitumor efficacy of (MSNs@CaO2-ICG)@LA-mediated CDT/PDT synergistic therapy in vivo was further conducted in MCF-7 tumor-bearing mice. Figure 5a exhibited the blood circulation curve of (MSNs@CaO2-ICG)@LA in mice by measuring the concentrations of Mn2+ in the blood at different time points after tail vein injection, and the fairly long circulation time was favorable for effective tumor accumulation of (MSNs@CaO2-ICG)@LA in tumor site due to the EPR effect which was observed in Fig. 5b. After irradiation by 808 nm laser for 10 min, the tumor-site temperature of (MSNs@CaO2-ICG)@LA-treated mice gradually increased to 46.8 °C, which exceeded the melting point temperature of LA, whereas the control group treated with 808 nm laser-irradiation only did not display obvious temperature change in tumor site (Supplementary Figure 14). We also investigated the MRI property of (MSNs@CaO2-ICG)@LA in vivo. As shown in Fig. 5c, the brightness in tumor site of mice treated with MSNs was obviously enhanced than the control group, indicating the MSNs could degrade to Mn2+ by GSH in vivo. As for (MSNs@CaO2-ICG)@LA-treated mice, the T1 signal intensity became very strong after irradiation of an 808 nm laser, confirming the thermo-responsive property of the nanosystem again. The in vivo MRI property of (MSNs@CaO2-ICG)@LA provided a powerful tool for guiding and monitoring therapy. Then, we divided the MCF-7 tumor-bearing nude mice into six group (n = 5) to investigate the antitumor efficacy of different treatments. The tumor volumes of all groups were recorded every 2 days and the tumors were collected at 14 days. As show in Fig. 5d and Supplementary Figure 15, relatively stable body weight and histopathological normal hematoxylin and eosin (H&E) staining of main organs in all groups suggested the negligible side effects of these treatments on mice. The 808 nm laser irradiation alone displayed little inhibition effect on tumor growth similar to the control group (Fig. 5e, f). The MSNs and showed some tumor repression effects owing to the CDT therapeutic efficacy. The tumors were significantly suppressed in the group of (MSNs-ICG)@LA and an 808 nm laser due to the therapeutic effect of CDT/PDT, and the tumors in the group treated with (MSNs@CaO2-ICG)@LA and an 808 nm laser irradiation were completely eliminated owing to the strongest ROS generation in H2O2/O2 self-supplying CDT/PDT (Fig. 5g and Supplementary Figure 16). The corresponding H&E and TUNEL staining of tumor slides also displayed the maximum tumor necrosis and apoptosis (Fig. 5h). These results indicated that (MSNs@CaO2-ICG)@LA have excellent antitumor effects due to combined PDT/CDT with H2O2 and O2 self-supply.
Fig. 5. In vivo demonstration of synergistic CDT/PDT.
a Blood circulation and b biodistribution of (MSNs@CaO2-ICG)@LA by measuring Mn concentrations over a span of 24 h after intravenous injection. Data are presented as mean ± SD (n = 3). c In vivo MRI of MCF-7 tumor-bearing mice with different treatments after 24 h (Laser: 808 nm, 0.64 W cm−2, 10 min). d The body weight and e the relative cancer volume changes of MCF-7 tumor-bearing mice after various treatments in 14 days. The mean value was calculated by the two-tailed t test (mean ± SD, n = 5). **P < 0.01 and ***P < 0.001, compared with the indicated group. f Representative photos of the mice with different treatments and corresponding tumor tissues collected from different groups at 14 days. g DCFH (green)- and DAPI (blue)-costained tumor tissues collected from different groups at 24 h post injection. Scale bar: 200 μm. h H&E- or TUNEL-stained tumor slices collected from different groups at 14 days. Scale bar: 100 μm.
Discussion
In summary, we rationally designed a thermo-responsive nanosystem through simple assembly method for overcoming the insufficient supply of O2 and H2O2 in antitumor PDT and CDT. The (MSNs@CaO2-ICG)@LA nanosystem was constructed by decorating CaO2 and ICG on the MSN-support with further surface modification of phase-change material LA. We demonstrated that phase-change material LA could be melted owing to the photothermal effect of excited ICG under irradiation, and then CaO2 could be exposed to react with water to release O2 and H2O2 to enhance the ROS generation in ICG-mediated PDT and Mn2+-involved CDT, respectively, acting as an open source strategy for ROS production. The Fenton-like agent Mn2+ released out from MSNs depleted ROS scavenger GSH, which further reduced ROS wastage termed reduce expenditure. Our results showed excellent tumor eradication effect of (MSNs@CaO2-ICG)@LA owing to the combined PDT/CDT with O2/H2O2 self-supply and GSH depletion. This work demonstrated a promising open source and reduce expenditure strategy for ROS generation enhancement in ROS-involved cancer therapies.
Methods
Instruments
The morphologies of samples were characterized by TEM (HT7700, Hitachi, Japan). XRD was conducted by a Bruker D8 ADVANCE X. DSC measurements were conducted by DSC Q2000 (TA Instruments, USA) under nitrogen atmosphere. Dissolved oxygen measurement was conducted by JPBJ-608 (Rex, INESA Scientific Instrument). The cell fluorescence imaging was obtained by confocal laser scanning fluorescence microscope (CLSM, FV1200, Olympus, Japan). In vitro and in vivo MRI was performed by an animal MRI scanner (BioSpec70/20USR, Bruker, Germany) at 7.0 T with a gradient echo sequence (TR = 299 ms and TE = 6.01 ms).
Synthesis of manganese silicate nanoparticles (MSNs)
The previously reported dendritic mesoporous silica nanoparticles33 were used as self-killing templates to synthesize MSNs. First, dendritic mesoporous silica nanoparticles (50 mg), MnCl2·4H2O (158.34 mg) and NH4Cl (534.9 mg) were dispersed in 40 mL of water. Then NH3·H2O (28% wt., 1 mL) was added dropwise into the mixture under continuously stirring. After stirring for 30 min, the mixture was transferred into a Teflon-lined autoclave and maintained at 180 °C for 24 h. The precipitates were washed with water and finally dried at 60 °C.
PEGylation of MSNs
In brief, MSNs (1 mg) were mixed with methoxy PEG silane (Mw = 2000, 5 mg) in 10 mL of ethanol under magnetic stirring at 60 °C for 24 h. Then, the MSNs-PEG were washed with ethanol and ultrapure water several times.
Synthesis of (MSNs@CaO2-ICG)@LA
The CaO2 nanoparticles were synthesized according to previous literature34. In brief, ultrapure water (3 mL), calcium chloride (2.7 mmol), ammonia solution (1.5 mL, 1 m) and PEG400 (12 mL) were successively added in the round bottom flask under stirring. Then H2O2 (35 wt. %, 1.5 mL) was dropwise added to the mixture within 50 min. After further stirring for 2 h at 900 rpm at room temperature, the pH of mixture was adjusted to 11.5 using NaOH solution (0.1 m). The precipitate was washed three times with NaOH (0.1 m) and with distilled water until the pH of supernatant reached to 8.4. The precipitate was dried in vacuo at 80 °C for 2 h, then resuspended in ethanol and filtered using a Millex Filter Unit (0.45 μm). The resulting filtrate was concentrated and dried to achieve CaO2 nanoparticles. MSNs (1 mg) and CaO2 (1 mg) were mixed in methanol (5 mL) and stirred for 12 h. Then, ICG (1 mg) was added to the mixture under stirring. After 2 h, lauric acid (0.1 g) was added and the mixture was stirred for another 5 h. The precipitates were washed with methanol for three times. The as-obtained composites were denoted as (MSNs@CaO2-ICG)@LA. The loading capacity of ICG was determined by dissolving the (MSNs@CaO2-ICG)@LA in methanol, and the characteristic absorption at 788 nm was measured by UV-1800 spectrophotometer (Shimadzu, Japan).
Extracellular measurement of H2O2 generation
The H2O2 generation from CaO2 was measured by Cu (II)-neocuproine spectrophotometric method41. In brief, 49 μL of PBS solution (10 mm, pH = 7.4, 6.5 or 5.5) of CaO2 or CaO2@LA ([CaO2]=1 mg mL−1, 1 μL), 25 μL of 0.01 m CuSO4 and 25 μL of 0.01 m neocuproine solution were added to a 96-well plate sequentially. Then the 96-well plate was shaken at room temperature or at 46 °C. At appointed time, the absorbance at 450 nm was measured by a microplate reader. The standard curve of H2O2 was obtained by measuring the absorbance of a known concentration of H2O2 (0~1000 μm) in the same way and then the H2O2 concentration of samples could be calculated.
Extracellular measurement of O2 concentration
Three mL of CaO2 or CaO2@LA methanol solution (1 mg mL−1) were added to 27 mL PBS (10 mm, pH = 7.4) under vigorous stirring at room temperature or at 46 °C. Then we monitored the O2 concentration of solution by a portable dissolved oxygen meter (JPBJ-608, Rex, INESA Scientific Instrument) in real time.
Extracellular measurement of 1O2 generation
DPBF solution (10 μL, 10 mm in DMSO) was added to the sample solution (100 μg mL−1, 2 mL) under irradiation (808 nm, 0.64 W cm−2) and the absorbance of DPBF solution at 420 nm was recorded every minute.
Extracellular measurement of photothermal property
The temperature of different concentration of (MSNs@CaO2-ICG)@LA (0, 25, 125 or 250 mg mL−1, aqueous dispersion) was recorded by an OMEGA 4-channel datalogger thermometer under irradiation of 808 nm laser (0.64 W cm−2, 10 min).
Extracellular measurement of NIR-triggered ICG release
The aqueous solution of (MSNs@CaO2-ICG)@LA (200 μg mL−1, 1 mL) was irradiated by laser (808 nm, 0.64 W cm−2) with different time (0, 2, 4, 6, and 8 min) or was not exposed to the NIR laser. Then, the sample solution was centrifuged. The released ICG in supernatant solution was characterized by measuring the absorption of ICG at 788 nm.
In vitro MRI imaging property
In all, 100 μL MSNs or (MSNs@CaO2-ICG)@LA ([Mn]: 5 mm) was added into 900 μL PBS solution (10 mm, pH = 7.4) containing 25 mm NaHCO3/5% CO2 without or with GSH (10 mm). After shaken at 37 °C for 1 h, MRI images and the T1 relaxation time of diluted supernatant (Mn concentration: 0, 0.1, 0.2, 0.4, and 0.5 mm) were measured by MRI system.
Cell cytotoxicity
The human MCF-7 breast cancer cells, human A549 adenocarcinoma alveolar basal epithelial cells and NHDF normal human dermal fibroblasts cells were purchased from Cell Bank, the Committee of Type Culture Collection of Chinese Academy of Sciences. NHDF, MCF-7, and A549 cells were seeded in 96-well plates (104 cells per well) respectively and incubated in DMEM medium containing 10% FBS and 1% antibiotics (penicillin−streptomycin, 10,000 U mL−1) at 37 °C under 5% CO2 for 12 h. Then the cells were incubated with OPTI-MEM solution containing (MSNs@CaO2-ICG)@LA at desired concentrations for 4 h, washed with PBS (10 mm, pH = 7.4) for three times and further incubated with DMEM medium for another 24 h. Finally, culture medium containing 10% of CCK-8 was added to each well. After incubation at 37 °C for 1 h, the absorbance at 450 nm of each well was obtained by microplate reader.
Intracellular antitumor performance
MCF-7 cells were seeded in 96-well plates (104 cells per well) for 12 h, the cells for hypoxia cytotoxicity evaluation were incubated in hypoxic chamber (1% O2, 5% CO2, and 94% N2) for another 4 h. Then the cells were incubated with OPTI-MEM containing PBS, MSNs, (MSNs-ICG)@LA or (MSNs@CaO2-ICG)@LA ([MSNs]=25 μg mL−1) for 4 h. After replaced with fresh DMEM medium, the cells were irradiated with laser (808 nm, 0.64 W cm−2) for 10 min and then further incubated for 24 h. For CCK-8 cytotoxicity assay, cells in each well were incubated with the culture medium containing 10% of CCK-8 for 1 h and measured by microplate reader.
Intracellular hypoxia relief and ROS measurement
Adherent MCF-7 cells were incubated in normoxia (5% O2, 21% CO2, and 74% N2) or hypoxia chamber (1% O2, 5% CO2, and 94% N2) for 4 h to build normoxic or hypoxic environment. Then the cells were incubated with OPTI-MEM solution containing PBS (10 mM, pH = 7.4), MSNs, (MSNs-ICG)@LA or (MSNs@CaO2-ICG)@LA for 4 h ([MSNs] = 25 μg mL−1), washed with PBS (10 mm, pH = 7.4) and co-stained with 1 μL of DCFH-DA (10 mm) and 1 μL of hypoxia detection probe (1 mm) (Hypoxia Detection Kit, Enzo) for 15 min. After washed with PBS (10 mm, pH = 7.4) for three times, the cells were irradiated by a 808 nm laser (0.64 W cm−2) for 10 min and observed by CLSM.
In vivo antitumor performance
MCF-7 cancer-bearing female Balb/c mice (4 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and used in accordance with the guidelines of the Department of Laboratory Animal Science of Peking University Health Science Center. All animal experiments were conducted and agreed with the Institutional Animal Care and Use Committee of the Beijing Institute of Basic Medical Science (Beijing, China). The tumor-bearing mouse model was built by subcutaneous injection of MCF-7 cells (2 × 107 mL−1, 100 μL) into the right axilla of each mouse. The mice were randomly distributed into six groups for in vivo experiments (5 mice in each group) when the tumor volumes reached about 50 mm3 and intravenously injected with different formulations ([MSNs]= 5 mg kg−1): (1) PBS (control group); (2) 808 nm laser; (3) (MSNs@CaO2-ICG)@LA; (4) PEG-modified MSNs; (5) (MSNs-ICG)@LA + 808 nm laser; (6) (MSNs@CaO2-ICG)@LA + 808 nm laser. The 808 nm laser-irradiation was conducted after injection for 24 h (0.64 W cm−2, 10 min). The tumor size (V) was calculated as follows: V = width2 × length/2 and measured every 2 days. After 2 weeks, tumors and main organs were collected from the killed mice for further analysis.
In vivo blood circulation and biodistribution
MCF-7 cancer-bearing female mice were intravenously injected with (MSNs@CaO2-ICG)@LA (10 mg kg−1). At indicated time points (0.1, 0.5, 1, 2, 4, 6, 11, 24 h), we collected 50 μL blood from the tail of each mouse. After intravenous injection for 24 h, the mice were killed to measure the Mn amount in liver, spleen, kidney, heart, lung, tumor as well as blood samples by ICP-OES.
In vivo MRI imaging
The in vivo MRI imaging experiments were conducted on MCF-7 tumor-bearing female mice when the tumor volume reached about 100 mm3. MSNs or (MSNs@CaO2-ICG)@LA ([MSNs] = 5 mg kg−1) were intratumorally injected into the tumor sites. After 4 h, one of the (MSNs@CaO2-ICG)@LA-injected mice were irradiated at the tumor sites by 808 nm-laser for 10 mim. After 12 h, T1-weighted MR images were recorded with an animal MRI scanner (BioSpec70/20USR, Bruker, Germany).
Statistical analysis
Data were calculated and processed as mean ± SD. Comparison analysis between groups was conducted by student’s t test (two tailed).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The work was supported by National Natural Science Foundation of China (21874008, 21727815) and Special Foundation for State Major Research Program of China (2019YFC1606603), the work was also supported by Major Program of National Natural Science Foundation of China (21890740, 21890742); the Fundamental Research Funds for the Central Universities (FRF-TP-18-007B1, FRF-TP-17-050A1) and Beijing Municipal Science and Technology Commission (z131102002813058).
source data
Author contributions
C.L. and H.D. designed the present work. H.D. and X.Z. supervised the project. C.L. synthesized and characterized the nanomaterials, analyzed the data, and wrote the manuscript. C.L., D.W., and Y.CH. performed extracellular and intracellular experiments. C.L. and Y.CA. performed the animal experiments. T.X. and L.S. provided useful suggestions to this work. All the authors contributed to the discussion during the whole project.
Data availability
All relevant data are available from the authors. The source data underlying Figs. 3a–c, 3f, 4a, c, f, 5a, b as well as Supplementary Figs 4, 5b, c, 6, 7, 9, 10, 16, and Supplementary Table 1 are provided as a Source Data file.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Lele Li and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueji Zhang, Email: zhangxueji@ustb.edu.cn.
Haifeng Dong, Email: hfdong@ustb.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15591-4.
References
- 1.Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 2.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, et al. Heterodimers made of upconversion nanoparticles and metal–organic frameworks. J. Am. Chem. Soc. 2017;139:13804–13810. doi: 10.1021/jacs.7b07302. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat. Commun. 2018;9:5044. doi: 10.1038/s41467-018-07197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, et al. Nd3+-sensitized upconversion metal-organic frameworks for mitochondria-targeted amplified photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:2634–2638. doi: 10.1002/anie.201911508. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew. Chem. Int. Ed. 2016;55:2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 8.Huo M, Wang L, Chen Y, Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LS, et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 2018;57:4902–4906. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 10.Ma B, et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2018;141:849–857. doi: 10.1021/jacs.8b08714. [DOI] [PubMed] [Google Scholar]
- 11.Lin LS, et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019;141:9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 13.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 14.Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano. 2018;12:11819–11837. doi: 10.1021/acsnano.8b07635. [DOI] [PubMed] [Google Scholar]
- 15.Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions. Angew. Chem. Int. Ed. 2019;58:946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, et al. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano. 2019;13:4267–4277. doi: 10.1021/acsnano.8b09387. [DOI] [PubMed] [Google Scholar]
- 17.Dong S, et al. Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem. Sci. 2019;10:4259–4271. doi: 10.1039/C9SC00387H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, et al. All-in-one theranostic nanoagent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication. ACS Nano. 2018;12:4886–4893. doi: 10.1021/acsnano.8b01893. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12:12181–12192. doi: 10.1021/acsnano.8b05860. [DOI] [PubMed] [Google Scholar]
- 20.Li M, et al. Near-infrared light-initiated molecular superoxide radical generator: rejuvenating photodynamic therapy against hypoxic tumors. J. Am. Chem. Soc. 2018;140:14851–14859. doi: 10.1021/jacs.8b08658. [DOI] [PubMed] [Google Scholar]
- 21.Fan W, Huang P, Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016;45:6488–6519. doi: 10.1039/C6CS00616G. [DOI] [PubMed] [Google Scholar]
- 22.Gong N, et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 2019;14:379–387. doi: 10.1038/s41565-019-0373-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, et al. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 24.Fan H, et al. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem. Int. Ed. 2016;55:5477–5482. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, et al. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc. 2017;139:10992–10995. doi: 10.1021/jacs.7b05559. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, et al. An adenosine triphosphate-responsive autocatalytic fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18:7609–7618. doi: 10.1021/acs.nanolett.8b03178. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y, et al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015;6:8785. doi: 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao H, et al. An assembled nanocomplex for improving both therapeutic efficiency and treatment depth in photodynamic therapy. Angew. Chem. Int. Ed. 2018;57:7759–7763. doi: 10.1002/anie.201802497. [DOI] [PubMed] [Google Scholar]
- 29.Li W-P, Su C-H, Chang Y-C, Lin Y-J, Yeh C-S. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome. ACS Nano. 2016;10:2017–2027. doi: 10.1021/acsnano.5b06175. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, et al. Reactive oxygen species–activatable liposomes regulating hypoxic tumor microenvironment for synergistic photo/chemodynamic therapies. Adv. Funct. Mater. 2019;29:1905013. doi: 10.1002/adfm.201905013. [DOI] [Google Scholar]
- 31.Wang H, et al. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: a kinetics study. Chem. Eng. J. 2016;303:450–457. doi: 10.1016/j.cej.2016.05.123. [DOI] [Google Scholar]
- 32.Goi A, Viisimaa M, Trapido M, Munter R. Polychlorinated biphenyls-containing electrical insulating oil contaminated soil treatment with calcium and magnesium peroxides. Chemosphere. 2011;82:1196–1201. doi: 10.1016/j.chemosphere.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, et al. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013;135:2427–2430. doi: 10.1021/ja3116873. [DOI] [PubMed] [Google Scholar]
- 34.Sheng Y, et al. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release. 2017;264:333–340. doi: 10.1016/j.jconrel.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, et al. “Manganese Extraction” strategy enables tumor-sensitive biodegradability and theranostics of nanoparticles. J. Am. Chem. Soc. 2016;138:9881–9894. doi: 10.1021/jacs.6b04299. [DOI] [PubMed] [Google Scholar]
- 36.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, et al. Localized electric field of plasmonic nanoplatform enhanced photodynamic tumor therapy. ACS Nano. 2014;8:11529–11542. doi: 10.1021/nn5047647. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Light-responsive nanoparticles for highly efficient cytoplasmic delivery of anticancer agents. ACS Nano. 2017;11:12134–12144. doi: 10.1021/acsnano.7b05214. [DOI] [PubMed] [Google Scholar]
- 39.Zhu C, et al. A eutectic mixture of natural fatty acids can serve as the gating material for near-infrared-triggered drug release. Adv. Mater. 2017;29:1703702. doi: 10.1002/adma.201703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao P, et al. Study on phase diagram of fatty acids mixtures to determine eutectic temperatures and the corresponding mixing proportions. Appl. Energy. 2014;115:483–490. doi: 10.1016/j.apenergy.2013.10.048. [DOI] [Google Scholar]
- 41.Park S, Park KM. Hyperbaric oxygen-generating hydrogels. Biomaterials. 2018;182:234–244. doi: 10.1016/j.biomaterials.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Feng L, et al. Magnetic targeting, tumor microenvironment-responsive intelligent nanocatalysts for enhanced tumor ablation. ACS Nano. 2018;12:11000–11012. doi: 10.1021/acsnano.8b05042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the authors. The source data underlying Figs. 3a–c, 3f, 4a, c, f, 5a, b as well as Supplementary Figs 4, 5b, c, 6, 7, 9, 10, 16, and Supplementary Table 1 are provided as a Source Data file.