Alzheimer’s disease (AD) is a progressive neurodegenerative disorder associated with cognitive impairment in older adults. The accumulation of insoluble forms of amyloid-β (Aβ) in plaques in extracellular spaces and the aggregation of hyperphosphorylated microtubule-associated protein tau in neurofibrillary tangles in neurons are considered to be central pathological features of AD [1, 2]. Before the cognitive symptoms and pathological signs are present, preclinical synaptic and neuronal injury begins to occur, and this continues for 10–20 years. Currently, one focus of AD research is to discern the preclinical stage of AD, when Aβ deposition has begun to occur but before clear cognitive impairment, as well as to develop therapeutic interventions at this stage to prevent AD progression [3].
Sleep disturbance is recognized as a common and often highly disruptive behavioral symptom associated with AD. It has been reported that Aβ deposition pathology itself can alter sleep architecture [4]. Moreover, recent evidence supports a role of sleep disturbance in the occurrence of AD. Sleep promotes the clearance of Aβ and tau to maintain homeostasis. Sleep disturbance significantly increases the levels of Aβ and tau and promotes senile plaque formation in AD mice [5, 6]. Based on this initial evidence, it has been proposed that the relationship between sleep and Aβ deposition is bidirectional: sleep disturbance leads to Aβ deposition and Aβ deposition in turn leads to sleep disruption. To thoroughly test this hypothesis, it is necessary to know how sleep is disrupted across the different stages of AD pathogenesis. Now, Zhang et al. report new findings that systematically address this issue: they found that sleep disturbance occurs before the cognitive decline and even precedes the pre-pathological stage of AD [7].
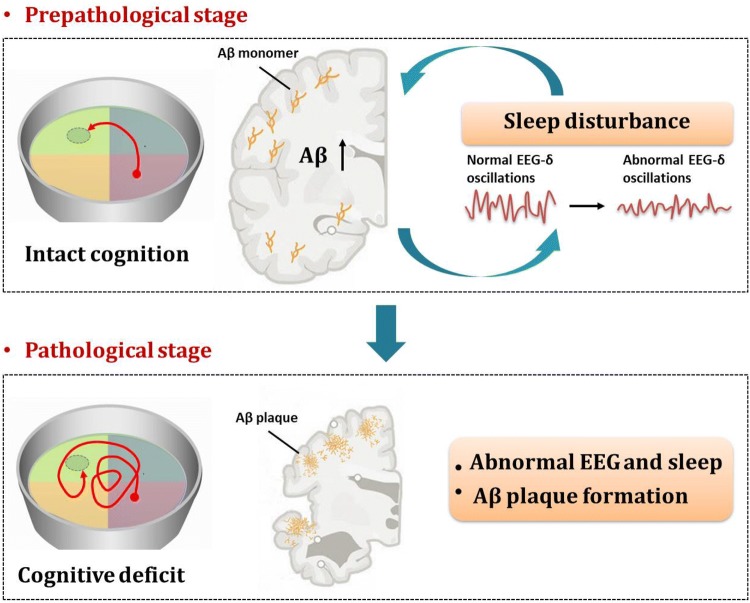
Using a battery of behavioral tests including the Morris water maze and novel object recognition tests, Zhang et al. initially investigated cognitive functions in APPswe/PS1ΔE9 AD mice at 3, 4, and 6 months of age. The authors found that AD mice at 6 months but not 3 and 4 months display a reduction in learning and memory. Moreover, Aβ plaque deposition and tau hyperphosphorylation appeared at 6 months but not at 3 and 4 months in these mice, suggesting that transgenic AD mice at 3 and 4 months of age are in a pre-pathological stage.
Next, Zhang et al., monitored the sleep-wake cycle in APPswe/PS1ΔE9 AD mice at 3, 4, and 6 months of age. The AD mice already spend more time in wakefulness and less time in slow-wave sleep during the dark period at 3 months. The AD mice at 4 and 6 months spend less time in slow-wave sleep during the light period, but these animals spend more time in slow-wave sleep during the dark period. Interestingly, the electroencephalogram (EEG) of AD mice exhibit lower delta rhythm power during sleep than WT mice. Altogether, these results demonstrate that disturbed sleep quality and architecture precede cognitive deficits and AD-like pathology (Fig. 1).
Fig. 1.
Decreases in slow-wave sleep time and EEG δ-power occur before the cognitive decline and pathological stage of AD, indicating that sleep disturbance serves as a valuable early sign of AD.
Several studies from animals have reported that sleep architecture and the EEG are disrupted at the pathological stage of AD [8, 9]. Consistent with these animal findings, monitoring has revealed changes in sleep EEG in patients with mild cognitive impairment (MCI) or mild-to-moderate AD [10, 11]. Patients with mild-to-moderate AD present abnormal theta oscillations in both rapid eye-movement (REM) and slow-wave sleep [11]. Another study quantified the EEG during REM sleep in patients with MCI, and found that the MCI subgroup shows EEG slowing in fronto-lateral regions compared to controls [10]. Zhang et al. provide evidence that sleep disturbance already occurs before the cognitive decline and even precedes the pre-pathological stage of AD [7]. These findings strongly suggest that sleep EEG changes may serve as a valuable early sign of AD in the preclinical stage, which would be beneficial for preclinical evaluation and therapeutic intervention to prevent progression to symptomatic AD.
The report by Zhang et al. further demonstrated a bidirectional relationship between deep sleep disorders and AD pathology. On one hand, a slight increase in Aβ in the AD animal model at the early stage disrupts sleep. On the other hand, the sleep-wake cycle influences Aβ and tau levels. High neural activity during wakefulness produces high levels of Aβ and tau, while sleep promotes the clearance of these waste products [5, 6]. The lower delta rhythm power during sleep in AD mice reported by Zhang et al. indicates high cortical neural activity, which could cause more Aβ and tau production. Furthermore, less sleep might also disrupt the clearance of these waste products. Through these dual mechanisms, sleep disorders trigger a vicious cycle to AD pathogenesis. Epidemiological studies have shown that up to 45% of patients with AD have various kinds of sleep disturbance. Consistent with this idea, sleep disturbance is positively correlated with the severity of cognitive and pathological impairment. AD patients with poor sleep quality have a greater Aβ burden and exhibit more severe cognitive impairment [12, 13].
Overall, the study by Zhang et al. provides crucial new insights into the changes in sleep in preclinical AD, as well as offering the possibility of preventing or delaying the occurrence of AD through interventions in sleep. Future studies will be helpful to test the causal role of sleep disturbance in the pathogenesis of AD by monitoring the sleep changes in patients. Moreover, it is also important to clarify how sleep-wakefulness is disrupted during the progression of AD. Multiple slow wave sleep-controlling nuclei, including the preoptic area, zona incerta, parafacial zone, and perioculomotor nucleus, and wakefulness-promoting nuclei, such as the paraventricular thalamus, basal forebrain, hypothalamus, and locus coeruleus, have been identified [14, 15]. Overexpressing Aβ protein in an AD animal model causes sleep-wakefulness disturbance preceding the cognitive deficit, indicating that the sleep-wakefulness-controlling systems are more vulnerable to Aβ than the cognition-related systems. Indeed, it has been reported that Aβ plaques develop in the basal forebrain before they appear in the hippocampus, and the locus coeruleus is affected by abnormal tau pathology during pre-pathological stages [16]. Despite these contributions, however, the field still lacks a comprehensive understanding of the cellular and molecular injuries underlying the sleep-wakefulness disturbance in AD and further studies are needed.
Contributor Information
Chao He, Email: hechaochongqing@163.com.
Zhian Hu, Email: zhianhu@aliyun.com.
Chenggang Jiang, Email: jcgaqq@126.com.
References
- 1.Gao Y, Liu Q, Xu L, Zheng N, He X, Xu F. Imaging and spectral characteristics of amyloid plaque autofluorescence in brain slices from the APP/PS1 mouse model of Alzheimer’s disease. Neurosci Bull. 2019;35:1126–1137. doi: 10.1007/s12264-019-00393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei YP, Ye JW, Wang X, Zhu LP, Hu QH, Wang Q, et al. Tau-induced Ca(2+)/calmodulin-dependent protein kinase-IV activation aggravates nuclear Tau hyperphosphorylation. Neurosci Bull. 2018;34:261–269. doi: 10.1007/s12264-017-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Zhong R, Li S, Fu Z, Wang R, Wang T, et al. Alteration in sleep architecture and electroencephalogram as an early sign of Alzheimer’s disease preceding the disease pathology and cognitive decline. Alzheimers Dement. 2019;15:590–597. doi: 10.1016/j.jalz.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Jyoti A, Plano A, Riedel G, Platt B. EEG, activity, and sleep architecture in a transgenic AbetaPPswe/PSEN1A246E Alzheimer’s disease mouse. J Alzheimers Dis. 2010;22:873–887. doi: 10.3233/JAD-2010-100879. [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Baldauf K, Wetzel W, Reymann KG. Behavioral and EEG changes in male 5xFAD mice. Physiol Behav. 2014;135:25–33. doi: 10.1016/j.physbeh.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Brayet P, Petit D, Frauscher B, Gagnon JF, Gosselin N, Gagnon K, et al. Quantitative EEG of rapid-eye-movement sleep: a marker of amnestic mild cognitive impairment. Clin EEG Neurosci. 2016;47:134–141. doi: 10.1177/1550059415603050. [DOI] [PubMed] [Google Scholar]
- 11.Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, et al. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol Psychol. 2011;87:334–339. doi: 10.1016/j.biopsycho.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol. 1990;45:M131–M138. doi: 10.1093/geronj/45.4.M131. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhong P, Hu F, Barger Z, Ren Y, Ding X, et al. An excitatory circuit in the perioculomotor midbrain for non-REM sleep control. Cell. 2019;177(1293–1307):e1216. doi: 10.1016/j.cell.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Ren S, Wang Y, Yue F, Cheng X, Dang R, Qiao Q, et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362:429–434. doi: 10.1126/science.aat2512. [DOI] [PubMed] [Google Scholar]
- 16.Neurodegeneration Goedert M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]