Dear Editor,
Understanding the primitives of visual perception is a fundamental question in the study of vision. To address this question, a theory of topology-based functional hierarchy in visual perception has been proposed [1, 2]. This theory suggests that the extraction of topological properties (TPs) serves as the starting point of object perception. The TP of a figure is the holistic identity which remains constant across various smooth shape-changing transformations of an image [2]. For example, the shape of a rubber sheet can be changed through bending and twisting without changing its TP as long as the sheet does not tear. The number of holes in a geometrical object is a TP because it is retained during such rubber-sheet deformations. Thus the TP of an object is thought to be a basic attribute that is crucial for the stability of perception from variable visual input and is processed with priority during visual perception [2]. The “early topological perception” hypothesis was first demonstrated by a finding showing that the visual system is more sensitive to topological differences than non-topological differences [1, 2]; this hypothesis has been widely tested in the past decades and is backed substantially by evidence from human infants and adults [3–5], other mammals [6], and even insects [7]. However, it is unclear how the TP is rapidly processed. Previous human fMRI data showed that the inferior temporal cortex, which is associated with the late stages of visual processing, is involved in the processing of topological recognition [8, 9]. These neuroimaging results seem to contradict behavioral evidence supporting the early topological perception hypothesis. To address this contradiction, we hypothesized that topological perception is processed in a fast subcortical visual pathway that stems from the superior colliculus (SC).
We used mice to test the subcortical hypothesis. If topological perception is relatively conserved across species, topological perception in animals may be innate and be processed through a conserved subcortical pathway. Here, we present behavioral evidence for innate topological perception and c-fos activation evidence in the SC for subcortical processing of the TP in mice.
A looming visual stimulus is usually presented as an expanding black disk, to mimic the shadow of an approaching aerial predator, and has been extensively adopted to study the innate defensive behaviors of animals and humans [10, 11]. When mice are presented with an upper field looming stimulus, they demonstrate conserved flight-to-nest behavior. The read-out parameters for defensive behavior induced by looming include (i) flight latency: the time from the onset of a looming stimulus to the onset of escape to the nest, (ii) time to the nest: the time taken to escape to the nest, and (iii) time in the nest: the time that a mouse spends in the nest after exposure to a stimulus. As reported in previous studies of mice [12], the flight latency to a looming stimulus is an important and sensitive index reflecting the magnitude of the defensive response to an incoming threat. In the looming paradigm of the present study, a topological shape change was inserted briefly into the continuous looming stimulus, to investigate the processing of the TP and the impact of topological shape change on the innate defensive behavior of mice.
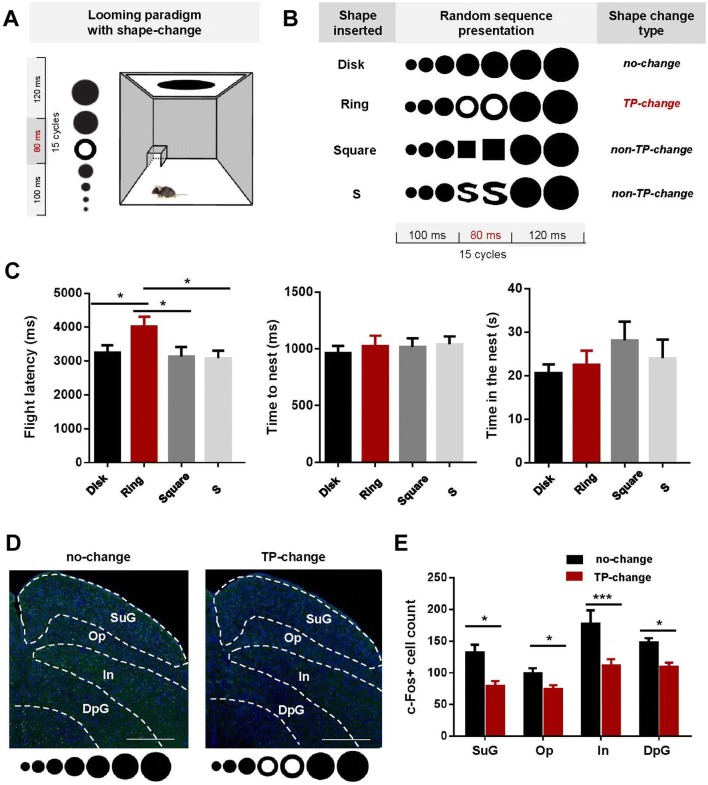
The study was approved by the Ethics Committees at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. Eighty adult (6–8 weeks old) male C57BL/6J mice were used in the behavioral experiments. Mice were placed in a closed Plexiglas box with a dark sheltered nest in one of the corners (Fig. 1A). The looming stimulus was presented on an LCD monitor in the ceiling. Four types of looming stimulus were presented (Fig. 1B): normal looming (no-change, Disk), topologically-changing looming (TP-change, Ring), and two non-topologically-changing looming (non-TP-change, Square and S). The Disk, Square, and S stimuli had the same TP (no hole), so the shape transformations between them were non-TP changes. The Ring stimulus (one hole) was topologically different from the other three, hence a TP-change looming. The S-like figure was specially designed to control the figure area and other potential confounding factors (spatial frequency and perimeter length). Therefore, the main difference between the S (and the Square) and the TP-change (Ring) stimulus was the hole in the ring, i.e., the topological property. The normal looming stimulus was a black disk expanding from 2° to 30° during a 300-ms cycle that was repeated 15 times. The TP-change looming stimulus was almost the same except that during each cycle, an 80-ms topologically different figure—a black ring—was inserted to replace the disk 100 ms after stimulus onset. Similarly, in the non-TP-change looming, a square or an S-like figure was introduced after the expanding disk had been shown for 100 ms and lasted for 80 ms before transforming back to the disk shape. The parameters flight latency, time to nest, and time in nest, were analyzed separately using one-way ANOVAs with stimulus type as the factor (for detailed materials and methods, please refer to the Supplementary Material). We found that flight latency showed a significant main effect (F(3,168) = 2.921, P = 0.035). Specifically, the average flight latency (Fig. 1C) was higher for the TP-change looming than the other conditions (all t > 2.22, P < 0.05), while the two non-TP-change looming stimuli had no significant impact on the flight latency compared to normal looming (both t < 0.49, P > 0.6). Time to nest and time in the nest showed no significant difference between these conditions (both F(3,168) < 0.894, P > 0.445). These results suggested that the defensive response of mice was weakened by the TP change but was not affected by non-TP shape changes in the looming stimuli.
Fig. 1.
Topological changes increase flight latency and decrease neuron activation in the SC of mice. A Schematic of the looming testing environment. B The four visual stimuli. C Flight latency, time to nest, and time in nest in response to the four stimuli (80 mice). D, E Representative immunohistochemistry and quantification showing less c-fos immunoreactivity after TP-change looming stimuli (12 sections) than normal looming stimuli (18 sections) in all layers of the SC (scale bars in (D), 500 μm). Graphs show the mean ± SEM; *P < 0.05, ***P < 0.001, Student’s t-test with Holm-Sidak’s correction.
Previously published evidence shows that the SC is involved in the defensive response [13] and the early stage of topological processing [14]. The effects of a TP change on defensive response may first occur in the SC, so we tested whether c-fos activation in the different layers of the SC decreased for topologically-changing looming stimuli. Mice were exposed to either a no-change or the TP-change looming stimulus. C-fos-positive cells within the SC were manually counted by an individual experimenter blind to the experimental groups. The number of c-fos-positive cells (18 sections for no-change conditions, 12 for the TP-change condition) was submitted to two-way ANOVA with topological change and SC layer as the factors. We found significant main effects for both topological change and SC layer (both F > 8.298, P < 0.001), and no significant interaction (F(3,112) = 1.012, P = 0.390). Further t-tests showed a significant decrease in neuron activation in all layers of the SC for the TP-change looming compared to the normal looming stimuli (Fig. 1D–E, all t > 2.4, P < 0.02). This result indicated that activation of the entire SC was reduced by the topological change, which is in agreement with the weakened defensive response for the TP-change looming stimulus during the behavioral experiment.
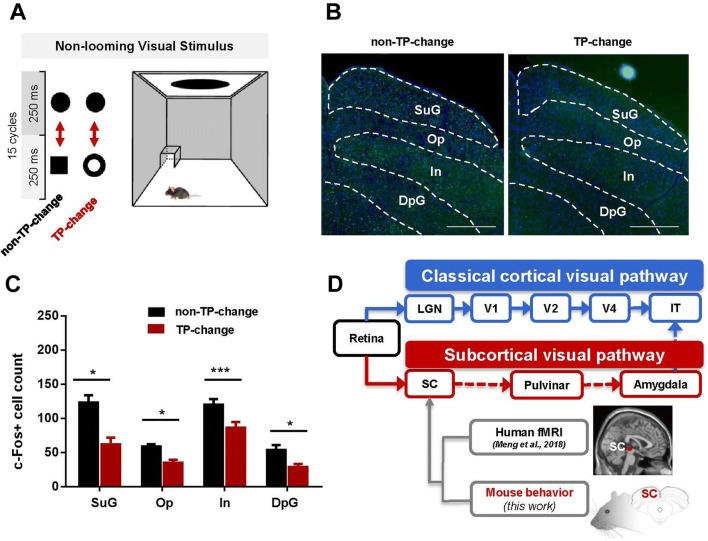
It seems plausible that topological change-detection itself takes place in the SC, because a topological change can affect the defensive response to looming stimuli and also affect neuronal activation in the SC. To test this hypothesis, we used constant-sized stimuli, instead of looming stimuli that grow rapidly in time and are associated with danger. Two types of stimuli were used (Fig. 2A). The TP-change stimulus was a transformation back and forth between a disk and an area-matched ring, and the non-TP-change was between a disk and an area-matched square. We found a significant decrease in neuron activation in all SC layers in mice exposed to the TP-change stimulus (26 sections) compared with mice exposed to the non-TP-change stimulus (22 sections, see Fig. 2, all t > 2.86, P < 0.006). These results suggest that the SC is involved in the detection of topological differences.
Fig. 2.
Continuous non-looming topological changes decrease neuron activation in the SC. A Schematic of the non-topologically changing stimulus and the topologically changing stimulus. Note that these two visual stimuli were unlikely to be perceived as threatening and therefore did not trigger a defensive response in mice. B, C Representative immunohistochemistry and quantification showing that mice had lower c-fos immunoreactivity in all SC layers following the topologically-changing stimulus (26 sections) than the non-topologically changing stimulus (22 sections) (scale bar in (B), 500 μm; graphs in (C) show the mean ± SEM; *P < 0.05, ***P < 0.001, Student’s t-tests with Holm-Sidak’s corrections). D Schematic of the subcortical hypothesis of topological processing and supporting evidence from human-brain imaging [14] and innate mouse behavior.
Although the theory of early topological perception has been discussed for decades to address the question of the primitives of vision [1]. the processing pathway of topological perception remains unclear. The subcortical hypothesis of early topological perception posits that the TP is processed through a fast subcortical pathway that stems from the SC and ends at the inferior temporal cortex instead of being processed through the classical slow cortical visual pathway [15–17]. Our previous work [14] provided human-brain imaging evidence for this subcortical hypothesis, demonstrating that the TP of an unconscious visual stimulus is processed in the SC and the pulvinar rather than in the lateral geniculate nucleus and the primary visual cortex. In the current study, there were two fundamental questions: (1) whether topological detection in rodents is an innate ability, and (2) whether topological detection occurs at the subcortical level. We used a visual-induced defensive paradigm to test the effect of a topological change inserted into a threatening looming stimulus and compared the results with those using a non-topological change insertion. We found that a topological change in a looming stimulus significantly decreased the magnitude of defensive responses. Our results suggest that topological perception is innate and capable of modifying a well-conserved defensive behavior in mice. This finding provides more evidence supporting the conclusion that topological perception is conserved across species [6, 7]. Furthermore, looming-evoked defensive responses are relatively conserved across rodents [11], monkeys [18] and humans [10]. It is intriguing that perception of a topological change affects a biological function and modulates innate defensive behavior. A possible explanation is that a topological change inserted into a looming stimulus impairs object continuity and reduces the dangerousness of visual stimulation, thus weakening the defensive response in mice.
A topological change in a looming stimulus not only weakened the behavioral defensive responses but also reduced c-fos activation in the SC, which has been reported to be a key nucleus in the subcortical pathway responsible for looming-evoked defensive behaviors. Furthermore, we found that topological changes in non-threatening visual stimuli also modulated activation of the SC in mice even when they were presented with non-alerting visual signals. It is widely accepted that the superficial layer of the SC is associated with visual information-processing and is responsible for the detection of visual motion, and deeper layers are involved in saccades and orienting responses [19]. Our present c-fos activation measurements showed that all the SC layers were less active when mice were exposed to the topologically-changing stimulus than when exposed to non-topologically-changing stimuli in both the looming and non-looming paradigms. Further studies deciphering the detailed mechanism of signal processing by/between different layers of the SC in response to looming stimuli may help to answer questions such as which layers of the SC are first responsible for the modulation of a topological change in looming-evoked behavior.
The present study provides, for the first time, behavioral evidence for innate topological perception in rodents and its influence on conserved defensive behavior. And the c-fos results provide further evidence for subcortical processing of the TP and imply a critical function of subcortical processing of the TP in visual perception. Dissecting the subcortical pathways for topological perception provides access to the mechanisms underlying the fast processing of the basic visual primitives and also sheds light on the mechanism of interaction between visual perception and innate defensive responses. Furthermore, several brain diseases, such as schizophrenia and autism, have been reported to be associated with dysfunction of subcortical pathways [20]. Future work with varying stimulus parameters such as size, time of change, and speed may help us understand how the SC processes topological perception.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by the Shenzhen Science and Technology Research Funding Program, China (JCYJ20170818161400180 and JCYJ20180508152336419), the National Natural Science Foundation of China (81425010, 31630031, and 31971072), and the Key Laboratory of Brain Connectome of Guangdong Province, China (2017B030301017).
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Yan Huang and Lei Li have contributed equally to this work.
Contributor Information
Yan Huang, Email: yan.huang@siat.ac.cn.
Liping Wang, Email: lp.wang@siat.ac.cn.
References
- 1.Chen L. Topological structure in visual perception. Science. 1982;218:699–700. doi: 10.1126/science.7134969. [DOI] [PubMed] [Google Scholar]
- 2.Chen L. The topological approach to perceptual organization. Vis Cogn. 2005;12:553–637. doi: 10.1080/13506280444000256. [DOI] [Google Scholar]
- 3.Huang Y, Zhou T, Chen L. The precedence of topological change over top-down attention in masked priming. J Vis. 2011;11:9. doi: 10.1167/11.12.9. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, He L, Wang W, Meng Q, Zhou T, Chen L. What determines the object-level visual masking: The bottom-up role of topological change. J Vis. 2018;18:3. doi: 10.1167/18.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Lin YL, Chien SHL, Hu S. An early sensitivity for detecting differences in visual topological property in 0-to 4- day-old human neonates. Pediatric Dimensions. 2016;1:29–33. doi: 10.15761/PD.1000107. [DOI] [Google Scholar]
- 6.Zhu J, Guo XY, Ma Y, Ren F. Different topological properties pattern recognition in mice. Prog Biochem Biophys. 2010;37:613–617. doi: 10.3724/SP.J.1206.2009.00698. [DOI] [Google Scholar]
- 7.Chen L, Zhang S, Srinivasan MV. Global perception in small brains: Topological pattern recognition in honey bees. Proc Natl Acad Sci U S A. 2003;100:6884–6889. doi: 10.1073/pnas.0732090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou K, Luo H, Zhou T, Zhuo Y, Chen L. Topological change disturbs object continuity in attentive tracking. Proc Natl Acad Sci U S A. 2010;107:21920–21924. doi: 10.1073/pnas.1010919108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo Y, Zhou TG, Rao HY, Wang JJ, Meng M, Chen M, et al. Contributions of the visual ventral pathway to long-range apparent motion. Science. 2003;299:417–420. doi: 10.1126/science.1077091. [DOI] [PubMed] [Google Scholar]
- 10.King SM, Dykeman C, Redgrave P, Dean P. Use of a distracting task to obtain defensive head movements to looming visual stimuli by human adults in a laboratory setting. Perception. 1992;21:245–259. doi: 10.1068/p210245. [DOI] [PubMed] [Google Scholar]
- 11.Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JND. Rats maintain an overhead binocular field at the expense of constant fusion. Nature. 2013;498:65–69. doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018;28:859–871. doi: 10.1016/j.cub.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–1477. doi: 10.1126/science.aaa8694. [DOI] [PubMed] [Google Scholar]
- 14.Meng Q, Huang Y, Cui D, He L, Chen L, Ma Y, et al. The dissociations of visual processing of “hole” and “no‐hole” stimuli: An functional magnetic resonance imaging study. Brain Behav 2018, 8: e00979. [DOI] [PMC free article] [PubMed]
- 15.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 16.Duan J, Fu H, Zhang J. Activation of parvalbumin-positive neurons in both retina and primary visual cortex improves the feature-selectivity of primary visual cortex neurons. Neurosci Bull. 2017;33:255–263. doi: 10.1007/s12264-016-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erskine D, Taylor JP, Thomas A, Collerton D, McKeith I, Khundakar A, et al. Pathological changes to the subcortical visual system and its relationship to visual hallucinations in dementia with lewy bodies. Neurosci Bull. 2019;35:295–300. doi: 10.1007/s12264-019-00341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiff W, Caviness JA, Gibson JJ. Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science. 1962;136:982–983. doi: 10.1126/science.136.3520.982. [DOI] [PubMed] [Google Scholar]
- 19.White BJ, Munoz DP. The Superior Colliculus. Oxford University Press, 2011.
- 20.Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.