Abstract
The objective of this study was to evaluate the effect of yellow mealworm (Tenebrio molitor L.) exuviae (ME) given as a prebiotic in 20% of the diet fed to BALB/c mice. Analysis of the ME revealed that it was mostly composed of crude protein (52.94%), crude fiber (10.70%), and moisture (10.54%). When ME was fed to mice for 8 weeks, the number of intestinal lactic acid bacteria increased, reaching similar numbers (4.50 ± 0.80 CFU/mL) to those (4.70 ± 0.80 CFU/mL) of the control group not fed ME. Microbiome analysis showed that 8 weeks feeding of ME promoted the growth of Bifidobacteriaceae and Lactobacillaceae compared to the POS group, indicating the positive effects of feeding 20% ME on the intestinal microbiota of mice. These results suggest that ME can be considered as a dietary prebiotics to improve human gut microbial population, but further application study to human is necessary.
Keywords: Mealworm, Exuviae, Microbiome, Lactic acid bacteria, Bifidobacteriaceae
Introduction
About 1700 insects are consumed as food in the world (Siemianowska et al., 2013). Many countries are still consuming insects as important protein sources. However, only a few people accept insects as foods because many people in developed countries perceive insects as “disgusting stuff”. Although many insects seem to be unacceptable to consumers in the commercial market, the nutritional value of insects is too important to be neglected. Actually, insects are valuable sources of protein, fat, mineral, and vitamins (Kim et al., 2014). Among the valuable nutrients, common edible insects contain protein contents of around 9–25%, similar to that of meat, which is 15–22% (Yi et al., 2013). Due to increase in the world population, the demand for valuable protein sources is also increasing. Only limited animal protein can be generated because land for domestic animals is limited. For this reason, many people try to cultivate edible insects. Some edible insects are also reared in Korea. Only a few types of insects are cultivated and consumed as pet feed in the Korean commercial market, unlike in America and Africa. Due to this global trend, the Korean government is trying to support the edible insect market as part of the agricultural economy. It seems that the attitude of Korean consumers can be refined to accept edible insects. Then, various products related to edible insects could be developed.
The yellow mealworm (Tenebrio molitor L.), a pest of flour and grain, is one of the main insects that can be used as a food or feedstuff. Yellow mealworm larvae contain high-quality protein and fat. Due to their nutritional value, they are commonly used in food formulations (Ravzanaadii et al., 2012). Depending on the lifecycle, insects can be classified as either holometabolous or hemimetabolous insects. The yellow mealworm is a holometabolous insect that undergoes a true metamorphosis from egg or embryo to larva to pupa to adult (Rumpold and Schluter, 2013). During the metamorphosis of larvae into yellow mealworm pupa, many exuviae (insect exoskeletons) are generated. They are mainly composed of chitin (Kim et al., 2014; Song et al., 2018) which is the second most abundant biopolymer. Since chitin has a wide range of biological activities, both the food and cosmetic industries use chitin to improve their quality, as well as their shelf-life. Moreover, chitin is considered a source of dietary fiber which provides a beneficial environment for bacteria in the gastrointestinal tract (Hamed et al., 2016; Ringø et al., 2012). For this reason, chitin from yellow mealworm exuviae may have potential as a prebiotic source. Thus, the objective of this study was to evaluate the potential of mealworm exuviae (ME) as a prebiotic by analyzing the gastrointestinal microbiota in BALB/c mice using next-generation sequencing (NGS) after feeding it to mice.
Materials and methods
Mealworm exuviae preparation
All mealworms for ME production were cared at Gochang Insect Farm (Gochang, Jeollabukdo, Korea) under the guideline of RDA (2013), and ME was collected once a week for 16 weeks and then stored at − 70 °C until used. All MEs were roughly washed with double distilled water (DDW) for 5 min (1:10,000 w/v), dried at 60 °C for 24 h and then powdered for BALB/c animal model diet manufacturing.
Chemical composition, fiber, and total caloric analysis of ME
To analyze the chemical composition of ME, the moisture, crude protein, crude ash, crude fat and crude fiber contents of ME were measured according to the method of AOAC (1995). The results are presented as percentages (%).
Animal model
Five-week-old BALB/c male mice (15–20 g) were purchased from Samtako Co., Ltd. (Osan, Korea) and raised at 22 ± 2 °C with 50 ± 5% of humidity. All mice were fed an AIN-76A diet (Research Diets, Inc., New Brunswick, NJ, USA) and sterilized water ad libitum for a week as an adaptation period. The mice were then randomly divided into three different groups (n = 20 per group), and four mouse per group were housed in three different cage. All mouse were freely accessed either AIN-74 (CON), the positive control (POS), or the mealworm exuviae (MWE) diet for 4 or 8 weeks. The nutritional compositions of the diets are shown in Table 1. The control diet contained only 50 g/kg cellulose (Vitcel L 600, J. Retenmaier & Söhne, Rosenberg, Germany) but the POS and mealworm exuviae (MWE) diets had 200 g/kg cellulose or ME, respectively. Two different sacrification time was used in each group (4 and 8 weeks, n = 10 each). Each mice was sacrificed via CO2 asphyxiation. The average mice weights for two different scarification were 22.14 ± 0.75 g at 4 weeks (p > 0.05) and 26.49 ± 0.78 g at 8 weeks (p > 0.05), respectively. The small intestines and ceca were collected for microbial analysis. Only 7 cecum samples were used for gut microbiota analysis, and each cecum sample was selected based on average weight at final sacrification week. To enumerate the bacteria, each small intestine was mixed with peptone water for 2 min, then the total bacteria (TBC), lactic acid bacteria (LAB), total coliforms (TCF), and Escherichia coli (ECL) were counted. All cecum samples were prepared for NGS analysis. The experimental protocols in this study were approved by the Institutional Animal Care and Use Committee (IACUC) for the care and use of laboratory animals of the Berry & Biofood Research Institute (BBRI-IACUC-17006).
Table 1.
Dietary composition
| Nutritional composition | CONa | POS | MWE | |||
|---|---|---|---|---|---|---|
| g | Kcal | g | Kcal | g | Kcal | |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 |
| Methionine | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 150 | 600 | 90 | 360 | 90 | 360 |
| Sucrose | 500 | 2000 | 290 | 1160 | 290 | 1160 |
| Cellulose | 50 | 0 | 200 | 0 | 200 (MWE) | 0 |
| Corn oil | 50 | 450 | 170 | 1530 | 170 | 1530 |
| Mineral | 35 | 0 | 35 | 0 | 35 | 0 |
| Vitamin | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 |
| Total | 1000 | 3902 | 1000 | 3902 | 1000 | 3902 |
aCON = negative Control; POS = positive Control; mealworm exuviae = MWE
Microbial enumeration
The small intestine samples mixed with peptone water described previously were serially (tenfold) diluted and 1 mL of each diluent was plated onto 3 M™ Petrifilm™ (3MSt. Paul, MN, USA), followed by incubation at 30 or 37 °C for 48 h. 3 M™ Petrifilm™ Aerobic Count Plates were used for the TBC, LAB, TCF, and ECL counts. After incubation, the typical colonies on each Petrifilm were manually counted.
Gut microbiota analysis
Total DNA was extracted from the cecum samples using the PowerSoil DNA Isolation Kit (Mo Bio Laboratories Inc., Solana Beach, CA, USA). The amplification of partial sequences of the 16S rRNA genes was performed based on the 16S rRNA amplification protocol of the Earth Microbiome Project (Gilbert et al., 2010). For each sample, the 16S rRNA genes were amplified using the 515F/806R primer set (including an adapter sequence) for amplification of the V4 region (515F forward primer: 5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG GTG CCA GCM GCC GCG GTA A-3′; 806R reverse primer: 5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGG ACT ACH VGG GTW TCT AAT-3′). To attach dual indices and adapters to the amplified polymerase chain reaction (PCR) products, an index PCR was performed using AmpONE™ α-Pfu DNA polymerase (GeneAll, Seoul, Korea) and a Nextera® XT Index Kit v2 (Illumina, San Diego, CA, USA). After amplification, the PCR products were purified using Expin™ PCR SV (GeneAll). Sequencing of the partial bacterial 16S rRNA genes was performed using the MiSeq Reagent Kit V3 (600 cycles) and MiSeq platform (Illumina).
Prior to the analysis of the 16S rRNA sequences, the BCL files were converted into raw FASTQ files, including read1, index and read2 sequences, using CASAVA-1.8.2. After pre-processing (quality filtering and trimming steps using the FASTX-Toolkit), the sequences were assigned to operational taxonomic units (OTUs; 97% identity). Representative sequences were selected using QIIME 1.7.0 software (Caporaso et al., 2010), followed by analyses of the taxonomic composition, alpha diversity, and beta diversity. Linear discriminant analysis (LDA) effect size (LEfSe) was used to estimate the taxonomic abundance and characterize differences between the groups (Segata et al., 2011). A heat map of functional gene abundance was generated using MultiExperiment Viewer (MEV) software (ver. 4.8.1; http://www.tm4.org/).
Statistical analyses
All values are expressed as mean ± standard deviation (SD) for each group. The mean values were statistically analyzed by analysis of variance (ANOVA) at p < 0.05 with Duncan’s multiple range test using SPSS (version 12.0, SPSS Inc, IBM Co., Armonk, NY, USA). The gut microbiota relative abundance analyses were performed using LEfSe based on the Kruskal–Wallis and Wilcoxon tests and significance was defined as p < 0.05. The threshold of the logarithmic LDA score was set as 3.0. Statistical significance was assessed by one-way analysis of variance followed by Duncan’s post hoc test. All statistical analyses were performed using RStudio (RStudio Inc., Boston, MA, USA). Statistical significance was considered at p< 0.05.
Results and discussion
Characteristics of mealworm exuviae (ME)
The approximate composition of ME cast off during mealworm larva growth is shown in Table 2. The most abundant compound in ME was crude protein (54.18%), followed by crude fiber (26.48%), and moisture (9.26%). Ravzanaadii et al. (2012) reported that ME was mainly composed of crude protein, moisture, crude fiber, crude fat, and crude ash at percentages of 32.87, 13.02, 25.96, 3.59, and 3.22%, respectively, indicating higher moisture and fat contents than those found in the present study.
Table 2.
General composition of mealworm exuviae
| Moia (%) | C.P. | C. Ash | C. Fat | C. Fib | |
|---|---|---|---|---|---|
| MWEb | 9.26 ± 0.07 | 54.18 ± 0.17 | 4.21 ± 0.02 | 2.97 ± 0.04 | 26.48 ± 0.14 |
aMoi = moisture; C.P = crude protein; C. Ash = crude ash; C. Fat = crude fat; C. Fib = crude fiber
bMWE = mealworm exuviae
Total bacteria, total coliform, E. coli, and lactic acid bacteria count
BALB/c mice small intestines were used for the TBC, TCF, ECL, and LAB counts (Table 3). For 4 weeks feeding, the highest TBC count (3.5 ± 0.5 log CFU/mL) was found in the POS group. However, no significant difference in TBC was observed between the CON and POS groups. A similar trend in the TCF and ECL counts was observed between the CON and POS groups. Significantly reduced numbers of TCF and ECL were found in BALB/c mice fed 20% ME diet for 4 weeks compared to the POS mice. There was no significant difference in LAB numbers between the groups. Prebiotics that are nondigestible in the small intestine but are fermentable in the colon by endogenous bacteria can stimulate only limited bacterial species, including Lactobacilli and Bifidobacteria (Figueroa-Gonzalez et al., 2011; Quigley and Quera, 2006; Schrezenmeir and de Vrese, 2001). Since chitin has prebiotic effects (Hamed et al., 2016; Ringø et al., 2012), the chitin in ME (Song et al., 2018) seems to be able to promote the growth of LAB in BALB/c mice. As a result, the TCF and ECL numbers decreased due to environmental changes in the small intestine. In this study, both the POS and MWE diets had higher corn oil contents than the CON diet and feeding the ME supplement resulted in lower numbers of TCF and ECL in the MWE mice compared to those of the POS mice. Supplementation with 20% ME reduced both the TCF and ECL numbers, which subsequently decreased the TBCs as a result.
Table 3.
Total bacteria, total coliform, E. coli, and lactic acid bacteria composition of small intestines of BALB/c mice fed with 20% mealworm exuviae for 4 and 8 weeks
| (Log CFU/mL) | ||||
|---|---|---|---|---|
| TBC1 | TCF | ECL | LAB | |
| 4 week feeding | ||||
| CON2 | 3.3 ± 0.8ab | 1.9 ± 1.2ab | 1.9 ± 1.2ab | 2.9 ± 0.9 |
| POS | 3.5 ± 0.5a | 2.2 ± 0.6a | 2.2 ± 0.6a | 2.9 ± 0.5 |
| MWE | 3.0 ± 0.5b | 1.3 ± 1.3b | 1.3 ± 1.3b | 3.1 ± 0.7 |
| 8 week feeding | ||||
| CON | 4.1 ± 0.9a | 2.0 ± 1.2 | 2.0 ± 1.2 | 4.7 ± 0.8a |
| POS | 3.5 ± 0.9ab | 1.5 ± 0.7 | 1.4 ± 0.8 | 3.4 ± 0.9b |
| MWE | 3.1 ± 0.7b | 1.9 ± 0.6 | 1.9 ± 0.6 | 4.5 ± 0.8a |
1TBC = total bacteria; TCF = total coliforms; ECL = E. coli; LAB = lactic acid bacteria
2CON = negative control; POS = positive control; MWE = mealworm exuviae
a,bValues followed by different letters are significantly different (p < 0.05) according to Duncan’s multiple range test
For 8 weeks of feeding, mice in the CON groups had higher numbers of TBC than the MWE mice, while a significant difference was not found between the CON and POS groups (Table 3). Unlike the 4 weeks feeding, no difference in TCF and ECL numbers was found between the CON, POS, and MWE groups fed for 8 weeks, probably due to the extended feeding period. Higher LAB counts were observed when the CON and MWE diets were fed to mice for 8 weeks compared to the POS group. However, the number of LAB between the CON and MWE-fed mice was not significantly different, indicating that 20% ME mitigated the high corn oil content effect of the mice diet and strongly influenced the population of LAB in the small intestine of the MWE mice. Based on the results for the two different feeding periods, the chitin of ME, a prebiotic, influenced the BALB/c small intestine over the 8 weeks feeding period. The environment in the small intestine was modulated during the first 4 weeks feeding period to reduce the TCF and ECL numbers and provide conditions favorable to microorganisms, including Lactobacilli species, by 8 weeks (Manning and Gibson, 2004). Therefore, high numbers of LAB (4.5 ± 0.8 log CFU/mL) were found in the small intestine of mice fed MWE for 8 weeks compared mice fed ME for 4 weeks (3.1 ± 0.7 log CFU/mL).
Analysis of gut microbiota
A total of 137,898 ± 22,591 bacteria in reading number was acquired. Lower average bacterial numbers were present when each experimental diet was supplied to mice for 8 weeks compared to 4 weeks (Fig. 1A). No significant difference in diversity was observed among the groups through Shannon-diversity plots from alpha-diversity analysis (Fig. 1B). Since alpha-diversity indicates the richness of the species diversity, these results indicate that there was no difference in the diversity of the microbial communities among the groups tested in this study. Unlike alpha-diversity, beta-diversity showed different compositional diversity of bacteria in the MWE samples of mice fed 20% ME for 4 or 8 weeks (Fig. 1C). The plots showed that all MWE samples were below 0.0 on the PC2 axis compared to the other groups and were aligned parallel to the PC1 axis. Moreover, the 8 weeks MWE samples (MWE8) were mostly over 0.0 on the PC1 axis, revealing high dissimilarity in the gut microbial community of mice fed ME for 4 or 8 weeks.
Fig. 1.
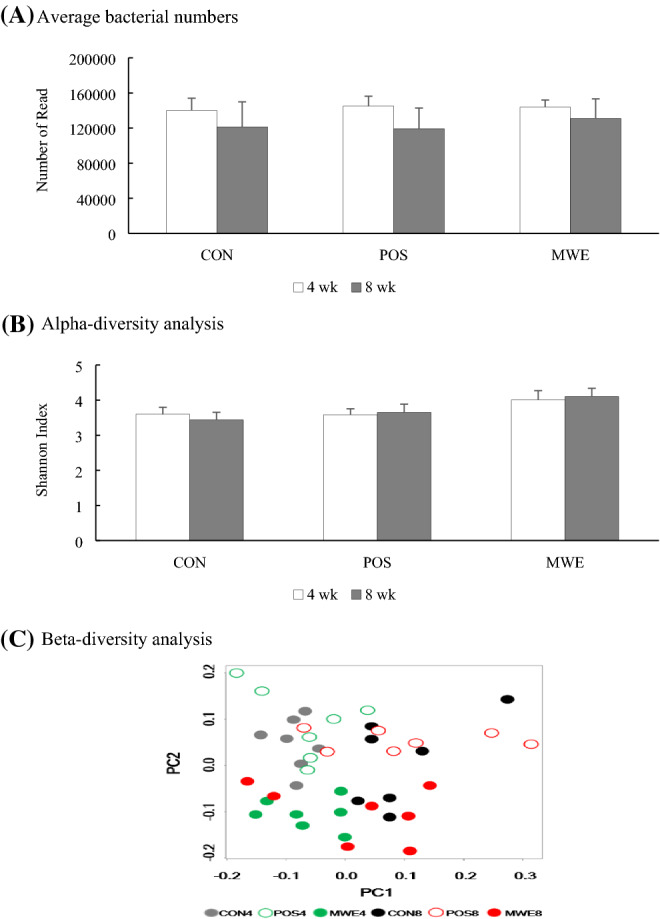
The total number of bacteria counted for next-generation sequencing (NGS) and alpha- and beta-diversity analysis depending on the groups and feeding periods. (A) The total number of bacteria counted for NGS. (B) Alpha-diversity analysis. (C) Beta-diversity analysis
Figure 2A shows that the cecal microbiota were dominated by Bacteroidetes (69.6 and 57.7%), Firmicutes (17.3 and 23.6%), and Proteobacteria (4.1 and 8.8%) in mice fed ME for 4 or 8 weeks, respectively. The Bacteroidetes, Firmicutes, and Proteobacteria phyla accounted for 90–91% of the entire phyla in this study, similar to the results of de Oliveira et al. (2016) and Kim et al. (2017). Unlike mice, Scott et al. (2013) reported that Bacteroidetes, Firmicutes, and Actinobacteria were three major phyla found in the human large intestine. The only difference was Actinobacteria. This difference might be due to the different subject samples used for microbiome analyses. Deferribacteres and others were minor abundant phyla in the mice small intestines. The ratio of Firmicutes to Bacteriodetes is shown on Fig. 2B, and it seems to associate with people with obesity (Lin et al., 2019; Wang et al., 2019). Both Yatsunenko et al. (2012) and Ley et al. (2006) reported that obese people had high Firmicutes to Bacteriodetes ratios, and the Firmicutes to Bacteriodtes ratio seems to ameliorate glucose and fat metabolism (Lin et al., 2019; Wang et al., 2019). The Bacteriodetes increased when they lost weight. However, when ME was supplied to mice for 4 to 8 weeks, the number of Firmicutes sharply increased and the Firmicutes to Bacteroidetes ratio increased about twofold in mice fed ME for 8 weeks (Fig. 2B). The average ratio of Firmicutes to Bacteroidetes is still under the average of POS ratio, which provide high fat diet to mouse for 4 and 8 weeks.
Fig. 2.
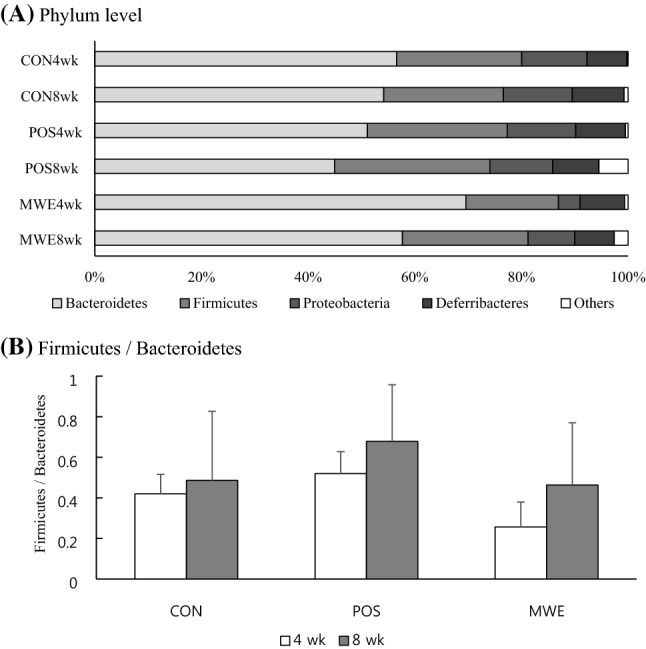
The relative abundance of the major bacteria in BALB/c mice small intestines distinguished by groups and feeding periods. (A) Phylum level bacteria. (B) Firmicutes to Bacteroidetes ratio
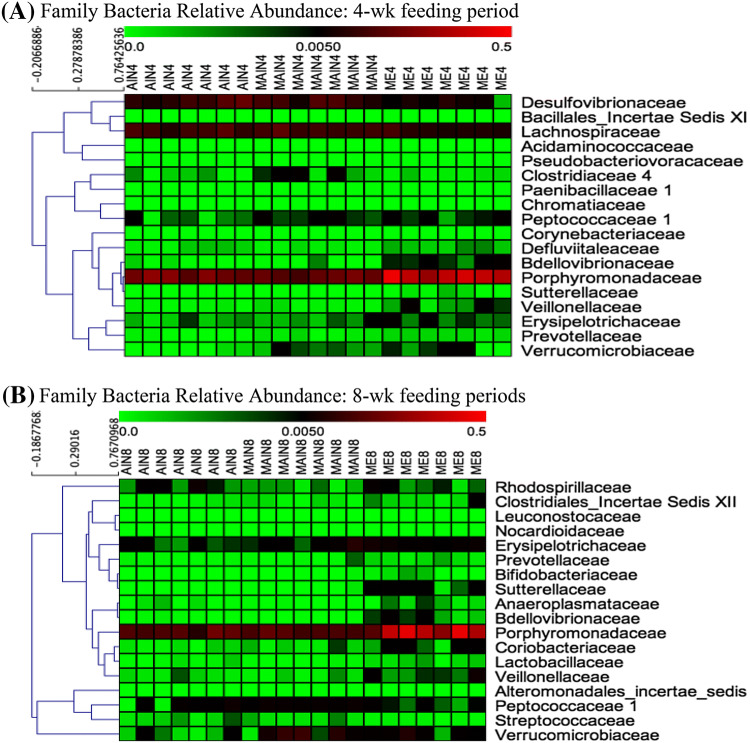
The relative abundance of different families of bacteria found after 4 and 8 weeks feeding periods is shown in Fig. 3. Among 18 total bacterial families identified after 4 weeks of feeding, only 8 bacterial families remained after an additional 4 weeks of feeding. They were Peptococcaceae, Bdellovibrionaceae, Porphyromonadaceae, Sutterellaceae, Veillonellaceae, Erysipelotrichaceae, Prevotellaceae, and Verrucomicrobiaceae. Moreover, 10 bacterial families diminished, while 10 new bacterial families appeared after 20% ME chow diet was fed to mice for 8 weeks. In mice fed 20% ME diet for 8 weeks, bacteria from the Bifidobacteriaceae and Lactobacillaceae families were present. Many studies have insisted that diets can strongly affect intestinal microbiota diversity and composition (Ringø et al., 2012; Scott et al., 2013). According to de Oliveira et al. (2016), microbes in the human gut are normally influenced by the percentage of calories from carbohydrates and lipids. Although calories in this study were fixed at 3902 kcal, the changes in both microbial diversity and composition were due to calories from either carbohydrates or lipids. Both the POS and MWE diets had higher contents of corn oil than the CON diet and the major microbial differences between the CON and both the POS and MWE diets seem to be due to the low carbohydrate calories and high lipids in the POS and MWE diets. In addition, another reason for the different microbial content in the small intestine of the POS and MWE mice might be the ME content in the mice diet. Specifically, the chitin of ME has an acetamido group structure and the acetamido groups at C2 of chitin are converted into amino groups which are electrostatically charged (BeMiller, 2018; Do et al., 2018). Such charges may change the intestinal environmental conditions by binding substances, including fatty acids and bile acids, which facilitates the growth of both Bifidobacteriaceae and Lactobacillaceae (Aranaz et al., 2009).
Fig. 3.
Relative abundance of major bacteria in BALB/c mice small intestine distinguished by groups and feeding periods. (A) Family level bacteria in the small intestine of BALB/c mice fed an experimental diet for 4 weeks (B) Family level bacteria in the small intestine of BALB/c mice fed an experimental diet for 8 weeks. AIN4: CON4, MAIN4: POS4, MIE4: MWE4, AIN8: CON8, MAIN8: POS8, MIE8: MWE8
In conclusion, this study analyzed the composition of mealworm exuviae which was mainly composed of protein, moisture, and fat. Also, the present results showed that ME feeding increased the LAB content in mice small intestines. Moreover, feeding ME to mice for 8 weeks promoted the growth of both Bifidobacteriaceae and Lactobacillaceae, indicating that ME feeding could positively influence the murine small intestine environment. Thus, this study suggests the potential of ME as a prebiotic for human gut health. However, further study is necessary to define how ME provides environmental conditions that promote the growth of both Bifidobacteriaceae and Lactobacillaceae in the small intestine.
Acknowledgements
This study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through Technology Commercialization Support Program and the High Value-added Food Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (817025-03-2-HD030) and (No. 315056-03), Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest related to this study to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gyoo Taik Kwon, Email: kgt486@naver.com.
Hyun-Gyun Yuk, Email: yukhg@ut.ac.kr.
Su Jung Lee, Email: 2-su@hanmail.net.
Yi Hyung Chung, Email: foodie@jib.re.kr.
Han Su Jang, Email: jhs@jib.re.kr.
Jong-Sang Yoo, Email: jsyou@daehanfeed.co.kr.
Kyung-Hoon Cho, Email: khcho@daehanfeed.co.kr.
Hyunseok Kong, Email: hskong0813@gmail.com.
Daekeun Shin, Email: aceflavor@hotmail.com.
References
- AOAC. Official methods of analysis. 16th ed. Association of official analytical chemists. Washington DC., USA (1995)
- Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, Galed G, Heras A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009;3:203–230. [Google Scholar]
- BeMiller JN. Carbohydrate chemistry for food scientists. 3rd ed. Woodhead Publishing. p. 342 (2018)
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira FP, Mendes RH, Dobbler PT, Mai V, Pylro VS, Waugh SG, Vairo F, Refosco LF, Roesch LF, Schwartz IVD. Phenylketonuria and gut microbiota: a controlled study based on next-generation sequencing. PLoS One. 2016;11:e0157513. doi: 10.1371/journal.pone.0157513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AR, Cho SJ, Cho YY, Kwon EY, Choi JY, Lee JH, Han Y, Kim YS, Piao Z, Shin YC. Choi MS Antiobesity effects of short-chain chitosan in diet-induced obese mice. J. Med. Food. 2018;21:927–934. doi: 10.1089/jmf.2017.4115. [DOI] [PubMed] [Google Scholar]
- Figueroa-Gonzalez I, Quijano G, Ramirez G, Cruz-Guerrero A. Probiotics and prebiotics–perspectives and challenges. J. Sci. Food Agr. 2011;91(8):1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Meyer F, Antonopoulos D, Balaji P, Brown CT, Desai N, Eisen JA, Evers D, Field D, Feng W, Huson D, Jasson J, Knight R, Knight J, Kolker E, Konstantindis K, Kostka J, Kyrpides N, Mackelprang R, McHardy A, Quince C, Raes J, Sczyrba A, Shade A, Stevens R. Meeting report: the terabase metagenomics workshop and the vision of an earth microbiome project. Stand. Genom. Sci. 2010;3:243–248. doi: 10.4056/sigs.1433550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed I, Ozogul F, Regenstein JM. Industrial applications of crustacean by-products (chitin, chitosan and chitooligosaccharides): a review. Trends Food Sci. Technol. 2016;48:40–50. doi: 10.1016/j.tifs.2015.11.007. [DOI] [Google Scholar]
- Kim SG, Kim JE, Oh HK, Kang SJ, Koo HY, Kim HJ, Choi HC, Sun SS. Feed supplementation of yellow mealworms (Tenebrio molitor L.) improves blood characteristics and meat quality in broiler. J. Agric. Sci. Technol. 49: 9-18 (2014)
- Kim SA, Park SH, Lee SI, Owens CM, Ricke SC. Assessment of chicken carcass microbiome responses during processing in the presence of commercial antimicrobials using a next generation sequencing approach. Sci. Rep. UK. 2017;7:43354. doi: 10.1038/srep43354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lin S, Wang Z, Lam K-L, Zeng S, Tan BK, Hu J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019;63:1518. doi: 10.29219/fnr.v63.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning TS, Gibson GR. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004;18:287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Quigley EMM, Quera R. Small intestinal bacterial overgrowth: role of antibiotics, prebiotics and probiotics. Gastroenterology. 2006;130:S78–S90. doi: 10.1053/j.gastro.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Ravzanaadii N, Kim S-H, Choi WH, Hong S-J, Kim NJ. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol. 2012;25:93–98. [Google Scholar]
- Ringø E, Zhou Z, Olsen RE, Song SK. Use of chitin and krill in aquaculture—the effect on gut microbiota and the immune system: a review. Aquac. Nutr. 2012;18(2):117–131. doi: 10.1111/j.1365-2095.2011.00919.x. [DOI] [Google Scholar]
- Rumpold BA, Schluter OK. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013;17:1–11. doi: 10.1016/j.ifset.2012.11.005. [DOI] [Google Scholar]
- Rural Development Administration (RDA). Rearing standards and specifications for beneficial insect [I]. Elim. Suwon, Korea. pp. 250-269 (2013)
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics and synbiotics—approaching a definition. Am. J. Clin. Nutr. 2001;73:361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemianowska E, Kosewska A, Aljewicz M, Skibniewska KA, Polak-Juszczak L, Jarocki A, Jedras M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 4: 287-291 (2013)
- Song Y-S, Kim M-W, Moon C, Seo D-J, Han YS, Jo YH, Noh MY, Park Y-K, Kim S-A, Kim YW, Jung W-J. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm. Tenebrio molitor. Entomol. Res. 2018;48:227–233. doi: 10.1111/1748-5967.12304. [DOI] [Google Scholar]
- Wang Z, Lam K-L, Hu J, Ge S, Zhou A, Zheng B, Zeng S, Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7:579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Lakemond CMM, Sagis LMC, Eisner-Schadler V, van Huis A, van Boekel MAJS. Extraction and characterization of protein fractions from five insect species. Food Chem. 2013;141:3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]