Abstract
Orexins comprise two neuropeptides produced by orexin neurons in the lateral hypothalamus and are released by extensive projections of these neurons throughout the central nervous system. Orexins bind and activate their associated G protein-coupled orexin type 1 receptors (OX1Rs) and OX2Rs and act on numerous physiological processes, such as sleep-wake regulation, feeding, reward, emotion, and motivation. Research on the development of orexin receptor antagonists has dramatically increased with the approval of suvorexant for the treatment of primary insomnia. In the present review, we discuss recent findings on the involvement of the orexin system in the pathophysiology of psychiatric disorders, including sleep disorders, depression, anxiety, and drug addiction. We discuss the actions of orexin receptor antagonists, including selective OX1R antagonists (SORA1s), selective OX2R antagonists (SORA2s), and dual OX1/2R antagonists (DORAs), in the treatment of these disorders based on both preclinical and clinical evidence. SORA2s and DORAs have more pronounced efficacy in the treatment of sleep disorders, whereas SORA1s may be promising for the treatment of anxiety and drug addiction. We also discuss potential challenges and opportunities for the application of orexin receptor antagonists to clinical interventions.
Keywords: Orexin, Insomnia, Depression, Anxiety, Drug addiction
Introduction
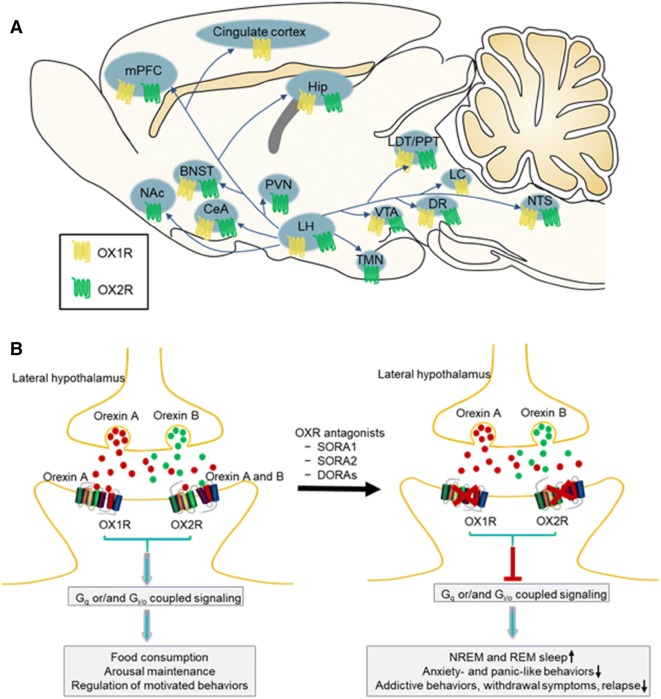
Orexins and hypocretins were almost simultaneously discovered by Sakurai and de Lecea, respectively, in 1998 and were later shown to be the same neuropeptide [1, 2]. There are two types of orexins: orexin-A (hypocretin-1) and orexin-B (hypocretin-2). Both are produced from the same precursor peptide, prepro-orexin, by neuronal cleavage in the lateral and posterior hypothalamus, and orexin projections are found throughout the brain [1–3]. Orexin peptides are ligands for G protein-coupled orexin type 1 receptors (OX1Rs) and OX2Rs. Orexin-A activates both OX1Rs and OX2Rs with approximately equal potency, while orexin-B preferentially activates OX2Rs [4, 5]. The anatomical distribution of OX1Rs and OX2Rs in the brain shows partially overlapping and partially distinct patterns [4, 6–8] (Fig. 1A). Both receptors are expressed in the lateral hypothalamus (LH), medial prefrontal cortex (mPFC), hippocampus (Hip), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), dorsal raphe (DR), ventral tegmental area (VTA), laterodorsal tegmental nucleus (LDT)/pedunculopontine nucleus (PPT), and nucleus of the solitary tract (NTS). OX1Rs are selectively expressed in the locus coeruleus (LC) and cingulate cortex, while OX2Rs are selectively expressed in the tuberomammillary nucleus (TMN), hypothalamic paraventricular nucleus (PVN), and nucleus accumbens (NAc). These brain regions are major effector sites of orexin neurons in the LH, which is involved in feeding, sleep, and motivated behaviors [9–11].
Fig. 1.
Orexin receptor distribution and potential application of orexin receptor antagonists for the treatment of psychiatric disorders. A Orexin neurons in the lateral hypothalamus send extensive projections to brain areas associated with feeding, sleep-wake regulation, and motivated and emotional behaviors. The anatomical distribution of OX1Rs and OX2Rs in these regions is shown. B Orexin A and orexin B are hypothalamic neuropeptides. Their actions are mediated by Gq- and/or Gi/o-coupled OX1Rs and OX2Rs, playing important roles in many physiological processes, such as feeding, the sleep-to-wakefulness transition, and motivation. Antagonism of the orexin system increases NREM and REM sleep, decreases anxiety- and panic-like behaviors, and inhibits the reinforcing and motivational properties of addictive drugs. Orexin receptor antagonists may be promising for the treatment of psychiatric disorders, such as insomnia, anxiety, and drug addiction. BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DORA, dual OX1/2R antagonist; DR, dorsal raphe; Hip, hippocampus; LC, locus coeruleus; LDT/PPT, laterodorsal tegmental nucleus/pedunculopontine nucleus; LH, lateral hypothalamus; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; NREM, non-rapid-eye-movement; NTS, nucleus of the solitary tract; OX1R, orexin type 1 receptor; OX2R, orexin type 2 receptor; PVN, hypothalamic paraventricular nucleus; REM, rapid-eye-movement; SORA1, selective OX1R antagonist; SORA2, selective OX2R antagonist; TMN, tuberomammillary nucleus; VTA, ventral tegmental area.
Fasting has been shown to increase prepro-orexin mRNA levels, and the central administration of orexin-A and orexin-B stimulates food consumption, suggesting that they play important physiological roles in regulating feeding behavior [2]. Satiety activates anorexigenic pro-opiomelanocortin (POMC)-containing neurons and inhibites orexin-producing neurons in the hypothalamus, thus suppressing feeding and/or stimulating energy expenditure [12, 13]. However, orexin-A and OX1R stimulation have been shown to inhibit satiety-inducing POMC neurons and increase the motivation for food [14, 15]. The exogenous administration of orexin-A into the LH increases wakefulness in rats [16]. The selective optogenetic activation of orexin neurons in the LH increases the probability of the sleep-to-wakefulness transition, and OX1Rs and OX2Rs play differential roles in the regulation of rapid-eye-movement (REM) sleep and non-REM (NREM) sleep [17]. NREM sleep is mainly regulated by OX2Rs, but there are important synergistic effects of OX1R and OX2R signaling in this regulation [18, 19]. Many reviews have indicated that the orexin system is involved in the regulation of reward, motivated behaviors, and stress-related psychiatric disorders [6, 9, 20, 21].
Given the critical role of orexin in multiple physiological processes and the wide distribution of orexin receptors throughout the brain, compounds that target orexin receptors may be beneficial for the treatment of various disorders. A growing number of patents for orexin receptor antagonists has emerged for use in the areas of sleep disorders, anxiety, panic, and addictive disorders[22]. Orexin receptor antagonists are classified into selective OX1R antagonists (SORA1s), selective OX2R antagonists (SORA2s), and dual OX1/2R antagonists (DORAs), based on their binding affinities. Several DORAs and SORA2s have been translated from basic research to clinical interventions. They have been shown to have sustained clinical efficacy and are well-tolerated for the management of primary insomnia [23, 24]. The DORA suvorexant was the first orexin receptor antagonist approved by United States Food and Drug Administration (FDA) for the treatment of primary insomnia [25]. Another DORA, lemobrexant, has been shown to be effective for the treatment of insomnia disorder in randomized controlled clinical trials [23, 26]. In addition, orexin receptor antagonists have been extensively investigated for use in psychiatric disorders associated with the dysregulation of orexin function [10, 20, 21, 27] (Fig. 1B). In this review, we discuss the potential involvement of the orexin system in the pathophysiology of several psychiatric disorders, including sleep disorders, anxiety, and drug addiction, and summarize the progress of recent research on orexin receptor antagonists for the treatment of these disorders, from basic research to clinical studies.
From the Laboratory to the Clinic: Orexin Receptor Antagonists for the Treatment of Psychiatric Disorders
Although orexin neurons are concentrated only in the LH and adjacent areas, their nerve fibers project widely to much of the central nervous system, from the brainstem to the cortex. These widespread connections are involved in the regulation of feeding, sleep/wake states, stress, and reward processing [6, 9, 28, 29]. Specific hypothalamic nuclei, such as the LH and PVN, are often referred to as “centers” for hunger and satiety, and the activation of orexin projections to the NTS and NAc has been shown to increase food intake [9, 12, 30]. The effects of orexin neuropeptides on sleep-wake regulation may be reflected by their innervation of noradrenergic neurons in the LC, histaminergic neurons in the TMN, serotonergic neurons in the DR, and cholinergic neurons in the LDT/PPT [11, 31]. Orexin terminals are also expressed in the CeA, BNST, mPFC, and Hip, which are critical for the regulation of stress and anxiety-related responses [10, 32]. Orexin neurons also send projections to dopamine neurons in the VTA and NAc, which are well known to modulate reward processing [9, 21]. Accumulating evidence indicates that the orexin system contributes to the etiology of several psychiatric disorders. Orexin receptor antagonists have shown promise for the treatment of these disorders.
Sleep Disorders, Especially Primary Insomnia
The discovery of selective orexin neuron loss in narcolepsy sparked great research interest in the sleep field and raised the possibility of an alternative approach of treating the symptoms of insomnia with orexin receptor antagonists [11]. The number of orexin neurons is selectively reduced in human narcolepsy, and canine narcolepsy is caused by a mutation of the OX2R gene [33, 34]. Intranasal administration of orexin peptide, orexin neuron transplants, and orexin gene therapy are promising approaches to the treatment of narcolepsy [35, 36]. The selective OX2R agonist YNT-185 has been shown to ameliorate cataplexy-like episodes in a mouse model of narcolepsy [37]. OX2R agonists (a modified orexin-B peptide) have also been reported to promote resilience to stress and have anxiolytic and anti-depressive effects [38, 39]. Inactivation of each orexin receptor subtype is required to induce narcoleptic effects, but OX1Rs and OX2Rs play distinct roles in gating NREM and REM sleep. OX2Rs are the pivotal receptors that regulate wakefulness and NREM sleep, whereas REM sleep is controlled by both receptor subtypes [19]. The selective inhibition of OX2Rs results in an increase in NREM sleep and a decrease in the latency to NREM sleep, but no effect on REM or NREM sleep occurs when OX1Rs are selectively inhibited [40–42]. Although OXR1s do not have a direct action on regulating sleep, almorexant has been shown to enhance the total REM sleep time in OX1R-knockout mice compared with wild-type mice, thus indicating that OX1Rs play additional roles in REM sleep [43]. Knockdown of OX1Rs in the LC selectively increases the time spent in REM sleep during the dark period [44]. The SORA1 JNJ-54717793 has minimal effects on spontaneous sleep in rats and wild-type mice, but JNJ-54717793 administration in OX2R-knockout mice selectively reduces REM sleep latency and increases REM sleep duration [45]. Pharmacological experiments have also shown that co-administration of an OX1R antagonist and an OX2R antagonist reduces REM sleep latency and increases REM sleep duration [18]. However, another study have showed that the inhibition of OX1Rs attenuates the increase in REM sleep time that is induced by selective OX2R antagonism, which appeared to be correlated with dopaminergic neurotransmission [46]. These reports suggest that OX2Rs play a dominant role in regulating sleep, while OX1Rs alone only make a minimal contribution to sleep induction but exert synergistic effects with OX2Rs to regulate REM sleep.
DORAs block the activity of both OX1Rs and OX2Rs and result in a rapid transition to sleep. Administered orally, the DORA suvorexant significantly and dose-dependently reduces locomotor activity and promotes both NREM and REM sleep in rats, dogs, and rhesus monkeys [47]. Suvorexant promotes sleep primarily by increasing REM sleep duration and reducing the latency to REM sleep [48]. It also increases NREM sleep and improves the impairment in glucose tolerance in an animal model of diabetes mellitus [49]. These studies suggest that DORAs effectively improve sleep without significantly disrupting its architecture. Treatment with the DORA MK6096 and the SORA2 MK1064 but not a SORA1 stabilizes sleep and improves sleep-dependent memory function [50]. Fos immunohistochemistry showed that the DORA almorexant does not block the sleep deprivation-induced activation of wake-promoting cell groups in the LH, TMN, or basal forebrain, which suggests a mechanism by which almorexant causes less cognitive impairment than the benzodiazepine receptor agonist zolpidem [51].
Consistent cross-species evidence for the sleep-promoting effects of orexin receptor antagonists spurred the pharmaceutical development of new orexin-targeted treatments for insomnia. Almorexant was the first-in-class compound that targeted the orexin system and the first DORA to proceed to Phase II sleep disorder studies in 2007, but investigations of almorexant were halted during Phase III trials for undisclosed reasons. Currently, the most advanced DORA is suvorexant, which was approved by the FDA in 2014 for the treatment of insomnia [25]. Several meta-analyses and reviews of clinical trials support the efficacy of suvorexant for the treatment of insomnia [52–55]. Randomized controlled clinical trials have reported that suvorexant generally improves sleep maintenance and onset over 3 months of nightly treatment and is well-tolerated in both adult and elderly patients with primary insomnia [56–59]. DORA treatment results in significant and dose-related improvements in both subjective and objective sleep [26, 60, 61]. A meta-analysis and several clinical trials found that suvorexant is associated with significant improvements in subjective time to sleep onset, subjective total sleep time, and subjective sleep quality [24, 62–64]. Statistically significant sleep-promoting effects on both REM and NREM sleep have been reported, and sleep architecture and the power spectral profile are not disrupted in patients with primary insomnia or healthy people who are treated with suvorexant [23, 60, 65, 66]. The DORA SB-649868 has been shown to increase total sleep time and REM sleep duration and reduce waking after sleep onset, the latency to persistent sleep, and the latency to REM sleep in healthy men in a model of situational insomnia and men with primary insomnia [67, 68]. Another DORA, lemborexant, improves sleep efficiency, decreases the latency to persistent sleep, decreases the latency to subjective sleep onset, and decreases waking after sleep onset in adults and elderly individuals with insomnia disorder [26].
In addition to treating primary insomnia, the DORA suvorexant has been evaluated for the treatment of insomnia symptoms in patients with psychosis and physical disease. Suvorexant is effective for the treatment of insomnia in patients with Alzheimer’s disease, but whether it influences Alzheimer’s disease itself remains unclear [69]. Suvorexant significantly increases sleep efficiency and decreases glucose levels in individuals with insomnia symptoms and type 2 diabetes mellitus, and these effects are consistent with the effects of suvorexant in an animal model [49, 70]. Combination therapy with suvorexant and ramelteon improves subjective sleep quality without inducing delirium in acute stroke patients [71]. Some case reports have also suggested that suvorexant treatment is useful in patients with schizophrenia [72] and Parkinson’s disease [73], but its efficacy in these patients remains unclear and needs further validation. Overall, DORAs have shown efficacy for the treatment of primary insomnia and insomnia symptoms. Several studies have focused on suvorexant for the treatment of patients with sleep apnea, but it lacks clinically important respiratory effects during sleep [74–76]. The SORA2 seltorexant has also been found to decrease the latency to persistent sleep and increase total sleep time and sleep efficiency in patients with major depressive disorder (MDD) and persistent insomnia who are being treated with antidepressants and individuals with insomnia disorder without psychiatric comorbidity [77, 78].
Data from a Japanese post-marketing survey suggested that adverse effects of suvorexant are rare, with an incidence of 8.8%. Next-day somnolence is the most commonly reported side-effect, which may be an inevitable consequence of pharmacological interventions that promote sleep in some patients [79]. Suvorexant is generally effective and well tolerated. However, compared with traditional sleep agents (e.g., benzodiazepines), the efficacy of suvorexant requires further validation [80, 81]. Some studies have reported infrequent but notable side-effects of suvorexant in the treatment of insomnia disorder, such as abnormal dreams, sleep paralysis, over-sedation, the acute worsening of depressive symptoms, and suicidal thoughts [82–84].
Overall, several SORA2s and DORAs that are clinically used for patients with primary insomnia have been shown to be safe and effective (Table 1). Additional orexin-targeted drugs are expected to be approved by the FDA for clinical use. Further studies are needed to evaluate the action of orexin receptor antagonists in the treatment of other sleep disorders and mental illnesses.
Table 1.
Summary of recent findings on orexin receptor antagonists for the treatment of sleep disorders.
| Manipulation and target | Subjects | Findings | References |
|---|---|---|---|
|
Filorexant (MK-6096) DORA |
Rats and dogs | Dose-dependently reduced locomotor activity and increased sleep in rats and dogs. | Winrow et al. [151] |
| Mice | Stabilized sleep and improved sleep-dependent memory function. | Li et al. [50] | |
| Patients (18–65 years old) with primary insomnia | Increased SE, decreased WASO, and decreased the latency to persistent sleep onset. | Connor et al. [61] | |
|
Suvorexant (MK-4305) DORA |
Rats, dogs, and rhesus monkeys | Reduced locomotor activity and promoted sleep in rats, dogs, and rhesus monkeys. | Winrow et al. [47] |
| Mice | Disturbed sleep architecture by selectively increasing REM sleep and decreasing wake time. | Hoyer et al. [48] | |
| Type 2 diabetic db/db mice | Increased NREM sleep and improved impairment in glucose tolerance in db/db mice. | Tsuneki et al. [49] | |
| Non-elderly patients with primary insomnia | Increased SE, decreased LPS, and decreased WASO | Herring et al. [152] | |
| Patients with primary insomnia | Showed greater efficacy than placebo in improving subjective TST and subjective TSO; was generally safe and well tolerated over 1 year of nightly treatment. | Michelson et al. [64] | |
| Nonelderly and elderly patients with insomnia. | Improved sleep onset and maintenance over 3 months of nightly treatment and was generally safe and well tolerated. | Herring et al. [57] | |
| Patients with insomnia | Increased TST (average increase ≤ 3.9% in REM sleep) and reduced REM sleep latency. | Snyder et al. [66] | |
| Elderly patients with insomnia | Generally improved sleep maintenance and onset over 3 months of nightly treatment and was well-tolerated. | Herring et al. [56] | |
| Elderly and nonelderly insomnia patients | Generally effective and well tolerated in both women and men with insomnia. | Herring et al. [59] | |
| Psychiatric inpatients with insomnia | Overall improvement in the quality of sleep and the severity of anxiety and depression. | Nakamura et al. [89] | |
| Adolescent patients with insomnia | Improved sleep quality in adolescent insomnia. | Kawabe et al. [62] | |
| Japanese elderly patients with chronic insomnia | Appeared to be more cost-effective than the alternative zolpidem in a virtual cohort. | Nishimura and Nakao [153] | |
| Elderly and non-elderly insomnia patients | Improved sleep to a greater extent than placebo as assessed by the ISI. | Herring et al. [63] | |
| Insomnia patients | Reduced WASO by reducing the number and time spent in long wake bouts. | Svetnik et al. [154] | |
| Healthy young men (18–45 years old) | Decreased LPS and wake after sleep onset time and increased SE; did not affect EEG frequency bands. | Sun et al. [60] | |
| Patients with primary insomnia | Limited effects on EEG power spectral density compared with placebo. | Ma et al. [65] | |
| Alzheimer’s disease patients with insomnia | Adequate efficacy for the treatment of insomnia in patients with Alzheimer’s disease. | Hamuro et al. [69] | |
| Patients with type 2 diabetes mellitus and insomnia | Increased TST and SE and decreased glucose levels. | Toi et al. [70] | |
| COPD patients | Did not have an overt respiratory depressant effect. | Sun et al. [76] | |
| Healthy adult men and women | Lacked clinically important respiratory effects during sleep. | Uemura et al. [74] | |
| Patients (18–65 years old) with OSA | Did not appear to have clinically important respiratory effects during sleep. | Sun et al. [75] | |
|
Almorexant (ACT-078573) DORA |
Healthy male participants | Equivalent to zolpidem with regard to subjectively assessed alertness. | Hoever et al. [155] |
| Mice | Increased REM and NREM sleep in wild-type mice but not in OX2R-knockout mice. | Mang et al. [43] | |
| Mice | Promoted sleep and exacerbated cataplexy in a mouse model of narcolepsy. | Black et al. [156] | |
| Rats | Promoted sleep without impairing memory performance. | Morairty et al. [157] | |
| Rats | Promoted sleep but was permissive for the activation of wake-promoting systems. | Parks et al. [51] | |
| Male and female adult patients with primary insomnia | Decreased subjective WASO, decreased objective and subjective LPS, decreased the latency to sleep onset, and increased objective and subjective TST. | Black et al. [158] | |
| Elderly patients with primary insomnia | Increased TST, decreased WASO, and decreased LPS. | Roth et al. [159] | |
|
SB-649868 DORA |
Healthy male volunteers | Increased TST and REM sleep duration, decreased WASO and LPS, and decreased the latency to REM sleep; did not affect SWS or EEG power spectra in NREM sleep. | Bettica et al. [67] |
| Male patients with primary insomnia | Decreased LPS, decreased WASO, increased TST, increased REM sleep, and decreased REM sleep latency. | Bettica et al. [68] | |
|
Lemborexant (E2006) DORA |
Mice | Efficacy demonstrated in an in vivo study that used objective sleep parameter measurements. | Yoshida et al. [160] |
| Adults and elderly subjects with insomnia | Improved SE and subjective SE, decreased LPS and subjective sleep onset latency, and decreased WASO and subjective WASO. | Murphy et al. [26] | |
|
Seltorexant (JNJ-42847922 and MIN 202) SORA2 |
Rats, mice, and healthy humans | Reduced latency to NREM sleep and prolonged NREM sleep duration in rats but not in OX2R-knockout mice; increased somnolence in healthy humans. | Bonaventure et al. [102] |
| Individuals with insomnia | Increased TST, decreased LPS, and decreased WASO in individuals with insomnia without psychiatric comorbidity. | De Boer et al. [78] | |
| MDD patients with persistent insomnia | Decreased LPS and increased TST and SE, accompanied by a tendency to subjectively improved mood in antidepressant-treated MDD patients with persistent insomnia. | Brooks et al. [77] | |
|
MK-1064 SORA2 |
Rats, dogs, and healthy humans | Increased NREM and REM sleep. | Gotter et al. [42] |
|
JNJ-54717793 SORA1 |
Rats and mice | Minimal effects on spontaneous sleep in rats and wild-type mice; selectively promoted REM sleep in OX2R-knockout mice. | Bonaventure et al. [45] |
|
IPSU SORA2 |
Mice | Influenced sleep only during the active phase and induced sleep by increasing NREM sleep. | Hoyer et al. [48] |
|
Compound 5 DORA |
Rats | Decreased locomotor activity and the time awake and increased REM sleep and delta sleep. | Whitman et al. [161] |
|
3,9-diazabicyclo[4.2.1]nonanes DORA |
Rats | Better oral bioavailability and exerted sleep-promoting activity in a rat EEG model. | Coleman et al. [162] |
COPD, chronic obstructive pulmonary disease; DORA, dual orexin receptor 1/2 antagonist; E2006, (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide; EEG, electroencephalogram; IPSU, 2-([1H-Indol-3-yl]methyl)9-(4-methoxypyrimidin-2-yl)-2,9-diazaspiro[5.5]undecan-1-one; ISI, Insomnia Severity Index; LPS, latency to persistent sleep; MDD, major depressive disorder; NREM, non-rapid-eye-movement; OSA, obstructive sleep apnea; OX1R, orexin type 1 receptor; OX2R, orexin type 2 receptor; REM, rapid-eye-movement; SE, sleep efficiency; SORA1, selective orexin receptor 1 antagonist; SORA2, selective orexin receptor 2 antagonist; TSO, time to sleep onset; TST, total sleep time; WASO, wake after sleep onset.
Mood Disorders
Orexin receptor antagonists have been implicated in the treatment of depression and anxiety (Table 2). Acute restraint stress activates orexin neurons in the LH and increases orexin-A levels in the VTA [85]. OX1R gene variants are associated with the development of MDD and depressive symptom severity [86]. Systemic administration of the SORA2 LSN2424100 has dose-dependent antidepressant-like effects in a delayed-reinforcement of low-rate assay in rodents, and the SORA2 seltorexant has a tendency to improve mood in antidepressant-treated MDD patients with persistent insomnia [77, 87]. The DORA almorexant prevents chronic unpredictable mild stress-induced depressive-like behavior in mice but does not affect hippocampal cell proliferation or neurogenesis [88]. Suvorexant has also been shown to improve sleep quality and mood in psychiatric inpatients with insomnia symptoms [89]. Another Phase 2 clinical trial reported inconsistent results, in which 6 weeks of treatment with the DORA filorexant did not have significant effects in patients with MDD, but this study had limitations with regard to enrollment challenges and insufficient statistical power, the results of which should be interpreted with caution [90].
Table 2.
Summary of recent findings on orexin receptor antagonists for the treatment of depression and panic/anxiety.
| Disorder | Manipulation | Target | Subjects | Findings | References |
|---|---|---|---|---|---|
| Depression | LSN2424100 | OX2R | Rats | Systemic administration dose-dependently produced antidepressant-like activity. | Fitch et al. [87] |
| Seltorexant | OX2R | MDD patients with persistent insomnia | Induced a tendency toward subjective improvements in mood in antidepressant-treated MDD patients with insomnia. | Brooks et al. [77] | |
| Almorexant | dual OX1/2R | Mice | Induced a robust antidepressant-like effect in the CUMS model of depression, and this effect was neurogenesis-independent. | Nollet et al. [88] | |
| Filorexant (MK-6096) | dual OX1/2R | Patients with MDD | No significant difference was found in the primary endpoint of change after 6 weeks of treatment. | Connor et al. [61] | |
| Suvorexant | dual OX1/2R | Psychiatric patients with insomnia | Overall improvement in the quality of sleep and the severity of anxiety and depression. | Nakamura et al. [89] | |
| Panic/anxiety | JNJ-54717793 | OX1R | Rats | Attenuated CO2- and sodium lactate-induced panic-like behaviors and cardiovascular responses. | Bonaventure et al. [45] |
| Compound 56 | OX1R | Rats | With a DORA, attenuated CO2-induced anxiety-like behaviors; Compound 56 attenuated CO2-induced cardiovascular responses; the SORA2 JnJ10397049 had no effect on panic-like responses. | Johnson et al. [101] | |
| SB-334867 | OX1R | Rats | Intracerebroventricular injections decreased seizures and anxiety in pentylenetetrazol-kindled rats. | Kordi Jaz et al. [97] | |
| Compound 56 | OX1R | Rats and mice | Attenuated sodium lactate-induced panic-like behaviors and cardiovascular responses. | Bonaventure et al. [102] | |
| SB-334867 | OX1R | Rats | Systemic administration blocked panic-like responses. | Johnson et al. [94] | |
| SB-334867 | OX1R | Rats | Systemic administration attenuated CO2-induced pressor and anxiety-like responses. | Johnson et al. [96] | |
| SB-334867 | OX1R | Rats | Attenuated FG-7142-induced anxiety-like behaviors and increased heart rate. | Johnson et al. [100] | |
| SB-334867 | OX1R | Rats | Microinjection into the trigeminal nucleus caudalis inhibited orofacial pain-induced anxiety-like behavior in rats. | Bahaaddini et al. [99] | |
| SB-334867 | OX1R | Rats | Systemic administration had opposite effects on arousal in adolescent and adult males and had no effect on anxiety-like behavior. | Blume et al. [98] |
OX1R, orexin type 1 receptor; OX2R, orexin type 2 receptor; MDD, major depressive disorder; CUMS, chronic unpredictable mild stress; CO2, carbon dioxide; SORA2, selective orexin receptor 2 antagonist; DORA, dual orexin receptor 1/2 antagonist.
Orexin modulates neuronal circuitry that is implicated in the expression and extinction of conditioned fear, suggesting that it may be a promising target for the treatment of anxiety disorders [10, 91]. Panic disorder is a common disabling anxiety disorder characterized by recurrent panic attacks. The orexin system in the medial hypothalamus and orexin-related molecular mechanisms in the amygdala–hippocampus–prefrontal cortex pathway are involved in the behavioral expression of panic disorder [92, 93]. Orexin-related pharmacological targets may be promising for the treatment of panic attacks. Orexin neurons are activated in a rat model of panic disorder, and the orexin levels in cerebrospinal fluid (CSF) increase in humans with panic-related anxiety [94]. OX1R gene variations are associated with the development of panic disorder and the treatment response to cognitive behavioral therapy [95]. Systemic administration of the SORA1 SB-334867 and silencing the orexin gene in the hypothalamus block panic responses and anxiety-like behavior in a rat model of CO2- and sodium lactate-induced panic [94, 96]. Intracerebroventricular injection of SB-334867 decreases seizures and anxiety in pentylenetetrazol-kindled rats [97]. Systemic SB-334867 administration has no effect on anxiety-like behavior in the open field, whereas microinjection of SB-334867 into the trigeminal nucleus caudalis reduces orofacial pain-induced anxiety-like behavior in rats [98, 99]. SB-334867 treatment also attenuates FG-7142-induced anxiety-like behavior, increases heart rate, and increases neuronal activation in anxiety- and stress-related brain regions, such as the CeA and BNST [100].
The novel brain-penetrant SORA1 JNJ-54717793 attenuates CO2- and sodium lactate-induced panic-like behavior and cardiovascular responses [45]. The SORA1 compound 56 also attenuates this behavior and cardiovascular responses without altering baseline locomotor or autonomic activity, while the SORA2 JNJ10397049 has no effects on panic-like responses [101, 102]. The antagonism of OX1Rs in the hypothalamus has been shown to treat panic disorder. However, a previous study showed that OX1R transcript levels increase and OX2R transcripts decrease in the basolateral amygdala in chronic social defeat-susceptible mice. Knockdown of either orexin receptor in the BLA has no effects on depressive-like behavior, whereas OX2R knockdown in the BLA increases anxiety-like behavior [103]. Therefore, the roles of the two orexin receptor subtypes in depressive- and anxiety-like behaviors may be brain region-specific.
Drug Addiction
Some addictive drugs act both locally on orexin neurons in the LH and at orexin terminals in the VTA. Orexin system activation promotes drug-seeking by stimulating postsynaptic OX1Rs and modulating the drug-induced glutamatergic synaptic plasticity in dopamine neurons in the VTA [21]. Hypothalamic orexin neurons co-release orexin and dynorphin in the VTA, and the SORA1 SB334867 has been shown to decrease reward thresholds, impulsive-like behavior, and cocaine self-administration, and these effects are reversed by dynorphin blockade with nor-binaltorphimine [21, 104]. Cocaine has been shown to modulate the σ1 receptor–corticotropin-releasing factor-1 receptor–OX1R complex. The SORA1 SB-334867 blocks the reinstatement of cocaine-induced conditioned place preference (CPP) that is induced by an intra-VTA microinjection of orexin-A [85, 105]. A recent study showed that intermittent access to cocaine produces a multi-endophenotype addiction-like state and persistently increases the number of orexin neurons and orexin neuron activity in the LH. The pharmacological blockade of OX1Rs with SB-334867 and the selective knockdown of orexin neurons in the LH reduce the motivation for cocaine and attenuate the addiction-like phenotype [106]. SB-334867 decreases cocaine self-administration in both rats and female rhesus monkeys. It also reduces the motivation for cocaine-associated cues and blocks the cue-induced reinstatement of cocaine-seeking behavior [107–109]. SB-334867 and almorexant, but not the SORA2 4PT, attenuate the cocaine-induced inhibition of dopamine uptake and reduce cocaine self-administration [110]. Prolonged access to cocaine self-administration enhances γ-aminobutyric acid neurotransmission in the medial CeA, and intra-CeA microinjections of SB-334867 reduce cocaine intake and attenuate the yohimbine-induced reinstatement of cocaine-seeking [111]. Systemic administration of the SORA2 RTIOX-276 decreases responding for cocaine under high-effort conditions and attenuates the cocaine-induced inhibition of dopamine uptake [112]. The DORA suvorexant attenuates the motivational and hedonic properties of cocaine and impulsive cocaine-seeking [113, 114]. The overall abuse liability of suvorexant has been shown to be low in both rodents and healthy recreational polydrug users with a history of sedative and psychedelic drug use [115, 116].
The orexinergic system has been implicated in the motivational effects of alcohol and opioids as well as other addictions [21, 117]. The SORA1s SB-334867 and GSK1059865 decrease alcohol intake and preference and reduce relapse to alcohol drinking but have no effects on sucrose intake [118–120]. SB-334867 selectively reduces alcohol self-administration and cue-induced reinstatement in highly-motivated rats [121]. SB-334867 inhibits the acquisition and expression of locomotor sensitization induced by morphine and amphetamine [122, 123]. SB-334867 also significantly attenuates morphine-induced CPP but does not affect morphine-induced hyperactivity [124]. SB-334867 reduces the motivation for the opioid remifentanil and reduces the cue-induced reinstatement of remifentanil-seeking in low takers [125]. Systemic SB-334867 administration significantly attenuates naloxone-induced morphine withdrawal symptoms and blocks activation of the NAc shell [126]. Intra-LC microinjections of SB-334867 significantly attenuate the somatic signs of morphine withdrawal, and this is blocked by the GABAA receptor antagonist bicuculline [127, 128]. Intra-VTA microinjections of SB-334867 and the DORA2 TCS-OX2-29 significantly attenuate the acquisition and expression of morphine-induced CPP [129]. Intra-dentate gyrus administration of SB-334867 and TCS-OX2-29 attenuates morphine priming-induced reinstatement and the acquisition and expression of LH stimulation-induced CPP in rats [130, 131].
Alcohol intake increases the mRNA levels of OX2Rs but not OX1Rs in the anterior paraventricular thalamus, and microinjections of TCS-OX2-29 but not SB-334867 into this nucleus decrease intermittent-access alcohol drinking [132]. Extended access to intravenous heroin self-administration increases OX2R mRNA levels in the CeA, and systemic administration of the SORA2 NBI-80713 dose-dependently decreases heroin self-administration in prolonged-access rats [133]. The DORA almorexant interferes with the expression of morphine-induced locomotor sensitization, attenuates the expression of cocaine- and amphetamine-induced CPP, and decreases alcohol self-administration [134, 135]. Almorexant does not potentiate the cognitive-impairing effects of alcohol in humans and does not affect motor performance in rats [136, 137]. Sleep disturbances occur during both alcohol exposure and withdrawal. Suvorexant decreases the latency to sleep onset but also exacerbates sleep fragmentation in rats exposed to chronic intermittent alcohol vapor and protracted withdrawal, which may help prevent relapse to alcohol use [138, 139].
Nicotine withdrawal increases the activation of orexin neurons in the LH and activates the PVN. Intra-PVN microinjections of the SORA1 SB334867 attenuate the somatic signs of nicotine withdrawal, but the SORA2 TCS-OX2-29 has no effect [140]. A genome-wide association study showed that the OX2R gene polymorphism Val308Ile is associated with nicotine dependence [141]. The DORA TCS1102 has no significant effects on palatable food self-administration or reinstatement in either hungry or sated rats and does not affect the reinstatement of nicotine-seeking [142, 143]. Administration of the SORA1 SB-334867 and knockdown of the orexin gene in the LH decrease responding for food under both variable-ratio and progressive-ratio schedules of reinforcement (i.e., impair the reinforcing and motivational properties of food) [144]. Contingent self-administration of the synthetic cannabinoid agonist WIN55,212-2 increases the activation of orexin neurons in the LH, and systemic administration of the SORA1 SB334867 but not SORA2 TCS-OX2-29 reduces the reinforcing and motivational properties of WIN55,212-2 [145].
Overall, OX1R antagonists may be useful for the treatment of cocaine and other drug addictions [146, 147] (Table 3).
Table 3.
Summary of recent findings on orexin receptor antagonists for the treatment of drug addiction.
| Addictive drug | Manipulation | Target | Subjects | Findings | References |
|---|---|---|---|---|---|
| Cocaine | Suvorexant | dual OX1/2R | Rats | Attenuated acute cocaine-induced impulsivity when administered systemically or directly into the ventral tegmental area. | Gentile et al. [113] |
| Suvorexant | dual OX1/2R | Rats | Attenuated motivational and hedonic properties of cocaine. | Gentile et al. [114] | |
| SB-334867 | OX1R | Rats | Reduced the acquisition and expression of cocaine self-administration and expression of amphetamine-induced conditioned place preference. | Hutcheson et al. [108] | |
| SB-334867 | OX1R | Rats | Systemic administration decreased cocaine intake specifically in prolonged-access rats; microinjections into the central nucleus of the amygdala reduced cocaine intake in prolonged-access rats and attenuated yohimbine-induced reinstatement of cocaine seeking. | Schmeichel et al. [111] | |
| SB-334867 | OX1R | Mice | Intra-ventral tegmental area microinjections of orexin-A reinstated extinguished cocaine-induced conditioned place preference in wildtype mice, which was blocked by SORA1 SB-334867. | Tung et al. [85] | |
| SB-334867 | OX1R | Mice | Intra-ventral tegmental area injections decreased cocaine self-administration, lateral hypothalamus self-stimulation, and impulsive-like behavior, which were reversed by dynorphin antagonism. | Muschamp et al. [104] | |
| SB-334867 | OX1R | Rats | Reduced the motivation for cocaine and attenuated the addiction phenotype. | James et al. [106] | |
| SB-334867 | OX1R | Female rhesus monkeys | Decreased cocaine self-administration. | Foltin et al. [107] | |
| SB-334867 | OX1R | Rats | Reduced cocaine demand in the presence of cocaine-associated cues and blocked cue-induced reinstatement. | Bentzley et al. [109] | |
| SB-334867 | OX1R | Rats | SB-334867 but not the SORA2 4PT attenuated the cocaine-induced inhibition of dopamine uptake and reduced cocaine self-administration. | Prince et al. [110] | |
| Almorexant | dual OX1/2R | Rats | Attenuated the cocaine-induced inhibition of dopamine uptake and reduced cocaine self-administration. | Prince et al. [110] | |
| RTIOX-276 | OX1R | Rats | Systemic administration decreased high-effort responding for cocaine and reduced the inhibition of cocaine-induced dopamine uptake. | Levy et al. [112] | |
| Alcohol | Suvorexant | dual OX1/2R | Rats | Dose-dependently decreased the latency to REM sleep and SWS onset and produced REM sleep and SWS fragmentation in rats exposed to chronic intermittent alcohol and protracted withdrawal. | Sanchez-Alavez et al. [138] |
| Almorexant | dual OX1/2R | Healthy humans | Did not potentiate impairing effects of alcohol. | Hoch et al. [137] | |
| Almorexant | dual OX1/2R | Rats | Systemic or intra-ventral tegmental area administration decreased alcohol self-administration. | Srinivasan et al. [135] | |
| Almorexant | dual OX1/2R | Rats | Did not interfere with forced motor performance or grip strength in rats and did not further increase the sedative effects of alcohol. | Steiner et al. [136] | |
| SB-334867 | OX1R | Rats | Intra-nucleus accumbens infusions decreased alcohol intake and preference. | Mayannavar et al. [118] | |
| SB-334867 | OX1R | Rats | Reduced alcohol relapse drinking. | Dhaher et al. [120] | |
| SB-334867 | OX1R | Rats | Attenuated alcohol self-administration and the cue-induced reinstatement of alcohol-seeking in highly motivated rats. | Moorman et al. [121] | |
| GSK1059865 | OX1R | Mice | Reduced alcohol drinking in alcohol-dependent mice but had no effect on sucrose intake. | Lopez et al. [119] | |
| TCS-OX2-29 | OX2R | Rats | Microinjections of TCS-OX2-29 but not SB-334867 into the anterior paraventricular nucleus of the thalamus decreased intermittent-access alcohol drinking. | Barson et al. [132] | |
| Opioids | SB-334867 | OX1R | Mice | Inhibited the acquisition of morphine-induced locomotor sensitization. | Łupina et al. [123] |
| SB-334867 | OX1R | Rats | Intra-locus coeruleus microinjections significantly attenuated somatic signs of naloxone-induced morphine withdrawal, which was blocked by the GABAA receptor antagonist bicuculline. | Davoudi et al. [127] | |
| SB-334867 | OX1R | Rats | Intra-locus coeruleus microinjections attenuated the expression of glutamate-induced morphine withdrawal during the active phase. | Hooshmand et al. [128] | |
| SB-334867 | OX1R | Mice | Systemic administration significantly attenuated naloxone-induced morphine withdrawal symptoms. | Sharf et al. [126] | |
| SB-334867 | OX1R | Mice | Significantly attenuated morphine-induced conditioned place preference but not morphine-induced hyperactivity or sensitization. | Sharf [144] | |
| SB-334867 | OX1R | Rats | Intra-ventral tegmental area microinjections significantly attenuated the acquisition and expression of morphine-induced conditioned place preference. | Farahimanesh et al. [129] | |
| SB-334867 | OX1R | Rats | Intra-dentate gyrus administration dose-dependently attenuated morphine priming-induced reinstatement. | Ebrahimian et al. [130] | |
| SB-334867 | OX1R | Rats | Reduced motivation and the cue-induced reinstatement of remifentanil-seeking in low takers. | Porter-Stransky et al. [125] | |
| NBI-80713 | OX2R | Rats | Systemic administration dose-dependently decreased heroin self-administration in prolonged-access rats and had no effect on food pellet self-administration. | Schmeichel et al. [133] | |
| TCS-OX2-29 | OX2R | Rats | Intra-ventral tegmental area microinjections significantly attenuated the acquisition and expression of morphine-induced conditioned place preference. | Farahimanesh et al. [129] | |
| TCS-OX2-29 | OX2R | Rats | Intra-dentate gyrus administration dose-dependently attenuated morphine priming-induced reinstatement. | Ebrahimian et al. [130] | |
| Amphetamine | Almorexant | dual OX1/2R | Rats | Attenuated the expression of cocaine- and amphetamine-induced conditioned place preference but not morphine-induced conditioned place preference; interfered with the expression of morphine-induced locomotor sensitization but not cocaine- or amphetamine-induced locomotor sensitization. | Steiner et al. [134] |
| SB-334867 | OX1R | Rats | Reduced amphetamine-induced dopamine outflow in the nucleus accumbens shell and decreased the expression of amphetamine sensitization. | Quarta et al. [122] | |
| Cannabis | SB334867 | OX1R | Mice | Systemic SB334867 administration but not the SORA2 TCSOX229 reduced the reinforcing and motivational properties of the synthetic cannabinoid agonist WIN55,212-2. | Flores et al. [145] |
| Nicotine | TCS 1102 | dual OX1/2R | Rats | No effect on the reinstatement of nicotine seeking. | Khoo et al. [143] |
| SB334867 | OX1R | Mice | SB334867 but not the SORA2 TCS-OX2-29 attenuated somatic signs of nicotine withdrawal. | Plaza-Zabala et al. [140] |
OX1R, orexin type 1 receptor; OX2R, orexin type 2 receptor; REM, rapid-eye-movement; SWS, slow-wave sleep; SORA1, selective orexin receptor 1 antagonist; SORA2, selective orexin receptor 2 antagonist; DORA, dual orexin receptor 1/2 antagonist.
Further Challenges and Unique Opportunities for the Application of Orexin Receptor Antagonists to Treating Psychiatric Disorders
Significant progress has been made in understanding the role of the orexin system in physiological processes and pathological behaviors. Hypothalamic orexin neurons have extensive projections throughout the central nervous system and act as regulators of sleep-to-wake transitions, emotion, and motivation [6]. Technical advances have allowed more precise manipulation of orexin neurons and their target brain regions, revealing the dysregulation of orexin function in various psychiatric disorders. Targeting the orexin system may be a promising strategy for the treatment of such disorders. Suvorexant has been found to promote sleep and was the first orexin receptor antagonist to be approved by the FDA in 2014 for the treatment of primary insomnia [25, 148]. The approval of suvorexant for the treatment for insomnia has helped pave the way for the development of more orexin receptor antagonists for the treatment of other disorders, such as anxiety and addiction. Further research is needed to optimize orexin receptor antagonists across diverse structural classes with better physicochemical properties (e.g., better solubility, higher functional and binding potencies at orexin receptors), and better pharmacokinetics (e.g., slower blood clearance, faster distribution, higher oral bioavailability, a plasma half-life that is appropriate for rapid sleep onset and maintenance, brain penetration, and in vivo activity) [149].
Hypothalamic orexin neurons have extensive projections to stress-sensitive brain regions, including the amygdala, BNST, and mPFC. The orexin system plays a critical role in the pathophysiology of stress-related psychiatric disorders [20, 32]. Patients with panic-related anxiety exhibit elevated orexin-A levels in the CSF, and patients with depression have low CSF orexin-A levels [20]. Given the role of the orexin system in the modulation of stress reactivity and fear responses, orexin-related pharmacological targets may represent promising opportunities for the treatment of depression and anxiety. However, clinical trials have reported inconsistent effects in the case of depression. Suvorexant attenuates anxiety and depressive symptoms in psychiatric patients with insomnia, and seltorexant has a tendency to subjectively improve depressive symptoms in antidepressant-treated MDD patients with persistent insomnia, whereas filorexant is not effective in patients with MDD [77, 89, 90]. Orexin system function is downregulated under conditions of chronic stress [20, 150], which may limit the application of orexin receptor antagonists for depression treatment. Nonetheless, SORA1s have been reported to consistently reduce panic- and anxiety-like behaviors in rodents. Further clinical trials are warranted to evaluate their efficacy in patients with anxiety and panic disorders.
Accumulating evidence indicates that the orexin system plays an important role in cocaine, opioid, alcohol, and other addictions. Orexin receptor antagonists, especially SORA1s, reduce drug-induced CPP and decrease the reinforcing and motivational properties of addictive drugs and relapse. Exposure to addictive drugs activates orexin neurons in the LH and regulates glutamatergic or GABAergic neurotransmission within orexin neuron-projecting brain regions, such as the VTA and CeA [104, 106, 111]. Orexin receptor antagonism in these regions also attenuates drug-seeking behavior and relapse. However, most of the currently available data have been derived from preclinical studies, and future work is needed to evaluate the efficacy of orexin receptor antagonists for the clinical treatment of addiction.
In conclusion, orexin receptor antagonism appears to be a very promising therapeutic avenue for the treatment of insomnia and other psychiatric disorders. Targeting the orexin system represents a breakthrough in our understanding of sleep disorders and the treatment of anxiety and drug addiction, although more preclinical and clinical studies are clearly needed.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81701312 and 81521063) and the Interdisciplinary Medicine Seed Fund of Peking University (BMU2018MX024).
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Ying Han and Kai Yuan have contributed equally to this work.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuki T, Sakurai T. Orexins and orexin receptors: from molecules to integrative physiology. Results Probl Cell Differ. 2008;46:27–55. doi: 10.1007/400_2007_047. [DOI] [PubMed] [Google Scholar]
- 5.Boss C, Roch C. Recent trends in orexin research–2010 to 2015. Bioorg Med Chem Lett. 2015;25:2875–2887. doi: 10.1016/j.bmcl.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Nevarez N, de Lecea L. Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Res 2018, 7: F1000 Faculty Rev-1421. [DOI] [PMC free article] [PubMed]
- 7.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 8.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 10.Flores A, Saravia R, Maldonado R, Berrendero F. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci. 2015;38:550–559. doi: 10.1016/j.tins.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nat Rev Neurosci. 2019;20:83–93. doi: 10.1038/s41583-018-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klockars A, Levine AS, Olszewski PK. Hypothalamic integration of the endocrine signaling related to food intake. Curr Top Behav Neurosci. 2018 doi: 10.1007/7854_2018_54. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa M, Parsons MP, Alberto CO. Interaction between orexins and the mesolimbic system for overriding satiety. Rev Neurosci. 2007;18:383–393. doi: 10.1515/revneuro.2007.18.5.383. [DOI] [PubMed] [Google Scholar]
- 14.Morello G, Imperatore R, Palomba L, Finelli C, Labruna G, Pasanisi F, et al. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proc Natl Acad Sci U S A. 2016;113:4759–4764. doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cun Y, Tang L, Yan J, He C, Li Y, Hu Z, et al. Orexin A attenuates the sleep-promoting effect of adenosine in the lateral hypothalamus of rats. Neurosci Bull. 2014;30:877–886. doi: 10.1007/s12264-013-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8:28. doi: 10.3389/fnins.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James MH, Campbell EJ, Dayas CV. Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr Top Behav Neurosci. 2017;33:197–219. doi: 10.1007/7854_2016_56. [DOI] [PubMed] [Google Scholar]
- 21.Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172:334–348. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boss C, Roch C. Orexin research: patent news from 2016. Expert Opin Ther Pat. 2017;27:1123–1133. doi: 10.1080/13543776.2017.1344221. [DOI] [PubMed] [Google Scholar]
- 23.Coleman PJ, Gotter AL, Herring WJ, Winrow CJ, Renger JJ. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509–533. doi: 10.1146/annurev-pharmtox-010716-104837. [DOI] [PubMed] [Google Scholar]
- 24.Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2017;35:1–7. doi: 10.1016/j.smrv.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan C. Insomniacs get new mechanism sleep drug Belsomra. Nat Biotechnol. 2014;32:968. doi: 10.1038/nbt1014-968. [DOI] [PubMed] [Google Scholar]
- 26.Murphy P, Moline M, Mayleben D, Rosenberg R, Zammit G, Pinner K, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13:1289–1299. doi: 10.5664/jcsm.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 2014;8:36. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93:747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XY, Yu L, Zhuang QX, Zhu JN, Wang JJ. Central functions of the orexinergic system. Neurosci Bull. 2013;29:355–365. doi: 10.1007/s12264-012-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores A, Maldonado R, Berrendero F. Cannabinoid-hypocretin cross-talk in the central nervous system: what we know so far. Front Neurosci. 2013;7:256. doi: 10.3389/fnins.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Sargin D. The role of the orexin system in stress response. Neuropharmacology. 2019;154:68–78. doi: 10.1016/j.neuropharm.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 34.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takenoshita S, Sakai N, Chiba Y, Matsumura M, Yamaguchi M, Nishino S. An overview of hypocretin based therapy in narcolepsy. Expert Opin Investig Drugs. 2018;27:389–406. doi: 10.1080/13543784.2018.1459561. [DOI] [PubMed] [Google Scholar]
- 36.Nepovimova E, Janockova J, Misik J, Kubik S, Stuchlik A, Vales K, et al. Orexin supplementation in narcolepsy treatment: a review. Med Res Rev. 2019;39:961–975. doi: 10.1002/med.21550. [DOI] [PubMed] [Google Scholar]
- 37.Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114:5731–5736. doi: 10.1073/pnas.1700499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res 2018; e-pub ahead of print 24 December 2018; 10.1016/j.brainres.2018.12.036. [DOI] [PMC free article] [PubMed]
- 39.Staton CD, Yaeger JDW, Khalid D, Haroun F, Fernandez BS, Fernandez JS, et al. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology. 2018;143:79–94. doi: 10.1016/j.neuropharm.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M, Gee CE, et al. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56:7590–7607. doi: 10.1021/jm4007627. [DOI] [PubMed] [Google Scholar]
- 41.Etori K, Saito YC, Tsujino N, Sakurai T. Effects of a newly developed potent orexin-2 receptor-selective antagonist, compound 1 m, on sleep/wakefulness states in mice. Front Neurosci. 2014;8:8. doi: 10.3389/fnins.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gotter AL, Forman MS, Harrell CM, Stevens J, Svetnik V, Yee KL, et al. Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci Rep. 2016;6:27147. doi: 10.1038/srep27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mang GM, Durst T, Burki H, Imobersteg S, Abramowski D, Schuepbach E, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35:1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, et al. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci. 2010;32:1528–1536. doi: 10.1111/j.1460-9568.2010.07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonaventure P, Dugovic C, Shireman B, Preville C, Yun S, Lord B, et al. Evaluation of JNJ-54717793 a novel brain penetrant selective orexin 1 receptor antagonist in two rat models of panic attack provocation. Front Pharmacol. 2017;8:357. doi: 10.3389/fphar.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 47.Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 48.Hoyer D, Durst T, Fendt M, Jacobson LH, Betschart C, Hintermann S, et al. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7:235. doi: 10.3389/fnins.2013.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuneki H, Kon K, Ito H, Yamazaki M, Takahara S, Toyooka N, et al. Timed inhibition of orexin system by suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology. 2016;157:4146–4157. doi: 10.1210/en.2016-1404. [DOI] [PubMed] [Google Scholar]
- 50.Li SB, Nevarez N, Giardino WJ, de Lecea L. Optical probing of orexin/hypocretin receptor antagonists. Sleep. 2018 doi: 10.1093/sleep/zsy141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parks GS, Warrier DR, Dittrich L, Schwartz MD, Palmerston JB, Neylan TC, et al. The dual hypocretin receptor antagonist almorexant is permissive for activation of wake-promoting systems. Neuropsychopharmacology. 2016;41:1144–1155. doi: 10.1038/npp.2015.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68:1429–1441. doi: 10.1111/ijcp.12568. [DOI] [PubMed] [Google Scholar]
- 53.Kishi T, Matsunaga S, Iwata N. Suvorexant for primary insomnia: a systematic review and meta-analysis of randomized placebo-controlled trials. PLoS One. 2015;10:e0136910. doi: 10.1371/journal.pone.0136910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee-Iannotti JK, Parish JM. Suvorexant: a promising, novel treatment for insomnia. Neuropsychiatr Dis Treat. 2016;12:491–495. doi: 10.2147/NDT.S31495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia – critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239–247. doi: 10.2147/NSS.S76910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25:791–802. doi: 10.1016/j.jagp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79:136–148. doi: 10.1016/j.biopsych.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12:1215–1225. doi: 10.5664/jcsm.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Clinical profile of suvorexant for the treatment of insomnia over 3 months in women and men: subgroup analysis of pooled phase-3 data. Psychopharmacology (Berl) 2017;234:1703–1711. doi: 10.1007/s00213-017-4573-1. [DOI] [PubMed] [Google Scholar]
- 60.Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connor KM, Mahoney E, Jackson S, Hutzelmann J, Zhao X, Jia N, et al. A phase II dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychopharmacol. 2016;19:pyw022. doi: 10.1093/ijnp/pyw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawabe K, Horiuchi F, Ochi M, Nishimoto K, Ueno SI, Oka Y. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27:792–795. doi: 10.1089/cap.2016.0206. [DOI] [PubMed] [Google Scholar]
- 63.Herring WJ, Connor KM, Snyder E, Snavely DB, Morin CM, Lines C, et al. Effects of suvorexant on the Insomnia Severity Index in patients with insomnia: analysis of pooled phase 3 data. Sleep Med. 2019;56:219–223. doi: 10.1016/j.sleep.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:461–471. doi: 10.1016/S1474-4422(14)70053-5. [DOI] [PubMed] [Google Scholar]
- 65.Ma J, Svetnik V, Snyder E, Lines C, Roth T, Herring WJ. Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects. Sleep. 2014;37:1609–1619. doi: 10.5665/sleep.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snyder E, Ma J, Svetnik V, Connor KM, Lines C, Michelson D, et al. Effects of suvorexant on sleep architecture and power spectral profile in patients with insomnia: analysis of pooled phase 3 data. Sleep Med. 2016;19:93–100. doi: 10.1016/j.sleep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012;37:1224–1233. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bettica P, Squassante L, Zamuner S, Nucci G, Danker-Hopfe H, Ratti E. The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. Sleep. 2012;35:1097–1104. doi: 10.5665/sleep.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamuro A, Honda M, Wakaura Y. Suvorexant for the treatment of insomnia in patients with Alzheimer’s disease. Aust N Z J Psychiatry. 2018;52:207–208. doi: 10.1177/0004867417747402. [DOI] [PubMed] [Google Scholar]
- 70.Toi N, Inaba M, Kurajoh M, Morioka T, Hayashi N, Hirota T, et al. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Transl Endocrinol. 2019;15:37–44. doi: 10.1016/j.jcte.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawada K, Ohta T, Tanaka K, Miyamura M, Tanaka S. Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients. J Stroke Cerebrovasc Dis. 2019;28:142–148. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki H, Hibino H, Inoue Y, Mikami A, Matsumoto H, Mikami K. Reduced insomnia following short-term administration of suvorexant during aripiprazole once-monthly treatment in a patient with schizophrenia. Asian J Psychiatr. 2017;28:165–166. doi: 10.1016/j.ajp.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Tabata H, Kuriyama A, Yamao F, Kitaguchi H, Shindo K. Suvorexant-induced dream enactment behavior in parkinson disease: a case report. J Clin Sleep Med. 2017;13:759–760. doi: 10.5664/jcsm.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uemura N, McCrea J, Sun H, Donikyan M, Zammit G, Liu R, et al. Effects of the orexin receptor antagonist suvorexant on respiration during sleep in healthy subjects. J Clin Pharmacol. 2015;55:1093–1100. doi: 10.1002/jcph.523. [DOI] [PubMed] [Google Scholar]
- 75.Sun H, Palcza J, Card D, Gipson A, Rosenberg R, Kryger M, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2016;12:9–17. doi: 10.5664/jcsm.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun H, Palcza J, Rosenberg R, Kryger M, Siringhaus T, Rowe J, et al. Effects of suvorexant, an orexin receptor antagonist, on breathing during sleep in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109:416–426. doi: 10.1016/j.rmed.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Brooks S, Jacobs GE, de Boer P, Kent JM, Van Nueten L, van Amerongen G, et al. The selective orexin-2 receptor antagonist seltorexant improves sleep: An exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol. 2019;33:202–209. doi: 10.1177/0269881118822258. [DOI] [PubMed] [Google Scholar]
- 78.De Boer P, Drevets WC, Rofael H, van der Ark P, Kent JM, Kezic I, et al. A randomized Phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32:668–677. doi: 10.1177/0269881118773745. [DOI] [PubMed] [Google Scholar]
- 79.Asai Y, Sano H, Miyazaki M, Iwakura M, Maeda Y, Hara M. Suvorexant (Belsomra((R)) tablets 10, 15, and 20 mg): Japanese drug-use results survey. Drugs R D. 2019;19:27–46. doi: 10.1007/s40268-018-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6:189–195. doi: 10.1177/2042098615595359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340–2372. doi: 10.1016/j.clinthera.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Hatano M, Kamei H, Inagaki R, Matsuzaki H, Hanya M, Yamada S, et al. Assessment of switching to suvorexant versus the use of add-on suvorexant in combination with benzodiazepine receptor agonists in insomnia patients: a retrospective study. Clin Psychopharmacol Neurosci. 2018;16:184–189. doi: 10.9758/cpn.2018.16.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sutton EL. Profile of suvorexant in the management of insomnia. Drug Des Devel Ther. 2015;9:6035–6042. doi: 10.2147/DDDT.S73224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrous J, Furmaga K. Adverse reaction with suvorexant for insomnia: acute worsening of depression with emergence of suicidal thoughts. BMJ Case Rep 2017, 2017: bcr-2017-222037. [DOI] [PMC free article] [PubMed]
- 85.Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, et al. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat Commun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cengiz M, Karaj V, Kocabasoglu N, Gozubatik-Celik G, Dirican A, Bayoglu B. Orexin/hypocretin receptor, Orx1, gene variants are associated with major depressive disorder. Int J Psychiatry Clin Pract. 2019;23:114–121. doi: 10.1080/13651501.2018.1551549. [DOI] [PubMed] [Google Scholar]
- 87.Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes MM, et al. LSN2424100: a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Front Neurosci. 2014;8:5. doi: 10.3389/fnins.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37:2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura M, Nagamine T. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov Clin Neurosci. 2017;14:30–37. [PMC free article] [PubMed] [Google Scholar]
- 90.Connor KM, Ceesay P, Hutzelmann J, Snavely D, Krystal AD, Trivedi MH, et al. Phase II proof-of-concept trial of the orexin receptor antagonist filorexant (MK-6096) in patients with major depressive disorder. Int J Neuropsychopharmacol. 2017;20:613–618. doi: 10.1093/ijnp/pyx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dustrude ET, Caliman IF, Bernabe CS, Fitz SD, Grafe LA, Bhatnagar S, et al. Orexin depolarizes central amygdala neurons via orexin receptor 1, phospholipase c and sodium-calcium exchanger and modulates conditioned fear. Front Neurosci. 2018;12:934. doi: 10.3389/fnins.2018.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park SC, Kim YK. A novel bio-psychosocial-behavioral treatment model of panic disorder. Psychiatry Investig. 2019;16:4–15. doi: 10.30773/pi.2018.08.21.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abreu AR, Molosh AI, Johnson PL, Shekhar A. Role of medial hypothalamic orexin system in panic, phobia and hypertension. Brain Res 2018. 10.1016/j.brainres.2018.09.010. [DOI] [PubMed]
- 94.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gottschalk MG, Richter J, Ziegler C, Schiele MA, Mann J, Geiger MJ, et al. Orexin in the anxiety spectrum: association of a HCRTR1 polymorphism with panic disorder/agoraphobia, CBT treatment response and fear-related intermediate phenotypes. Transl Psychiatry. 2019;9:75. doi: 10.1038/s41398-019-0415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37:1911–1922. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kordi Jaz E, Moghimi A, Fereidoni M, Asadi S, Shamsizadeh A, Roohbakhsh A. SB-334867, an orexin receptor 1 antagonist, decreased seizure and anxiety in pentylenetetrazol-kindled rats. Fundam Clin Pharmacol. 2017;31:201–207. doi: 10.1111/fcp.12249. [DOI] [PubMed] [Google Scholar]
- 98.Blume SR, Nam H, Luz S, Bangasser DA, Bhatnagar S. Sex- and age-dependent effects of orexin 1 receptor blockade on open-field behavior and neuronal activity. Neuroscience. 2018;381:11–21. doi: 10.1016/j.neuroscience.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahaaddini M, Khatamsaz S, Esmaeili-Mahani S, Abbasnejad M, Raoof M. The role of trigeminal nucleus caudalis orexin 1 receptor in orofacial pain-induced anxiety in rat. Neuroreport. 2016;27:1107–1113. doi: 10.1097/WNR.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 100.Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, et al. Orexin 1 and 2 receptor involvement in CO2 -induced panic–associated behavior and autonomic responses. Depress Anxiety. 2015;32:671–683. doi: 10.1002/da.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, et al. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015;352:590–601. doi: 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, et al. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 2014;40:17–26. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Navarro G, Quiroz C, Moreno-Delgado D, Sierakowiak A, McDowell K, Moreno E, et al. Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J Neurosci. 2015;35:6639–6653. doi: 10.1523/JNEUROSCI.4364-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2019;85:925–935. doi: 10.1016/j.biopsych.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foltin RW, Evans SM. Hypocretin/orexin antagonists decrease cocaine self-administration by female rhesus monkeys. Drug Alcohol Depend. 2018;188:318–327. doi: 10.1016/j.drugalcdep.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- 109.Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine–associated cues. Eur J Neurosci. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmeichel BE, Herman MA, Roberto M, Koob GF. Hypocretin neurotransmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol Psychiatry. 2017;81:606–615. doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, Espana RA. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology (Berl) 2017;234:2761–2776. doi: 10.1007/s00213-017-4673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, et al. Effects of suvorexant, a dual orexin/hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology. 2018;43:1001–1009. doi: 10.1038/npp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gentile TA, Simmons SJ, Barker DJ, Shaw JK, Espana RA, Muschamp JW. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol. 2018;23:247–255. doi: 10.1111/adb.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schoedel KA, Sun H, Sellers EM, Faulknor J, Levy-Cooperman N, Li X, et al. Assessment of the abuse potential of the orexin receptor antagonist, suvorexant, compared with zolpidem in a randomized crossover study. J Clin Psychopharmacol. 2016;36:314–323. doi: 10.1097/JCP.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 116.Born S, Gauvin DV, Mukherjee S, Briscoe R. Preclinical assessment of the abuse potential of the orexin receptor antagonist, suvorexant. Regul Toxicol Pharmacol. 2017;86:181–192. doi: 10.1016/j.yrtph.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 117.Anderson RI, Moorman DE, Becker HC. Contribution of dynorphin and orexin neuropeptide systems to the motivational effects of alcohol. Handb Exp Pharmacol. 2018;248:473–503. doi: 10.1007/164_2018_100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mayannavar S, Rashmi KS, Rao YD, Yadav S, Ganaraja B. Effect of Orexin A antagonist (SB-334867) infusion into the nucleus accumbens on consummatory behavior and alcohol preference in Wistar rats. Indian J Pharmacol. 2016;48:53–58. doi: 10.4103/0253-7613.174528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez MF, Moorman DE, Aston-Jones G, Becker HC. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016;1636:74–80. doi: 10.1016/j.brainres.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, et al. The Orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in alcohol-preferring (P) rats. J Addict Med. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2017;1654:34–42. doi: 10.1016/j.brainres.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 123.Lupina M, Tarnowski M, Baranowska-Bosiacka I, Talarek S, Listos P, Kotlinska J, et al. SB-334867 (an Orexin-1 Receptor Antagonist) effects on morphine-induced sensitization in mice-a view on receptor mechanisms. Mol Neurobiol. 2018;55:8473–8485. doi: 10.1007/s12035-018-0993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res. 2010;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2017;22:303–317. doi: 10.1111/adb.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davoudi M, Azizi H, Mirnajafi-Zadeh J, Semnanian S. The blockade of GABAA receptors attenuates the inhibitory effect of orexin type 1 receptors antagonist on morphine withdrawal syndrome in rats. Neurosci Lett. 2016;617:201–206. doi: 10.1016/j.neulet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 128.Hooshmand B, Azizi H, Javan M, Semnanian S. Intra-LC microinjection of orexin type-1 receptor antagonist SB-334867 attenuates the expression of glutamate-induced opiate withdrawal like signs during the active phase in rats. Neurosci Lett. 2017;636:276–281. doi: 10.1016/j.neulet.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 129.Farahimanesh S, Zarrabian S, Haghparast A. Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides. 2017;66:45–51. doi: 10.1016/j.npep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 130.Ebrahimian F, Naghavi FS, Yazdi F, Sadeghzadeh F, Taslimi Z, Haghparast A. Differential roles of orexin receptors within the dentate gyrus in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Behav Neurosci. 2016;130:91–102. doi: 10.1037/bne0000112. [DOI] [PubMed] [Google Scholar]
- 131.Parsania S, Moradi M, Fatahi Z, Haghparast A. Involvement of orexin-1 and orexin-2 receptors within the dentate gyrus of the hippocampus in the acquisition, expression and extinction of lateral hypothalamic-induced conditioned place preference in the rats. Brain Res. 2016;1639:149–160. doi: 10.1016/j.brainres.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 132.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, et al. Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology. 2015;40:1123–1129. doi: 10.1038/npp.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steiner MA, Lecourt H, Jenck F. The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol. 2013;16:417–432. doi: 10.1017/S1461145712000193. [DOI] [PubMed] [Google Scholar]
- 135.Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, et al. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One. 2012;7:e44726. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steiner MA, Lecourt H, Strasser DS, Brisbare-Roch C, Jenck F. Differential effects of the dual orexin receptor antagonist almorexant and the GABA(A)-alpha1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology. 2011;36:848–856. doi: 10.1038/npp.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoch M, Hay JL, Hoever P, de Kam ML, te Beek ET, van Gerven JM, et al. Dual orexin receptor antagonism by almorexant does not potentiate impairing effects of alcohol in humans. Eur Neuropsychopharmacol. 2013;23:107–117. doi: 10.1016/j.euroneuro.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 138.Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL. Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep 2019, 42: zsz020. [DOI] [PMC free article] [PubMed]
- 139.Campbell EJ, Marchant NJ, Lawrence AJ. A sleeping giant: suvorexant for the treatment of alcohol use disorder? Brain Res 2018; e-pub ahead of print 3 August 2018; 10.1016/j.brainres.2018.08.005. [DOI] [PubMed]
- 140.Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry. 2012;71:214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 141.Nishizawa D, Kasai S, Hasegawa J, Sato N, Yamada H, Tanioka F, et al. Associations between the orexin (hypocretin) receptor 2 gene polymorphism Val308Ile and nicotine dependence in genome-wide and subsequent association studies. Mol Brain. 2015;8:50. doi: 10.1186/s13041-015-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khoo SY, Clemens KJ, McNally GP. Palatable food self-administration and reinstatement are not affected by dual orexin receptor antagonism. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:147–157. doi: 10.1016/j.pnpbp.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 143.Khoo SY, McNally GP, Clemens KJ. The dual orexin receptor antagonist TCS1102 does not affect reinstatement of nicotine-seeking. PLoS One. 2017;12:e0173967. doi: 10.1371/journal.pone.0173967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Flores A, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry. 2014;75:499–507. doi: 10.1016/j.biopsych.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 146.Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, Espana RA. Hypocretin receptor 1 involvement in cocaine-associated behavior: Therapeutic potential and novel mechanistic insights. Brain Res. 2018 doi: 10.1016/j.brainres.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 147.Perrey DA, Zhang Y. Therapeutics development for addiction: orexin-1 receptor antagonists. Brain Res. 2018 doi: 10.1016/j.brainres.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dubey AK, Handu SS, Mediratta PK. Suvorexant: The first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 2015;6:118–121. doi: 10.4103/0976-500X.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coleman PJ, Cox CD, Roecker AJ. Discovery of dual orexin receptor antagonists (DORAs) for the treatment of insomnia. Curr Top Med Chem. 2011;11:696–725. doi: 10.2174/1568026611109060696. [DOI] [PubMed] [Google Scholar]
- 150.Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 151.Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, et al. Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62:978–987. doi: 10.1016/j.neuropharm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 152.Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 153.Nishimura S, Nakao M. Cost-effectiveness analysis of suvorexant for the treatment of Japanese elderly patients with chronic insomnia in a virtual cohort. J Med Econ. 2018;21:698–703. doi: 10.1080/13696998.2018.1466710. [DOI] [PubMed] [Google Scholar]
- 154.Svetnik V, Snyder ES, Tao P, Scammell TE, Roth T, Lines C, et al. Insight Into reduction of wakefulness by suvorexant in patients with insomnia: analysis of wake bouts. Sleep. 2018 doi: 10.1093/sleep/zsx178. [DOI] [PubMed] [Google Scholar]
- 155.Hoever P, de Haas S, Winkler J, Schoemaker RC, Chiossi E, van Gerven J, et al. Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther. 2010;87:593–600. doi: 10.1038/clpt.2010.19. [DOI] [PubMed] [Google Scholar]
- 156.Black SW, Morairty SR, Fisher SP, Chen TM, Warrier DR, Kilduff TS. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36:325–336. doi: 10.5665/sleep.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morairty SR, Wilk AJ, Lincoln WU, Neylan TC, Kilduff TS. The hypocretin/orexin antagonist almorexant promotes sleep without impairment of performance in rats. Front Neurosci. 2014;8:3. doi: 10.3389/fnins.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Black J, Pillar G, Hedner J, Polo O, Berkani O, Mangialaio S, et al. Efficacy and safety of almorexant in adult chronic insomnia: a randomized placebo-controlled trial with an active reference. Sleep Med. 2017;36:86–94. doi: 10.1016/j.sleep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 159.Roth T, Black J, Cluydts R, Charef P, Cavallaro M, Kramer F, et al. Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 2017 doi: 10.1093/sleep/zsw034. [DOI] [PubMed] [Google Scholar]
- 160.Yoshida Y, Naoe Y, Terauchi T, Ozaki F, Doko T, Takemura A, et al. Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluor opyridin-2-yl)cyclopropanecarboxamide (E2006): a potent and efficacious oral orexin receptor antagonist. J Med Chem. 2015;58:4648–4664. doi: 10.1021/acs.jmedchem.5b00217. [DOI] [PubMed] [Google Scholar]
- 161.Whitman DB, Cox CD, Breslin MJ, Brashear KM, Schreier JD, Bogusky MJ, et al. Discovery of a potent, CNS-penetrant orexin receptor antagonist based on an n,n-disubstituted-1,4-diazepane scaffold that promotes sleep in rats. ChemMedChem. 2009;4:1069–1074. doi: 10.1002/cmdc.200900069. [DOI] [PubMed] [Google Scholar]
- 162.Coleman PJ, Schreier JD, Roecker AJ, Mercer SP, McGaughey GB, Cox CD, et al. Discovery of 3,9-diazabicyclo[4.2.1]nonanes as potent dual orexin receptor antagonists with sleep-promoting activity in the rat. Bioorg Med Chem Lett. 2010;20:4201–4205. doi: 10.1016/j.bmcl.2010.05.047. [DOI] [PubMed] [Google Scholar]