Abstract
The present work aimed to estimate the possible anti-fatigue effect and potential mechanism of Isochrysis galbana (IG) in mice. The anti-fatigue activity of IG (100, 200, and 400 mg/kg) was elucidated by a weight-loaded forced swimming test, and the potential mechanism was explored by determination of fatigue-related biochemical parameters. The results showed that pretreatment with IG significantly extended the exhaustive swimming time and increased the levels of liver glycogen, muscle glycogen and blood glucose in a dose-dependent manner. Besides, the increased levels of alanine aminotransferase, aspartate aminotransferase, blood lactic acid, lactic dehydrogenase, creatine kinase, and blood urea nitrogen by exhausted swimming, were dramatically attenuated by pretreatment with IG. Furthermore, supplementation with IG significantly enhanced the glutathione peroxidase and superoxide dismutase levels, while attenuated the level of malonaldehyde. Taken together, IG possessed appreciable efficacy to alleviate fatigue, and the mechanism might be associated with favorably modulating the process of energy consumption, metabolism, and attenuating oxidative stress injury.
Keywords: Isochrysis galbana, Anti-fatigue activity, Antioxidant activity, Swimming time, Biochemical indicator
Introduction
Fatigue is thought to be physical and/or mental weariness that negatively affects athletic ability, work achievement, family relation, and social interaction (Mehta and Agnew, 2012). Physical fatigue is commonly defined as lack of ability to maintain voluntary activities, which is considered to be related to physiological decline and usually caused by excess exercise accompanied by degeneration in exercise performance (Tanaka et al., 2008). It has been reported that long-term cumulative physical fatigue can lead to multiple serious fatigue-related syndromes (Fischer et al., 2014). Several attractive theories, including the “exhaustion theory”, “radical theory”, “clogging theory” and some others have been presented to explain the mechanisms of physical fatigue (Zhong et al., 2017). According to these theories, energy exhaustion, radical accumulation and metabolic clogging are well established as the major inducers of fatigue (Zhao et al., 2015). However, few effective pharmacological drugs or therapies are currently available to eliminate fatigue (Gupta et al., 2009). Therefore, it is worthwhile to develop safe and effective anti-fatigue functional foods to enhance exercise endurance, postpone the generation or promote the elimination of fatigue.
Due to the high contents of protein, vitamins, polysaccharides, polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), and photosynthetic pigments (Matos et al., 2017), microalgae became one of the most prospective providers of ingredients and bioactive compounds for new food products to elevate the nutritional and gustatory value of foods and promote human health (Nuno et al., 2013). Isochrysis galbana (IG) is an important species of marine microalgae. It contains rich bioactive materials and is recognized as a rich source of PUFAs, which makes it of significant medicinal value, and potentially promising for the food industry (Bandarra et al., 2003). Researches have indicated that PUFAs play a beneficial role in endurance exercise (Shei et al., 2014), and oxidative stress (Devos et al., 2006). Although the bioactive properties of IG biomass and/or its extract has been demonstrated (Raposo et al., 2013), little attention has been devoted to exploring the potential pharmacological activities of IG. Therefore, the present study was conducted in pioneering endeavor to investigate the possible anti-fatigue activity and mechanism of IG as a potential nutraceutical.
The anti-fatigue effect of IG and spirulina (Cyanophyceae) was comparatively investigated from the prospect of energy consumption, metabolite accumulation, cell damage and intracorporal oxidative state. Specifically, the anti-fatigue effect of IG was intuitively evaluated by measuring the swimming endurance time of mice. And some biochemical parameters intimately related to fatigue were determined to illustrate the potential mechanisms underlying its anti-fatigue effect.
This is the first study to report the anti-fatigue potency of IG and the microalgae of Prymnesiophyceae. The results provided enlightening insight into the anti-fatigue effect of IG, which contributed to buttressing the modern application and pharmacodynamic activity of this marine microalgae. The promising anti-fatigue activity of IG suggests that it holds a promising potential to be developed into a marine dietary supplement for relieving fatigue.
Materials and methods
Materials and reagents
IG and spirulina were provided by freshwater Algae Culture Collection at the Institute of Hydrobiology (FACHB-collection) of Chinese Academy of Sciences (Wuhan, China). The kits for biochemical analysis of lactic acid (LA), hepatic glycogen, muscle glycogen, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) were obtained from Jiancheng Bioengineering Institute (Nanjing, China). All reagents utilized in the experiment were at least of analytical grade.
Preparation of ultrafine powder of IG
IG and spirulina were processed into ultrafine powder for animal experiments according to previous studies (Luo et al., 2017). Briefly, samples were dried at 50 °C for 24 h, smashed by a ball mill and sift successively through the mesh size of 80 and 500 to obtain the satisfactory particle size of IG and spirulina. IG and spirulina were suspended in normal saline for oral administration.
Animal
A total of 120 male and 10 female Kunming (KM) mice (20–25 g) were obtained from the Medical Experiment Animal Center of Guangzhou University of Chinese Medicine (GZUCM, Guangzhou, China). All animals were housed adaptively for 7 days under a controlled temperature (23 ± 1 °C) and 40–60% humidity before the experiment. The experiment was conducted abiding by the guidelines provided by the Animal Care Committee and approved by the Institutional Ethics Committee of GZUCM (No. 2016047) and conducted in accordance with the National Institutes of Health (NIH) guidelines. All institutional and national guidelines for the care and use of laboratory animals were followed.
Acute oral toxicity of IG
Acute oral toxicity study on IG was performed to provide preliminary safety information according to “up and down procedure (UDP)” recommended by the OECD guideline (OECD, 2008). Briefly, two experimental groups of mice (5 male mice and 5 female mice in each group, respectively) were treated orally with IG at a single dose of 0 and 5000 mg/kg bodyweight. Mice receiving the vehicle (saline) served as control. Mice were observed for a period of 14 days. Food and water were provided throughout the experiment. For 14 days the animals were weighed and the number of deaths noted. If more than 3 animals of each sex survive in IG-pretreatment group, the LD50 is regarded to be greater than the limit administration dose (5000 mg/kg).
Animal experimental design
The experiment (Fig. 1) was carried out as previously described with slight modifications (Duan et al., 2017; Zamanian et al., 2017). Mice were assigned to 6 groups: normal control group (NC), model group (MC), positive control group (spirulina, 800 mg/kg), and IG-pretreatment groups (100, 200 and 400 mg/kg). 20 mice were randomly assigned to each group while 10 mice were assigned to the NC group [10 mice per group except the NC group were subjected to forced swimming test to record the swimming time (n = 10), and the rest 10 mice in each group were given a 90-min swim for the measurement of biochemical parameters associated with fatigue (n = 10)]. The doses of IG were chosen based on our preliminary experiments. NC and MC groups were intragastrically administrated with normal saline, while other groups were pretreated with corresponding agents for 14 consecutive days, respectively.
Fig. 1.
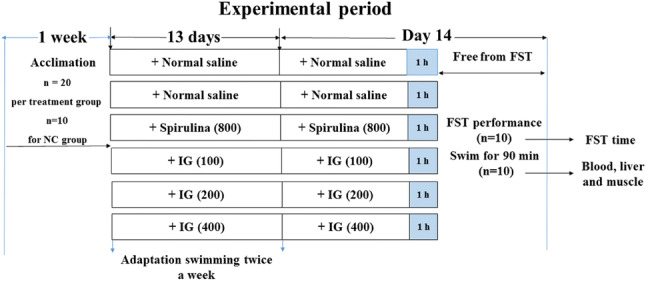
Schematic representation of the experimental design and treatment schedule for different groups. Experimental mice were randomly divided into 6 groups (n = 20 in all groups except n = 10 in NC group) and pretreated with vehicle, spirulina and IG, respectively. Weight-loaded forced swimming test (FST) (n = 10) and a 90-min swimming (n = 10) were performed, then the swimming time was recorded and samples were collected
Swimming exercise training
Swimming exercise training was carried out twice a week with 20 min each time. Briefly, all groups of mice were performed based on a regular and moderate swimming program without weight loading in a swimming pool with a smooth surface (40 cm × 40 cm × 35 cm). The water in the pool was 30 cm deep and maintained at 25 ± 2 °C.
Weight-loaded forced swimming test
The weight-loaded forced swimming test (FST) was proceeded as mentioned anteriorly with mild modifications (Zhong et al., 2017). Briefly, 30 min post the last administration, 10 mice in each group except the NC group were subjected to FST. Mice were loaded 5% of their corresponding body weight fixed to the tail base and forced to swim in the same conditions with swimming exercise training. Exhaustion was defined by observable unbalance of motility and failure to return to the surface within 10 s, and the endurance time was recorded immediately. The duration for flotage, struggle, and necessary movements of mice were measured in the swimming endurance until exhaustion and possible sinking.
Sample collection
The rest of mice of each group (n = 10) free from FST, were taken out for the measurement of biochemical parameters associated with fatigue. Briefly, 30 min post the final supplement of IG, mice were placed in the swimming pool and forced to swim continuously without loads for 90 min, and then immediately anesthetized. Subsequently, the blood of animals was collected in heparinized tubes by removing the eyeballs and placed at room temperature for 30 min. Serum was subjected to centrifugation at 1000×g at 4 °C for 15 min and stored at − 80 °C for further analysis of levels of blood glucose (Glu), LA, lactic dehydrogenase (LDH), creatine kinase (CK), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), MDA, GSH-Px, and SOD. Liver and gastrocnemius muscles were taken out and washed in ice-cold saline solution and kept at − 80 °C until the determination of glycogen content was conducted.
Measurement of fatigue-related biochemical parameters
The levels of BUN, Glu, CK, LDH, ALT, and AST were measured by Hitachi -7180 type biochemical analyzer following the standard methods. The levels of SOD, GSH-Px, MDA, LA, hepatic glycogen and muscle glycogen were estimated by commercial assay kits following the manufacturer’s guide.
Statistical analysis
Data are presented as mean ± standard deviation (SD), and analyzed by SPSS 19.0 software (IBM, Armonk, NY, USA). The differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by LSD multiple comparison test and Dunnett T3 test. p < 0.05 was considered statistically significant.
Results and discussion
Fatigue is mainly comprised of two branches: mental fatigue and physical fatigue. Physical fatigue is accompanied by a sense of extreme physiological weariness, which resulted from excessive stress and intensive physical work such as forced exercise or swimming (Chen et al., 2009). Since pharmacological medications and therapies for fatigue were limited and failed to satisfy people’s needs, athletic physiologists and health scholars have focused attention on the quest of natural anti-fatigue remedies in recent decades (Zhao et al., 2015). Microalgae has been recognized as one of the most prospective and innovative sources for nutrient food and functional additives due to its considerable natural resource of highly valuable bioactive compounds (Matos et al., 2017), which might contribute to postponing fatigue, promoting the release of metabolites related to fatigue, and improve exercise performance. IG, an important member of microalgae contains valuable source of omega-3 PUFAs, which has been reported to relieve chronic fatigue syndrome (Maes et al., 2005). However, the potential pharmacological activities of IG have seldomly been explored, and whether IG possesses anti-fatigue activity remains to be explored. Therefore, this work was performed to investigate the anti-fatigue effect and the potential underlying mechanism of IG.
Acute toxicity evaluation
In the acute toxicity test, IG was administered once by gavage to 10 male and 10 female KM mice at dose levels of 0 and 5000 mg/kg, respectively. Animals of both sexes did not manifest any significant abnormal signs and alternations, behavioral changes, water or food consumption, bodyweight variations, macroscopic findings at any time of observation. No deaths of mice occurred during the 14 days of study. Autopsy results exhibited no significant change or lesion in the viscera of any animal. Therefore, IG was considered to have a wide safety margin with LD50 higher than 5000 mg/kg.
Effect of IG on body weight of mice
The change of body weight of mice throughout the experiment was shown in Table 1. There was no statistically significant difference for the initial or final body weights between the IG or spirulina groups and the MC group (all p > 0.05), indicating that IG and spirulina had no obvious effect on the body weight of mice.
Table 1.
The body weight of mice in all groups (mean ± SD)
| Group | N | Dose (mg/kg) | Initial (g) | Final (g) |
|---|---|---|---|---|
| NC | 10 | – | 22.36 ± 0.67 | 26.40 ± 1.44 |
| MC | 10 | – | 22.10 ± 0.35 | 26.34 ± 1.54 |
| Spirulina | 10 | 800 | 21.78 ± 0.71 | 27.28 ± 1.48 |
| IG | 10 | 100 | 21.90 ± 0.82 | 27.52 ± 1.29 |
| IG | 10 | 200 | 21.87 ± 0.73 | 27.27 ± 1.60 |
| IG | 10 | 400 | 22.62 ± 0.58 | 27.49 ± 0.88 |
Effect of IG on FST capacity
For anti-fatigue experiments, the weight-loaded forced swimming test (FST) is standard and accurate, and therefore has been utilized widely to assess the performance and endurance capacity for exercise in animals by most of researchers (Zhang et al., 2011). The improvement in exercise endurance indicates the performance on anti-fatigue activity. Therefore, the swimming endurance time is recorded as an indicator of athletic capability in mice, which could intuitively mirror the capacity of resistance to fatigue (Liu and Liu, 2016). In our present study, as shown in Fig. 2, the time of swimming to exhaustion was prolonged by pretreatment with IG. Moreover, supplementation with spirulina (800 mg/kg) and IG (200 and 400 mg/kg) remarkably increased swimming time compared to the MC group (all p < 0.01), which may indicate a favorable anti-fatigue activity of IG.
Fig. 2.
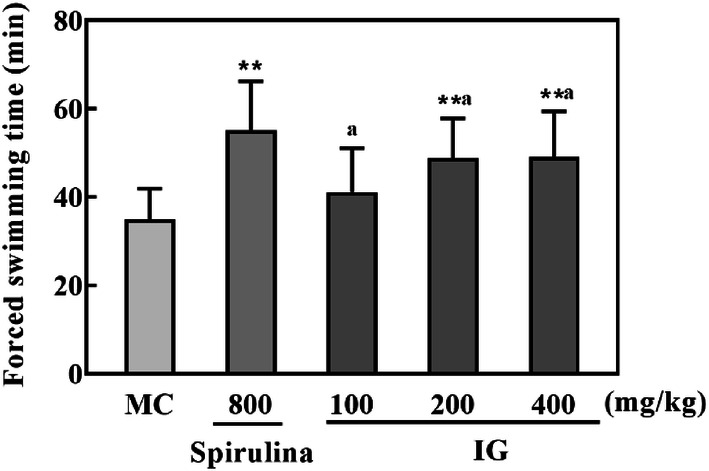
Effect of IG on FST in mice (n = 10). Results are expressed as mean ± SD and analyzed by ANOVA followed by LSD multiple comparison test and Dunnett T3 test. **p < 0.01 versus MC group. Statistical difference between IG groups was indicated by alphabetical superscripts, and data in columns designated by the same letter are not significantly different at p = 0.05
Effect of IG on fatigue-related biochemical parameters in mice
Effect of IG on energy consumption in mice
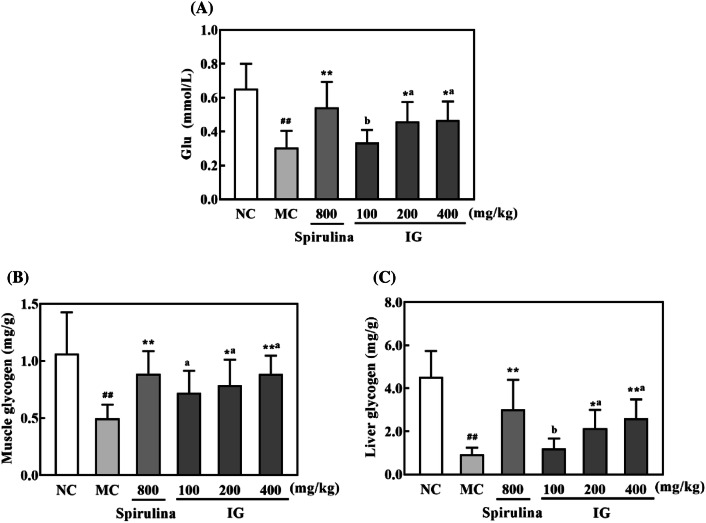
Exhaustion theory indicates that depletion of energy source and accumulation of excessive metabolite are two major causes of fatigue (Nybo, 2003; You et al., 2011). It is well-known that physiological fatigue could be crucially induced by energy deficiency, which resulted from rapid ATP consumption due to muscular exercise (Belluardo et al., 2001). Therefore, energy depletion in the process of muscular activity determines the level of physical fatigue. When encountering high-intensity exercise, Glu is primarily used to meet the needs of energy and soon depleted since it is rapidly available and able to be immediately oxidized to offer ATP in the blood (Bergstrom et al., 1967). Blood glycogen is a secondary form to store energy for long term, which could swiftly migrate to respond to a sudden shortage of glucose during exercise and to remain the level within the physiological state in blood (Ortenblad et al., 2013). And increasing levels of hepatic glycogen and muscle glycogen could intensify endurance during exhausted exercise.
In the current work (Fig. 3), relative to the NC group, the serum levels of Glu, hepatic glycogen and muscle glycogen were all significantly decreased (p < 0.01) in the MC group. However, the decreased levels of the above parameters were significantly raised by spirulina and IG in a dose-dependent manner. Supplementation with IG at 100 mg/kg failed to achieve statistically significant difference, as compared to the MC group. Noteworthily, the level of muscle glycogen in IG-supplemented (400 mg/kg) mice was comparable to that in spirulina-supplemented group. These results suggested that IG may be helpful to postpone the consumption of energy during the exhausted exercise.
Fig. 3.
Effect of IG on the contents of serum Glu (A), muscle glycogen (B), and liver glycogen (C) in mice (n = 10) after exhausted swimming. Results are expressed as mean ± SD and analyzed by ANOVA followed by LSD multiple comparison test and Dunnett T3 test. ##p < 0.01 versus NC group, *p < 0.05, **p < 0.01 versus MC group. Statistical difference between IG groups was indicated by alphabetical superscripts, and data in columns designated by the same letter are not significantly different at p = 0.05
Effect of IG on metabolism in mice
Recovery from exercise-elicited fatigue requires accelerating excretion of the metabolite accumulated in the body and/or prompting repair of the potential damage occurred during exercise (Xu et al., 2013). LA, the metabolic product of glycolysis, is generated through the process of anaerobic glycolysis of muscle during strenuous exercise. A rapid elimination of LA has been reported to alleviate fatigue (Ding et al., 2011). BUN can definitely indicate the catabolism of protein and amino acids, and increased BUN activity generally symbolizes the proteolysis, which will imperil the muscle contractility and ultimately bring about fatigue (Jia and Wu, 2008).
As cytosolic enzymes, both LDH and CK are sufficient in the cells of organs under the physiological condition. LDH is a crucial enzyme involved in anaerobic glycolysis and gluconeogenesis, which catalyzes lactic acid to transform into pyruvate (Jin and Wei, 2011). By connecting the sites between ATP production and utilization, CK plays the essential part in maintaining the balance of intracellular energy (Wang et al., 2011). Intense exercise induces the excessive production of oxygen radicals, which brings about damage of myocyte and infiltration of LDH and CK into plasma. Therefore, high levels of LDH and CK in serum are considered to be important indicators of cellular necrosis and tissue damage (Ding et al., 2011). Increased levels of serum LDH and CK suggests that muscle damage has formed or is being initiated (Zamanian et al., 2017). Therefore, the accumulation of LA, BUN, LDH and CK in serum can exposit the advance of fatigue development and the workload intensity. Besides, excessive physical exercise can lead to the sharp shrinkage of liver cells, and further trigger the dysfunction of liver (Latour et al., 1999). Over-accumulation of ALT and AST are important indicators of liver cell damage, therefore representing typical responses to exhaustive exercise (Praphatsorn et al., 2010).
As shown in Fig. 4, when exposed to exhausted swimming, the levels of LA, BUN, LDH, and CK in the MC group were all significantly elevated compared with the NC group (p < 0.01). Nevertheless, supplementation with spirulina and IG significantly decreased the levels of LA, BUN and CK in a dose-related manner (p < 0.05 or p < 0.01), especially for 200 and 400 mg/kg IG (p < 0.01). However, the LDH level in 100 mg/kg IG pretreatment group failed to reach statistical difference compared to the MC mice (p > 0.05). In addition, exposure to FST resulted in the elevation of serum ALT and AST levels in mice (p < 0.01vs. NC group). However, these alterations were significantly suppressed by pretreatment with IG (p < 0.01 vs. MC group in all doses). These results suggested that IG might improve the body’s endurance of exercise and exert its anti-fatigue effect, at least in part, via reducing the metabolism of energy and protein and protecting cells from damage during excessive exercise.
Fig. 4.
Effect of IG on the serum levels of LA (A), BUN (B), LDH (C), CK (D), ALT (E) and AST (F) in mice (n = 10) after exhausted swimming. Results are expressed as mean ± SD and analyzed by ANOVA followed by LSD multiple comparison test and Dunnett T3 test. ##p < 0.01 versus NC group, *p < 0.05, **p < 0.01 versus MC group. Statistical difference between IG groups was indicated by alphabetical superscripts, and data in columns designated by the same letter are not significantly different at p = 0.05
Effect of IG on antioxidant status in mice
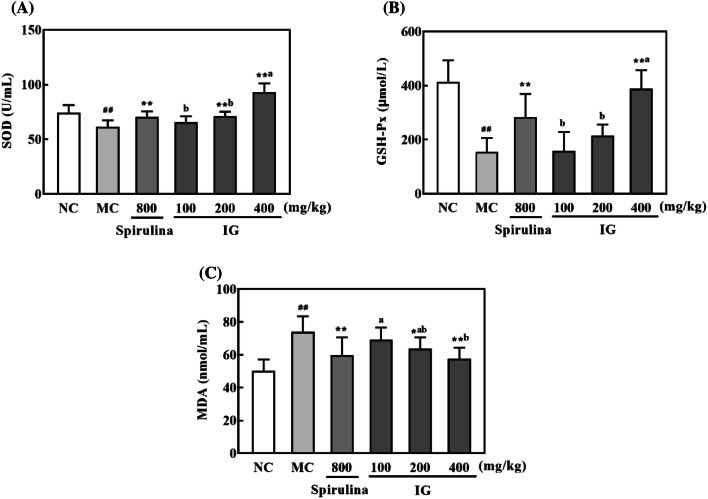
Oxidative stress plays a significant role in physical fatigue (Coombes et al., 2002). The accumulation of reactive free radicals, which was caused by exhaustive exercise, will lead to tissue damage (You et al., 2011). MDA is one of the most important products of membrane lipid peroxidation. And it is one of the main outcomes of free radical-mediated lesion which directly reflects the oxidative damage of cell membrane. Exhaustive exercise can increase MDA level (Zhao et al., 2014). On the other hand, SOD and GSH-Px, as two main antioxidases, play the positive roles in resistance of fatigue and protection of cells, by decreasing the production of active oxygen radicals and eliminating products of metabolism from oxidative stress injury (Shan et al., 2011).
The effect of IG on the serum levels of SOD, MDA, and GSH-Px was shown in Fig. 5. After mice were forced to swim, the SOD and GSH-Px activities in the MC group were markedly lessened while the content of MDA was significantly augmented. However, IG was observed to substantially elevate the SOD and GSH-Px activities, while decrease the MDA level in a dose-dependent manner, which was consistent with previous reports that IG and its bioactive ingredient possessed remarkable antioxidant capacities (Sun et al., 2014). Noticeably, the antioxidant capacity of IG was stronger than spirulina, an important microalgae widely reported to possess antioxidant property (Nuno et al., 2013) and exert anti-fatigue effect (Zhao et al., 2015). These findings suggested that the anti-fatigue effect of IG might be intimately associated with its antioxidant activity.
Fig. 5.
Effects of IG on the serum levels of SOD (A), GSH-Px (B), and MDA (C) in mice (n = 10) after exhausted swimming. Results are expressed as mean ± SD and analyzed by ANOVA followed by LSD multiple comparison test and Dunnett T3 test. ##p < 0.01 versus NC group, *p < 0.05, **p < 0.01 versus MC group. Statistical difference between IG groups was indicated by alphabetical superscripts, and data in columns designated by the same letter are not significantly different at p = 0.05
In conclusion, our present study showed that IG possessed favorable anti-fatigue effect in mice for the first time, which was superior to spirulina. The anti-fatigue effect might be partly explained by its contributions of decreasing energy consumption, reducing the accumulation of metabolic products, especially, exerting appreciable antioxidant activity. Although more in-depth studies were needed to explore the anti-fatigue mechanism of IG, our findings suggested IG might serve as a promising candidate dietary supplement for relieving fatigue.
Acknowledgements
This work was supported by grants from the key lab of Zhanjiang for R&D marine microbial resources in the Beibu Gulf Rim (No. 2012E02), the natural science foundation of Guangdong Province (No. 2015A030310121), and the public service platform of south China Sea for R&D marine biomedicine resources (No. 2017C8A).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenjie Li, Email: dglwj.135@163.com.
Chaodan Luo, Email: 752334715@qq.com.
Yongmei Huang, Email: 727148105@qq.com.
Jingting Zhan, Email: 575590143@qq.com.
Jinli Lei, Email: 1038415724@qq.com.
Ning Li, Email: lining201@163.com.
Xiaoqi Huang, Email: huangxiaoqi@gzucm.edu.cn.
Hui Luo, Email: luohui-gdmu@outlook.com.
References
- Bandarra NM, Pereira PA, Batista I, Vilela MH. Fatty acids, sterols and alpha-tocopherol in Isochrysis galbana. J. Food Lipids. 2003;10:25–34. doi: 10.1111/j.1745-4522.2003.tb00003.x. [DOI] [Google Scholar]
- Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, Caniglia G, Pastoris O, Grassi F, Ibanez CF. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol. Cell Neurosci. 2001;18:56–67. doi: 10.1006/mcne.2001.1001. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Chen JR, Wang TJ, Huang HY, Chen LJ, Huang YS, Wang YJ, Tseng GF. Fatigue reversibly reduced cortical and hippocampal dendritic spines concurrent with compromise of motor endurance and spatial memory. Neuroscience. 2009;161:1104–1113. doi: 10.1016/j.neuroscience.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely RA, Powers SK. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur J Appl Physiol. 2002;87:272–277. doi: 10.1007/s00421-002-0631-3. [DOI] [PubMed] [Google Scholar]
- Devos M, Poisson L, Ergan F, Pencreac’h G. Enzymatic hydrolysis of phospholipids from Isochrysis galbana for docosahexaenoic acid enrichment. Enzyme Microb. Tech. 2006;39:548–554. doi: 10.1016/j.enzmictec.2005.08.040. [DOI] [Google Scholar]
- Ding JF, Li YY, Xu JJ, Su XR, Gao XA, Yue FP. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocolloid. 2011;25:1350–1353. doi: 10.1016/j.foodhyd.2010.12.013. [DOI] [Google Scholar]
- Duan FF, Guo Y, Li JW, Yuan K. Antifatigue effect of Luteolin-6-C-Neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Longev: Oxid. Med. Cell; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DB, William AH, Strauss AC, Unger ER, Jason L, Marshall GD, Jr, Dimitrakoff JD. Chronic Fatigue Syndrome: The Current Status and Future Potentials of Emerging Biomarkers. Fatigue. 2014;2:93–109. doi: 10.1080/21641846.2014.906066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. 2009;214:33–39. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Jia JM, Wu CF. Antifatigue activity of tissue culture extracts of Saussurea involucrata. Pharm. Biol. 2008;46:433–436. doi: 10.1080/13880200802055909. [DOI] [Google Scholar]
- Jin HM, Wei P. Anti-Fatigue Properties of Tartary buckwheat Extracts in Mice. Int. J. Mol. Sci. 2011;12:4770–4780. doi: 10.3390/ijms12084770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour MG, Brault A, Huet PM, Lavoie JM. Effects of acute physical exercise on hepatocyte volume and function in rat. Am. J. Physiol. 1999;276:R1258–R1264. doi: 10.1152/ajpregu.1999.276.5.R1258. [DOI] [PubMed] [Google Scholar]
- Liu YY, Liu CJ. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J. Food Drug Anal. 2016;24:738–745. doi: 10.1016/j.jfda.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DD, Qu C, Zhang ZB, Xie JH, Xu LQ, Yang HM, Li CL, Lin GS, Wang HF, Su ZR. Granularity and laxative effect of ultrafine powder of Dendrobium officinale. J. Med. Food. 2017;20:180–188. doi: 10.1089/jmf.2016.3827. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Leunis JC. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol. Lett. 2005;26:745–751. [PubMed] [Google Scholar]
- Matos J, Cardoso C, Bandarra NM, Afonso C. Microalgae as healthy ingredients for functional food: a review. Food Funct. 2017;8:2672–2685. doi: 10.1039/C7FO00409E. [DOI] [PubMed] [Google Scholar]
- Mehta RK, Agnew MJ. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Eur. J. Appl. Physiol. 2012;112:2891–2902. doi: 10.1007/s00421-011-2264-x. [DOI] [PubMed] [Google Scholar]
- Nuno K, Villarruel-Lopez A, Puebla-Perez AM, Romero-Velarde E, Puebla-Mora AG, Ascencio F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Func. Foods. 2013;5:106–115. doi: 10.1016/j.jff.2012.08.011. [DOI] [Google Scholar]
- Nybo L. CNS fatigue and prolonged exercise: Effect of glucose supplementation. Med. Sci. Sports Exerc. 2003;35:589–594. doi: 10.1249/01.MSS.0000058433.85789.66. [DOI] [PubMed] [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. (2008)
- Ortenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J. Physiol-London. 2013;591:4405–4413. doi: 10.1113/jphysiol.2013.251629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praphatsorn P, Thong-Ngam D, Kulaputana O, Klaikeaw N. Effects of intense exercise on biochemical and histological changes in rat liver and pancreas. Asian biomed. 2010;4:619. doi: 10.2478/abm-2010-0078. [DOI] [Google Scholar]
- Raposo MFD, de Morais R, de Morais A. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013;93:479–486. doi: 10.1016/j.lfs.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Shan X, Zhou J, Ma T, Chai Q. Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int. J. Mol. Sci. 2011;12:1081–1088. doi: 10.3390/ijms12021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shei RJ, Lindley MR, Mickleborough TD. Omega-3 polyunsaturated fatty acids in the optimization of physical performance. Milit. Med. 2014;179:144–156. doi: 10.7205/MILMED-D-14-00160. [DOI] [PubMed] [Google Scholar]
- Sun YY, Wang H, Guo GL, Pu YF, Yan BL. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014;113:22–31. doi: 10.1016/j.carbpol.2014.06.058. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Baba Y, Kataoka Y, Kinbara N, Sagesaka YM, Kakuda T, Watanabe Y. Effects of (-)-epigallocatechin gallate in liver of an animal model of combined (physical and mental) fatigue. Nutrition. 2008;24:599–603. doi: 10.1016/j.nut.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang S, Gao YS, Chen Z, Zhou HM, Yan YB. Dissimilarity in the folding of human cytosolic creatine kinase isoenzymes. Plos One 6 (2011) [DOI] [PMC free article] [PubMed]
- Xu C, Lv JL, Lo YM, Cui SW, Hu XZ, Fan MT. Effects of oat beta-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr. Polym. 2013;92:1159–1165. doi: 10.1016/j.carbpol.2012.10.023. [DOI] [PubMed] [Google Scholar]
- You LJ, Zhao MM, Regenstein JM, Ren JY. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011;124:188–194. doi: 10.1016/j.foodchem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Zamanian M, Hajizadeh MR, Esmaeili Nadimi A, Shamsizadeh A, Allahtavakoli M. Antifatigue effects of troxerutin on exercise endurance capacity, oxidative stress and matrix metalloproteinase-9 levels in trained male rats. Fundam. Clin. Pharmacol. 2017;31:447–455. doi: 10.1111/fcp.12280. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Ren F, Huang W, Ding RT, Zhou QS, Liu XW. Anti-fatigue activity of extracts of stem bark from Acanthopanax senticosus. Molecules. 2011;16:28–37. doi: 10.3390/molecules16010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZH, Zheng XW, Fang F. Ganoderma lucidum polysaccharides supplementation attenuates exercise-induced oxidative stress in skeletal muscle of mice. Saudi J. Biol. Sci. 2014;21:119–123. doi: 10.1016/j.sjbs.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XN, Liang JL, Chen HB, Liang YE, Guo HZ, Su ZR, Li YC, Zeng HF, Zhang XJ. Anti-fatigue and antioxidant activity of the polysaccharides isolated from Millettiae speciosae champ. Leguminosae. Nutrients. 2015;7:8657–8669. doi: 10.3390/nu7105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Zhao LY, Yang FM, Yang WJ, Sun Y, Hu QH. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice. J. Int. Soc. Sport Nutr. 2017;14:10. doi: 10.1186/s12970-017-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]