Abstract
Propolis is known to have multiple biological and pharmacological properties including the regulation of energy homeostasis. Although phenolic compounds are considered to be the major active components in propolis, there is little information available about their mechanisms underlying the regulation of energy homeostasis. In this study, the effects of five phenolic compounds in propolis, chrysin, pinocembrin, galangin, pinobanksin, and caffeic acid phenethyl ester (CAPE) were evaluated on the activation of free fatty acid receptor 4 (FFA4), which are involved in the control of energy homeostasis by enhancing insulin signaling, increasing glucose uptake, and regulating adipogenesis. The results showed that three phenolic compounds exhibited the activation of FFA4, which were ranked in the order of pinocembrin, CAPE and pinobanksin in FFA4-expressing cells. These results suggest that some phenolic compounds in propolis, particularly pinocembrin, may affect the control of energy homeostasis via the activation of FFA4.
Keywords: Free fatty acid receptor 4, Energy homeostasis, Propolis phenolic compounds, Pinocembrin
Introduction
Propolis is an adhesive and resinous substance, commonly collected by honeybees (Apis mellifera L.) from resin present in cracks of tree barks and leaf buds (Burdock, 1998). Propolis is known to have multiple biological and pharmacological properties including the regulation of energy homeostasis (Fuliang et al., 2005; Kitamura et al., 2013; Nakajima et al., 2016). However, the mechanisms underlying the regulation of energy homeostasis by propolis have not been fully elucidated. Although more than 300 compounds have been found in propolis which include phenolic acids, cinnamic acids, caffeic acids and their esters, flavonoids (flavones, flavanones, flavonols, dihydroflavonols, and chalcones), terpenes, aromatic aldehydes and alcohols, fatty acids, stilbenes, and steroids (Akyol et al., 2013; Li et al., 2016), studies of propolis components on the mechanisms of energy homeostasis regulation are very limited until now. Chrysin, pinocembrin, galangin, pinobanksin and caffeic acid phenethyl ester (CAPE) were reported as bioactive components in propolis (Huang et al., 2014). Moreover, the five components were abundantly present in the variety of propolis. The contents of chrysin, pinocembrin, galangin, pinobanksin and CAPE have been reported as 169.7–9940.3 µg, 361.2–13,992 µg, 22.4–2589 µg, 82.1–1235 µg, and 37.7–402.4 µg in 1 g of dry extract, respectively (Kasiotis et al., 2017). We focused on the five components as they are representative components in bioactivity and contents in propolis.
Luminal long-chain fatty acids (LCFAs) are sensed by free fatty acid receptor (FFA) 4, previously known as G protein-coupled receptor 120, located on the enteroendocrine cells in the proximal and distal intestine, lung, adipocyte, pancreas and the brain (Depoortere et al., 2015; Hara et al., 2014). The activation of FFA4 peripherally and centrally controls physiological processes associated with energy homeostasis, including gastrointestinal peptide hormone secretion, islet function, food preference, appetite control, adipogenic differentiation, insulin sensitization and anti-obesity effects (Auguste et al., 2016; Im, 2018; Liu et al., 2015). Naturally occurring dietary LCFAs, including α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, have been identified as FFA4 agonists (Moniri, 2016).
Based on the aforementioned reports which support the regulatory activity of propolis on multiple functions, we hypothesized that propolis components could activate FFA4. In this study, the effects of five phenolic compounds, chrysin, galangin, pinocembrin, pinobanksin and CAPE were evaluated on the activation of FFA4 in the cells stably transfected with human FFA4.
Materials and methods
Cell culture
Human FFA4 cells, which are human embryonic kidney (HEK) 293 cells stably transfected with human FFA4 and Galpha16, were purchased from Charles River Laboratories Inc. (San Diego, CA, USA). HEK 293 cells were obtained from the American Type Culture Collection (ATCC No. CCL-1573; Manassas, VA, USA). FFA4 and HEK 293 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco Co., Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco Co.), 100 IU/mL penicillin (Gibco Co.) and 100 mg/mL streptomycin (Gibco Co.) at 37 °C in 5% CO2, respectively.
Measurement of cytotoxicity in cells
The cytotoxic effects of test agents on FFA4 and HEK 293 cells were examined by measuring the release of lactate dehydrogenase (LDH; a stable cytosolic enzyme) into the supernatant of the cells exposed to test agents, respectively. The cell densities plated in microplates were same as in the measurements of FFA4 activation mentioned below. The incubation time with test agents was 1 h in the cells. The cell-free culture supernatant was collected and incubated with the reaction mixture from LDH cytotoxicity detection kit (Clontech laboratories, California, USA). Cytotoxicity was calculated as the relative release (%) of LDH after exposure to test agents compared the total LDH (100%) released upon treatment with a lysis reagent (Nakajima et al., 2014).
Measurement of changes in intracellular Ca2+ concentration in FFA4 and HEK 293 cells
In vitro calcium flux assay serves to assess agonistic activity of test agents against the human FFA4. The assay utilized HEK 293 cells stably transfected to express the human FFA4 (FFA4 cells). FFA4 and HEK 293 cells were seeded at 2.0 × 104 cells/well in 96-well clear-bottomed black plates (Corning Inc., Bedford, MA, USA) for 48 h at 37 °C, respectively. Then the cells were loaded with the calcium-sensitive dye from the FLIPR Calcium 5 Assay Kit (Molecular Devices, Sunnyvale, CA, USA) for 1 h at 37 °C, respectively. Upon stimulation, intracellular Ca2+ released can bind to the dye and alter its fluorescence intensity. This change in fluorescence signal, and thus the flux in the concentration of intracellular Ca2+ ([Ca2+]i) is detected and quantitated by fluorescence imaging using a FLIPR reader. Fluorescence values of excitation at 485 nm and emission at 525 nm were monitored at 2-s intervals for 120 s using a FLIPR reader FlexStation 3 (Molecular Devices) at 25 °C. The values of each well were detected and results were calculated as ΔRFU (delta relative fluorescent units), i.e., (maximum fluorescent value) - (minimum fluorescent value). The data are reported as the mean ± SEM of the ΔRFU (Kim et al., 2014).
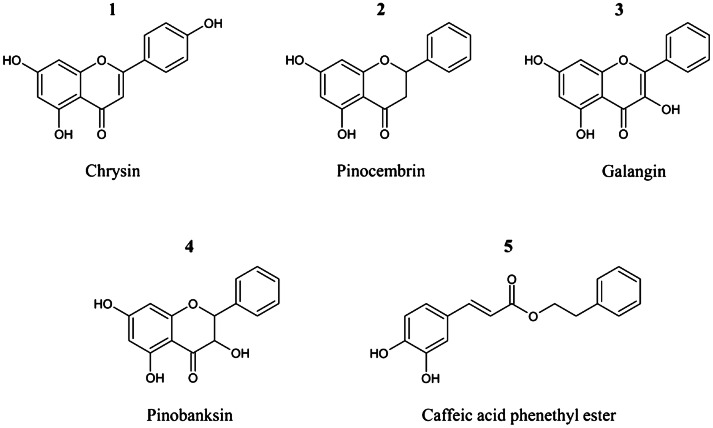
Chrysin (5,7-dihydroxyflavone), pinocembrin (5,7-dihydroxyflavanone), galangin (3,5,7-trihydroxyflavone), pinobanksin (3,5,7-trihydroxyflavanone), CAPE (Fig. 1) and alpha (α)-linolenic acid was from Sigma Aldrich Co. (St. Louis, MO, USA). The chemicals were first dissolved in dimethyl sulfoxide (DMSO) and diluted to a final concentration of 0.1% (v/v) DMSO in cells.
Fig. 1.
Structures of five phenolic compounds in propolis
Statistical analysis
Experiments were performed at least three separate experiments. All values are expressed as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey’s test and Student’s t-test was used for statistical analysis (IBM SPSS statistics 20 software, IBM Corp., Armonk, NY, USA).
Results and discussion
To examine whether the tested compounds induce cell lysis, LDH release was measured in FFA4, and HEK 293 cells. The propolis compounds and α-linolenic acid at the concentrations from 0.01 to 100 μM were applied to FFA4, and HEK 293 cells for 1 h. None of the tested compounds induced LDH release (data not shown). Therefore, we added the tested compounds at the concentrations from 0.01 to 100 μM for the calcium flux assay using FFA4 and HEK 293 cells.
To evaluate whether the five compounds activate FFA4, we tested for their ability to induce the elevation of [Ca2+]i in FFA4 cells. The treatment with α-linolenic acid, an agonist of FFA4, significantly increased [Ca2+]i in FFA4 cells, compared to the treatment in HEK 293 cells that were not transfected with FFA4 (Fig. 2). Chrysin and galangin slightly increased [Ca2+]i in FFA4 cells in a dose-independent manner at the concentrations from 0.01 to 10 µM, and the half maximal effective concentration (EC50) of FFA4 activity could not be calculated. Pinocembrin, pinobanksin, and CAPE significantly increased [Ca2+]i in FFA4 cells in a dose-dependent manner at the concentrations from 1 to 100 µM (Fig. 2), and displayed a EC50 of 13.27 µM, 206.81 µM, and 124.49 µM (Table 1), respectively. Pinocembrin was particularly interesting as it exhibited comparable FFA4 potency as α-linolenic acid.
Fig. 2.
Changes in intracellular calcium concentrations stimulated by the five phenolic compounds in propolis and α-linolenic acid in FFA4 cells (HEK 293 cells stably expressing FFA4 and Gα16). Values are expressed as mean ± SEM from three separate experiments performed in triplicate (n = 15). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with a group treated with one of the five phenolic compounds or α-linolenic acid in HEK 293 cells. Means with different letters indicate significantly different (P < 0.05) by Tukey’s test within a group treated with one of the five phenolic compounds or α-linolenic acid in FFA4 cells
Table 1.
FFA4 activity of five phenolic compounds of propolis
| Common name | IUPAC name | CAS No. | Molecular formula | Molecular weight (g/mol) | EC50 |
|---|---|---|---|---|---|
| FFA4 activity (µM)a | |||||
| Chrysin | 5,7-dihydroxy-2-phenylchromen-4-one | 480-40-0 | C15H10O4 | 254.24 | NEb |
| Pinocembrin | (2S)-5,7-dihydroxy-2-phenyl-2,3-dihydrochromen-4-one | 480-39-7 | C15H12O4 | 256.253 | 13.27 |
| Galangin | 3,5,7-trihydroxy-2-phenylchromen-4-one | 548-83-4 | C15H10O5 | 270.237 | NE |
| Pinobanksin | (2R,3R)-3,5,7-trihydroxy-2-phenyl-2,3-dihydrochromen-4-one | 548-82-3 | C15H12O5 | 272.25 | 206.81 |
| Caffeic acid phenethyl ester | 2-phenylethyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | 104,594-70-9 | C17H16O4 | 284.306 | 124.49 |
aEC50 for FFA4 activity was calculated when the response to 10 µM α-linolenic acid was considered to be the maximum
bNE: Could not be estimated
This study provided information on the FFA4 agonistic activities of three phenolic compounds in propolis, pinocembrin, pinobanksin, and CAPE. In particular, pinocembrin exhibited FFA4 activity as potent as α-linolenic acid. The findings may suggest that three phenolic compounds in propolis might control energy homeostasis via FFA4 activation. Moreover, the multiple functions of propolis could be due in part to the activation of FFA4 of the three compounds.
Among five tested compounds, four compounds have been shown to have pharmacological activities involved in the regulation of energy homeostasis. Chrysin had anti-diabetic (Ahad et al., 2014; Mani and Natesan, 2018) and anti-inflammatory effects (Lee and Park, 2015). Pinocembrin inhibited inflammation (Giri et al., 2016; Pei and Sun, 2018; Zhou et al., 2015), and improved lipid profiles (Granados-Pineda et al., 2018; Sang et al., 2012). Galangin improved insulin resistance, reduced lipid levels and body weight in rats (Kumar and Alagawadi, 2013; Sivakumar et al., 2010), and inhibited inflammation (Choi et al., 2017). CAPE inhibited inflammation (Huang et al., 2014), and decreased the levels of glucose, cholesterol and triglyceride (Celik et al., 2009; Hassan et al., 2014). Pinobanksin has not been reported concerning the control of energy homeostasis. From our results, FFA4 activation may be responsible for the regulatory effects of pinocembrin and CAPE, but not responsible for the effects of chrysin and galangin on energy homeostasis.
Concerning the FFA4 activity of the tested five compounds in this study, pinocembrin (a flavanone) exhibited the highest followed by CAPE (a hydroxycinnamic acid) and pinobanksin (a flavanone). Chrysin and galangin (flavones) showed weak activities. Pinocembrin and pinobanksin are flavanones, chrysin and galangin are flavones, and CAPE is a hydroxycinnamic acid of phenolic acid which structure is similar to flavonoids. The structure of flavanone differs from that of flavone by the lack of the double bond of C ring. This difference makes the nuclear skeleton of flavanone non-planar. In the case of this study, two tested flavanones exhibited higher FFA4 activities than two tested flavones. The FFA4 agonistic activities of phenolic compounds have not been reported from other studies. For the information about the FFA4 agonistic activities of phenolic compounds are limited at this time, the structure-activity relationship is not resolved in this study. Therefore, the significant amount of data available on the FFA4 agonistic activities of phenolic compounds could be needed to analyze the structure-activity relationship.
Acknowledgements
This work was supported by a Grant from Small Grant for Exploratory Research of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2016R1D1A1A02937328).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyunnho Cho, Email: Nh3d@hotmail.com.
Kyong Kim, Email: kim_kyong@hanmail.net.
Nayeon Kim, Email: yeon0124@naver.com.
Minji Woo, Email: 07917@kfri.re.kr.
Hye Young Kim, Email: khyey@kfri.re.kr.
References
- Ahad A, Ganai AA, Mujeeb M, Siddiqui WA. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014;279:1–7. doi: 10.1016/j.taap.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR. In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr. Cancer. 2013;65:515–526. doi: 10.1080/01635581.2013.776693. [DOI] [PubMed] [Google Scholar]
- Auguste S, Fisette A, Fernandes MF, Hryhorczuk C, Poitout V, Alquier T, Fulton S. Central agonism of GPR120 acutely inhibits food intake and food reward and chronically suppresses anxiety-like behavior in mice. Int. J. Neuropsychopharmacol. 2016;19:1–10. doi: 10.1093/ijnp/pyw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdock G. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem. Toxicol. 1998;36:347–363. doi: 10.1016/S0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- Celik S, Erdogan S, Tuzcu M. Caffeic acid phenethyl ester (CAPE) exhibits significant potential as an antidiabetic and liver-protective agent in streptozotocin-induced diabetic rats. Pharmacol. Res. 2009;60:270–276. doi: 10.1016/j.phrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Choi MJ, Lee EJ, Park JS, Kim SN, Park EM, Kim HS. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: critical role of PPAR-γ signaling pathway. Biochem. Pharmacol. 2017;144:120–131. doi: 10.1016/j.bcp.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Depoortere I. Taste receptors in the gut tune the release of peptides in response to nutrients. Peptides. 2015;66:9–12. doi: 10.1016/j.peptides.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol. Res. 2005;51:147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Giri SS, Sen SS, Sukumaran V, Park SC. Pinocembrin attenuates lipopolysaccharide-induced inflammatory responses in Labeo rohita macrophages via the suppression of the NF-κB signalling pathway. Fish Shellfish Immunol. 2016;56:459–466. doi: 10.1016/j.fsi.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Granados-Pineda J, Uribe-Uribe N, García-López P, Ramos-Godinez M, Rivero-Cruz J, Pérez-Rojas J. Effect of Pinocembrin Isolated from Mexican Brown Propolis on Diabetic Nephropathy. Molecules. 2018;23:852. doi: 10.3390/molecules23040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta Mol. Cell Biol. Lipids 1841: 1292-1300 (2014) [DOI] [PubMed]
- Hassan NA, El-Bassossy HM, Mahmoud MF, Fahmy A. Caffeic acid phenethyl ester, a 5-lipoxygenase enzyme inhibitor, alleviates diabetic atherosclerotic manifestations: effect on vascular reactivity and stiffness. Chem-Biol. Interact. 2014;213:28–36. doi: 10.1016/j.cbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19:19610–19632. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS. FFA4 (GPR120) as a fatty acid sensor involved in appetite control, insulin sensitivity and inflammation regulation. Mol. Aspects Med. 2018;64:92–108. doi: 10.1016/j.mam.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Kasiotis KM, Anastasiadou P, Papadopoulos A, Machera K. Revisiting Greek propolis: chromatographic analysis and antioxidant activity study. PloS one. 2017;12:e0170077. doi: 10.1371/journal.pone.0170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Park M, Lee YM, Rhyu MR, Kim HY. Ginsenoside metabolite compound K stimulates glucagon-like peptide-1 secretion in NCI-H716 cells via bile acid receptor activation. Arch. Pharmacal. Res. 2014;37:1193–1200. doi: 10.1007/s12272-014-0362-0. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Naoe Y, Kimura S, Miyamoto T, Okamoto S, Toda C, Shimamoto Y, Iwanaga T, Miyoshi I. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2013;2:227–236. doi: 10.4161/adip.25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Alagawadi KR. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm. Biol. 2013;51:607–613. doi: 10.3109/13880209.2012.757327. [DOI] [PubMed] [Google Scholar]
- Lee JY, Park W. Anti-inflammatory effect of chrysin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Biotechnol. Bioprocess Eng. 20: 1026-1034 (2015) [DOI] [PMC free article] [PubMed]
- Li H, Wu F, Tan J, Wang K, Zhang C, Zheng H, Hu F. Caffeic acid phenethyl ester exhibiting distinctive binding interaction with human serum albumin implies the pharmacokinetic basis of propolis bioactive compounds. J. Pharm. Biomed. Anal. 2016;122:21–28. doi: 10.1016/j.jpba.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Liu HD, Wang WB, Xu ZG, Liu CH, He DF, Du LP, Li MY, Yu X, Sun JP. FFA4 receptor (GPR120): a hot target for the development of anti-diabetic therapies. Eur. J. Pharmacol. 2015;763:160–168. doi: 10.1016/j.ejphar.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Moniri NH. Free-fatty acid receptor-4 (GPR120): cellular and molecular function and its role in metabolic disorders. Biochem. Pharmacol. 2016;110:1–15. doi: 10.1016/j.bcp.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Arimatsu K, Minagawa T, Matsuda Y, Sato K, Takahashi N, Nakajima T, Yamazaki K. Brazilian propolis mitigates impaired glucose and lipid metabolism in experimental periodontitis in mice. BMC Complementary Altern. Med. 2016;16:329. doi: 10.1186/s12906-016-1305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Hira T, Yahagi A, Nishiyama C, Yamashita T, Imagi J, Hara H. Unsaturated aldehydes induce CCK secretion via TRPA1 in STC-1 cells. Mol. Nutr. Food Res. 2014;58:1042–1051. doi: 10.1002/mnfr.201300412. [DOI] [PubMed] [Google Scholar]
- Pei B, Sun J. Pinocembrin alleviates cognition deficits by inhibiting inflammation in diabetic mice. J. Neuroimmunol. 2018;314:42–49. doi: 10.1016/j.jneuroim.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Sang H, Yuan N, Yao S, Li F, Wang J, Fang Y, Qin S. Inhibitory effect of the combination therapy of simvastatin and pinocembrin on atherosclerosis in apoE-deficient mice. Lipids Health Dis. 2012;11:166. doi: 10.1186/1476-511X-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar AS, Viswanathan P, Anuradha CV. Dose-dependent effect of galangin on fructose-mediated insulin resistance and oxidative events in rat kidney. Redox Rep. 2010;15:224–232. doi: 10.1179/135100010X12826446921545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LT, Wang KJ, Li L, Li H, Geng M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3 K/Akt/NF-κB pathway. Eur. J. Pharmacol. 2015;761:211–216. doi: 10.1016/j.ejphar.2015.06.003. [DOI] [PubMed] [Google Scholar]