Abstract
Dietary advanced glycation end products (AGEs) are involved in the pathogenesis of diabetic complications, atherosclerosis, and kidney disease. Formation of Nε-(carboxymethyl)lysine (CML), a well-known AGEs, was evaluated from the reaction of casein from bovine milk with different reducing sugars (glucose, tagatose, and xylose) at various sugar concentrations and heating temperatures (75 and 120 °C) used in food processing to determine the best sweetener to be used in dairy products. The concentration of CML was measured using an enzyme-linked immunosorbent assay. Additionally, SDS-PAGE was carried out to observe the changes in the molecular weight of casein. The results reveal that tagatose leads to a lower CML concentration at 75 °C than glucose or xylose, whereas no significant differences are observed at 120 °C. We conclude that it would be more appropriate to use tagatose rather than glucose or xylose as a sweetener, considering the AGEs contents in heat-treated dairy products.
Keywords: Advanced glycation end products, Casein, Nε-(Carboxymethyl)lysine, Maillard reaction, Reducing sugar
Introduction
Sweeteners, which are nowadays considered to be ingredients that should be avoided in the food manufacturing process, have attracted increasing attention as a possible cause of metabolic diseases including obesity (Rippe and Angelopoulos, 2016; Swithers and Shearer, 2017). Nε-(carboxymethyl)lysine (CML), a glyoxal-derived advanced glycation end product (AGEs), has been the most widely used biomarker of oxidative stress in long-lived tissue proteins (Shaw et al., 2002) and a marker for AGEs in food analysis (Poulsen et al., 2013). CML is formed in food products via a non-enzymatic Maillard reaction during their thermal processing and storage (Charissou et al., 2007). The formation of CML depends on the food ingredients and processing conditions such as the temperature and heating time (Lund and Ray, 2017; Nguyen et al., 2016). Common sources of dietary CML include grilled meat, cereals, potato chips, and several types of cheese processed through heat-treatment (Hull et al., 2012). Dietary CML might pose a risk to human health, as it enhances the oxidative stress and initiates inflammatory responses that are implicated in the pathogenesis of diabetes mellitus and other metabolic disorders (Uribarri et al., 2015; Vlassara and Uribarri, 2014).
Therefore, it is important to reduce dietary CML content in processed food toward improving both human health and food quality (Kellow and Savige, 2013). In order to control the quantity of CML in processed food, it is crucial to quantitatively study its formation. Gaining detailed understanding of the kinetics of the reactions under individual reaction conditions would allow for an accurate description and prediction of the formation of CML.
In this study, a comprehensive formation model of CML via the Maillard reaction between casein and monosaccharides such as glucose, xylose, and tagatose was developed at both low (75 °C) and high (120 °C) temperatures under aqueous conditions, which are the conditions relevant to food processing. These sugars have been selected to determine the effect of the structural differences (e.g. aldose vs ketose and hexose vs pentose) on CML formation. The chosen heating temperature corresponds to the pasteurization or sterilization temperatures usually employed in the food industry. Further, enzyme-linked immunosorbent assay (ELISA) was used to identify and confirm the formation of CML. Additionally, the reaction model and temperature-dependence of the formation of CML was investigated. The results provide a further understanding of the formation of CML in a casein–monosaccharide Maillard reaction system at low and high temperatures. Moreover, this study provides some guidance on low-CML forming conditions and suitable sweetening agents for dairy products.
Materials and methods
Chemicals and reagents
Casein from bovine milk, glucose, tagatose, and xylose were purchased from Sigma-Aldrich (St. Louis, MO, USA). A CML-ELISA kit and protein ladder were purchased from CircuLex Ltd. (Nagano, Japan) and Thermo Scientific (Waltham, MA, USA), respectively. All other reagents were of analytical grade from Sigma-Aldrich.
Preparation of the reaction mixtures
Casein mixtures that were reacted with glucose, tagatose, and xylose are termed as CGR, CTR, and CXR, respectively. Briefly, to considering a solubility of casein–monosaccharide reactants (CMRs), casein (0.01 M) and a reducing sugars (0.01, 0.05, or 0.25 M) were dissolved in a sodium phosphate buffer (50 mM, pH 7.5) at a molar ratio of casein to monosaccharide of 1:5. Then, the CMR mixtures were heated at different temperatures (75 and 120 °C) for varying times (20, 40, 60, 80, 80, 100 and 120 min) in a Block Heater (QBH2, Grant instrument, Cambridge, England) using a low-temperature circulating bath with a reflux condenser. After a given heating time, the CMR samples were centrifuged at 2630×g and 10 °C for 15 min. The CML content in the separated supernatants was then determined immediately. All experiments were carried out in triplicate.
CML quantification
CML was quantified using a CML-ELISA kit. In brief, CMR mixed with an anti-CML monoclonal antibody was added to the wells of the ELISA plate. Then, the anti-CML-bovine serum albumin (BSA) antibody was immobilized and allowed to react at 23 ± 2 °C for 1 h. After washing the wells with the wash buffer provided in the kit, a horseradish peroxidase (HRP)-conjugated detection antibody was added and allowed to react at 23 ± 2 °C for 1 h. After washing the wells again with the wash buffer, tetramethylbenzidine was added and the plate was incubated for 10 min to induce coloration. After the completion of reaction, the samples were analyzed by ELISA in triplicate at 450 nm using a spectrophotometer. The CML concentration was then calculated from the absorbance values using a calibration curve created with known concentrations of CML-HSA (0.109–14.000 μg/mL).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE analysis was performed according to a previously reported method (Oh et al., 2018) using a 10% acrylamide separating gel and a 5% stacking gel. The reacted sample (20 μL) was mixed with ultrapure water (5 μL) and 10 μL of 2× SDS sample buffer (100 mM Tris, pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, and 200 mM 2-mercaptoethanol) and the resulting mixture was then heated for 10 min at 95 °C. A small aliquot (15 μL) of each sample was loaded into the designated well for electrophoresis at 200 mV with a Tris-HEPES-SDS running buffer (100 mM Tris, 100 mM HEPES, 3 mM SDS, pH 8.0). The gels were stained with Coomassie brilliant blue R-250 for protein visualization. After ~ 2 h of staining, the gels were rinsed with distilled water and destained with a destaining solution [10% acetic acid (v/v)] for 24 h. Finally, the gels were photographed using an imaging system and the protein fractions were identified using a prestained protein ladder.
Statistical analysis
All experiments were conducted at least in triplicate unless otherwise stated. The acquired data are expressed as mean ± standard deviation (SD) and the samples were analyzed via a one-way analysis of variance using SAS, version 8.1 (SAS Institute, Cary, USA). Differences with a P value < 0.05 were considered significant.
Results and discussion
Our previous study on the formation of CML through the Maillard reaction focused on liquid-models of the milk protein, casein and sugar reduction via a response surface method (Oh et al., 2018). Milk casein is one of the most studied protein source on Maillard reaction and CML formation (Lima et al., 2010). The results from these studies indicate that casein gives rise to pro-inflammatory CML. However, whether such an AGEs is a health risk factor has been disputable (Takeuchi et al., 2015).
Differences of CML formation between casein and reducing sugars
Common methods to determine the levels of CML in serum, tissues, and food include mass spectrometry (MS) and ELISA. Based on the CML levels assessed using ELISA, a database of food products and their AGEs concentrations has been created (Uribarri et al., 2010). As can be observed, processed or cooked food contains various AGEs and their formation depends on the ingredients and heat-treatment methods.
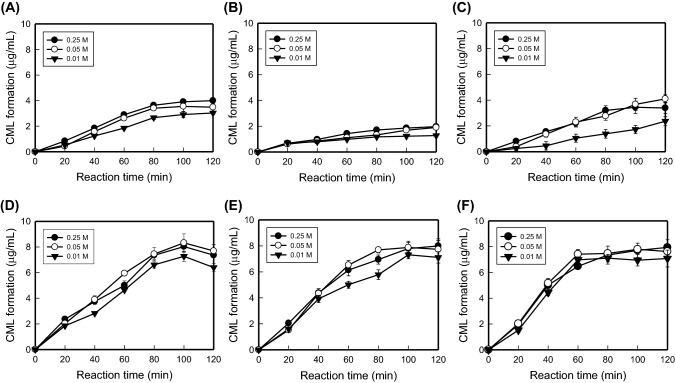
The results from this study indicate that, with increasing glucose concentration, an increased amount of CML is produced from the reaction of casein (0.01 M) and glucose at 75 °C for 120 min. At glucose levels of 0.01, 0.05, and 0.25 M, the amount of CML was found to be 3.13 ± 0.03, 3.49 ± 0.05, and 4.00 ± 0.02 μg/mL, respectively (Fig. 1A). These results suggest that glucose concentrations at 0.25 M or less affect the CML production. Interestingly, the amount of CML formed 0.01 M casein with glucose concentrations of 0.01, 0.05, and 0.25 M increased to 6.40 ± 0.02, 7.69 ± 0.01, and 7.37 ± 0.82 μg/mL, respectively, when the temperature was increased to 120 °C, with the difference at this temperature not being significant (Fig. 1D). In contrast, it was found that both CTR and CXR were more affected by the reaction temperature and time than by the concentration of the reducing sugar (Fig. 1B–F).
Fig. 1.
Experimental data of aqueous reaction systems consisting of casein (0.01 M) and reducing sugars (0.01, 0.05, and 0.25 M) heated at 75 and 120 °C. Reaction of casein with (A) glucose, (B) tagatose, and (C) xylose at 75 °C; (D) glucose, (E) tagatose, and (F) xylose at 120 °C. Mean ± standard deviations were used to plot the figures (n = 3)
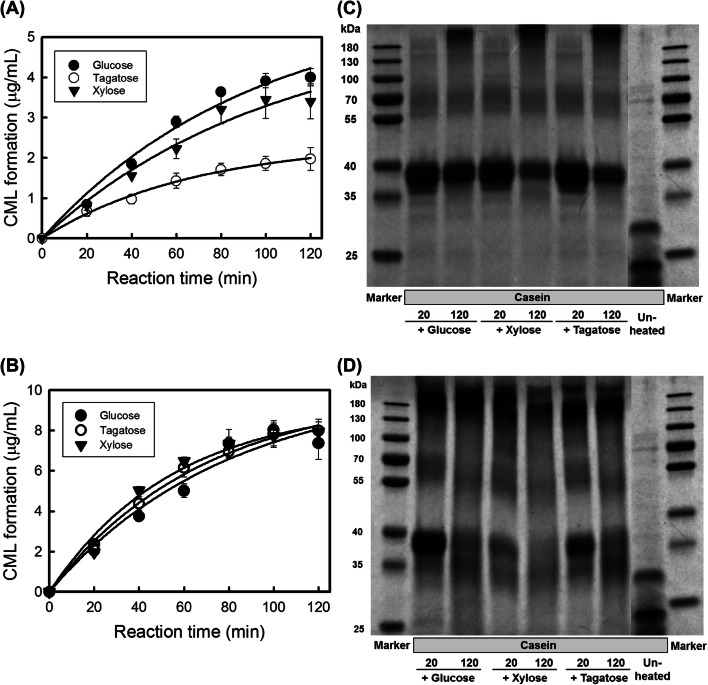
Interestingly, at 75 °C, 3.99 ± 0.22 and 3.39 ± 0.43 μg/mL of CML were produced in reactions of casein (0.01 M) with 0.25 M of glucose and xylose, respectively, with no significant difference. However, when 0.25 M tagatose was used as the reducing sugar, 1.97 ± 0.08 μg/mL CML was produced, which is almost half the amount of CML produced in the reaction with the other two sugars (Fig. 2A). However, no significant difference was found in the amount of CML formation in the 120 °C reaction model among the sugars (Fig. 2B).
Fig. 2.
Changes in the CML concentration when casein reacts with different reducing sugars at 75 and 120 °C. Reaction of casein (0.01 M) with 0.25 M glucose, tagatose, and xylose at (A) 75 °C and (B) 120 °C. Protein patterns of casein-reducing sugar reactants as a function of different heating temperatures: (C) 75 and (D) 120 °C. Mean ± standard deviations were used to plot the figures (n = 3)
Tagatose (ketose) is an isomer of galactose (aldose) that is often used as a low-calorie sweetener for replacing sugar (Delidovich and Palkovits, 2016) and has sweetness equivalent to approximately 92% of the sweetness of sugar (Guerrero-Wyss et al., 2018). Although the reactivity of pentoses is faster than that of hexoses (Hellwig and Henle, 2014), the difference in the amount of CML produced during the Maillard reaction is not related to their molecular weights. Compared to the reactions involving glucose (aldohexose) and xylose (aldopentose), the reaction of tagatose (ketohexose) led to decreased CML formation, which might be due to the structural differences between the ketoses and aldoses.
This study compares the amount of CML produced in the Maillard reaction of casein (the main protein in milk) with glucose, xylose, and tagatose (sweetening components). Considering the amount of CML formed in the experiments presented herein, we conclude that it is more appropriate to use tagatose rather than glucose or xylose as a sweetener in heat-treated dairy products. However, it is necessary to conduct further studies on the effects of the sugar structure on the amount of AGEs produced and develop possible methods to limit the formation of AGEs.
Changes in protein pattern of casein-reducing sugar reactants
Therefore, SDS-PAGE was performed to determine the changes in the molecular weight of CMRs, which represent the reactants in the Maillard reaction of casein with glucose, xylose, and tagatose, respectively. The reaction was carried out at 75 °C (lower heat-treatment condition) for 20 or 120 min. The results from the SDS-PAGE indicate that CGR, CXR, and CTR show bands at 35–40 kDa (Fig. 2C). When compared to the molecular weight of casein (20–25 kDa), it can be concluded that the sugars are bound to the molecules of CGR, CXR, and CTR.
A similar SDS-PAGE was performed on the samples obtained from the same reaction carried out at 120 °C (higher heat-treatment condition) for 20 or 120 min (Fig. 2D). For a reaction time of 20 min, the bands of CGR and CTR were observed at 35–40 kDa, similar to those obtained in the reaction at 75 °C. In contrast, the band of CXR was found to be weak under these reaction conditions. In addition, all reactants were shown a band near 180 kDa and it seemed denatured product of casein which formed under heat treatment. With the increase of heat-treated time, the band at 35–40 kDa was disappeared. Based on the report that a complex polymer forms and aggregates through a conversion to a high-molecular-weight derivative during the Maillard reaction, (Kang et al., 2015), the reactions were conducted different kinds of sugar for 120 min at 120 °C. However, no bands for CGR, CXR, or CTR were observed following these reaction conditions.
This study demonstrates that the formation of CML from casein can be modulated by using a specific reducing sugar and that tagatose leads to a lower CML concentration at 75 °C than glucose or xylose. The reaction condition was determined to understand the quantitative changes in CML production from casein under heat-treating conditions. The results indicate that it would be more appropriate to use tagatose rather than glucose or xylose as a sweetener in heat-treated dairy products. Although the underlying mechanism for the formation of AGEs could not be unraveled by this systematic study, it extends our knowledge on the formation of CML with different types of sweetener under typical processing conditions in complex food systems.
Acknowledgements
This research was supported by Main Research Program (E0164402-03) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ho-Young Park, Email: hypark@kfri.re.kr.
Mi-Jin Oh, Email: mjoh@kfri.re.kr.
Yongkon Park, Email: ykpark@kfri.re.kr.
Yoonsook Kim, Email: kimyus@kfri.re.kr.
References
- Charissou A, Ait-Ameur L, Birlouez-Aragon I. Kinetics of formation of three indicators of the Maillard reaction in model cookies: influence of baking temperature and type of sugar. J. Agric. Food Chem. 2007;55:4532–4539. doi: 10.1021/jf063024j. [DOI] [PubMed] [Google Scholar]
- Delidovich I, Palkovits R. Catalytic isomerization of biomass-derived aldoses: a review. ChemSusChem. 2016;9:547–561. doi: 10.1002/cssc.201501577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Wyss M, Duran Aguero S, Angarita Davila L. d-tagatose is a promising sweetener to control glycaemia: a new functional food. BioMed. Res. Int. 2018;2018:8718053. doi: 10.1155/2018/8718053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig M, Henle T. Baking, ageing, diabetes: a short history of the Maillard reaction. Angew Chem. Int. Ed. Engl. 2014;53:10316–10329. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- Hull GLJ, Woodside JV, Ames JM, Cuskelly GJ. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012;131:170–174. doi: 10.1016/j.foodchem.2011.08.055. [DOI] [Google Scholar]
- Kang H, Uddin MA, Lee C, Kim K-H, Nguyen TL, Lee W, Li Y, Wang C, Woo HY, Kim BJ. Determining the role of polymer molecular weight for high-performance all-polymer solar cells: its effect on polymer aggregation and phase separation. J. Am. Chem. Soc. 2015;137:2359–2365. doi: 10.1021/ja5123182. [DOI] [PubMed] [Google Scholar]
- Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur. J. Clin. Nutr. 2013;67:239–248. doi: 10.1038/ejcn.2012.220. [DOI] [PubMed] [Google Scholar]
- Lima M, Assar SH, Ames JM. Formation of Nε-(carboxymethyl)lysine and loss of lysine in casein glucose − fatty acid model systems. J. Agric. Food Chem. 2010;58:1954–1958. doi: 10.1021/jf903562c. [DOI] [PubMed] [Google Scholar]
- Lund MN, Ray CA. Control of Maillard reactions in foods: strategies and chemical mechanisms. J. Agric. Food Chem. 2017;65:4537–4552. doi: 10.1021/acs.jafc.7b00882. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, van der Fels-Klerx HJ, van Boekel MAJS. Kinetics of Nε-(carboxymethyl)lysine formation in aqueous model systems of sugars and casein. Food Chem. 2016;192:125–133. doi: 10.1016/j.foodchem.2015.06.110. [DOI] [PubMed] [Google Scholar]
- Oh M-J, Kim Y, Lee SH, Lee K-W, Park H-Y. Prediction of CML contents in the Maillard reaction products for casein–monosaccharides model. Food Chem. 2018;267:271–276. doi: 10.1016/j.foodchem.2017.07.141. [DOI] [PubMed] [Google Scholar]
- Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Rippe JM, Angelopoulos TJ. Sugars, obesity, and cardiovascular disease: results from recent randomized control trials. Eur. J. Nutr. 2016;55:45–53. doi: 10.1007/s00394-016-1257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JN, Baynes JW, Thorpe SR. N epsilon-(carboxymethyl)lysine (CML) as a biomarker of oxidative stress in long-lived tissue proteins. Methods Mol. Biol. 2002;186:129–137. doi: 10.1385/1-59259-173-6:129. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Shearer J. Obesity: Sweetener associated with increased adiposity in young adults. Nat. Rev. Endocrinol. 2017;13:443–444. doi: 10.1038/nrendo.2017.71. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Takino J, Furuno S, Shirai H, Kawakami M, Muramatsu M, Kobayashi Y, Yamagishi S. Assessment of the concentrations of various advanced glycation end-products in beverages and foods that are commonly consumed in Japan. PLoS One. 2015;10:e0118652. doi: 10.1371/journal.pone.0118652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macias-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, Portero-Otin M, Rojas A, Sampaio GR, Wrobel K, Garay-Sevilla ME. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr. Diab. Rep. 2014;14:453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]