Abstract
Carbon steel is strong primarily because of carbides with the most well-known one being θ-Fe3C type cementite. However, the formation mechanism of cementite remains unclear. In this study, a new metastable carbide formation mechanism was proposed as ω-Fe3C → ω′-Fe3C → θ′-Fe3C based on the transmission electron microscopy (TEM) observation. Results shown that in quenched high-carbon binary alloys, hexagonal ω-Fe3C fine particles are distributed in the martensite twinning boundary alone, while two metastable carbides (ω′ and θ′) coexist in the quenched pearlite. These two carbides both possess orthorhombic crystal structure with different lattice parameters (aθ′ = aω′ = aω = aα-Fe = 4.033 Å, bθ′ = 2 × bω′ = 2 × cω = aα-Fe = 4.94 Å, and cθ′ = cω′ = aω = 6.986 Å for aα-Fe = 2.852 Å). The θ′ unit cell can be constructed simply by merging two ω′ unit cells together along its bω′ axis. Thus, the θ′ unit cell contains 12 Fe atoms and 4 C atoms, which in turn matches the composition and atomic number of the θ-Fe3C cementite unit cell. The proposed theory in combination with experimental results gives a new insight into the carbide formation mechanism in Fe-C martensite.
Subject terms: Engineering, Materials science
Introduction
The main phase constituents in carbon steels are ferrites (α-Fe) and carbides according to the equilibrium binary Fe-C phase diagram. Therefore carbides have long been considered as a critical phase in strengthening carbon steels. Among all the carbides, the most well-known one is θ-type Fe3C cementite, which possesses orthorhombic crystal structure (space group Pnma) with its lattice parameter being aθ = 4.524 Å, bθ = 5.088 Å and cθ = 6.741 Å1,2. Although the θ-Fe3C cementite has been studied extensively due to its importance and popularity in carbon steels3–16, its formation mechanism remains unclear. This is particularly true for the θ-Fe3C formation during martensitic transformation. One possible reason for this is its ultra-fine particle size, which makes it difficult for the normal characterization techniques to detect the earlier stage of the carbide formation.
Thus far, several types of carbides, which are thought to be the precursors of cementite, have been investigated17–25. However, detailed crystal structural relationship between these carbides has not been explained yet. To explain the formation mechanism of the cementite in martensitic structure, a martensite decomposition mechanism (martensite → ε-Fe carbide → cementite) has been proposed previously during tempering at low temperature around 200 °C23,26–29. However, most of the alloys used for studying carbide formation were ternary (such as Fe-Ni-C) alloys or other complex alloy systems, which may complicate the analysis and interpretation of carbide formation mechanism. In order to study the fundamental formation mechanism of cementite, the simple binary Fe-C is more appropriate.
Each unit cell of the θ-Fe3C cementite with the formula Fe3C contains 12 Fe atoms and 4 C atoms, leading to a ratio of Fe to C being 31,2,30. Interestingly, a recently discovered ω-Fe phase located in the martensite twin boundary has three iron atoms in its unit cell as well31,32. If one interstitial carbon atom were to join this ω-Fe unit cell, the product would have the formula ω-Fe3C. The possibility that there exists certain relationship between the ω-Fe3C and θ-Fe3C stimulates the investigation into the possible unknown carbides formed earlier than θ-Fe3C cementite in the binary Fe-C system.
Metastable hexagonal ω-Fe3C phase particles, which are 1 to 2 nm big in size, distribute only at the body-centered cubic (BCC) {112}<111>-type twinning boundary region in twinned high-carbon Fe-C martensite33–40. It was observed by in-situ heating transmission electron microscopy (TEM) that these twinning boundary ω-Fe3C particles eventually transformed into θ-Fe3C carbides41–44. However, the ω → θ transition speed is too fast for any details to be recorded. Thus, indirect approach is needed to figure out the formation mechanism of these metastable carbides that might exist in the quenched high carbon Fe-C alloys in which several types of ultra-fine carbides with pearlite-like structures have been observed45,46. Furthermore, as mentioned above, it is difficult to characterize the crystal structures of ultra-fine carbides via tilting TEM specimens since their particle size is approximately 1–2 nm. Another difficulty comes from the co-existence of several types of fine carbides in localized region. This situation often causes superimposition of relevant selected area electron diffraction (SAED) patterns from several carbide phases.
Therefore, in this paper, we study the metastable carbides by comparative analysis of theoretical and experimental TEM data. The structural models of new carbides were built based on the hexagonal ω-Fe structure and their electron diffraction patterns were simulated using the commercial software, which is designed to simulate the crystal structure, including electron and X-ray diffraction patterns. Then, the calculated diffraction patterns were compared with the observed experimental results. It was confirmed that new kind of metastable carbide, θ′-Fe(C) with 12 Fe atoms and 4 C or less C atoms in its unit cell, existed in the quenched high carbon Fe-C alloys. This carbide has a quite similar crystal structure and the same chemical composition with that of the well-known cementite (θ-Fe3C). Thus, understanding the formation mechanism of the θ′-Fe(C) will help us to explore the nature of θ-Fe3C cementite.
Materials and experiment
A Fe-1.6 C (wt.%) binary ingot was prepared in Ar atmosphere within a high-vacuum induction furnace. The ingot was hot-forged into 20 mm-thick plates. Thin plates (approximately 10 mm × 10 mm × 1.0 mm) were then mechanically cut from the hot-forged plates and austenitized at 1150 °C for 30 mins under flowing Ar atmosphere, followed by quenching in water. TEM specimens were prepared from the water-quenched thin plates. The specimens were mechanically ground, polished, and finally ion-milled at room temperature. An ion-mill device (Fischione Model 1050 TEM Mill) was used to prepare the specimens at 4 kV. Sample microstructure was observed using a JEM 2000FX TEM operated at 200 kV. Electron diffraction patterns were calculated using the commercial CrystalMaker software. All electron diffraction patterns shown in the present work were calculated such that the spot intensity saturation was 100 in the software.
Results and discussion
TEM observations revealed that ultra-fine ω-Fe3C particles exist at twinning boundary region in twinned Fe-C martensite, and the ω-Fe3C has a hexagonal crystal structure with lattice parameters of a = aω = aα-Fe = 4.033 Å, cω = 1/2 × aα-Fe = 2.47 Å for aα-Fe = 2.852 Å31,33,35–38. The ω-Fe3C unit cell structure can be seen from Fig. 1(a).
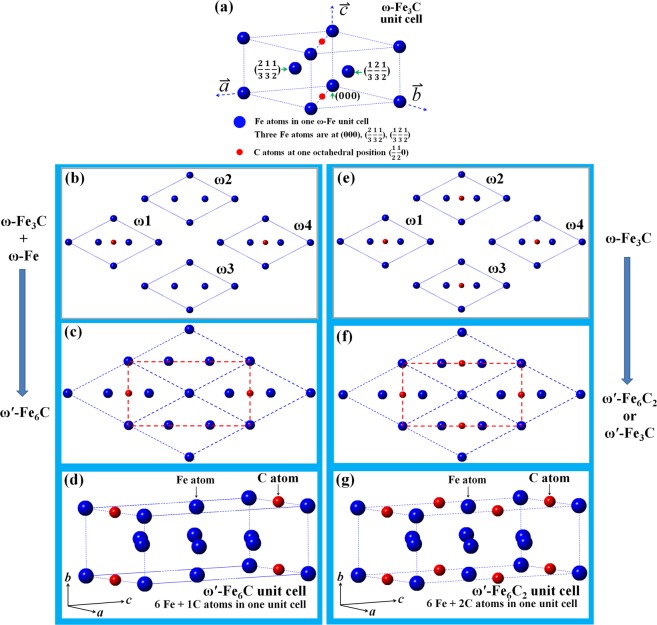
Figure 1.
Atomic structure of various carbides. (a) Unit cell of ω-Fe3C crystal structure. (b) Two ω-Fe3C and two ω-Fe unit cells projected along their c axes. (c) Coarsening of the four unit cells of the ω-Fe3C and ω-Fe in (b) results in the formation of new carbide (ω′-Fe6C) outlined by red dashed lines. (d) The ω′-Fe6C unit cell can have an orthorhombic structure and lattice parameters (aω′ = 4.033 Å, bω′ = 2.47 Å, and cω′ = 6.986 Å for aα-Fe = 2.852 Å), and C atom at (0.5 0 0). (e) Four ω-Fe3C unit cells. (f) Coarsening of the four ω-Fe3C unit cell in (e) results in the formation of a new carbide (ω′-Fe6C2 or ω′-Fe3C) with the same crystal structure and lattice parameters as the ω′-Fe6C. (g) The ω′-Fe6C2 atomic structure in one unit cell.
ω′-variants
As an interstitial atom, the position of carbon atoms in crystals determines carbide structure. Two different coarsening behaviors of the ultra-fine ω-Fe3C particles are illustrated in Fig. 1. Figure 1(a) shows the atomic structure of one ω-Fe3C unit cell. The coarsening route (Fig. 1(b)) will generate a new kind of carbide, with its unit cell outlined by red dashed lines in Fig. 1(c). Its corresponding three-dimensional (3D) atomic structure is shown in Fig. 1(d). There are six iron atoms and one carbon interstitial atom in this unit cell, which has been designated as ω′-Fe6C in our previous study46. On the other hand, if the positions of two ω-Fe3C (ω1, ω4) and two ω-Fe (ω2, ω3) in Fig. 1b exchange, the ω′-Fe6C will has the carbon atom at (0 0 0.5) as shown in Fig. 2a. Obviously, ω′-Fe6C has two forms because of the different carbon atom position as shown in Figs. 1d and 2a.
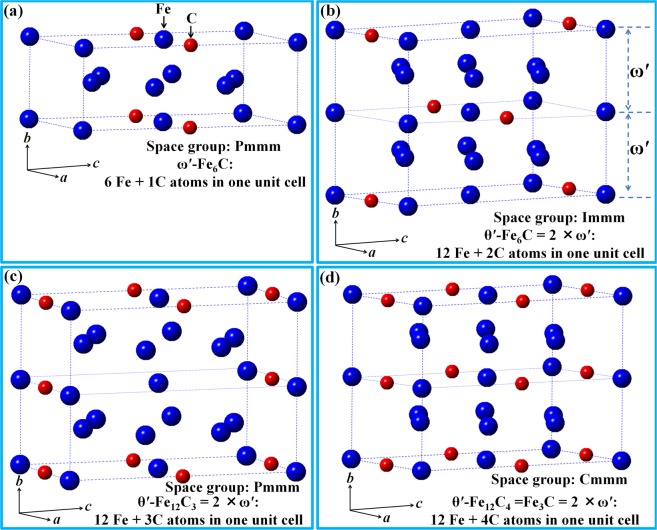
Figure 2.
Atomic structure of various carbides. (a) Unit cell of one ω′-Fe6C variant with one C atom at (0 0 0.5). (b) New θ′ variant (θ′-Fe12C2 or θ′-Fe6C) formed by merging the ω′-Fe6C variant with one C atom at (0.5 0 0) (Fig. 1(d)) and the ω′-Fe6C variant with one C atom at (0 0 0.5) in (a) along b axis. (c) New θ′ (θ′-Fe12C3 or θ′-Fe4C) variant. (d) New θ′ variant of θ′-Fe12C4 or θ′-Fe3C formed by doubling the ω′-Fe6C2 in Fig. 1(f) along b axis. All θ′ have an orthorhombic unit cell with lattice parameters of aθ′ = 4.033 Å, bθ′ = 2 × 2.47 Å = 4.94 Å, and cθ′ = 6.986 Å for aα-Fe = 2.852 Å).
When the coarsening of the ultra-fine ω-Fe3C particles follows the route shown in Fig. 1(e–g), new carbide consisting of six iron atoms and two carbon atoms in its unit cell will form as shown in Fig. 1(g), with its formula being ω′-Fe6C2 or ω′-Fe3C. There is no any difference in the calculated electron diffraction patterns between the ω′-Fe6C2 and ω-Fe3C carbides since both carbide crystals have the exact same atomic positions. As can be seen in Fig. 1, the transformation of the ω-Fe3C hexagonal structure to an orthorhombic structure depends on the carbon content and/or positons alone. Once the ordering of carbon atoms occurs, the orthorhombic structure can form in a spontaneous way. The electron diffraction spots associated with such an ordering has been observed in the ω′-Fe6C carbide46.
Since there is no obvious difference in the calculated electron diffraction patterns between ω-Fe3C carbide and ω-Fe phase45, the three phases (ω-Fe, ω-Fe3C, ω′-Fe6C2 (ω′-Fe3C)) would have similar electron diffraction patterns, which may cause difficulty in charactering the carbides experimentally. Simply speaking, there are three phases (hexagonal ω-Fe, orthorhombic ω-Fe3C and ω′-Fe6C2 (ω′-Fe3C)) present theoretically. However, it is difficult to distinguish among them since they show similar electron diffraction pattern experimentally. Formation of this new ω′-Fe3C carbide can actually explain why the ultra-fine ω-Fe3C particles never grow big in real materials. The ω-Fe3C particle size is just only 1–2 nm.
The carbide coarsening can be achieved via the several fine ω-Fe3C particles merged together. The driving force for the movement of fine ω-Fe3C particles comes from the recrystallization of ultra-fine α-Fe matrix grains. There are two crystalline phases, namely fine α-Fe as a matrix grain and fine ω-Fe3C particles at the α-Fe twinning boundaries that co-exist in the twinned martensitic structure. Thus, the coarsening behavior is actually controlled by the recrystallization process of the α-Fe matrix grains upon tempering. The α-Fe recrystallization results in a movement of the α-Fe grain boundaries and/or twinning boundaries, which promotes the ω-Fe3C particles at the boundaries to move and meet other ω-Fe3C particles. The coarsening behavior of the fine ω-Fe3C particles and the recrystallization process of the ultra-fine α-Fe grains have been experimentally confirmed and explained in our previous work35,40,42–44.
θ′-variants
Following the same coarsening mechanism explained in Fig. 1, new carbide, here designated as θ′, can be formed by combining two variants of ω′. The atomic structures of possible θ′ variants are shown in Fig. 2. Figure 2(a) shows one of the ω′ variants, while the other two ω′ variants are shown in Fig. 1(d,g). After two variants of ω′ merge together along its b axis, three θ′ variants (θ′-Fe12C2 or θ′-Fe6C (Fig. 2(b)), θ′-Fe12C3 or θ′-Fe4C (Fig. 2(c)), θ′-Fe12C4 or θ′-Fe3C (Fig. 2(d))) can be formed. Thus, the θ′ carbides possess lattice parameter of aθ′ = 4.033 Å, bθ′ = 2 × 2.47 Å = 4.94 Å, and cθ′ = 6.986 Å and retain an orthorhombic crystal structure. During the coarsening of fine ω′ particles, one ω′ particle with the crystal structure in Fig. 1(g) may combine with another ω′ particle with the same crystal structure along its bω′ axis. When this occurs, it is possible for a θ′-Fe3C carbide particle to form.
The formation of θ′-Fe12C4 or θ′-Fe3C variant involves merging two ω′-Fe6C2 or ω′-Fe3C carbide particles together alone without any atomic movement or variation in carbon content. It can be seen from Figs. 1 and 2 that the position of both Fe and C atoms during the ω → ω′ → θ′ transition are kept unchanged, meaning that this transition depends completely on the size of the ω-Fe3C carbide particle. The atomic positions of Fe and C atoms in the ω-Fe3C, ω′-Fe3C and θ′-Fe3C unit cells have been listed in the Tables 1–3, respectively.
Table 2.
The fraction coordination of six Fe atoms and two C atom of ω′-Fe3C in orthorhombic structure with the lattice parameters of aω′ = aα-Fe = 4.033 Å, bω′ = aα-Fe = 2.47 Å, cω′ = aα-Fe = 6.986 Å for aα-Fe = 2.852 Å. New variant of the ω′ carbide can be formed depending on the carbon concentration and positions.
| Atoms | aω′ | bω′ | cω′ |
|---|---|---|---|
| Fe1 | 0 | 0 | 0 |
| Fe2 | 1/2 | 0 | 1/2 |
| Fe3 | 1/2 | 1/2 | 1/6 |
| Fe4 | 1/2 | 1/2 | 5/6 |
| Fe5 | 0 | 1/2 | 1/3 |
| Fe6 | 0 | 1/2 | 2/3 |
| C1 | 1/2 | 0 | 0 |
| C2 | 0 | 0 | 1/2 |
Table 1.
The fraction coordination of three Fe atoms and one C atom of ω-Fe3C in hexagonal structure with the lattice parameters of aω = aα-Fe = 4.033 Å, cω = aα-Fe = 2.47 Å for aα-Fe = 2.852 Å.
| Atoms | aω | bω | cω |
|---|---|---|---|
| Fe1 | 0 | 0 | 0 |
| Fe2 | 2/3 | 1/3 | 1/2 |
| Fe3 | 1/3 | 2/3 | 1/2 |
| C | 1/2 | 1/2 | 0 |
Table 3.
The fraction coordination of twelve Fe atoms and four C atom of θ′-Fe3C in orthorhombic structure with the lattice parameters of aθ′ = aα-Fe = 4.033 Å, bθ′ = aα-Fe = 4.94 Å, cθ′ = aα-Fe = 6.986 Å for aα-Fe = 2.852 Å. Various variants are formed depending on the position and concentration of the intestinal carbon atoms.
| Atoms | aθ′ | bθ′ | cθ′ |
|---|---|---|---|
| Fe1 | 0 | 0 | 0 |
| Fe2 | 1/2 | 0 | 1/2 |
| Fe3 | 0 | 1/2 | 0 |
| Fe4 | 1/2 | 1/2 | 1/2 |
| Fe5 | 1/2 | 1/4 | 1/6 |
| Fe6 | 1/2 | 1/4 | 5/6 |
| Fe7 | 0 | 1/4 | 1/3 |
| Fe8 | 0 | 1/4 | 2/3 |
| Fe9 | 1/2 | 3/4 | 1/6 |
| Fe10 | 1/2 | 3/4 | 5/6 |
| Fe11 | 0 | 3/4 | 1/3 |
| Fe12 | 0 | 3/4 | 2/3 |
| C1 | 0 | 0 | 1/2 |
| C2 | 1/2 | 0 | 0 |
| C3 | 0 | 1/2 | 1/2 |
| C4 | 1/2 | 1/2 | 0 |
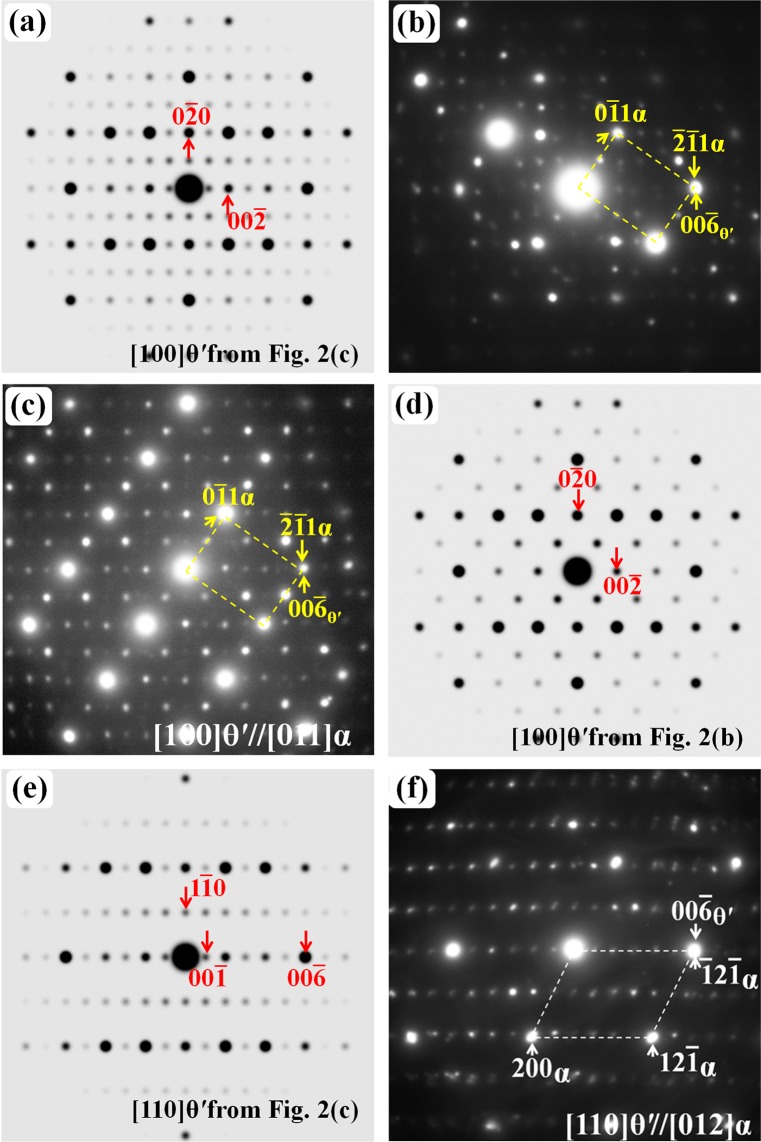
As explained in Figs. 1 and 2, ω′ and θ′ can have other variants with lower carbon content than that in the ω′-Fe6C2 (ω′-Fe3C) and θ′-Fe12C4 (θ′-Fe3C) unit cells. The ω′ variant (ω′-Fe6C) has been experimentally observed previously.46 Fig. 3 shows the evidence that there exist other type θ′ variants in the quenched high carbon Fe-C alloys. Simulated electron diffraction pattern of the θ′-Fe12C3 carbide with its [100] zone axis parallel to the electron beam is shown in Fig. 3(a), while the corresponding experimental electron diffraction pattern is shown in Fig. 3(b). The experimental diffraction pattern is composed of two sets of diffraction spots. One set is from [011] α-Fe zone axis and the other is from the [100] zone axis of the θ′-Fe12C3 carbide as shown in Fig. 3(a). The mixed electron diffraction patterns of α-Fe and fine carbides are frequently observed in the quenched Fe-C alloy with pearlite structure since both phases have ultra-fine particles (the region selected for experimental observations depends on the selected aperture size in TEM equipment, the smallest diameter size of the aperture is about 250 nm).
Figure 3.
Electron diffraction patterns of the θ′ variants: (a) Simulated [100] zone axis pattern of the θ′-Fe12C3 carbide. (b) Experimental pattern consisting of the diffraction spots from the [011] α-Fe zone axis and [100] θ′-Fe12C3 carbide. (c) Experimental pattern consisting of three sets of diffraction spots: [011] α-Fe zone axis, [100] θ′-Fe12C3 carbide and [100] zone axis of [100] θ′-Fe12C2 carbide. (d) Simulated [100] zone axis pattern of the θ′-Fe12C2 carbide. (e) Simulated [110] zone axis pattern of the θ′-Fe12C3 carbide. (f) Experimental electron diffraction patterns consisting of the spots from the [012] α-Fe zone axis and [110] θ′-Fe12C3 carbide.
Figure 3(c) shows a particular experimental pattern, which is composed of three sets of diffraction spots: (1) the strong spots from [011] α-Fe zone axis as outlined by the yellow dashed lines, (2) the spots shown in Fig. 3(a), which is from [100] zone axis of the θ′-Fe12C3 carbide, and (3) the spots [100] zone axis of the θ′-Fe12C2 carbide as shown in Fig. 3(d). Experimental diffraction patterns are usually obtained from an area of several hundreds of nanometers in diameter. Thus, the diffraction pattern is composed of several sets of diffraction spots, which come from various carbides present in the pearlite-like region. Figure 3(e) shows the simulated electron diffraction patterns of the θ′-Fe12C3 carbide along its [110] zone axis. This pattern can also be observed experimentally along the α-Fe [012] zone axis as shown in Fig. 3(f).
The results in Fig. 3 reveal that various θ′ variants can co-exist in the quenched sample, and the formation of different type of variants is dependent on carbon concentration and positions. The possible variants of both θ′ carbides and ω′ carbides are summarized and listed in Table 4. Both ω′ and θ′ carbides possess orthorhombic crystal structure. The unit cell of θ′ carbides is composed of two ω′ unit cells merged along its b axis. The formation mechanism of θ′ carbides is the variation in carbon atoms or concentration on different atomic planes, which causes an ordering structure of ω-Fe. Since the carbon atoms or concentration are the same in (001) planes of ω′-Fe6C2 (ω′-Fe3C) and θ′-Fe12C4 (θ′-Fe3C) and the electron diffraction patterns of ω-Fe3C, ω′-Fe6C2 (ω′-Fe3C) and θ′-Fe12C4 (θ′-Fe3C) are similar, no carbon-ordering diffraction spots could be observed. However, that is not to say the ω′-Fe6C2 (ω′-Fe3C) or θ′-Fe12C4 (θ′-Fe3C) does not exist in the sample.
Table 4.
The structural parameters and chemical composition of possible variants of the ω′ and θ′ carbides. The electron diffraction patterns of the ω′-Fe6C2 (ω′-Fe3C) and θ′-Fe12C4 (θ′-Fe3C) are the same with that of the ω-Fe3C.
| Metastable carbide | Variant composition | Notes |
|---|---|---|
|
ω′, orthorhombic aω′ = 4.033 Å, bω′= 2.47 Å, cω′ = 6.986 Å |
ω′-Fe6C | C atoms at different (001) atomic planes |
| ω′-Fe6C | ||
| ω′-Fe6C2 (ω′-Fe3C) | ||
|
θ′, orthorhombic aθ′ = 4.033 Å, bθ′= 4.94 Å, cθ′ = 6.986 Å |
θ′-Fe12C2 (θ′-Fe6C) | C concentration varies at different (001) atomic planes |
| θ′-Fe12C3 (θ′-Fe4C) | ||
| θ′-Fe12C4 (θ′-Fe3C) |
θ-Fe3C cementite
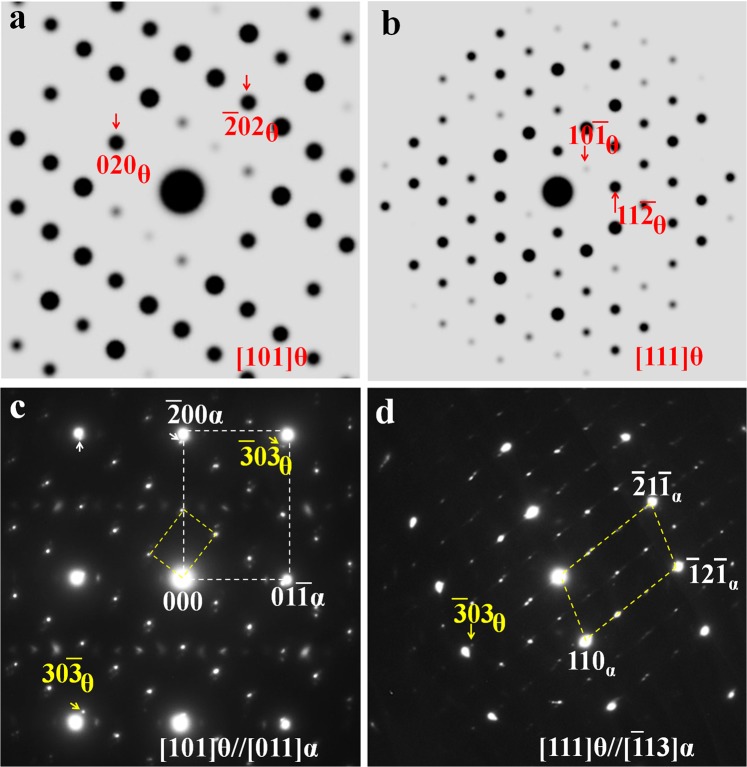
The diffraction patterns of θ-Fe3C cementite from two different zone axes ([101]θ in Fig. 4(a) and [111]θ in Fig. 4(b)) are shown here in comparison with that of previous carbides (ω-Fe3C, ω′-Fe6C2 (ω′-Fe3C) or ω′-Fe6C and various θ′-variants). It can be seen from Fig. 4(c), the experimental 03θ spot is completely separated from the α-Fe 1 spot, unlike the corresponding ω and ω′ or θ′ spots, which overlap perfectly with the corresponding α-Fe spots. This kind of separation can also be clearly observed in other direction as shown in Fig. 4(d). The results shown in Fig. 4 explain that the carbide with the well-known cementite structure has lost the perfect overlapping in diffraction spots compared with other carbides mentioned earlier.
Figure 4.
Simulated θ-Fe3C electron diffraction patterns: (a) [101]θ and (b) [111]θ. The corresponding experimental patterns observed along the zone axes of (c) [011]α and (d) [113]α.
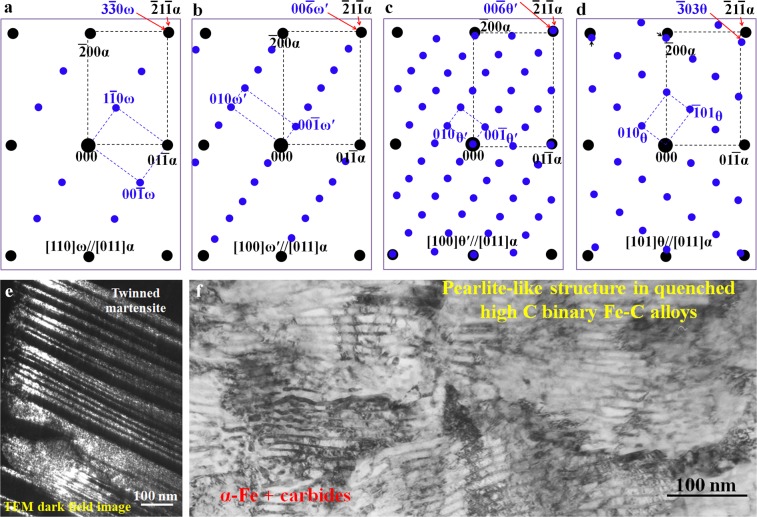
The electron diffraction patterns of ω-related carbide structures (ω, ω′, θ′ and θ) in quenched high-carbon binary Fe-C alloys are illustrated in Fig. 5. Figure 5(a–d) show the schematics of diffraction patterns based on the experimental results.45,46 All these patterns are obtained from the same α-Fe [011] zone axis. The pattern shown in Fig. 5(a) can only be observed within twinned martensite (Fig. 5(e)). The pattern (Fig. 5(a)) reveals a complete overlapping between the 211 α and the 330 ω spots. In Fig. 5(b), the ω-Fe3C diffraction pattern is converted into ω′-Fe6C with an ordering pattern and the original three spots (1 0ω, 2 0ω and 3 0ω) turn out to be six spots between the transmitted (central) and the 211 α diffraction spots. When two ω′-Fe6C unit cells merge together to form a θ′-Fe12C3 variant with its bθ′ = 2 bω′, an extra row of diffraction spots would occur in reciprocal space as shown in Fig. 5(c). The corresponding diffraction pattern from θ-Fe3C is shown in Fig. 5(d) for a comparison with that of the ω-Fe3C-related carbides to show a crystal structural similarity among these carbides. The patterns shown in Fig. 5(b–d) are normally observed in quenched pearlite-like microstructure like that shown in Fig. 5(f). Not only can ω′ carbides be observed in the pearlite-like microstructure, but θ′ and θ fine carbides can also be observed in the same pearlite-like region. Nevertheless, it is difficult to differentiate these carbides based on particle size or morphology alone since all of them are several nanometers in size.
Figure 5.
Schematic electron diffraction patterns between α-Fe and the (a) ω, (b) ω′, (c) θ′, and (d) the θ-Fe3C carbides. All patterns are along the [011]α zone axis.(e) Dark field TEM image revealing the twinned structure, which corresponds to the ω existing region. (f) TEM bright field image showing the pearlite-like structure corresponding to the existing region of the ω′, θ′, and θ fine carbide region.
In experimental TEM observations, these three carbides (ω, ω′ and θ′) can be identified easily based on the superimposition of certain diffraction spots on 211 α-Fe. Once these carbides start to transform into the well-known θ-Fe3C cementite, the separation of the 303 θ diffraction spots from 211 α-Fe spots can be clearly seen as shown in Fig. 5(d). Such a separation will produce complex diffraction patterns and cause difficulty in carbide characterization.
Conclusion
Ultra-fine carbides formed in quenched Fe-C alloys were investigated by comparing experimental results with simulated electron diffraction patterns.
Based on the unit cells of the ω′-Fe3C and its variants, an orthorhombic θ′ carbide structure with lattice parameter of a = 4.033 Å, b = 4.94 Å, and c = 6.986 Å was constructed and experimentally confirmed. The θ′ carbide can be: θ′-Fe12C2 (or θ′-Fe6C), θ′-Fe12C3 (or θ′-Fe4C) and θ′-Fe12C4 (or θ′-Fe3C) compounds.
A transition route (ω → ω′→ θ′) has been proposed during the coarsening of ultra-fine ω-Fe3C particles to explain the formation mechanism of the θ′ carbide with various variants. The transition occurs accompanying the variation in the position and concentration of carbon atoms, while the position of Fe atoms is kept unchanged.
It was observed that the ω′, θ′ and θ metastable carbides with ultra-fine particle size co-existed in the pearlite-like microstructure of quenched high carbon Fe-C alloys.
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFB0702100), JSPS KAKENHI Grant No. JP15H02304 and the National Natural Science Foundation of China (Nos. 51671043 and 51971159 and 51771026).
Author contributions
D.H.P. contributed to the experimental work, the result analysis and manuscript writing. H.P.X. and H.C. contributed to the simulation work, L.L.G., K.Gao, and X.L. contributed to the sample preparation and discussion.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. H. Ping, Email: ping.de-hai@nims.go.jp
X. Lu, Email: lu@djtu.edu.cn
References
- 1.Andrews KW. The structure of cementite and its relation to ferrite. Acta Metall. 1963;11:939–946. doi: 10.1016/0001-6160(63)90063-4. [DOI] [Google Scholar]
- 2.Elsukov EP, Dorofeev GA, Ulyanov AL, Vytovtov DA. On the problem of the cementite structure. Phy. Met. Metallogr. 2006;102:76–82. doi: 10.1134/S0031918X06070106. [DOI] [Google Scholar]
- 3.Cottrell AH. A theory of cementite. Mater. Sci. Tech. 1993;9:277–280. doi: 10.1179/mst.1993.9.4.277. [DOI] [Google Scholar]
- 4.Chiou WC, Jr, Carter EA. Structure and stability of Fe3C-cementite surfaces from first principles. Surf. Sci. 2003;530:87–100. doi: 10.1016/S0039-6028(03)00352-2. [DOI] [Google Scholar]
- 5.Wood IG, et al. Thermal expansion and crystal structure of cementite, Fe3C, between 4 and 600 K determined by time-of-flight neutron powder diffraction. J. Appl. Cryst. 2004;37:82–90. doi: 10.1107/S0021889803024695. [DOI] [Google Scholar]
- 6.Faraoun HI, Zhang YD, Esling C, Aourag H. Crystalline, electronic, and magnetic structures of θ-Fe3C, χ-Fe5C2, and η-Fe2C from first principle calculation. J. Appl. Phys. 2006;99:093508. doi: 10.1063/1.2194118. [DOI] [Google Scholar]
- 7.Jiang C, Srinivasan SG, Caro A, Maloy SA. Structure, elastic, and electronic properties of Fe3C from first principles. J. Appl. Phys. 2008;103:043502. doi: 10.1063/1.2884529. [DOI] [Google Scholar]
- 8.Lv ZQ, et al. First-principles study on the mechanical, electronic and magnetic properties of Fe3C. Comput. Mater. Sci. 2008;44:690–694. doi: 10.1016/j.commatsci.2008.05.006. [DOI] [Google Scholar]
- 9.Fang CM, van Huis MA, Zandbergen HW. Structure and stability of Fe2C phases from density-functional theory calculations. Scr. Mater. 2010;63:418–421. doi: 10.1016/j.scriptamat.2010.04.042. [DOI] [Google Scholar]
- 10.Fang CM, Sluiter MHF, van Huis MA, Ande CK, Zandbergen HW. Origin of predominance of cementite among iron carbides in steel at elevated temperature. Phys. Rev. Lett. 2010;105:055503. doi: 10.1103/PhysRevLett.105.055503. [DOI] [PubMed] [Google Scholar]
- 11.Leineweber A. Anisotropic microstrain broadening in cementite, Fe3C, caused by thermal microstress: Comparison between prediction and results from diffraction-line profile analysis. J. Appl. Cryst. 2012;45:944–949. doi: 10.1107/S0021889812036862. [DOI] [Google Scholar]
- 12.Garvik N, Carrez P, Cordier P. First-principles study of the ideal strength of Fe3C cementite. Mater. Sci. Eng. A. 2013;572:25–29. doi: 10.1016/j.msea.2013.02.028. [DOI] [Google Scholar]
- 13.Ghosh G. A first-principles study of cementite (Fe3C) and its alloyed counterparts: Elastic constants, elastic anisotropies, and isotropic elastic moduli. AIP Advances. 2015;5:087102. doi: 10.1063/1.4928208. [DOI] [Google Scholar]
- 14.Zhang X, et al. Structural transformations among austenite, ferrite and cementite in Fe-C alloys: A unified theory based on ab initio simulations. Acta Mater. 2015;99:281–289. doi: 10.1016/j.actamat.2015.07.075. [DOI] [Google Scholar]
- 15.Jia N, et al. Nanoscale spheroidized cementite induced ultrahigh strength-ductility combination in innovatively processed ultrafine-grained low alloy medium-carbon steel. Sci. Rep. 2017;7:2679. doi: 10.1038/s41598-017-02920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou YT, et al. Atomic structure of the Fe/Fe3C interface with the Isaichev orientation in pearlite. Phil. Mag. 2017;97:2375–2386. doi: 10.1080/14786435.2017.1332434. [DOI] [Google Scholar]
- 17.Hirotsu Y, Nagakura S. Crystal structure and morphology of the carbide precipitated from martensitic high carbon steel during the first stage of tempering. Acta Metall. 1972;20:645–655. doi: 10.1016/0001-6160(72)90020-X. [DOI] [Google Scholar]
- 18.Bauer-Grosse E, Frantz C, Le Caer G, Heiman N. Formation of Fe7C3 and Fe5C2 type metastable carbides during the crystallization of an amorphous Fe75C25 alloy. J. Non-Cryst. Solids. 1981;44:277–286. doi: 10.1016/0022-3093(81)90030-2. [DOI] [Google Scholar]
- 19.Ma CB, Ando T, Williamson DL, Krauss G. Chi-carbide in tempered high carbon martensite. Metall. Trans. A, 1983;14A:1033–1045. doi: 10.1007/BF02659852. [DOI] [Google Scholar]
- 20.Habibi Bajguirani HR. The effect of ageing upon the microstructure and mechanical properties of type 15-5 PH stainless steel. Mater. Sci. Eng. 2002;A338:142–159. doi: 10.1016/S0921-5093(02)00062-X. [DOI] [Google Scholar]
- 21.Clarke AJ, et al. Atomic and nanoscale chemical and structural changes in quenched and tempered 4340 steel. Acta Mater. 2014;77:17–27. doi: 10.1016/j.actamat.2014.05.032. [DOI] [Google Scholar]
- 22.Vieira I, et al. A dilatometric study of tempering complemented by Mössbauer spectroscopy and other characterization techniques. Sci. Rep. 2017;7:17337. doi: 10.1038/s41598-017-17654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor KA, Olson GB, Cohen M, Vander Sande JB. Carbide precipitation during stage I tempering of Fe-Ni-C martensites. Metall. Trans. A. 1989;20:2749–2765. doi: 10.1007/BF02670168. [DOI] [Google Scholar]
- 24.Meng F, Tagashira K, Azuma R. Role of eta-carbide precipitations in the wear resistance improvements of Fe-12Cr-Mo-V-1.4C tool steel by cryogenic treatment. ISIJ Int. 1994;34:205–210. doi: 10.2355/isijinternational.34.205. [DOI] [Google Scholar]
- 25.Bhadeshia HKDH. Cementite. Int. Mater. Rev. 2020;65:1–27. doi: 10.1080/09506608.2018.1560984. [DOI] [Google Scholar]
- 26.Hofer LJE, Cohn EM. Some reactions in the iron-carbon system: application to the tempering of martensite. Nature. 1951;167:977–978. doi: 10.1038/167977a0. [DOI] [Google Scholar]
- 27.Roberts CS, Averbach BL, Cohen M. The mechanism and kinetics of the 1st stage of tempering. Trans. ASM. 1953;45:576–604. [Google Scholar]
- 28.Lement BS, Averbach BL, Cohen M. Microstructural changes on tempering iron carbon alloys. Trans. ASM. 1954;46:851–881. [Google Scholar]
- 29.Mittemeijer EJ, Cheng L, van der Schaaf PJ, Brakman CM, Korevaar BM. Analysis of non-isothermal transformation kinetics-tempering of iron-carbon and iron-nitrogen martensites. Metall. Trans. A, 1988;19A:925–932. doi: 10.1007/BF02628377. [DOI] [Google Scholar]
- 30.Jack DH, Jack KH. Carbides and nitrides in steel. Mater. Sci. Eng. 1973;11:1–27. doi: 10.1016/0025-5416(73)90055-4. [DOI] [Google Scholar]
- 31.Ping DH, Geng WT. A popular metastable omega phase in body-centered cubic steels. Mater. Chem. Phys. 2013;139:830–835. doi: 10.1016/j.matchemphys.2013.02.040. [DOI] [Google Scholar]
- 32.Ping DH. Review on omega phase in body-centered cubic metals and alloys. Acta Metall. Sin. (Eng. Lett.) 2014;27:1–11. doi: 10.1007/s40195-013-0014-2. [DOI] [Google Scholar]
- 33.Liu TW, et al. A new nanoscale metastable iron phase in carbon steels. Sci. Rep. 2015;5:15331. doi: 10.1038/srep15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Chen Y, Xiao W, Ping D, Zhao X. Twin structure of the lath martensite in low carbon steel. Prog. Nat. Sci. Mater. Int. 2016;26:169–172. doi: 10.1016/j.pnsc.2016.03.004. [DOI] [Google Scholar]
- 35.Ping DH, et al. Microstructural evolution and carbides in quenched ultra-low carbon (Fe-C) alloys. ISIJ Int. 2017;57:1233–1240. doi: 10.2355/isijinternational.ISIJINT-2016-624. [DOI] [Google Scholar]
- 36.Ping DH, et al. A simple method for observing ω-Fe electron diffraction spots from <112>α-Fe directions of quenched Fe-C twinned martensite. ISIJ Int. 2018;58:159–164. doi: 10.2355/isijinternational.ISIJINT-2017-270. [DOI] [Google Scholar]
- 37.Ping DH, Ohnuma M. ω-Fe particle size and distribution in high-nitrogen martensitic steels. J. Mater. Sci. 2018;53:5339–5355. doi: 10.1007/s10853-017-1938-0. [DOI] [Google Scholar]
- 38.Liu TW, Ping DH, Ohmura T, Ohnuma M. Electron diffraction characterization of quenched Fe-C martensite. J. Mater. Sci. 2018;53:2976–2984. doi: 10.1007/s10853-017-1731-0. [DOI] [Google Scholar]
- 39.Chen Y, Ping D, Wang Y, Zhao X. An atomic mechanism for the formation of nanotwins in high carbon martensite. J. Alloys Compd. 2018;767:68–72. doi: 10.1016/j.jallcom.2018.07.099. [DOI] [Google Scholar]
- 40.Ping DH, et al. Lath formation mechanisms and twinning as lath martensite substructures in an ultra-low carbon iron alloy. Sci. Rep. 2018;8:14264. doi: 10.1038/s41598-018-32679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells MGH. An electron transmission study of the tempering of martensite in an Fe-Ni-C alloy. Acta Metall. 1964;12:389–399. doi: 10.1016/0001-6160(64)90009-4. [DOI] [Google Scholar]
- 42.Man TH, Liu TW, Ping DH, Ohmura T. TEM investigations on lath martensite substructure in quenched Fe-0.2C alloys. Mater. Char. 2018;135:175–182. doi: 10.1016/j.matchar.2017.11.039. [DOI] [Google Scholar]
- 43.Liu X, Ping DH, Xiang HP, Lu X, Shen J. Nanoclusters of α-Fe naturally formed in twinned martensite after martensitic transformation. J. Appl. Phys. 2018;123:205111. doi: 10.1063/1.5034146. [DOI] [Google Scholar]
- 44.Liu X, et al. In situ heating TEM observations on carbide formation and α-Fe recrystallization in twinned martensite. Sci. Rep. 2018;8:14454. doi: 10.1038/s41598-018-32896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ping DH, Xiang HP. Simulated electron diffraction patterns of ω-Fe in Fe-C martensite. J. Appl. Phys. 2019;125:045105. doi: 10.1063/1.5065432. [DOI] [Google Scholar]
- 46.Ping DH, et al. Metastable ω′-Fe3C carbide formed during ω-Fe3C particle coarsening in binary Fe-C alloys. J. Appl. Phys. 2019;125:175112. doi: 10.1063/1.5091935. [DOI] [Google Scholar]