Abstract
Effects of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) on the oxidative stability were determined in soybean oil–water system at different locations including at the interface of air–oil, in the middle of oil, and at the interface of oil–water. Also, profile changes of tocopherols were determined during UV irradiation for 18 days. Although no significant changes in tocopherol profiles were observed at three different locations irrespective of DOPC from 0 to 1250 μmol/kg oil, addition of DOPC increased total tocopherols, α-tocopherol, and δ-tocopherol whereas content of β + γ tocopherols did not increase at any locations. Moisture content in water–oil interface was higher than other locations while those were not consistent at different DOPC concentration. Added DOPC significantly decreased oxidative stability from 250 to 830 μmol/kg oil compared to controls (p < 0.05) whereas 1250 μmol/kg oil DOPC increased oxidative stability. Stabilities of tocopherols especially α-tocopherol were lower in oil–water system than those in bulk oil at UV irradiation.
Keywords: Interface, Tocopherol profile, Phospholipid, Oxidative stability, UV irradiation
Introduction
Lipid oxidation is a chemical reaction among unsaturated fatty acids and oxygen molecules. Many researches have conducted to determine the mechanism of lipid oxidation by chemical approaches using diverse model systems. Roles of the degree of unsaturation in fatty acids, types of oxygen molecules, matrix types such as oil-in-water (O/W) emulsions or bulk oils, and the presence of prooxidative metal ions, antioxidative compounds, and amphiphilic minor compounds have been elucidated in lipid oxidation (Ghnimi et al., 2017; Grosshagauer et al., 2019). However, current understanding on lipid oxidation mechanisms has some limitation explaining the phenomena of lipid oxidation (Budilarto and Kamal-Eldin, 2015a; 2015b; Laguerre et al., 2015).
Nowadays, interfaces of water–oil rather than air–oil are believed as major locations for lipid oxidation (Chaiyasit et al., 2007; Chen et al., 2012; McClements and Decker, 2000; Schwarz et al., 2000). A water soluble radical generator accelerated the rates of lipid oxidation than lipid soluble radical generator in bulk oils containing phospholipids and trace amount of water (Koga and Terao, 1995). Polar antioxidants could not migrate to the air–oil interface due to the lower dielectric constant of air (1.0) than oil (approximately 3) and most antioxidants did not decrease surface tension in hexadecane system (Chaiyasit et al., 2007). However, evidences of lipid oxidation for the oil–water interfaces are increasing in the literature (Budilarto and Kamal-Eldin, 2015a; 2015b; Laguerre et al., 2015).
Importance of amphiphilic minor compounds and moisture increases in lipid oxidation. Amphiphilic minor compounds including free fatty acids (FFAs), diacylglycerols (DAGs), monoacylglycerols (MAGs) and phospholipids (PLs) in bulk oils may form association colloids like reverse micelles or lamella structures (Chaiyasit et al., 2007; Laguerre et al., 2015). As lipid oxidation increases in bulk oil, moisture content tends to increase due to the migration of headspace moisture and increases in amphiphilic oxidized compounds like lipid hydroperoxides (Park et al., 2014). Also, mixtures of linoleic acid and deuterium oxide or corn oil and deuterium oxide produced volatiles containing deuterium during 60 °C oxidation, implying that moisture participated in the volatile formation under airtight conditions (Kim et al., 2014a; 2014b). Therefore, the balance of moisture and amphiphilic compounds are important factors influencing the rate of lipid oxidation.
DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), one of phosphatidylcholines (PC), is found in the membranes of cell organelles and has been used in preparing model lipid membranes or reverse micelles (Cui et al., 2014; Kittipongpittaya et al., 2014; Sun and Bandara 2019). Antioxidant and prooxidant properties of PC or DOPC have been studied in diverse matrix and model systems (Kim et al., 2015; Lee and Choe, 2009). PC acted as a prooxidant in canola oil under chlorophyll photosensitization (Lee and Choe, 2009). PC showed different activity toward oxidation depending on the stripping system. In stripped corn oil, PC acted as antioxidant whereas PC accelerated the rates of oxidation in non-stripped oil system (Kim et al., 2015). Chen et al. (2012) reported that phosphatidylcholine act as a prooxidant in bulk oil systems due to the formation of reverse micelles.
Although many studies have tried to understand the action of PLs in diverse model systems, the antioxidative or prooxidative effects of DOPC in oils at different sampling locations have not been reported in the literature.
The objective of this study was to determine the antioxidant or prooxidant properties of DOPC in soybean oil–water system at different sampling locations at oil–water interface, inside of oil, and oil–air interface. Different concentration of DOPC was added to soybean oil–water system and antioxidant profiles of tocopherol homologues were determined in different locations to find out correlation of phospholipids and location effects.
Materials and methods
Materials
DOPC was purchased from Avanti Lipid Polar (Alabaster, AL, USA). Standard tocopherols and tocopherol mixture were purchased from Sigma-Aldrich (St. Louis, MO, USA). Medium chain triacylglycerols (MCT) were purchased from Dongbang plastic (Seoul, Korea). HPLC grade solvents including n-hexane (95.0%), methylene chloride (99.8%), and 2-propanol (99.7%) were purchased from Fisher Scientific (J.T. Baker®, Fairlawn, NJ, USA). Soybean and corn oils were purchased from a local market (Suwon, Korea). Other reagent grade chemicals were obtained from Daejung Chemical (Seoul, Korea).
Sample preparation
DOPC was dissolved in n-hexane and added to soybean oil at the concentration of 0, 250, 650, 830, and 1250 μmol/kg oil. Solvent was removed using nitrogen flushing. Twenty gram of soybean oil containing DOPC was carefully added to 10 mL of deionized water in a 50-mL conical tube and let the tube stand without mixing for 24 h. To find out the location effects, 3 g of soybean oils at oil–water interface, inside of bulk oil, and oil–air interface in the soybean oil–water mixtures were taken carefully using a syringe. Specifically, sampling locations for oil–water interface, inside of oil, and oil–air interface were just above water, middle of height in oil phase, and just below air–oil interface. Soybean oil without water addition was prepared as controls. Samples were prepared in triplicate.
To observe the changes of tocopherol profiles, corn oil was first stripped to remove inherent tocopherols according to the methods of Yi et al. (2017). The stripped corn oil was mixed with MCT at the ratio of 1: 3 (w/w) and mixtures of tocopherols was added to the corn oil and MCT at a concentration of 5000 ppm. The 20 g oil mixture was carefully added to 10 mL deionized water in a 50-mL conical tube and let the tubes stand without mixing for 24 h in the dark. The conical tubes were placed in a light box containing UV lights with 253.7 and 352 nm at 25 °C chamber (JDECRS-2DAC, Jeung Do Bio & Plant, Seoul, Korea) for 18 days. The 0.1 g oils at three different locations were sampled everyday for 18 days and tocopherol profiles were analyzed by HPLC. Three conical tubes were prepared and HPLC was conducted three times for each sampled oil.
Tocopherol content analysis
An oil sample of 0.1 g was directly mixed with 1 mL n-hexane and filtered through a 0.4 polytetrafluorethylene (PTFE) membrane filter. Tocopherol content was analysed by HPLC (Jasco Pu-2089 plus, JASCO International Co. Ltd., Tokyo, Japan) with a fluorescence detector (Jasco Pu-2020 plus). Stationary phase was a μ-Porasil™ column (3.9 × 300 mm, 10 μm ID, Waters) and mobile phase was a mixture of n-hexane and isopropanol at a ratio of 99.8–0.2 (v/v) with an isocratic 0.7 mL/min velocity. The injection volume was 20 μL and the oven temperature was 35 °C. The tocochromanols were detected at 290 nm excitation and 330 nm emission wavelengths. Calibration curves were constructed using standard α-, γ-, and δ-tocopherols (Sigma-Aldrich) dissolved in n-hexane.
Moisture content analysis
The moisture content in oil was determined using a coulometric KF titrator (C20, Mettler-Toledo Intl., Columbus, OH, USA) according to the manufacturer’s instructions.
Oxidation induction time (OIT) by differential scanning calorimetry (DSC)
Differential Scanning Calorimetry (DSC) experiments were performed with a DSC 4000 from Perken Elmer (Waltham, Massachusetts, US) according to the modified of Velasco et al. (2004) and Simon et al. (2000). Briefly, soybean oil sample (5.0–8.0 mg) was weighed into aluminium DSC pan without a cap. An empty pan was used as reference. Samples were heated under isothermal conditions (130 °C) with an oxygen flow of 50 mL/min. Sudden decrease in DSC thermogram was calculated using the changes of regression slopes and the time to reach the sudden decrease was regarded as oxidation induction time.
Statistical analysis
Data for tocopherol content, oxidation induction time, moisture content, and size analysis were analyzed statistically by ANOVA and Duncan’s multiple range test using the SPSS software program version 22 (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered significant.
Results and discussion
Tocopherol content analysis
Total tocopherols, α-, β + γ-, and δ-tocopherols in soybean oil–water system with 0 and 1250 μmol DOPC/kg oil at oil–water interface, inside of oil, and oil–air interface are shown in Table 1. Significant difference in total tocopherols, α-tocopherol, β + γ-tocopherols, and δ-tocopherol at oil–water interface, inside of oil, and oil–air interface were not found at either 0 or 1250 μmol DOPC/kg oil (p > 0.05). However, addition of DOPC made significant increases in each tocopherol homologue compared to control soybean oil without addition of DOPC (p < 0.05). Specifically, addition of DOPC increased the detected content of total tocopherols, α-tocopherol, and δ-tocopherol at oil–water interface, inside of oil, and oil–air interface whereas content of β + γ tocopherols did not increase at any locations. It is quite interesting findings that addition of 1250 μmol DOPC could increase the detected concentration of lipophilic antioxidants. Chen (2011) found that α-tocopherol was associated with DOPC reverse micelles in bulk oil mainly at the oil–water interface. Reverse micelles made of DOPC may stabilize and concentrate α- and δ-tocopherols in bulk oil selectively than β + γ tocopherols, which makes higher concentration of α- and δ-tocopherols in Table 1.
Table 1.
Total tocopherols, α–, β + γ, and δ-tocopherols in soybean oil with 0 and 1250 μmol DOPC/kg oil at oil–water interface, inside of oil, and oil–air interface
| DOPC Concentration (μmol/kg oil) | |||||
|---|---|---|---|---|---|
| 0 | 1250 | 0 | 1250 | ||
| α-Tocopherol (ppm) | β + γ Tocopherols (ppm) | ||||
| Air1 | 143.0 ± 8.92aB3 | 221.7 ± 3.6aA | Air | 1630.4 ± 23.9aA | 1587.1 ± 25.5aA |
| Oil | 132.7 ± 4.3aB | 224.7 ± 5.3aA | Oil | 1534.3 ± 505.4aA | 1603.6 ± 32.5aA |
| Water | 171.7 ± 32.9aB | 226.0 ± 5.3aA | Water | 1612.7 ± 63.3aA | 1615.9 ± 44.2aA |
| δ-Tocopherol (ppm) | Total tocopherol (ppm) | ||||
| Air | 220.6 ± 19.5aB | 399.0 ± 8.5aA | Air | 1994.0 ± 37.7aB | 2207.7 ± 37.4aA |
| Oil | 198.7 ± 4.2aB | 403.2 ± 10.9aA | Oil | 1865.7 ± 506.5aB | 2231.5 ± 48.1aA |
| Water | 214.7.72 ± 30.8aB | 406.8 ± 17.2aA | Water | 1999.2 ± 46.8aA | 2248.6 ± 66.5aA |
1‘Air’, ‘Oil’, and ‘Water’ were samples from oil–water interface, inside of oil, and oil–air interface, respectively
2Mean ± standard deviation (n = 3)
3Different capital and small letters indicate significant differences in the same row and same column at 0.05, respectively
Even distribution of lipophilic α-tocopherol or hydrophilic Trolox was reported in oil between air–oil interface and middle of oil using freezing the sample (Chaiyasit et al., 2007). Current study did not freeze oil into solid state and continuous movement and mixing phenomena make insignificant difference in tocopherol homologues among locations.
Critical micelle concentration (CMC) of DOPC in stripped soybean oil was reported as 400 μmol/kg oil by tetracyanoquinodimethane (TCNQ) technique (Kittipongpittaya et al., 2014) whereas Chen (2011) reported 650 and 950 μmol/kg oil using interfacial tension and fluorescence spectrometry, respectively. Concentration of 1250 μmol DOPC/kg oil is much higher than reported CMC of DOPC and reverse micelles should be formed inside the bulk oil. Currently, main causes for the higher concentration of tocopherols in oil–water system than control oil is not fully understood yet.
Moisture content analysis
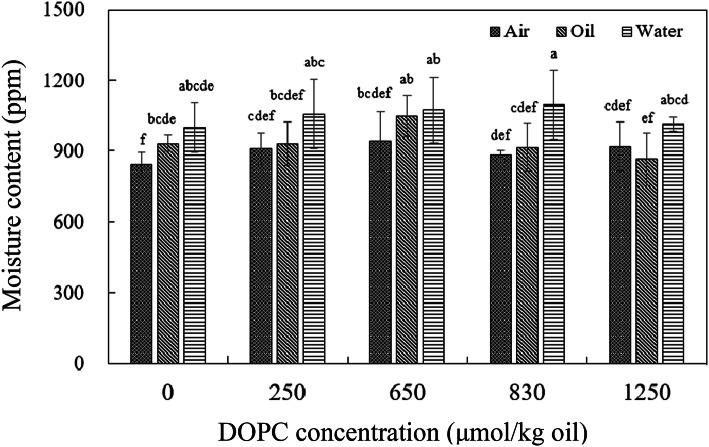
Moisture content in soybean oil at oil–water interface, inside of oil, and oil–air interface from oil–water mixture are shown in Fig. 1. Overall, oil–water interface showed higher moisture content than inside of oil and oil–air interface while significant difference was not observed (p > 0.05) except when DOPC was added at 830 μmol DOPC/kg oil. Moisture content at oil–water interface in control oil was significantly higher than that at oil–air interface (p < 0.05) while no significant difference with at inside of oil (p > 0.05). Moisture content in samples with 250, 650, 830, and 1250 μmol DOPC/kg oil DOPC were not significantly different irrespective of sampling locations (p > 0.05). It seems like that high concentration of DOPC distributes moisture in bulk oils more evenly.
Fig. 1.
Moisture content in soybean oil at oil–water interface, inside of oil, and oil–air interface from oil–water mixture. ‘Air’, ‘Oil’, and ‘Water’ indicate samples from oil–air interface, inside of oil, and oil–water interface, respectively. Different letters are significantly different at 0.05
Oxidation induction time assay by DSC
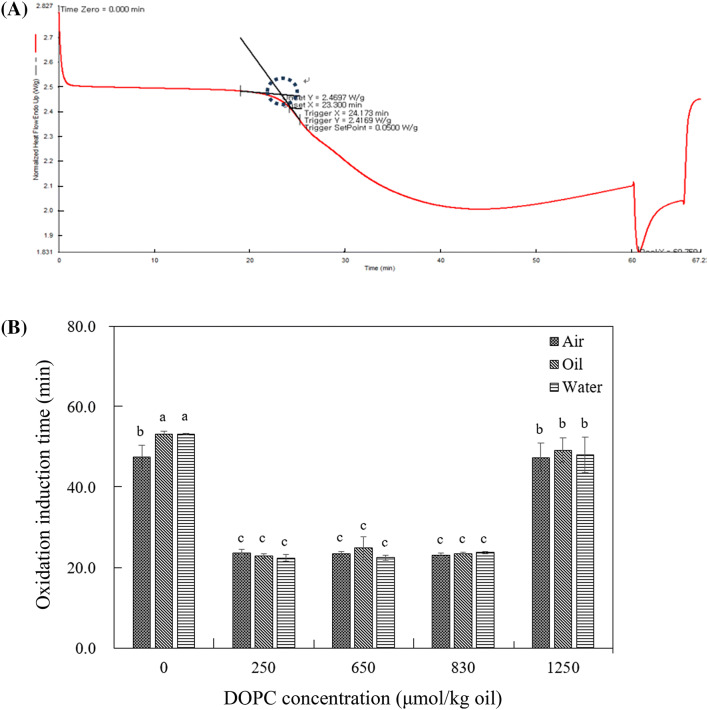
DSC thermogram (A) of soybean oil and DOPC mixtures and changes of oxidation induction time (OIT) in soybean oil with 0, 250, 650, 830, and 1250 μmol DOPC/kg oil at oil–water interface, inside of oil, and oil–air interface from oil–water mixture (B) are shown in Fig. 2. The principles for the determination of induction period by vegetable oils can be divided into two categories, which is sudden increase of oxidation products and of oxygen uptake. In DSC thermogram, a sudden decrease was observed in oils under continuous oxygen flow condition (Fig. 2A). This point may be formed due to the extra thermal energy required for the propagation steps of lipid oxidation (Velasco et al., 2004). The sudden decreasing point in DSC thermogram was named as OIT.
Fig. 2.
DSC thermogram (A) of soybean oil and DOPC mixtures to determine oxidation induction time (OIT) and changes of OIT in soybean oil with 0, 250, 650, 830, and 1250 μmol DOPC/kg oil at oil–water interface, inside of oil, and oil–air interface from oil–water mixture (B). Abbreviations are listed in the Fig. 1 legend. Different letters are significantly different at 0.05
For oils without DOPC, OIT at oil–air interface was significantly lower than that at the position of oil–water interface and inside of oil (p < 0.05), which indicates soybean oil near air oxidized faster than other positions. However, no significant difference was observed in OIT at oil–water interface, inside of oil, and oil–air interface (p > 0.05) in samples containing DOPC. The OIT from soybean oils with 250, 650, and 830 μmol DOPC/kg oil were significantly lower than those from oils with 0 and 1250 μmol DOPC/kg oil (p < 0.05), which implies DOPC acted as prooxidants at these concentration. OIT in soybean oils containing 1250 μmol DOPC/kg oil increased to the OIT value of oils without addition of DOPC (Fig. 2B). Addition of DOPC at lower concentration than CMC significantly decreased OIT, which implies prooxidative properties of DOPC. The results of OIT revealed that depending on the concentration of lecithin, oxidative stability of oils varied (Fig. 2B).
Antioxidant properties of PLs are affected by the presence of minor compounds in corn oil and especially lecithin near CMC showed strong antioxidant effects in non-stripped corn oil but not in stripped corn oil (Kim et al., 2019). Chen (2011) observed that depending on the concentration of α-tocopherol, effects of DOPC were changed in stripped soybean oil. DOPC (1000 μM) over CMC acted prooxidantly at 100 μM α-tocopherol whereas antioxidantly at 10 μM α-tocopherol in stripped soybean oil (Chen, 2011). In this study, different concentration of added DOPC affected differently on the stability of tocopherols and oxidative stability in bulk oils.
Antioxidative or prooxidative properties of phospholipids or DOPC have been reported in the literature. Soy lecithin added over its CMC showed better antioxidant activity in non-stripped corn oil than samples added with lower concentration of lecithin. However, this antioxidant effects were not observed clearly in stripped corn oil system (Kim et al., 2019). DOPC had prooxidative properties while 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) retarded the rates of lipid oxidation in stripped soybean oil with 100 μM α-tocopherol or trolox (Cui and Decker, 2016). Regeneration of α-tocopheryl quinone to α-tocopherol by phosphatidylethanolamine and phosphotidylserin was found whereas DOPC did not show such regeneration ability (Cui et al., 2015; Doert et al., 2012). DOPC had prooxidative properties in reverse micelles after the CMC whereas 1,2-dibutyryl-sn-glycero-3-phoshocholine did not make changes in the lipid oxidation (Chen et al., 2010). Added DOPC in stripped oils had antioxidative property whereas showed pro-oxidative property in non-stripped oils (Kim et al., 2015). These prooxidative effects of PC could be due to the participation on the formation of association colloids where lipid hydroperoxides and metals ions play important roles into the oil–water interface (Chen et al., 2012).
Tocopherol profile changes in different locations during UV irradiation
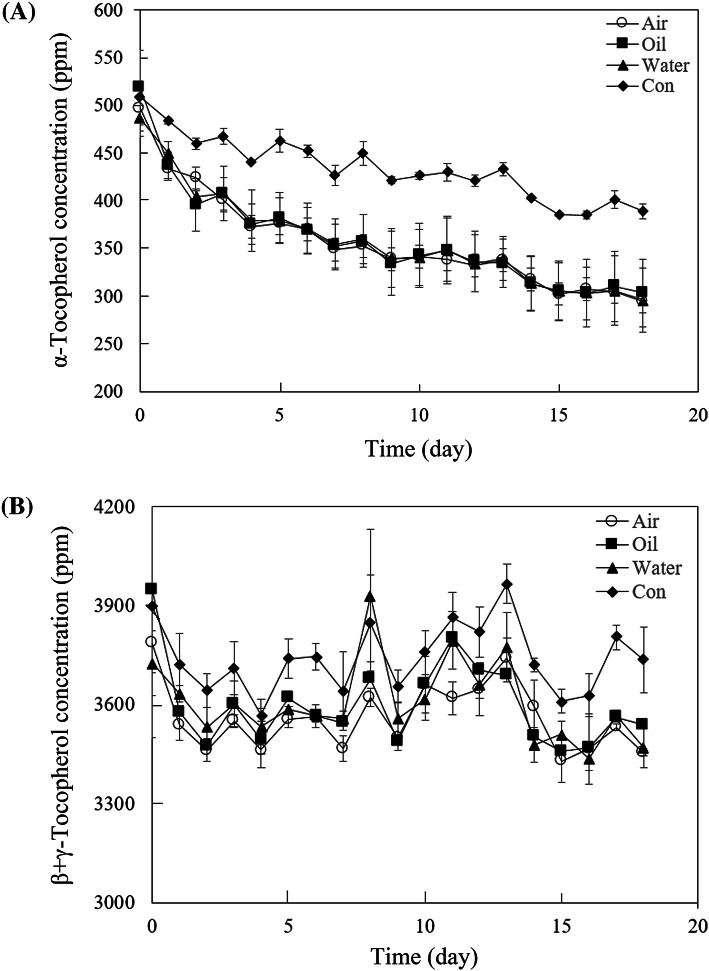
Changes of α-tocopherol (A) and β + γ tocopherols (B) in the mixtures of MCT, tocopherols, and stripped corn oil under UV irradiation for 18 days at oil–water interface, inside of oil, and oil–air interface are shown in Fig. 3. Generally, α-tocopherol content decreased in all the samples during UV irradiation for 18 days. α-Tocopherol content from control corn oil were significantly higher than those from three locations whereas there were no significant difference at three locations (p > 0.05) (Fig. 3A). However, this difference among samples was not observed in β + γ tocopherols (Fig. 3B). The presence of moisture accelerates the decomposition of α-tocopherol than β + γ tocopherols during UV irradiation, which implies that association colloids made of moisture and amphiphilic compounds play an important role in the instability of tocopherol homologues at non-thermal stress like UV irradiation. The stability of α-tocopherol was lower than other tocopherol homologues because interfaces of association colloids are believed as major lipid oxidation places and α-tocopherol is mainly located at the interfaces of reverse micelles made of DOPC (Chen, 2011).
Fig. 3.
Changes of α-tocopherol (A) and β + γ tocopherols (B) in the mixtures of medium chain triacylglycerol, tocopherol mixture, and stripped corn oil under UV irradiation for 18 days at oil–water interface, inside of oil, and oil–air interface. ‘Air’, ‘Oil’, and ‘Water’ indicate samples from oil–air interface, inside of oil, and oil–water interface from a mixture of medium chain triacylglycerol, tocopherol mixture, and stripped corn oil under UV irradiation, respectively whereas ‘Con’ was the mixture sample without addition of water
In conclusion, DOPC ranging from 250 to 830 μmol DOPC/kg oil accelerated the oxidation in soybean oil whereas 1250 μmol DOPC/kg oil did not show such prooxidative properties based on the results of OIT. CMC of DOPC was reported ranging from 650 to 830 μmol DOPC/kg oil, which implies that ratio of moisture and amphiphilic compounds like DOPC could affect the oxidative stability in oils. Profiles of tocopherol homologues were not different at three locations including oil–water interface, inside of oil, and oil–air interface in the soybean oil–water mixtures. Generally, presence of moisture decreased the stability of α-tocopherols in bulk oils more than other tocopherol homologues. DOPC could form association colloids with moisture in bulk oils and may affect stability of tocopherol homologues and oxidation of oils depending on the concentration of DOPC.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2B4002613) and Ottogi HamTaeHo Foundation.
Complice with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
YongJun Kwon, Email: yjkwon50@naver.com.
Seungbeen Jo, Email: tmdqlssla93@naver.com.
HeeSun Na, Email: aeiouai@naver.com.
SungHwa Kim, Email: plqaz0505@gmail.com.
Mi-Ja Kim, Email: mijakim@kangwon.ac.kr.
JaeHwan Lee, Email: s3hun@skku.edu.
References
- Budilarto ES, Kamal-Eldin A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015;117:1095–1138. doi: 10.1002/ejlt.201400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budilarto ES, Kamal-Eldin A. Water content and micelle size change during oxidation of sunflower and canola oils. Eur. J. Lipid Sci. Technol. 2015;117:1971–1977. doi: 10.1002/ejlt.201400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007;47(3):299–317. doi: 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- Chen BC. Minor components and their roles on lipid oxidation in bulk oil that contains association colloids. Ph. D. thesis, University of Massachusetts, Amherst, MA, USA (2011)
- Chen BC, Han A, McClements DJ, Decker EA. Physical structures in soybean oil and their impact on lipid oxidation. J. Agric. Food Chem. 2010;58:11993–11999. doi: 10.1021/jf102763p. [DOI] [PubMed] [Google Scholar]
- Chen BC, Panya A, McClements DJ, Decker EA. New insights into the role of iron in the promotion of lipid oxidation in bulk oils containing reverse micelles. J. Agric. Food Chem. 2012;60:3524–3532. doi: 10.1021/jf300138h. [DOI] [PubMed] [Google Scholar]
- Cui L, Decker EA. Phospholipids in foods: prooxidants or antioxidants? J. Sci. Food Agric. 2016;96:18–31. doi: 10.1002/jsfa.7320. [DOI] [PubMed] [Google Scholar]
- Cui L, Kittipongpittaya K, McClements DJ, Decker EA. Impact of phosphoethanolamine reverse micelles on lipid oxidation in bulk oils. J. Am. Oil Chem. Soc. 2014;91:1931–1937. doi: 10.1007/s11746-014-2544-9. [DOI] [Google Scholar]
- Cui L, McClements DJ, Decker. Impact of phosphatidylethanolamine on the antioxidant activity of α-tocopherol and trolox in bulk oil. J. Agric. Food Chem. 63: 3288-3294 (2015) [DOI] [PubMed]
- Doert M, Jaworska K, Moersel JT, Kroh LW. Synergistic effect of lecithins for tocopherols: lecithin-based regeneration of α-tocopherol. Eur. Food Res. Technol. 2012;235(5):915–928. doi: 10.1007/s00217-012-1815-7. [DOI] [Google Scholar]
- Ghnimi S, Budilarto E, Kamal-Eldin A. The new paradigm for lipid oxidation and insights to microencapsulation of omega-3 fatty acids. Compr. Rev. Food Sci. F. 2017;16:1206–1218. doi: 10.1111/1541-4337.12300. [DOI] [PubMed] [Google Scholar]
- Grosshagauer S, Steinschaden R, Pignitter M. Strategies to increase the oxidative stability of cold pressed oils. LWT-Food Sci. Technol. 2019;106:72–77. doi: 10.1016/j.lwt.2019.02.046. [DOI] [Google Scholar]
- Kim JY, Kim MJ, Lee JH. Role of moisture on the lipid oxidation determined by D2O in linoleic acid system. Food Chem. 2014;146:134–140. doi: 10.1016/j.foodchem.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim MJ, Lee JH. Effects of deuterium oxide on the oxidative stability and changes of headspace volatiles of corn oil. J. Am. Oil Chem. Soc. 2014;91:623–628. doi: 10.1007/s11746-013-2398-6. [DOI] [Google Scholar]
- Kim JY, Oh SM, Yi BR, Kim MJ, Lee JH. Synergism of phosphatidylcholine on the antioxidant properties of α-tocopherol in corn oils under different relative humidity. Int. J. Food Sci. Technol. 2015;50:1421–1428. doi: 10.1111/ijfs.12793. [DOI] [Google Scholar]
- Kim JS, Woo YS, Ryu JW, Kim MJ, Lee JH. Lecithin near its critical micelle concentration increases oxidative stability of non-stripped corn oil but not stripped corn oil. Eur. J. Lipid Sci. Technol. 2019;117:1800219. doi: 10.1002/ejlt.201800219. [DOI] [Google Scholar]
- Kittipongpittaya K, Panya A, McClements DJ, Decker EA. Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk. J. Am. Oil Chem. Soc. 2014;91:453–462. doi: 10.1007/s11746-013-2388-8. [DOI] [Google Scholar]
- Koga T, Terao J. Phospholipids increase radical scavenging activity of vitamin E in a bulk oil model system. J. Agric. Food Chem. 1995;43:1450–1454. doi: 10.1021/jf00054a007. [DOI] [Google Scholar]
- Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA, Villeneuve P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015;55(2):183–201. doi: 10.1080/10408398.2011.650335. [DOI] [PubMed] [Google Scholar]
- Lee J, Choe E. Effects of phosphatidylcholine and phosphatidylethanolamine on the photooxidation of canola oil. J. Food Sci. 2009;74:C481–C486. doi: 10.1111/j.1750-3841.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65:1270–1283. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Park JW, Kim JY, Kim MJ, Lee JH. Evaluation of oxygen limitation on lipid oxidation and moisture content in corn oil at elevated temperature. J. Am. Oil Chem. Soc. 2014;91:439–444. doi: 10.1007/s11746-013-2385-y. [DOI] [Google Scholar]
- Schwarz K, Huang SW, German JB, Tiersch B, Hartmann J, Frankel EN. Activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils. J. Agric. Food Chem. 2000;48(10):4874–4882. doi: 10.1021/jf991289a. [DOI] [PubMed] [Google Scholar]
- Simon P, Kolman L, Niklova I, Schmidt S. Analysis of the induction period of oxidation of edible oils by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2000;77:639–642. doi: 10.1007/s11746-000-0103-8. [DOI] [Google Scholar]
- Sun X, Bandara N. Applications of reverse micelles technique in food science: A comprehensive review. Trend Food Sci. Tech. 2019;91:106–115. doi: 10.1016/j.tifs.2019.07.001. [DOI] [Google Scholar]
- Velasco J, Andersen ML, Skibsted LH. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 85(4): 623-632 (2004)
- Yi BR, Kim MJ, Lee SY, Lee JH. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017;26(1):79–87. doi: 10.1007/s10068-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]