Abstract
Wheat bran (WB) is an abundant source of fiber, promoting the health for constipation, irritable bowel syndrome, and gastrointestinal disorders. However, the role of superfine-WB in improving the obesity, hyperglycemia, and hyperlipidemia needs to be revealed. The superfine-WB (low and high treatments) was studied on body-weight, blood sugar, serum, and liver lipids in a high-fat rat model for 5-weeks. The high-fat diet substantially increased body-weight, sugar levels, lipids, and malondialdehyde in serum and liver. In contrast, the superfine-WB treatments reduced food and energy intake, postprandial glucose, body-weight, blood and liver cholesterol, triglycerides, malondialdehyde, low-density lipoprotein, and increased the level of high-density lipoprotein. Additionally, when the two different concentrations were compared, the maximum impact was exhibited by the superfine-WB containing high concentration. These results suggest that the superfine-WB significantly improves the hyperglycemia, hyperlipidemia, and possibly also protecting against other acute, recurrent, or chronic diseases.
Keywords: Superfine wheat bran, High-fat rat model, Postprandial glucose, Lipid profile
Introduction
The wheat grain consists of the endosperm, germ, and bran. The outer layers (aleurone and pericarp) of the grain are part of the bran fraction, known as a by-product of the milling process and contain food as well as non-food applications (Onipe et al., 2015). In recent years, people are interested in adding WB to their diet, mainly due to it’s an excellent source of dietary fiber (DF). Although it is undigested in the upper part of the human gastrointestinal tract but contains health-beneficial components such as minerals, antioxidants, vitamins, and phenolic compounds (Leo et al., 2012). According to the solubility in water, the DF is classified as insoluble dietary fiber (IDF) and soluble dietary fiber (SDF). The IDF promotes mechanical peristalsis in the intestine, preventing irritable bowel syndrome, constipation, cardiovascular disease, colorectal cancer, diverticulitis, and other gastrointestinal disorders (Junejo et al., 2018). While the SDF, containing hydrophilic properties, has more significant health-promoting functions (Chawla and Patil, 2010). High-SDF diet may slow down the glucose homeostasis and type 2 diabetes (Sonnenburg and Bäckhed, 2016). Previous studies reported that the presence of SDF in the diet affects the starch and lipid digestibility through fat binding molecules, thereby reducing the glucose and cholesterol levels in the blood (Omole and Ighodaro, 2012). Besides this, Walker and Parkhill (2013) stated that high-SDF diet enhances the satiation hormones that help to decrease body-weight.
Therefore, in order to increase the SDF content in WB, the micro and nanotechnologies can be applied which has good potential in the manufacturing of functional foods. The raw WB subjected to such technology may significantly change its physical structure, surface area, and functional properties that further help to increase high solubility, dispersion, adsorption, chemical reactivity, and fluidity (Zhu et al., 2015). The high fiber solubility and absorbing constituents of superfine-WB powder bind sterol derivatives and cholesterol, thereby preventing the absorption of nutrients in the human body (Chen et al., 2014). When eating high-SDF food from superfine-WB, its resilient structure and physiochemical properties such as particle size, solubility, hydration properties, and viscosity may play a significant role in slowing down the digestibility of nutrients (Capuano, 2017). The rising global rates of overweight and obesity have prompted the need for new prevention and management strategies (Chan and Woo, 2010). Superfine-WB is a kind of low-energy acute fiber, which is likely to increase satiety, reduce food intake, and promote weight management. Although, the studies have proved that plant sources containing high-SDF may reduce the weight gain, hyperglycemia, and hypercholesterolemia (Chang et al., 2017) but there are very few studies that are showing the anti-obesity, anti-hyperglycemic, and anti-hyperlipidemic effects of superfine-WB. Therefore, in order to develop the health-promoting products from superfine-WB, the main objectives of the present study were to investigate the role of different concentrations (low and high-treatments) of the superfine-WB on body weight, serum, and liver lipid profiles in which obesity rat model was induced through a high-fat diet.
Materials and methods
Source of material
The WB (a variety of durum wheat 26) was obtained from Rui Fuxiang Food Co., Ltd. (Bozhou, Anhui Province). It was dried for 48 h at 60 °C. The dried sample was couriered to Qinhuangdao Tai-ji Central Nano Products Co., Ltd and ground into superfine-WB using a High-Energy Nano Impact Grinding (CJM-SY-B, Shandong, China). Throughout the 8 h of the milling process (Zhang et al., 2016), the temperature was kept below 4 °C using a mixture of alcohol, water, and glycol coolant.
WB particle size analysis
The raw and ground superfine-WB were analyzed for their particle size using the Mastersizer 3000 (Malvern Instruments Ltd., Solihull, England) according to the method previously described by Li et al. (2019). The specific amount of WB dispersed into distilled water and sonicated for 2 min. The dispersed sample was added drop-wise into the dispersion unit. When a laser beam detected more than 10% obscuration, the particle size was measured in the range from 0.02 to 2000 µm. The software recorded the data as D (0.1), D (0.5), and D (0.9), in the 10%, 50%, and 90% of particles.
Chemical analysis
The chemical properties of the unprocessed and superfine-WB particles were analyzed in triplicate. The dry matter and ash contents were analyzed according to AOAC (2000). The crude protein was determined by Kjeldahl instrument (VELP UDK159, Milan, Italy) using the nitrogen to the protein conversion factor of 6.25 (AOAC, 2000). The petroleum ether was used to determine crude fat (Li et al., 2015). The total starch was measured by colorimetry method (AOAC, 1998), and the DF was analyzed by an enzymatic gravimetric procedure (AOAC, 1995).
In vivo experimental design
An adult male Sprague–Dawley rat model (n = 28) with an average weight of 100 ± 10 g was obtained from the Animal Breeding and Research Center affiliated with Anhui Medical University (Hefei, China). Animals were placed in specific pathogen-free (SPF) and an environmentally controlled room with 40–60% relative humidity, 22–25 °C temperature, and a 12 h light/dark cycle. The Animal Ethical Committee of the Anhui Agricultural University approved this trial. Four groups of rats were prepared randomly, each group contained seven rats and named as normal chow (NC), high-fat diet (HFD), low treatment (LT), and high treatment (HT). After the acclimatization period of five days, the NC group was fed a chow diet containing 600 g/kg wheat, clover, corn, and broken rice, 330 g/kg soybean, chicken, and fish powder, 30 g/kg sunflower and soybean oil, and 40 g/kg minerals, vitamins, and amino acids. The HFD group was fed a diet based on 788 g/kg normal chow, 100 g/kg egg yolk powder, 100 g/kg pig oil, 10 g/kg cholesterol, and 2 g/kg bile salts, that has been reported by Yan et al. (2009), these two groups regarded as control groups until to the end of the study. However, the remaining two groups, in which LT group was fed 688 g/kg normal chow, 100 g/kg egg yolk powder, 100 g/kg pig oil, 10 g/kg cholesterol, and 2 g/kg bile salts containing 100 g/kg superfine WB. Whereas, the HT group was 588 g/kg normal chow, 100 g/kg egg yolk powder, 100 g/kg pig oil, 10 g/kg cholesterol, and 2 g/kg bile salts containing 200 g/kg superfine WB. All the diets and water were fed ad libitum on w/w basis and feeding diets were prepared commercially by Xietong Biological Engineering Co., Ltd, (Nanjing, China).
Oral glucose tolerance test (OGTT)
During the 5th week of the trial, the OGTT was performed on overnight starved rats using the protocol described by Tara et al. (2016). The sterilized 25% solution of glucose (2 g/kg body-weight) was administered orally. Blood glucose levels were measured using the one-touch ultra mini glucometer from blood drops taken from the tip of the tail vein at different time intervals of 0 (baseline), 15, 30, 60, and 120 min after gavage.
Separation of animal organs
Throughout the trial period, body-weight, food intake, and feces were recorded weekly. The research trial was terminated at the end of the 5th week. The rats were starved for 12 h, but given access to water. Anesthesia was performed before slaughter, and the blood was collected from the retro-orbital vein into the EDTA tube, then immediately centrifuged for 5 min at 3663×g (JW-2018H, Anhui province, China). The organs of the animal body were quickly removed, weighed, rinsed with saline water, and placed into liquid nitrogen. For further analysis, the collected samples were stored at − 80 °C. The weight of the body parts of all the groups was compared to each other.
Serum lipid analysis
Lipid profile was determined by the enzymatic method using commercial kits purchased online from Institute of Jiancheng Bioengineering (Nanjing, China) by using Automatic Analyzer (Spectrum MAX 190, Molecular Devices, USA). The levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-Ch), low density lipoprotein cholesterol (LDL-Ch), serum glucose, and malondialdehyde (MDA). Furthermore, very low density lipoprotein cholesterol (VLDL-Ch), and atherogenic index (AI) were calculated using formulas described by Junejo et al. (2018).
Liver lipids analysis
TC, TG, and MDA levels in liver were analyzed based on the methods mentioned by Folch et al. (1957). Liver tissue (150 mg) was homogenized in 0.025% calcium chloride 5 mL for 3 min. A solution containing 25 mL chloroform: methanol 2:1 (v/v) was mixed into a homogeneous substance and then again homogenized. The 1 min vortexed suspension was left for 1 h to form a layer. The upper layer was discarded, and the lipids containing layer was dried at 55 °C with the evaporation unit and nitrogen. Above serum lipid analysis procedure was used to determine TC, TG, and MDA levels. The content of protein was determined by the Bradford method with bovine serum albumin standard (Bradford, 1976).
Histopathological analysis of liver tissues
The tissues of the liver were taken from the liver right lobe and immersed in a 10% solution of formalin (Han et al., 2015). The aortic root specimens were taken and stained with blood lignin and eosin. The morphology and morphometry of liver tissues were observed under a microscope (Nikon 80i, Japan) at ×200 magnification.
Statistical analysis
The results are mean with standard deviation (SD). The IBM SPSS 21 was used for statistical analysis and one-way analysis of variance was used for multiple comparisons. The values were considered statistically significant at p < 0.05. The data graphs were prepared using GraphPad Prism (version 6.00).
Results and discussion
Physiochemical properties
The particle size data are represented in Table 1. After micronization of WB into superfine, its averaged particle size reduced to 11.63 ± 0.03 µm (p < 0.05). As the particle size decreased, the interaction of particles was observed that sufficiently increased the surface area, and the reactive components were released. The reduction of particle size effectively changed the structural, surficial, and functional properties of bran (Hemery et al., 2011). Moreover, the ratios of IDF and SDF in raw (43.60 ± 0.13, 9.65 ± 0.09) and superfine-WB (33.79 ± 0.19, 17.37 ± 0.20) were decreased to 22.50% and increased to 44.44%, respectively (p < 0.05). Besides this, after the reduction of particle size, no significant changes were noticed in the protein, starch, and ash content. While fat was observed significantly higher in superfine-WB (Table 1). The higher fat and SDF content are probably due to the breakage of cell wall components (Yan et al., 2015). Additionally, previous studies have shown that the SDF can be increased significantly in oats, rice, soybeans, and WB (Lebesi and Tzia, 2012). The quality of raw materials, physiological characteristics, processing technology, and particle size reduction might have played an essential role in improving SDF (Chen et al., 2014). The reason behind the decreased amount of the IDF can be the destruction of covalent and non-covalent bonds in carbohydrate and protein components, thus promoted more soluble molecular fragments (Rashid et al., 2015).
Table 1.
The physiochemical properties of WB analyzed on a dry basis
| Contents | Raw-WB | Superfine-WB |
|---|---|---|
| Dry matter (g/kg) | 96.24 ± 0.08a | 95.58 ± 0.19a |
| Protein (g/kg) | 18.11 ± 0.16a | 17.6 ± 0.09a |
| Starch (g/kg) | 24.58 ± 0.24a | 24.08 ± 0.09a |
| Fats (g/kg) | 2.12 ± 0.02a | 3.73 ± 0.10b |
| Ash (g/kg) | 4.32 ± 0.04a | 4.19 ± 0.02a |
| Insoluble fibre (g/kg) | 43.60 ± 0.13b | 33.79 ± 0.19a |
| Soluble fibre (g/kg) | 9.65 ± 0.09a | 17.37 ± 0.20b |
| Particles size (μm) | 1283.17 ± 1.30b | 11.63 ± 0.07a |
The given values are the average of three replications with ± SD. The values representing the same superscript letter within the row are not significant (p > 0.05)
Oral glucose tolerance test
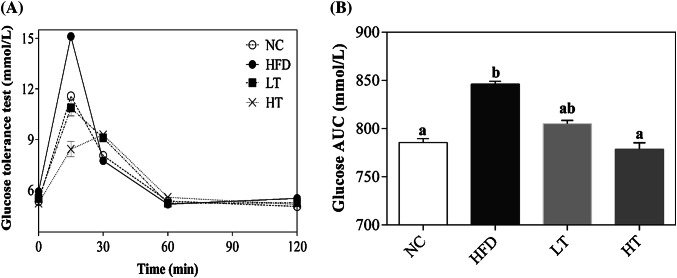
The mean baseline blood glucose was different amongst all groups. The HFD group displayed higher glucose absorption at 15 min peak point, while later, it decreased rapidly. However, compared to the HFD group the glucose absorption decreased in treatment groups, the most significant postprandial glucose effect revealed by the HT group, while no significant difference was recorded by the glucose tolerance test and AUC curve of the LT group (Fig. 1A, B). The glycaemic response was probably improved by physiological properties such as the viscous gel-forming, oil binding, and hydrating properties (Wanders et al., 2013). During the processing of the raw bran into superfine-WB, the intrinsic plant structure was destroyed, that increased the availability of the SDF. Moreover, in mixed foods, the presence of other components, such as the SDF, fat, and protein can reduce the postprandial glycemic response by encapsulating starch granules, the formation of starch-lipid or starch-protein complexes reduce the availability of starch to amylase (Venkatachalam et al., 2009).
Fig. 1.
Glucose tolerance (A) and OGTT-AUC (B) were analysed, data are represented as the mean ± SD (n = 7/group), fed a HFD diet with supplementation ratio of 10% (LT) and 20% (HT) wt:wt superfine-WB. In the OGTT-AUC, the same small letters above the column show that the groups are not significant (p > 0.05)
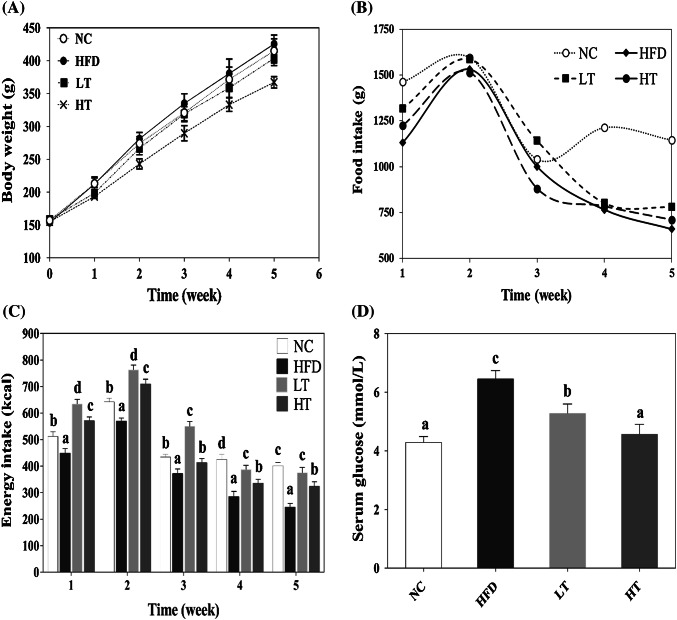
Food intake, energy intake, and body weight regulation
Metabolic syndrome is a cluster of metabolic dysfunction (obesity, hypertension, elevated blood sugar, high-triglycerides ratio, and low-HDL-Ch level) that increases the risk of type 2 diabetes and cardiovascular diseases (Han et al., 2015). The DF supports the human regulation; especially the SDF alters the composition of intestinal microflora to prevent obesity. When all the diets were compared, the average body-weight reduced in treatment groups while food intake remained higher, the HT group showed a significant decline in body-weight (Fig. 2A, B). During the first 2-weeks of the feeding period, the food and energy intake of treatment groups were higher than in the last 3-weeks (Fig. 2C), and treatment groups were noted more active. It is speculated that long-term intake of fiber minimized the food intake and energy levels. The weight loss may have occurred because fibers are not digested in the upper part of the digestive tract and affect the absorption of water. The stomach containing food is swelled that increased the satiation in the small intestine to lose weight (Rebello et al., 2016). The previous studies have shown that SDF may contribute to weight loss by reducing calorie intake and slowing down the digestion and absorption of nutrients in the small intestine (Han et al., 2015). The reduction in food, energy intake, and weight gain are also positively correlated to weight loss in the adipose tissue and liver (Table 2).
Fig. 2.
Body weight (A), food and energy intake (B, C), and serum glucose (D) were examined. Data are the mean with ± SD (n = 7/group), fed a HFD diet with supplementation ratio of 10% (LT), and 20% (HT) wt:wt superfine-WB. The same letters above the bar are indicating the nonsignificance (p > 0.05)
Table 2.
Weight of animal body parts
| Contents | NC | HFD | LT | HT |
|---|---|---|---|---|
| Initial body weight (g) | 156.91 ± 3.85a | 153.91 ± 1.64a | 158.54 ± 3.01a | 155.11 ± 1.06a |
| Final body weight (g) | 415.00 ± 18.44bc | 425.49 ± 13.61bc | 403.67 ± 7.52abc | 386.1 ± 10.47a |
| Body weight gain (g/d) | 7.37 ± 0.48bc | 7.76 ± 0.35c | 7.00 ± 0.21b | 6.05 ± 0.23a |
| Weight of liver (g) | 17.45 ± 1.02a | 20.57 ± 1.75b | 18.64 ± 1.37ab | 17.45 ± 0.82a |
| Liver % of body weight | 3.87 ± 0.22a | 4.84 ± 0.24c | 4.33 ± 0.25b | 4.89 ± 0.12c |
| Heart (g) | 1.46 ± 0.15a | 1.56 ± 0.22a | 1.56 ± 0.19a | 1.53 ± 0.16a |
| Kidney (g) | 2.76 ± 0.12c | 2.63 ± 0.09cb | 2.54 ± 0.18ab | 2.34 ± 0.14a |
| Adipose tissues (g) | 3.82 ± 0.41a | 10.23 ± 2.71b | 5.37 ± 1.47a | 8.33 ± 1.60b |
| Spleen (g) | 0.86 ± 0.06b | 0.90 ± 0.08b | 0.81 ± 0.12a | 0.83 ± 0.09a |
| Total length (cm) | 24.36 ± 0.58a | 24.57 ± 0.56a | 23.86 ± 0.58a | 24.07 ± 0.17a |
The animal groups represented as normal chow (NC), high-fat diet (HFD), low-treatment (LT) based on high-fat diet added with 100 g/kg superfine-WB, and high-treatment (HT) based on high-fat diet added with 200 g/kg superfine-WB were fed for 5-weeks. The data are an average (n = 7) with ± SD; the values with the same superscripted letter within the same row are not significant (p > 0.05)
Lipid profile
The hyperlipidemia is a primary endocrine disease, commonly found in the world population and is an independent risk factor for cardiovascular complications. A high-fat diet can easily transform hypercholesterolemia through adipose tissue and may significantly increase the risk of cardiovascular complications (Manna and Jain, 2015). The high-cholesterol diet and low physical activity are considered major causes of hyperlipidemia. Our results suggest that the low body weight, low blood sugar, and low inflammatory biomarkers are the major factors to control metabolic syndrome. The data analysis showed that with the addition of 10% and 20% concentrations of superfine-WB in the HFD diet, the serum glucose (Fig. 2D) and serum TC (3.75%, 19.03%), TG (10.34%, 23.77%), MDA (4.38%, 23.65%), LDL-Ch (13.45%, 18.07%) were reduced while HDL-Ch (20%, 34.18%) was improved respectively (Table 3). The maximum reducing effect was exhibited by 20% concentrated superfine-WB.
Table 3.
Effects of superfine-WB on lipid profile
| Contents | NC | HFD | LT | HT |
|---|---|---|---|---|
| Serum TC (mmol/L) | 2.34 ± 0.10a | 3.73 ± 0.14c | 3.59 ± 0.07c | 3.02 ± 0.25b |
| Serum TG (mmol/L) | 2.35 ± 0.15a | 3.87 ± 0.08d | 3.47 ± 0.22c | 2.95 ± 0.10b |
| Serum HDL (mmol/L) | 0.64 ± 0.04b | 0.52 ± 0.04a | 0.65 ± 0.03b | 0.79 ± 0.03c |
| Serum LDL (mmol/L) | 2.10 ± 0.13a | 2.38 ± 0.03b | 2.06 ± 0.11a | 1.95 ± 0.03a |
| Serum VLDL (mmol/L) | 0.47 ± 0.03a | 0.77 ± 0.02d | 0.69 ± 0.04c | 0.59 ± 0.02b |
| Atherogenic index (mmol/L) | 2.67 ± 0.29a | 6.12 ± 0.49c | 4.54 ± 0.28b | 2.85 ± 0.31a |
| Serum MDA (nmol/mL) | 5.15 ± 0.43a | 6.85 ± 0.63b | 6.55 ± 0.34b | 5.23 ± 0.79a |
| Liver TC (mmol/L) | 1.83 ± 0.11a | 2.31 ± 0.08b | 2.21 ± 0.11b | 1.95 ± 0.10a |
| Liver TG (mmol/L) | 0.74 ± 0.05a | 1.32 ± 0.08c | 0.78 ± 04ab | 0.84 ± 0.06b |
| Liver MDA (nmol/mg) | 3.17 ± 0.05a | 5.74 ± 0.38d | 4.41 ± 0.08c | 3.63 ± 0.15b |
| Liver protein (mg/mL) | 16.36 ± 0.19c | 13.61 ± 0.31a | 14.16 ± 0.24a | 15.07 ± 0.06b |
The analyzed data are means (n = 7) with ± SD and the values showing same superscripted letters within a row are not significant (p < 0.05)
The particular risk factors were explored using mechanisms through which SDF of superfine-WB played a specific role. Based on the gel formation structure, the viscous fiber affects the absorbability in the small intestine that possibly lowered the blood sugar and blood lipids. Such gels contributed to weight loss by slowing the stomach emptying and increasing the satiety (Ménard et al., 2018). Furthermore, gut microbiota can ferment the SDF and resistant starch, thereby producing short-chain fatty acids, which might have played an essential role in the reduction of TC (Qiu et al., 2013). Our findings revealed that the addition of 10% and 20% of superfine-WB reduced the TC, TG, and LDL-Ch in serum and liver. We suggest these results are attributing to the fibrous structure that binding sterol derivatives and cholesterol to inhibit their absorbance in the body. Moreover, the HDL-Ch may be contributed in the preventing cholesterol transportation, acting as an anti-inflammatory, stimulating the release of nitric oxide synthase, releasing the prostacyclin, and promoting the repair of endothelial tissues (Mohd et al., 2011). In addition, the MDA of HFD group was significantly increased compared to NC group, which may accelerate the lipid peroxidation by interfering with lipid metabolism that generates cellular oxidative stress in fatty liver. The obesity, hyperglycemia, and hyperlipidemia were significantly overcome by the different concentrations of superfine-WB, indicating the quality or quantity of physiochemical properties of the superfine-WB were improved after processing such as the capability of fiber to solubilize, retain, and swell in water. Moreover, the physiochemical and physiological properties of WB are affected by particles size, which influences the colonic fermentation, inflammatory markers, and intestinal microbiota (Suriano et al. 2018).
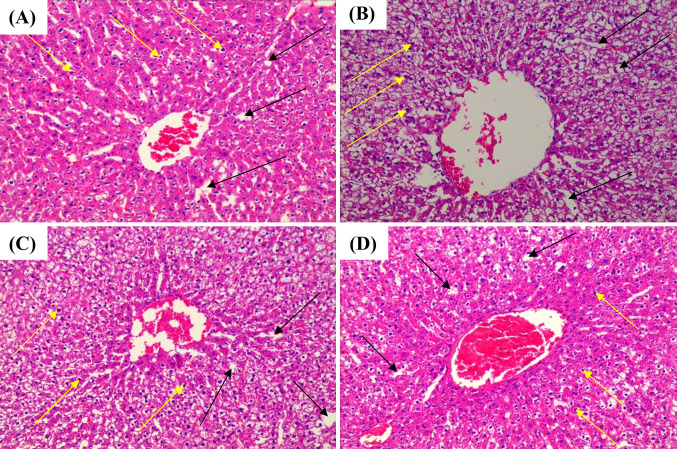
Liver histology
The observations of hematoxylin and eosin staining are shown in Fig. 3. A large number of fat droplets and deformed cells were observed in the liver tissues of the HFD group, showed the microvascular steatosis and fat changes, while the NC group had normal morphological characteristics and no steatosis. However, compared to the HFD group, the histopathological status of the LT and HT groups was improved. Moreover, the high-concentrated HT group elucidated the fine morphological structure, reduced-fat droplets, and hepatocyte degeneration than the low-concentrated LT group. These results support the previous study conducted by Han et al. (2015), who reported that long term intake of SDF from WB lowered the volume and size of adipocytes. It is concluded that high-concentrated superfine-WB exhibited to reduce the obesity, TC, TG, MDA, serum glucose, LDL-Ch, and increase the HDL-Ch in the serum, and liver. It is proposed that physiological properties (such as SDF, viscosity, and hydration properties) of superfine-WB after minimizing particle size contributed to the reduction of serum and liver lipid profiles. On account of these findings, it is confirmed that high-concentrated superfine-WB has the potential to regulate the body-weight, hyperglycemia, hyperlipidemia, and other health-related chronic diseases. Nevertheless, to explore the full range of superfine-WB health benefits, more research is needed related to its proportion of pectin and its molecular features such as molecular weight, methoxylation degree, and acetylation degree.
Fig. 3.
Histological observations of liver tissues were determined by H&E staining at 200×magnification of SD rats. (A) Normal chow group, (B) high-fat/cholesterol diet group, (C) high-fat diet +10% superfine-WB, (D) high-fat diet + 20% superfine-WB. The black and yellow arrows are representing the fat droplets and degeneration
Acknowledgements
We are expressing our gratitude to all the participants who contributed to this work. This research study was conducted with the support of the Anhui Natural Sciences Foundation (No. 11008761). It has also been supported by the Anhui Sciences and Technology Plant Project (No. 1704a07020098) and (No. Ilj20170144).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shahid Ahmed Junejo, Email: shahidfst42@gmail.com.
Huihui Geng, Email: m18326910755_1@163.com.
Songnan Li, Email: 837333606@qq.com.
Ajeet Kumar Kaka, Email: ajeetkumarkaka@gmail.com.
Alam Rashid, Email: rashidahmad00gilgit@gmail.com.
Yibin Zhou, Email: zhouyibin@ahau.edu.cn.
References
- AOAC. Total, soluble, and insoluble dietary fibre in foods. Cereal Foods. 991.43: 7-9 (1995)
- AOAC . Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- AOAC I. AOAC Official method 996.11 (1998)
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017;57:3543–3564. doi: 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- Chan RSM, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int. J. Environ. Res. Public Health. 2010;7:765–783. doi: 10.3390/ijerph7030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Cui X, Guo M, Tian Y, Xu W, Huang K, Zhang Y. Insoluble dietary fiber from pear pomace can prevent high-fat diet induced obesity in rats mainly by improving the structure of gut microbiota. Microbiol. Biotechnol. 2017;27:856–867. doi: 10.4014/jmb.1610.10058. [DOI] [PubMed] [Google Scholar]
- Chawla R, Patil GR. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010;9:178–196. doi: 10.1111/j.1541-4337.2009.00099.x. [DOI] [Google Scholar]
- Chen Y, Ye R, Yin L, Zhang N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014;120:1–8. doi: 10.1016/j.jfoodeng.2013.07.011. [DOI] [Google Scholar]
- Folch J, Lees M, Sloane GS. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Han S, Jiao J, Zhang W, Xu J, Wan Z, Zhang W, Gao X, Qin L. Dietary fiber prevents obesity-related liver lipotoxicity by modulating sterol-regulatory element binding protein pathway in C57BL/6J mice fed a high-fat/cholesterol diet. Sci. Rep. 2015;5:15256. doi: 10.1038/srep15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemery Y, Chaurand M, Holopainen U, Lampi AM, Lehtinen P, Piironen V, Sadoudi A, Rouau X. Potential of dry fractionation of wheat bran for the development of food ingredients, part I: influence of ultra-fine grinding. J. Cereal Sci. 2011;53:1–8. doi: 10.1016/j.jcs.2010.09.005. [DOI] [Google Scholar]
- Junejo SA, Zhang L, Yang L, Wang N, Zhou Y, Xia Y, Wang H. Anti-hyperlipidemic and hepatoprotective properties of wheat bran with different particle sizes. J. Sci. Food Agr. 2018;99:1990–1996. doi: 10.1002/jsfa.9397. [DOI] [PubMed] [Google Scholar]
- Lebesi DM, Tzia C. Use of endoxylanase treated cereal brans for development of dietary fiber enriched cakes. Innov. Food Sci. Emerg. Technol. 2012;13:207–214. doi: 10.1016/j.ifset.2011.08.001. [DOI] [Google Scholar]
- Leo S, Frankie P, Kathryn OS, Jenny W. Wheat bran: its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012;63:1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang B, Tan CP, Li C, Fu X, Huang Q. Octenylsuccinate quinoa starch granule-stabilized pickering emulsion gels: preparation, microstructure and gelling mechanism. Food Hydrocoll. 2019;91:40–47. doi: 10.1016/j.foodhyd.2019.01.001. [DOI] [Google Scholar]
- Li S, Zhou Y, Liu M, Zhang Y, Cao S. Nutrient composition and starch characteristics of Quercus glandulifera Bl. seeds from china. Food Chem. 2015;185:371–376. doi: 10.1016/j.foodchem.2015.03.147. [DOI] [PubMed] [Google Scholar]
- Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard O, Famelart MH, Deglaire A, Gouar YL, Guérin S, Malbert CH, Dupont D. Gastric emptying and dynamic in vitro digestion of drinkable yogurts: effect of viscosity and composition. Nutrients. 2018;10:1308. doi: 10.3390/nu10091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd EN, Abdul Kadir KK, Amom Z, Azlan A. Improving the lipid profile in hypercholesterolemia-induced rabbit by supplementation of germinated brown rice. J. Agric. Food Chem. 2011;59:7985–7991. doi: 10.1021/jf201323x. [DOI] [PubMed] [Google Scholar]
- Omole JO, Ighodaro OM. Comparative studies of the effects of egg yolk, oats, apple, and wheat bran on serum lipid profile of wistar rats. ISRN Nutr. 2012;2013:730479. doi: 10.5402/2013/730479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onipe OO, Jideani AIO, Beswa D. Composition and functionality of wheat bran and its application in some cereal food products. J. Food Sci. Technol. 2015;50:2509–2518. doi: 10.1111/ijfs.12935. [DOI] [Google Scholar]
- Qiu T, Ma X, Ye M, Yuan R, Wu Y. Purification, structure, lipid lowering and liver protecting effects of polysaccharide from Lachnum YM281. Carbohydr. Polym. 2013;98:922–930. doi: 10.1016/j.carbpol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Rashid S, Rakha A, Anjum FM, Ahmed W, Sohail M. Effects of extrusion cooking on the dietary fibre content and water solubility index of wheat bran extrudates. J. Food Sci. Technol. 2015;50:1533–1537. doi: 10.1111/ijfs.12798. [DOI] [Google Scholar]
- Rebello CJ, O’Neil CE, Greenway FL. Dietary fiber and satiety: the effects of oats on satiety. Nutr. Rev. 2016;75:131–147. doi: 10.1093/nutrit/nuv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535:6–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriano F, Neyrinck AM, Verspreet J, Olivares M, Leclercq S, Wiele TVD, Courtin CM, Cani PD, Bindels LB, Delzenne NM. Particle size determines the anti-inflammatory effect of wheat bran in a model of fructose over-consumption: implication of the gut microbiota. J Funct. Foods. 2018;41:155–162. doi: 10.1016/j.jff.2017.12.035. [DOI] [Google Scholar]
- Tara J, Victor H, Kristina Y, Michael AC, Mark WS. Dietary macronutrient composition directs ChREBP isoform expression and glucose metabolism in mice. PLoS One. 2016;11:e0168797. doi: 10.1371/journal.pone.0168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M, Kushnick MR, Zhang G, Hamaker BR. Starch-entrapped biopolymer microspheres as a novel approach to vary blood glucose profiles. J. Am. Coll. Nutr. 2009;28:583–590. doi: 10.1080/07315724.2009.10719790. [DOI] [PubMed] [Google Scholar]
- Walker AW, Parkhill J. Fighting obesity with bacteria. Science. 2013;341:1069–1070. doi: 10.1126/science.1243787. [DOI] [PubMed] [Google Scholar]
- Wanders AJ, Jonathan MC, Joost JGC, Borne VD, Mars M, Schols HA, Feskens EJM, Graaf CD. The effects of bulking, viscous and gel-forming dietary fibres on satiation. Br. J. Nutr. 2013;109:1330–1337. doi: 10.1017/S0007114512003145. [DOI] [PubMed] [Google Scholar]
- Yan H, Shao W, Xiao R, Xu K, Ma Z, Johnstone BH, Du Y. Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model. Exp. Gerontol. 2009;44:434–439. doi: 10.1016/j.exger.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Yan X, Ye R, Chen Y. Blasting extrusion processing: the increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015;180:106–115. doi: 10.1016/j.foodchem.2015.01.127. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiao W, Ji G, Chen X, Han L, Gao C. Effects on physicochemical properties of black tea by mechanical superfine and general grinding. Trans. Chin. Soc. Agric. Eng. 2016;32:295–301. [Google Scholar]
- Zhu F, Du B, Xu B. Superfine grinding improves functional properties and antioxidant capacities of bran dietary fibre from Qingke (hull-less barley) grown in Qinghai-Tibet Plateau, China. J. Cereal Sci. 2015;65:43–47. doi: 10.1016/j.jcs.2015.06.006. [DOI] [Google Scholar]