Abstract
Oligodendrocyte (OL) and myelin development are crucial for network integration and are associated with higher brain functions. Accumulating evidence has demonstrated structural and functional impairment of OLs and myelin in serious mental illnesses. However, whether these deficits contribute to the brain dysfunction or pathogenesis of such diseases still lacks direct evidence. In this study, we conditionally deleted Olig2 in oligodendroglial lineage cells (Olig2 cKO) and screened the behavioral changes in adult mice. We found that Olig2 ablation impaired myelin development, which further resulted in severe hypomyelination in the anterior cingulate cortex. Strikingly, Olig2 cKO mice exhibited an anxious phenotype, aberrant responses to stress, and cognitive deficits. Moreover, Olig2 cKO mice showed increased vulnerability to social avoidance under the mild stress of social isolation. Together, these results indicate that developmental deficits in OL and myelin lead to cognitive impairment and increase the risk of phenotypes reminiscent of mental illnesses.
Keywords: Oligodendrocyte, Olig2, Hypomyelination, Cognition, Social withdrawal
Introduction
The oligodendrocytes (OLs) and myelin that underly long-rang connectivity are crucial for complex forms of network integration and many aspects of higher brain function, such as motor-skill learning and the oscillation frequency and synchrony of neuronal activity [1, 2]. OL maturation and myelination are dynamic processes during developmental and early adulthood stages, notably in the cerebral cortex [3]. Notably, the critical period for OL and myelin development coincides with the peak onset of psychiatric disorders, which is concentrated in a very narrow age range from adolescence to young adulthood [4, 5]. In the adolescent brain, the structure and function of neural circuits, including the number and structure of synapses, the availability of neurotransmitters, and the expression of neurotransmitter receptors, are highly dynamic [4]. Furthermore, the function of neural circuits, which are crucial for adolescent emotions and behaviors, is strongly affected by the interactions between neurons and OLs [6, 7].
Accumulating evidence has demonstrated structural and functional impairment of myelin and OL in serious mental illnesses [8]. In schizophrenia, even before disease onset, reduced myelin integrity occurs in frontal areas and this advances in further stages of the disorder to more caudal and posterior regions [9, 10]. A recent postmortem study identified a decrease in the total number of OLs, but not progenitor cells, in the prefrontal cortex (PFC) of the schizophrenic brain [11]. Microarray analysis of dysregulated OL and myelin genes further suggests that OL differentiation defects are involved in the pathogenesis of schizophrenia [12]. In addition, patients with severe OL and myelin abnormalities, such as the leukodystrophies and multiple sclerosis, also show grave neurological symptoms reminiscent of psychiatric disorders [13, 14]. These findings suggest that OL/myelin developmental deficits are involved in the early pathogenesis of psychiatric disorders.
Notably, cognitive impairment is one of the pivotal common features of major psychiatric disorders [15]. Some evolutionary findings support the hypothesis that OL and myelin development is positively associated with cognition [5]. Multiple clinical studies have further shown that the number of OLs and myelin integrity are correlated with cognition in healthy individuals [16], as well as cognitive dysfunction in those with schizophrenia [17, 18]. Furthermore, our previous study showed that developmental deficits in OL and myelin cause aberrant synaptic transmission of pyramidal neurons in cortex [19], which could be tightly related to cognitive dysfunction in schizophrenia [20, 21]. However, whether and how these developmental deficiencies lead to cognitive deficits and increase the risk of psychiatric disorders still lack direct evidence. Therefore, we set out to determine the effect of OL and myelin deficits on cognitive function by using our previously reported mouse model with specific deletion of Olig2 in oligodendroglial lineage cells (CNPase-Cre).
Materials and Methods
Animals
Olig2-flox mice and CNP-Cre mice were as previously described [22, 23]. Olig2 loxP/loxP mice were crossed with CNP-Cre +/− mice (kindly provided by Dr. Klaus-Armin Nave, Max Planck Institute of Experimental Medicine, Göttingen, Germany) to specifically delete Olig2 in oligodendroglial lineage cells. All animal experiments were performed according to a protocol approved by the Third Military Medical University Institutional Animal Care and Use Committee.
Immunostaining
Immunostaining was performed as previously described [24]. Briefly, for immunofluorescence staining, free-floating sections were blocked with 5% BSA for 1 h, and then incubated with mouse anti-MBP (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C, followed by Alexa fluor 488-conjugated anti-mouse secondary antibody (1:2000; Invitrogen) for 1.5 h.
Western Blotting
Samples containing 50 μg of total protein were denatured in gel-loading buffer and separated on 10% SDS-PAGE gel. Protein samples were transferred to PVDF membranes and visualized by chemiluminescence (ECL plus, GE Healthcare). β-actin was used as a loading control. Quantification of band intensity was analyzed using Image-Pro Plus software 5.0 (Media Cybernetics, Silver Spring, MD). The following primary antibodies were used: mouse anti-MBP (1:1000) and mouse anti-β-actin (1:2000; Santa Cruz Biotechnology).
RNA Extraction and Analysis
RNA extraction, reverse-transcription and quantitative RT-PCR (RT-qPTR) were carried out as previously described [25]. The primers used were: MBP: forward 5′-ACACACGAGAACTACCCATTATGG-3′, reverse 5′-AGAAATGGACTACTGGGTTTTCATCT-3′; PLP: forward 5′-CCCACCCCTATCCGCTAGTT-3′, reverse 5′-CAGGAAAAAAAGCACCATTGTG-3′; MAG: forward 5′-GACTAAGCCCTAGCTCAATCAC-3′, reverse 5′-CCCTCGAGAAGCTGAAATCAT-3′; GAPDH: forward 5′-ACCCAGAAGACTGTGGATGG-3′, reverse 5′-CACATTGGGGGTAGGAACAC-3′.
Electron Microscopy
Electron microscopy was performed as previously described [24]. Briefly, the anterior cingulate cortex (ACC) was removed rapidly and fixed in fresh fixative overnight at 4°C. Tissue cubes were rinsed in PBS, postfixed in 1% OsO4 in PBS for 2 h, counterstained with uranyl acetate, dehydrated in a graded ethanol series, infiltrated with propylene oxide, and embedded in Epon. Ultrathin sections were cut on an ultramicrotome (LKB-V, LKB Produkter AB, Bromma, Sweden) and were viewed in a transmission electron microscope (HT7700, Hitachi, Japan). The g-ratios of myelinated fibers were calculated as the ratio of the diameter of the axon to the diameter of the axon with the myelin sheath using Image-Pro Plus software. Measurements were made on electron micrographs from three pairs of mice in all cases.
Behavioral Tests
Mice were housed in a controlled environment (25°C) with free access to food and water and maintained on a 12 h/12 h light/dark cycle. In the social isolation experiments, mice were singly housed for 3 to 8 weeks; otherwise, they were group housed (3–4 animals per cage). After each experiment, all apparatus was wiped clean with 70% ethanol to remove traces of the previous assay. In all the behavioral experiments, investigators were blinded to Olig2 cKO or wild-type (WT) mice.
Open field tests were performed in an open-field apparatus (Biowill, Shanghai, China) as previously described [26]. Briefly, mice were placed in the center of the open-field box (50 cm × 50 cm × 50 cm), then their activity was recorded for 10 min. The total and center-area distances traveled were measured and the time spent in the central area was recorded.
The forced swim test was used to assess the response to an acute stress. Mice were forced to swim in a glass cylinder (diameter 12 cm, height 24 cm) for 6 min. The first 2 min were regarded as adaptive training. The activity and total immobility times were recorded during the following 4 min.
The prepulse inhibition (PPI) test was conducted in a foam-lined (sound-damping) isolation chamber (Med Associates, Startle Reflex System ENV-022s). The chamber was equipped with an acoustic stimulator (ANL-925) and a platform with a transducer amplifier (PHM-255A and PHM-250B). The mouse was confined to the holder for 10 min under background noise (65 dB), then acclimatized to a startle pulse (120 dB, 40 ms) 5 times. PPI test sessions consisted of 30 trials: pulse-alone trials (120 dB, 40 ms), prepulse + pulse trials, and no-stimulus trials. Prepulse + pulse trials consisted of a prepulse of noise (75 dB, 20 ms) followed 100 ms after prepulse onset by a startle pulse (120 dB, 40 ms). Each kind of trial was given 10 times pseudorandomly with an interval of 10 s to 30 s. The percentage PPI induced by prepulse intensity was calculated as [1 − (startle amplitude on prepulse trial)/(startle amplitude on pulse alone)] × 100%. The startle amplitude on pulse-alone trials was recorded as an acoustic startle response.
The novel object recognition test (NORT) was conducted in an open-field apparatus (50 cm × 50 cm × 50 cm). In habituation training, the animal was exposed to the experimental apparatus in the absence of objects for 10 min per day for 2 days. The training session was conducted 24 h after the last habituation training. During the training session, mice were placed in the experimental apparatus in the presence of two identical objects and allowed to explore for 5 min. After a retention interval of 2 h, the mice were again placed in the apparatus; however, one of the objects was replaced with a novel one. Mice were allowed to explore for 5 min. The objects chosen for this experiment were a plastic cylinder and a plastic rectangular block of the same height. The time spent exploring each object was recorded. The NORT discrimination index was calculated as: time exploring novel object/(time exploring familiar object + time exploring novel object) × 100%.
The social interaction test was conducted as previously described with a slight modification [27]. Two identical plastic cylinders (each 8 cm in diameter, 12 cm in height) were placed in opposite corners of a box (50 cm × 50 cm × 50 cm). Each cylinder was perforated with multiple holes (0.5 cm diameter) to allow air exchange between the interior and exterior of the cylinder. The subject mouse was first placed at the center of the box. After a 10-min adaptation period in which the subject was free to explore each cylinder, a stranger mouse was placed in one of the cylinders. Containing the stranger mouse in a cylinder ensured that all social approach was initiated by the subject mouse without direct physical contact. The number of contacts and time spent by the subject mouse in approaching and exploring each cylinder was measured for 5 min. The social index was calculated as: time exploring stranger mouse cylinder/(time exploring stranger mouse cylinder + time exploring empty cylinder) × 100%.
Statistics
For statistical analyses we used GraphPad Prism 5 software. For between-group comparisons, we used the independent-samples t-test, and the Welch correction was applied when the variance was unequal. For the effect of genotype and social isolation on mouse sociability, two-way ANOVA and the Bonferroni post hoc test were used. We considered results to be significant at P < 0.05.
Results
Conditional Knockout of Olig2 in Oligodendroglia Leads to Myelin Deficits During Adolescence
The transcription factor Olig2 is crucial for OL development [28]. In a previous study, we demonstrated that specifically knocking out olig2 in myelinating OLs leads to robust impairment of myelination at the juvenile stage [24]. OL differentiation is a key process before myelin sheath formation [29]. To further test the effect of Olig2 loss on postnatal myelin development during adolescence, we stained for myelin basic protein (MBP), which is crucial for myelin membrane compaction and essential in initiating and driving the axonal wrapping process [30]. The myelin deficit was preferentially found in the cerebral cortex of Olig2 cKO mice through adolescence into young adulthood (P56) (Fig. 1A); MBP protein was markedly lower in the ACC (100.0% ± 18.9% in WT versus 7.1% ± 3.2% in Olig2 cKO, P = 0.001) and the corpus callosum (100.0% ± 17.3% in WT vs 52.0% ± 9.5% in Olig2 cKO, P = 0.032) in Olig2 cKO mice (Fig. 1B). Besides, the transcripts of myelin-related genes were robustly lower in the ACC of Olig2 cKO mice [MBP: 100.0% ± 8.6% in WT vs 8.5% ± 1.8% in Olig2 cKO, P < 0.001; PLP (proteolipid protein): 100.0% ± 16.4% in WT vs 15.2% ± 3.6% in Olig2 cKO, P = 0.007; and MAG (myelin-associated glycoprotein): 100.0% ± 12.4% in WT vs 21.8% ± 6.0% in Olig2 cKO, P < 0.001] (Fig. 1C). This further suggested that myelin gene transcriptional deficits could be responsible for the impairment of myelin development.
Fig. 1.
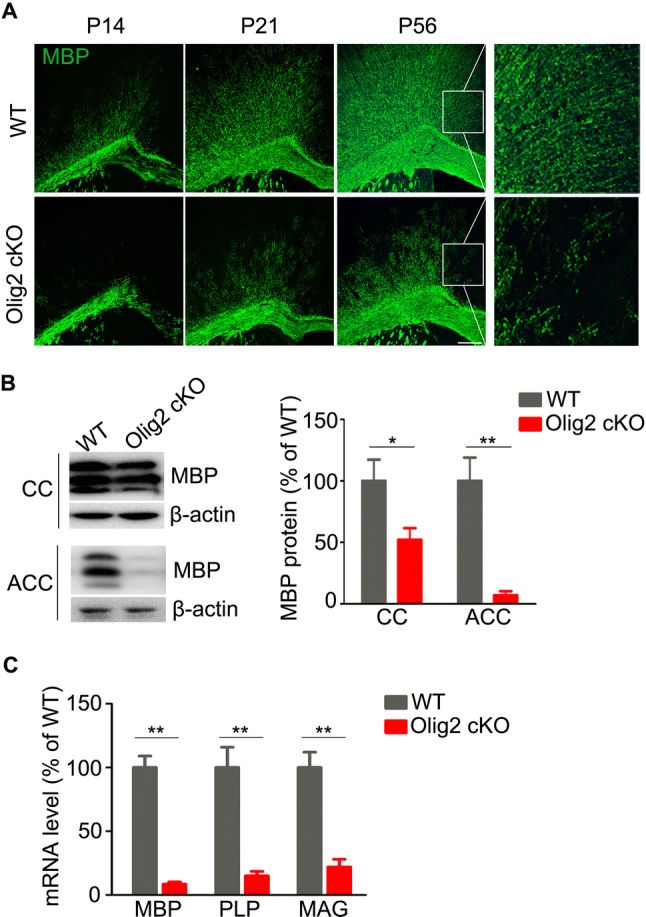
Impaired myelin development in cortex of Olig2 cKO mice. A Representative images of immunostaining for MBP showing myelin tracts in the cortex of WT and Olig2 cKO mice at P14, P21, and P56 (white squares enclose the ACC; scale bar, 200 μm). B Immunoblots and quantification of MBP in the ACC and corpus callosum of wild-type (WT) and Olig2 cKO mice (n = 5 per group). C Quantification of mRNA transcripts of MBP, PLP, and MAG in the ACC of WT and Olig2 cKO mice (n = 5 per group). Data are expressed as the mean ± SEM; *P < 0.05, **P < 0.01.
To access more details of myelin structure in the ACC, we analyzed the ultra-structural organization of myelin sheaths using a transmission electron microscope. We found that the density of myelinated axons was remarkably lower in the ACC of Olig2 cKO mice than in WT littermates (Fig. 2A, B; 100.0% ± 15.7% in WT vs 5.9% ± 2.7% in Olig2 cKO, P = 0.004). Furthermore, the remaining myelin sheaths in the ACC of Olig2 cKO mice were also thinner than those in WT controls (Fig. 2C; P < 0.001). These results clearly showed that loss of Olig2 caused myelin deficiency in the ACC of young adult mice.
Fig. 2.
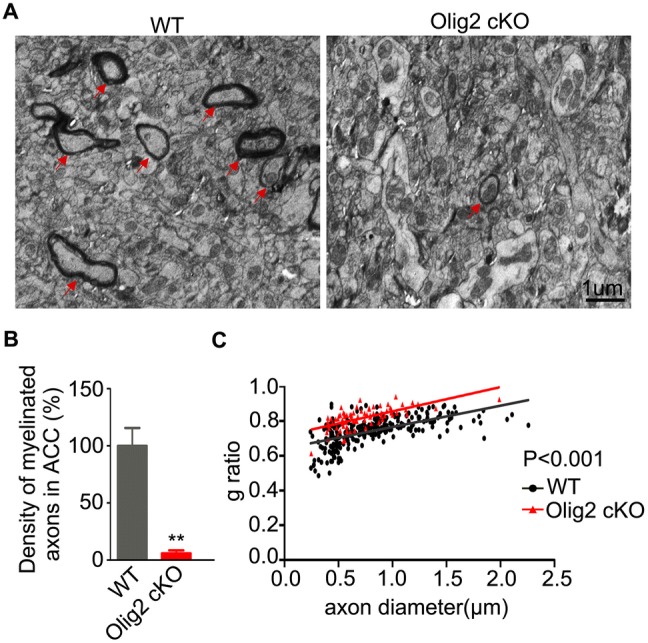
Analyses of myelin ultrastructure in the ACC of Olig2 KO and WT mice at P56. A Electron micrographs of myelin sheaths in the ACC from WT and Olig2 cKO mice (arrows indicate myelinated axons; scale bar, 1 μm.). B Quantification of the density of myelinated axons in ACC (n = 3 animals per group; > 17 000 μm2 per animal were randomly analyzed). Data are expressed as the mean ± SEM. C Scatter-plot of g ratio values of myelinated axons in WT (n = 253) and Olig2 cKO (n = 79) mice. **P < 0.01.
Anxiety and Altered Stress Response in Young Adult Olig2 cKO Mice
We next tested whether a de novo myelin deficit could cause maladaptive behaviors in young adult mice. First, we chose the open field test, a non-conditioned procedure commonly used for assessing locomotor activity in rodents [31]. In this test, Olig2 cKO mice showed normal locomotor activity by traveling a total distance during a 10-min test similar to WT littermates (Fig. 3A; 65.0 ± 3.6 m in WT vs 59.8 ± 3.4 m in Olig2 cKO, P = 0.296). Similar to juveniles as previously reported [24], young adult Olig2 cKO mice showed a lower ratio of travel distance within the central area to the total travel distance (Fig. 3B; 0.16 ± 0.01 in WT vs 0.1 ± 0.01 in Olig2 cKO, P = 0.001) and spent less time in the central area than WT controls (Fig. 3C; 69.3 ± 5.3 s in WT vs 49.2 ± 6.5 s in Olig2 cKO, P = 0.025). These results suggest that deleting Olig2 in oligodendroglia leads to anxiety-like behaviors at the young adult stage.
Fig. 3.
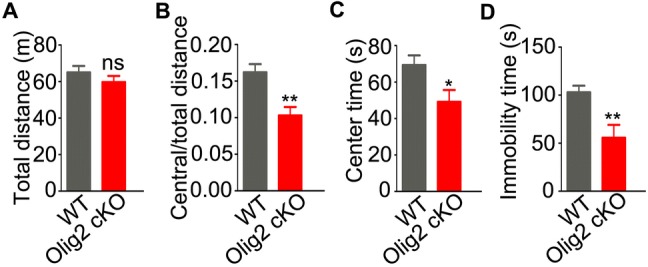
Anxiety-like behavior and stress response in young adult Olig2 cKO and WT mice. A–C Locomotor activity of WT (n = 12) and Olig2 cKO (n = 12) mice in the open field test: total distance (A), ratio of central to total distance (B), and center time (C). D Immobility time in the forced swim test in WT (n = 15) and Olig2 cKO (n = 11) mice. Data are expressed as the mean ± SEM; *P < 0.05; **P < 0.01; ns, no significant difference.
Stress sensitization may be critical in the development or relapse of schizophrenia [32]. The forced swimming test is a well-established paradigm to assess the stress-coping strategy in rodents [33]. In this test, young adult Olig2 cKO mice spent less time immobile than WT littermates (Fig. 3D; 102.9 ± 6.8 s in WT vs 55.6 ± 13.4 s in Olig2 cKO, P = 0.002), suggesting that deleting Olig2 in oligodendroglia leads to an aberrant response to acute stress in the young adult.
Behaviors of Young Adult Olig2 cKO Mice are Analogous to Cognitive Deficits
Cognitive impairment in schizophrenia includes problems in the speed of information processing, attention, learning, and memory [34]. PPI is the most common method to quantify information-processing deficits in schizophrenia with predictive and construct validity [35]. In the present study, we used the PPI test to directly assess the influence of hypomyelination on attention and information processing. First, no significant difference but only a decreasing trend was found in the acoustic startle response between WT and Olig2 cKO mice (Fig. 4A; 358.0 ± 48.9 in WT vs 248.4 ± 45.4 in Olig2 cKO, P = 0.112). Moreover, to our surprise, Olig2 cKO mice had lower PPI values than WT littermates (Fig. 4B; 44.0% ± 3.8% in WT vs 28.8% ± 5.1% in Olig2 cKO, P = 0.032), which demonstrated information-processing deficits in young adult Olig2 cKO mice.
Fig. 4.
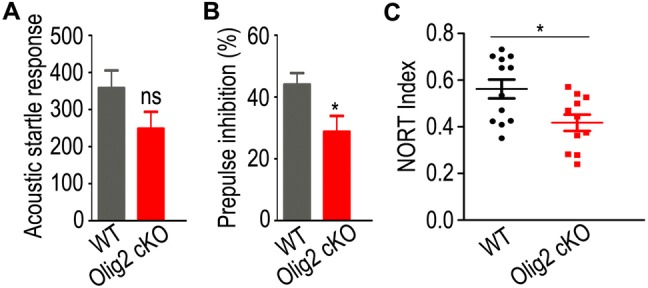
Analyses of cognitive behaviors in young adult Olig2 cKO and WT mice. A, B PPI test in young adult (P56) WT (n = 13) and Olig2 KO (n = 17) mice. A Acoustic startle response; B PPI of acoustic startle response. C Novel object recognition test in WT (n = 12) and Olig2 KO (n = 11) mice. Data are expressed as the mean ± SEM. *P < 0.05; ns, no significant difference.
Besides, the NORT is a relatively low-stress, efficient screening method for recognition memory in rodents, and is appropriate for the detection of neuropsychological changes following genetic manipulations [36]. In this test, Olig2 cKO mice had a lower novel object recognition index than WT littermates (Fig. 4C; 0.56 ± 0.04 in WT vs 0.42 ± 0.03 in Olig2 cKO, P = 0.014), suggesting a non-spatial memory deficit in the Olig2 cKO mice. Thus, these results demonstrated schizophrenia-like cognitive deficits in young adult Olig2 cKO mice.
Loss of Olig2 in Oligodendroglial Cells Increases the Susceptibility to Social Withdrawal after Adolescent Isolation
Recent clinical studies have clearly demonstrated social cognitive deficits in patients with schizophrenia [37]. The social interaction test is widely used to analyze sociability in mice [38]. In the present study, young adult Olig2 cKO mice had numbers of social contacts (Fig. 5A; 12.9 ± 1.3 in WT vs 13.6 ± 1.3 in Olig2 cKO, P = 0.719), durations of approaching stranger mice (Fig. 5B; 82.1 ± 6.2 s in WT vs 86.6 ± 6.9 s in Olig2 cKO, P = 0.635), and durations of contact with stranger mice (Fig. 5C; 65.1 ± 6.3 s in WT vs 86.5 ± 10.4 s in Olig2 cKO, P = 0.077) similar to WT littermates. These results suggest intact sociability in young adult Olig2 cKO mice.
Fig. 5.
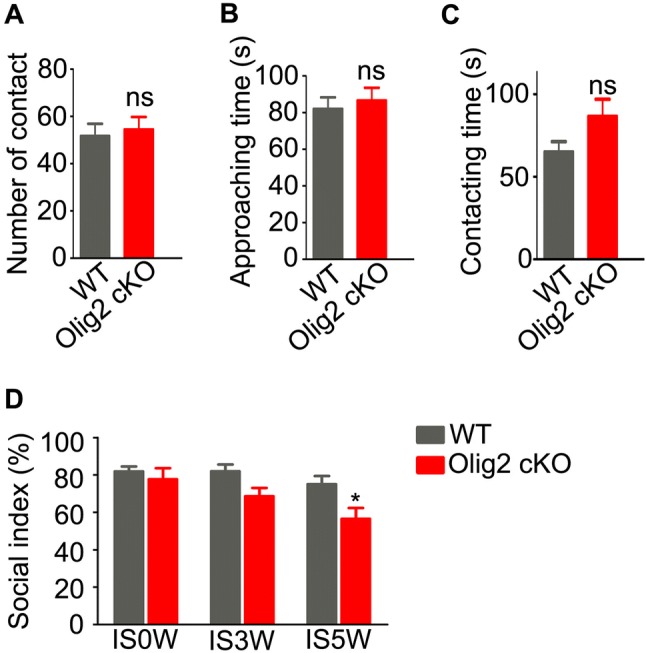
Sociability of young adult Olig2 cKO and WT mice in social interaction test. A Number of contacts; B duration of approaches to stranger mouse; C duration of contact in WT and Olig2 cKO mice. D Sociability of Olig2 cKO mice (n = 6–8) and WT littermates (n = 10–12) after 0, 3, and 5 weeks of social isolation. There were significant effects of genotype (F = 10.91, P = 0.002) and isolation duration (F = 5.16, P = 0.010), but not genotype–isolation interaction (F = 1.31, P = 0.280). Data are expressed as the mean ± SEM. ns, no significant difference. *P < 0.05 vs WT mice.
Social deprivation, which can induce mild neurocognitive impairment, impulsivity, and attentional and social deficits [39], is regarded as an environmental risk factor for schizophrenia. In the present study, social isolation was applied to WT and Olig2 cKO mice from P35 to P70 to test whether OL and myelin deficits affect vulnerability to environmental stress. To our surprise, both the genetic manipulation of Olig2 (F = 10.91, P = 0.002) and the duration of isolation (F = 5.16, P = 0.010) had significant effects on sociability (Fig. 5D), while no significant interaction of these two factors was found (F = 1.31, P = 0.280). Interestingly, Olig2 cKO mice were significantly more susceptible to social withdrawal than WT littermates after 5 weeks of isolation (Fig. 5D). These results suggest that ablating Olig2 in oligodendroglial cells may increase the risk of social withdrawal when under environmental stress during adolescence.
Discussion
In this study, we used a mouse model with de novo OL and myelin deficits by specific knockout of Olig2 in oligodendroglial cells. We examined mouse behaviors, especially cognitive-related phenotypes in young adults. Similar to our previously reported phenotypes of juvenile mice, young adult Olig2 cKO mice showed anxiety-like behaviors. Moreover, we clearly demonstrated cognitive deficits in Olig2 cKO mice. Strikingly, even though loss of Olig2 in OLs did not impair sociability, it significantly increased the vulnerability to social withdrawal under the mild stress of social isolation. These results demonstrated that developmental deficits in OL and myelin led to cognitive impairments and increased vulnerability to social withdrawal, providing new evidence that OL and myelin deficiency may be involved in brain dysfunction of psychiatric disorders.
In recent years, increasing numbers of risk genes have been identified by genome-wide association studies and rare mutation findings [40, 41]. Some risk genes, such as Olig2, have been recognized to play important roles in OL development and myelination [42]. Notably, two schizophrenia-related single-nucleotide polymorphisms in the Olig2 gene have been associated with the downregulation of Olig2 in the PFC of schizophrenic patients [43, 44], implying that loss-of-function of Olig2 increases the risk of psychiatric disorders by interfering with oligodendroglial development. In this study, we used a mouse model in which conditionally deleting Olig2 in oligodendroglial cells mimicked the genetic deficiency of Olig2. Following our previous study [24], we further confirmed that impaired myelin development caused by Olig2 deletion in oligodendroglial cells lasts throughout adolescence (Fig. 1), and this results in severe hypomyelination in the ACC of young adult mice (Fig. 2). Our finding is consistent with previous studies showing a high capacity of myelination in PFC to be regulated by neuronal activity and social interactions [45–47]. It is known that myelin plasticity, which is produced by mature OLs, occurs from early childhood to adulthood [48]. Mice at P56 may be roughly equivalent to human beings 16 years of age [49], which has been recognized as a key stage of transition from adolescence into young adulthood. Therefore, Olig2 cKO mice provide an ideal model in which to address the impact of de novo OL and myelin developmental deficits on brain function.
In our previous study, juvenile Olig2 cKO mice exhibited an anxious phenotype and deficits in behavioral inhibition [24]. Unfortunately, cognitive function was not addressed in the study, as the juvenile age (P21) is not suitable for the behavioral training process and cognitive testing in mice. In the present study, we found that young adult Olig2 cKO mice showed an anxious phenotype and an aberrant response to acute stress (Fig. 3), which further extended our understanding of the effect of OL and myelin deficits on adolescent emotions and actions. More importantly, young adult Olig2 cKO mice exhibited cognitive dysfunctions, such as deficits in information processing and non-spatial memory (Fig. 4), both of which are core features of psychiatric disorders [34]. To our knowledge, this is the first study to uncover the influence of de novo developmental deficits in OL and myelin on higher brain function from the viewpoint of neural development, even though altered myelin structure caused by genetic alterations and chemical toxicants has already been shown to induce brain dysfunction in adults and at older ages [50, 51]. These results may, at least in part, explain why environmental risk factors such as oxidative stress, which suppress OL and myelin development, may increase the risk of psychiatric diseases [52].
Moreover, a recent animal study showed that early social isolation during adolescence results in behavioral and cognitive dysfunctions which are correlated with myelin alterations in the PFC [46, 53, 54]. Specifically, the influence of social isolation on mouse behaviors occurs at a key period ranging from P21 to P35 [46]. In the present study, we isolated WT and Olig2 cKO mice from P35 to P70, beyond the previously-reported key period. Importantly, Olig2 cKO mice were more vulnerable to social withdrawal under the mild stress of social isolation during adolescence (Fig. 5). Together with other studies which demonstrated that social isolation significantly enhances the disruption of PPI in mice [55], these findings support the notion that genetic alterations underlying OL and myelin deficiency increase the vulnerability to psychiatric disorders.
Notably, the ablation of Olig2 in OLs was under the control of the Cnp promoter, which is not brain-region specific. As shown in Fig. 1, the ACC was not the only region with hypomyelination, so abnormal behaviors and cognitive deficits could also result from OL and myelin deficits in other brain regions. Future studies are needed to explore whether and how impaired OL and myelin development in specific regions, such as the PFC, affects brain function and contributes to the white matter etiology in psychiatric disorders. Moreover, a recent study showed that the OL myelination process is highly plastic, and this could finely remold and regulate circuit function according to neuron functional demand [7]. Since myelin plasticity is consistently required for higher brain functions in adults [56], we assumed that the hypomyelination caused by Olig2 ablation would also lead to abnormal behaviors in the adult. Besides, developmental deficits in OL and myelin cause a loss of synapses and aberrant synaptic function in adults [19], further providing several possible mechanisms by which OL and myelin deficits lead to behavioral consequences.
In conclusion, even though further investigations are needed to address the mechanisms by which developmental OL and myelin deficits affect the neural circuits responsible for the behavioral abnormalities, our results provide direct evidence for a role of impaired OL development and myelination in brain functions from the viewpoint of neural development, as well as vulnerability to social withdrawal. Our results further strengthen the hypothesis that OL and myelin deficits are involved in the pathogenesis of psychiatric disorders rather than simply accompaniments to pathological abnormalities.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31671117).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2014;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 4.Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, et al. Mental health. Adolescent mental health–opportunity and obligation. Science 2014, 346: 547–549. [DOI] [PMC free article] [PubMed]
- 5.Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Hoz L, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays. 2015;37:60–69. doi: 10.1002/bies.201400127. [DOI] [PubMed] [Google Scholar]
- 8.Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, et al. A combined DTI and structural MRI study in medicated-naive chronic schizophrenia. Magn Reson Imaging. 2014;32:1–8. doi: 10.1016/j.mri.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 11.Mauney SA, Pietersen CY, Sonntag KC, Woo TW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res. 2015;169:374–380. doi: 10.1016/j.schres.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radu A, Hristescu G, Katsel P, Haroutunian V, Davis KL. Microarray database mining and cell differentiation defects in schizophrenia. Adv Exp Med Biol. 2011;696:67–74. doi: 10.1007/978-1-4419-7046-6_7. [DOI] [PubMed] [Google Scholar]
- 13.Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol 1992, 49: 401–406. [DOI] [PubMed]
- 14.Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. The link between multiple sclerosis and depression. Nat Rev Neurol. 2014;10:507–517. doi: 10.1038/nrneurol.2014.139. [DOI] [PubMed] [Google Scholar]
- 15.Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 16.Muetzel RL, Mous SE, van der Ende J, Blanken LM, van der Lugt A, Jaddoe VW, et al. White matter integrity and cognitive performance in school-age children: A population-based neuroimaging study. Neuroimage. 2015;119:119–128. doi: 10.1016/j.neuroimage.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 18.Falkai P, Steiner J, Malchow B, Shariati J, Knaus A, Bernstein HG, et al. Oligodendrocyte and interneuron density in hippocampal subfields in schizophrenia and association of oligodendrocyte number with cognitive deficits. Front Cell Neurosci. 2016;10:78. doi: 10.3389/fncel.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Yang YJ, Yang N, Chen XJ, Huang NX, Zhang J, et al. Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron. 2018;99(689–701):e685. doi: 10.1016/j.neuron.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 23.Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, et al. A critical role for dorsal progenitors in cortical myelination. J Neurosci. 2006;26:1275–1280. doi: 10.1523/JNEUROSCI.4717-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Zhang W, Li T, Guo Y, Tian Y, Wang F, et al. Impairment of oligodendroglia maturation leads to aberrantly increased cortical glutamate and anxiety-like behaviors in juvenile mice. Front Cell Neurosci. 2015;9:467. doi: 10.3389/fncel.2015.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Ku L, Mei R, Liu G, Xu C, Wen Z, et al. Novel schizophrenia risk factor pathways regulate FEZ1 to advance oligodendroglia development. Transl Psychiatry. 2017;7:1293. doi: 10.1038/s41398-017-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu P, Xu H, Tang X, Xu L, Wang Y, Guo L, et al. Liver X receptor beta is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol Psychiatry. 2014;19:947–957. doi: 10.1038/mp.2014.60. [DOI] [PubMed] [Google Scholar]
- 27.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy RJ, Friedrich VL., Jr Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci. 1996;18:243–254. doi: 10.1159/000111414. [DOI] [PubMed] [Google Scholar]
- 30.Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourin M, Petit-Demouliere B, Dhonnchadha BN, Hascoet M. Animal models of anxiety in mice. Fundam Clin Pharmacol. 2007;21:567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 32.Yuii K, Suzuki M, Kurachi M. Stress sensitization in schizophrenia. Ann N Y Acad Sci. 2007;1113:276–290. doi: 10.1196/annals.1391.013. [DOI] [PubMed] [Google Scholar]
- 33.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 2006, 67 Suppl 9: 3–8; discussion 36–42. [PubMed]
- 35.Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 2017. [DOI] [PMC free article] [PubMed]
- 37.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- 38.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 39.Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 40.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511: 421–427. [DOI] [PMC free article] [PubMed]
- 41.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, et al. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40:925–935. doi: 10.1093/schbul/sbt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, et al. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2006;103:12469–12474. doi: 10.1073/pnas.0603029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitkus SN, Hyde TM, Vakkalanka R, Kolachana B, Weinberger DR, Kleinman JE, et al. Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res. 2008;98:129–138. doi: 10.1016/j.schres.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flurkey K, Currer JM. Pitfalls of animal model systems in ageing research. Best Pract Res Clin Endocrinol Metab. 2004;18:407–421. doi: 10.1016/j.beem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Poggi G, Boretius S, Mobius W, Moschny N, Baudewig J, Ruhwedel T, et al. Cortical network dysfunction caused by a subtle defect of myelination. Glia. 2016;64:2025–2040. doi: 10.1002/glia.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Li C, Mei F, Liu Z, Shen HY, Xiao L. Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit. Neurosci Bull. 2013;29:251–259. doi: 10.1007/s12264-013-1323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maas DA, Valles A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry. 2017;7:e1171. doi: 10.1038/tp.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull. 2015;31:207–219. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai H, Okuda H, Iwabuchi K, Sakurai E, Chen Z, Kato M, et al. Social isolation stress significantly enhanced the disruption of prepulse inhibition in mice repeatedly treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:257–266. doi: 10.1196/annals.1316.032. [DOI] [PubMed] [Google Scholar]
- 56.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, et al. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]


