Abstract
The rice flours were hydrolyzed using α-amylase (A), α-amylase and xylanase (AX), and α-amylase, xylanase and β-amylase (AXB). The effects of different enzymatic rice flour hydrolysates (ERH) on the quality of the fermented rice cake (FRC) were determined at 25 °C for 4 days. ERH had higher porosity, water absorption index, water solubility index and lower viscosity than the control. Moisture content of FRC center decreased significantly after 4 days. Specific volumes of fresh A-, AX- and AXB-FRC were higher than the control. Color of fresh A-FRC was closer to that of the control. AXB-FRC had lower hardness and firming rate than other samples during storage. After 4 days of storage, FRC with ERH had lower endotherm enthalpy and more uniform and clearer pore structure than the control. Therefore, the ERH with single or mixed enzymes could improve the structure of FRC, and extend its shelf-life.
Keywords: Carbohydrase, Fermented rice cake, Quality, Shelf-life, Staling
Introduction
Fermented rice cake (FRC) is a traditional rice fermented food with white color, soft structure and sweet, unique flavor and high nutritive value in East Asian country. Because the fermented rice cake is a rice product with high moisture and starch content, it will gradually be staled, harden and lose moisture in the course of production and distribution. Furthermore, its quality will be reduced rapidly, which cannot meet the requirements of consumers. Longer shelf-life of FRC can be achieved by preventing or reducing staling reactions, moisture loss and microbial growth (Jang et al., 2019; Kim et al., 1999). Enzymes have usually been used to improve the qualities of starchy foods (including bread) and to prolong their shelf-lives in different storage conditions. Matsushita et al. (2017) reported that the enzymatic treatments could drastically improve the qualities of whole wheat flour dough for bread. The addition of α-amylase and cyclodextrin glycosyltransferase improved the specific volume and crumb firmness of rice bread (Gujral et al., 2003). It has been described that xylanases may accelerate to break down polysaccharides in doughs. The addition of xylanase resulted in an increase in the specific volume and shelf life, and a decrease in hardness, brightness and staling of bread during storage (Courtin and Delcour, 2002; Ghoshal et al., 2013). Kaltsa et al. (2013) reported that α-amylase and xylanase influenced the crumb firmness, color and shape uniformity of cold stored breads. A Korean rice cake (non-fermented rice cake) with α-amylase, β-amylase and GP (glucoamylase + pullulanase) had different textural characteristics and higher sensory evaluation scores (Song and Park, 2003).
Even though previous works have investigated the anti-staling properties of enzymes to starchy foods (bread, cooked rice and steamed bread, etc.), little has been reported on the effects of enzymes on quality and shelf-life of FRC. The objectives of this study were to investigate the qualities of different enzymatic rice flour hydrolysates (ERH), and the effects of ERH on the quality characteristics of FRC during storage.
Materials and methods
Materials
The milled rice grains were soaked in water for 6 h, and ground to a powder after draining using a mixing mill (HMF-3080SS; Hanil Electric Co., Seoul, Korea) to obtain the wet rice flour (40% water content). Makgeolli (a traditional Korean rice wine as a starter culture), sugar and salt were provided by Donghae Gijung Co. (Gangneung, Korea). Porcine pancreatic α-amylase (cat. no. A-3176, Type VI-B, ≥ 5 U/mg), β-amylase from barley (cat. no. A7130, Type II-B, 20–80 U/mg), and xylanase from Thermomyces lanuginosus (cat. no. X2753, ≥ 2.5 U/mg) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of the enzymatic rice flour hydrolysate (ERH)
Three kinds of carbohydrases: 8 mg of α-amylase (A), 4 mg of α-amylase and 2 mg of xylanase (AX), and 4 mg of α-amylase, 2 mg of xylanase and 4 mg of β-amylase (AXB) were separately added to 338 g of wet rice flour, and then the mixture was mixed with 90 g of distilled water and stirred at room temperature for 25 min. The optimum hydrolysis temperatures of A, AX and AXB were 40, 50 and 45 °C, and the optimum hydrolysis times were 3, 4 and 4 h, respectively, which were obtained according to the method of Kunamneni and Singh (2005). After enzymatic hydrolysis, the enzymatic hydrolyzed rice batter was freeze-dried and ground into ERH to pass through a 500 μm sieve, and the unhydrolyzed rice flour treated in the same manner was used as a control.
Bulk density, true density and porosity
A 3 g of ERH in a 10 mL mass cylinder was tapped continuously until a constant volume was obtained. Bulk density (g/mL) was calculated as weight of sample (g) divided by the volume of sample (mL) (Oladele and Aina, 2007). A 1 g of ERH was put into a 10 mL graduated cylinder containing 5 mL of toluene. True density (g/mL) was calculated as weight of sample (g) divided by the increased volume (mL) (Yeboah-Awudzi et al., 2018). Porosity (%) was determined by subtracting bulk density from true density and dividing by true density then multiplying by 100 (Adedeji and Ngadi, 2011).
Water absorption and water solubility
Water absorption index (WAI) and water solubility index (WSI) were determined according to the method of Stojceska et al. (2008). A 3 g of ERH and 6 mL of distilled water were placed in a tube for 30 min at 25 °C and centrifuged at 3000×g for 15 min (VS-550 centrifuge, Vision Scientific Co., Buchon, Korea). WAI and WSI were the ratio of the weight of the residue obtained after removing the supernatant and the weight of the dry solid in the supernatant to the weight of the original sample, respectively.
Pasting properties
The amylogram of ERH was measured according to the modified method of Heyman et al. (2014) using a rapid dynamic rheometer with the standard stainless steel cone-plate geometry (diameter 40 mm, angle 2°) (AR 2000ex; TA Instruments, New Castle, DE, USA). The silicone oil was applied on its edge to prevent water evaporation. The rice paste obtained by mixing ERH and distilled water in 1:1.25 ratio was used as a test sample. The rice pastes were transferred onto the Peltier plate. Afterwards, the geometry was dropped to a gap of 200 μm from the Peltier plate, in which the excess extruded sample was cleared away from the periphery. Afterwards, the sample was heated from 25 to 85 °C at a heating rate of 12 °C/min, held at 85 °C for 2 min, and then cooled to 25 °C at the same rate. A shear rate of 100 s−1 was maintained during heating and cooling. Pasting temperature, peak and final viscosity were calculated using Rheology Advantage Software (Version 5.4.0; TA Instruments).
Preparation of fermented rice cake
FRC was prepared according to the modified method of Lim et al. (2017). The enzymatic hydrolyzed rice batter (approximately 428 g) obtained according to the above method was mixed with 34.5 g Makgeolli, 35 g sugar and 2.5 g salt, and then whisked for 5 min. The container with a batter was covered using aluminum foil with holes, and fermented at 25 °C for 6 h in a BOD incubator (Vision Scientific Co., Seoul, Korea). Afterwards, the fermented rice batter was whisked slowly for 5 min to exhaust the gas, and then poured into the molds (approximately 400 g per piece). The molds were covered with aluminum foil, and fermented at 25 °C for 6 h. The fermented rice cakes were cooled at room temperature for 30 min after steam-cooking at 100 °C for 18 min. The samples were packaged in oriented polypropylene/cast polypropylene bag (Totalpack Co., Yangju, Korea), in which the mixed gas (50% CO2 and 50% N2) was flushed using a vacuum and gas flushing machine (Airzero, Ansan, Korean), and then stored at 25 °C.
Specific volume and color
The volume was measured using a rapeseed displacement method (Ragaee and Abdel-Aal, 2006). Specific volume was determined as volume of FRC (mL) divided by its weight (g). Color of fresh FRC was measured according to the method of Ghoshal et al. (2013). L* (lightness), a* (redness), b* (yellowness) values of FRC surface were directly measured using a Chroma meter (CR-400; Konica Minolta Sensing, Inc., Osaka, Japan). The color values of the control sample were used as reference values. Total color difference (ΔE) was calculated using Eq. (1) from the International Commission on Illumination (Mokrzycki and Tatol, 2011).
| 1 |
Moisture content
The moisture contents (% wet basis) of the top surface (thickness 2 mm) and center sample which were rapidly separated from FRC by tweezer and scissor were determined using the convection oven method (Approved Method 44-15A, AACC International 2000).
Textural properties
The textural properties of FRC were measured using a SUN Rheometer (CR-100; Sun Scientific Company, Ltd., Tokyo, Japan) according to the method of Wang et al. (2011). A diameter of 50 mm probe was used to determine the overall hardness of the sample (length 50 mm, width 30 mm, height 35 mm), in which the parameter settings were: descent and return speed, 1 mm/s; maximum pressure, 10 kg; compress rate, 40%. A diameter of 3.0 mm probe was used to determine the hardness of the sample surface (thickness 2 mm), in which the parameter settings were: descent and return speed, 1 mm/min; maximum pressure, 2 kg; insertion depth, 2 mm. Avrami analysis was executed by Eq. (2) according to the method of Gujral et al. (2003). The firming rate was analyzed using a restricted model with a fixed Avrami exponent, n = 1 according to the method of Jiang et al. (2005).
| 2 |
where, θ was the fraction of uncrystallized material at time t expressed in terms of hardness; H0 was hardness of fresh FRC; Ht was hardness at time t; H∞ was limiting value of hardness at 4 days stored at 25 °C; k was rate constant; n was the Avrami exponent; and t was storage time.
Thermal properties
The thermal properties of FRC were determined according to the modified method of Ghoshal et al. (2013) using a differential scanning calorimeter (MDSC 2910; TA Instruments). Three mg of FRC sample was directly transferred into the aluminum crucible, and then heated from 40 to 100 °C at the speed of 10 °C/min. The endothermic curve was measured using the TA Instruments analysis software program. Straight line was drawn between between onset temperature (To) and conclusion temperature (Tc). The area enclosed by the straight line and the endothermic curve represents the enthalpy (ΔH).
Scanning electron microscope (SEM)
Preparation of samples was carried out according to the modified method of Sokolova et al. (2011). The samples were sliced into thin slices (thickness 10 mm), dehydrated with 90% alcohol for 24 h, dried at 40 °C for 4 days in a forced convection oven (JSOF-150; JS Research Inc., Gongju, Korea), and then coated with gold. The microstructure of FRC samples was determined using a field emission scanning electron microscope (Quanta 250; FEG FEI Company, Brno, Czech Republic) at an accelerating voltage of 10 kV and magnification of 1000×.
Sensory evaluation
Sensory characteristics was evaluated according to the modified method of Ariffin et al. (2015). In order to improve the accuracy of sensory evaluation, 30 panelists (15 men and 15 women, 20–50 year olds) were selected from students and faculty in food science and technology. The samples (size 4 × 3 cm) and a cup of plain water (used as a mouth rinse) were given to the panelists. A 9-point hedonic scale (9-like extremely, 8-like very much, 7-moderately like, 6-slightly like, 5-like/dislike, 4-dislike slightly, 3-dislike moderately, 2-dislike very much and 1-dislike extremely) was used to rank the preferences of A-, AX- and AX-FRC stored at 25 °C. Each panelist evaluated the product and awarded the scores according to appearance, texture, taste and flavor of FRC.
Statistical analysis
Data were statistically analyzed using SPSS software (version 18.0; SPSS Inc, Chicago, IL, USA) and Microsoft EXCEL software (version 2010; Microsoft, Redmond, WA, USA). Significant difference was determined using Duncan’s multiple range tests (P < 0.05).
Results and discussion
Properties of the enzymatic rice flour hydrolysate
Physical and pasting properties of ERH are presented in Table 1. The physical properties of ERH were significantly different (P < 0.05) from that of the control. The ERH with AX had lower bulk density and higher porosity than other samples. In general, ERH had higher water absorption index (WAI) and water solubility index (WSI) than the control, which might be related to its higher porosity. The hydrophilic nature was significantly dependent on both enzyme type and concentration, while hydrophobic nature depended only on the enzyme type (Benavent-Gil and Rosell, 2017). AX-ERH had the highest WAI and WSI, followed by A- and AXB-ERH. According to Benavent-Gil and Rosell (2017), the adsorption water capacity of starch was inversely proportional to the concentration of the enzyme. In addition, the cell wall structure of rice starch was decomposed by endo-xylanase during storage which led to a decrease in the viscosity of rice flour (Shibuya and Iwasaki, 1984). The enzymatic rice flour hydrolysates had lower peak and final viscosities than the control, which was in agreement with previous results (Benavent-Gil and Rosell, 2017; Dura et al., 2014). This may be due to the fact that amylase destroys α-(1–4) glycosidic linkages of the starch molecule and produces a dextrin or maltose which exhibits a lower swelling during gelatinization (Rocha et al., 2010). Hickman et al. (2008) also reported that the shortening of amylopectin external chains was the primary factor for such reduction. AX-ERH had higher peak viscosity than A- and AXB-ERH. This is related to the type and dosage of amylase. The α-amylase can hydrolyze the α-1,4-glycosidic bond inside the starch, and the hydrolyzed product is dextrin, oligosaccharide and monosaccharide, which can rapidly reduce the viscosity of the gelatinized starch. While the β-amylase hydrolyzes starch from the end of the starch molecule, and the viscosity of the gelatinized starch decreases slowly due to the presence of macromolecules. The addition of xylanase had a little effect on the viscosity of rice flour due to the lower hemicellulose content in rice flour. Meanwhile, low concentration of amylase led to insufficient hydrolysis and an increase in peak viscosity. On the other hand, the viscosity of ERH was inversely proportional to its water absorption capacity, and water molecules can enhance the fluidity of starch during heating. However, the peak temperatures had no significant difference (P > 0.05) among A, AX, AXB and the control. Therefore, α- and β-amylases, xylanase had a great influence on density, porosity, water absorption capacity and viscosity of rice flour, but did not affect significantly its gelatinization temperature.
Table 1.
Characteristics of different enzymatic rice flour hydrolysates
| Properties | Control | Enzymatic rice flour hydrolysates | ||
|---|---|---|---|---|
| A-ERH | AX-ERH | AXB-ERH | ||
| Physical properties | ||||
| Bulk density (g/ml) | 0.88 ± 0.026a | 0.76 ± 0.005c | 0.73 ± 0.005d | 0.77 ± 0.007b |
| True density (g/ml) | 1.59 ± 0.006b | 1.60 ± 0.007b | 1.63 ± 0.013a | 1.56 ± 0.014c |
| Porosity (%) | 44.62 ± 0.548d | 52.90 ± 0.237b | 55.22 ± 0.419a | 50.16 ± 0.457c |
| WAI (g/g) | 1.83 ± 0.005d | 2.28 ± 0.025b | 2.57 ± 0.023a | 2.22 ± 0.050c |
| WSI (g/100 g) | 1.27 ± 0.099c | 3.37 ± 0.437b | 4.68 ± 0.791a | 4.01 ± 0.225b |
| Pasting properties | ||||
| Peak viscosity (Pa s) | 61.42 ± 1.98a | 37.16 ± 0.68c | 43.97 ± 0.55b | 39.15 ± 0.42d |
| Pasting temperature (°C) | 70.93 ± 2.00a | 71.63 ± 1.10a | 72.97 ± 1.95a | 73.75 ± 1.15a |
| Final viscosity (Pa s) | 19.95 ± 0.85a | 7.83 ± 0.85c | 6.48 ± 0.21d | 10.15 ± 0.99b |
WAI water absorption index, WSI water solubility index, ERH enzymatic rice flour hydrolysate
a–dMeans in the same row with different superscripts are significantly different (P < 0.05)
Specific volume and color
Specific volume and color of fresh FRC are shown in Table 2. Fresh A-, AX- and AXB-FRC had higher specific volumes than the control. The volume of bread was determined by the level of bubble expansion, more extensible bubbles led to a larger volume (Ananingsih et al., 2013). The amylase might contribute to a considerable amount of degraded starch which was consumed by yeast to produce CO2, which could maintain the expansion of rice batter during steaming, resulting in the increase of specific volume. Ananingsih et al. (2013) also reported that lack of degraded starch resulted in lower specific volume of steamed bread. On the other hand, this might be related to a decrease in paste viscosity of rice batter. The intermediate or lower paste viscosity may be beneficial for the expansion of the batter during baking, resulting in a large specific volume (Renzetti and Arendt, 2009). However, the specific volumes of A-, AX and AXB-FRC were not significantly different, indicating that there was not significant correlation between specific volume and the viscosity of ERH. This might also be due to the similar content of degraded starch obtained by enzymatic hydrolysis of A, AX and AXB.
Table 2.
Specific volume and color of fresh fermented rice cakes, Avrami parameters for firming kinetics of fermented rice cakes during storage and melting enthalpies of fermented rice cake stored for 4 days
| Sample | Specific volume (mL/g) | Color | ∆E | Avrami parameters | Melting enthalpy (J/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | f0 (kg) | f∞ (kg) | f∞ − f0 (kg) | k (days−1) | R2 | ||||
| Control | 1.81 ± 0.04b | 68.2 ± 0.32a | − 0.1 ± 0.00a | 5.4 ± 0.12a | 0 | 1.81 ± 0.061 | 2.47 ± 0.041 | 0.66 ± 0.020 | 0.425 ± 0.025a | 0.987 | 3.5 ± 0.58a |
| A-FRC | 2.06 ± 0.03a | 68.6 ± 0.46a | − 0.3 ± 0.06b | 4.6 ± 0.17b | 0.90 | 1.36 ± 0.018 | 2.16 ± 0.034 | 0.74 ± 0.037 | 0.313 ± 0.013c | 0.944 | 1.5 ± 0.48b |
| AX-FRC | 2.03 ± 0.11a | 66.0 ± 1.74b | − 0.5 ± 0.12c | 3.6 ± 0.26c | 2.85 | 1.43 ± 0.041 | 2.08 ± 0.031 | 0.65 ± 0.011 | 0.337 ± 0.004b | 0.991 | 1.9 ± 0.42b |
| AXB-FRC | 2.05 ± 0.09a | 66.8 ± 1.31b | − 0.3 ± 0.21bc | 3.5 ± 0.71c | 2.40 | 0.93 ± 0.049 | 1.54 ± 0.052 | 0.61 ± 0.031 | 0.293 ± 0.020c | 0.927 | 1.6 ± 0.62b |
f0, f∞, and k are total hardness, final total hardness and rate constant, respectively
a–cMeans in the same column with different superscripts are significantly different (P < 0.05)
The surface of FRC with ERH exhibited lower a* and b* values than that of the control. The L* values of AX- and AXB-FRC surface were lower than that of the control, while L* values of A-FRC surface was not changed significantly as compared with the control. Researchers have different opinions about the effects of enzymes on bread color. Bread crust color did not changed significantly at high α-amylase levels despite the higher reducing sugars proportions in the system (Barrera et al., 2016). However, Hidalgo and Brandolini (2011) reported that the higher reducing sugars content of the dough resulted in a decrease in the brightness (L* value) of bread crust. In this study, the addition of AXB had greatest effect on the color of the FRC, while the color of A-FRC was similar to that of the control. The reason is not clear yet. Bread crust color is promoted by high temperatures (Maillard-reactions) and by dehydration (caramelization-reactions). However, the processing method of FRC is different from bread, this theory cannot be used to explain its color change. The coloring rate may depend on the moisture content and degree of fermentation, therefore rice batters with lower development during fermentation might result in surface wetness and less coloration (Barrera et al., 2016). In this present study, the use of AXB promoted the fermentation of rice batter and changed the color of the FRC surface.
Moisture content
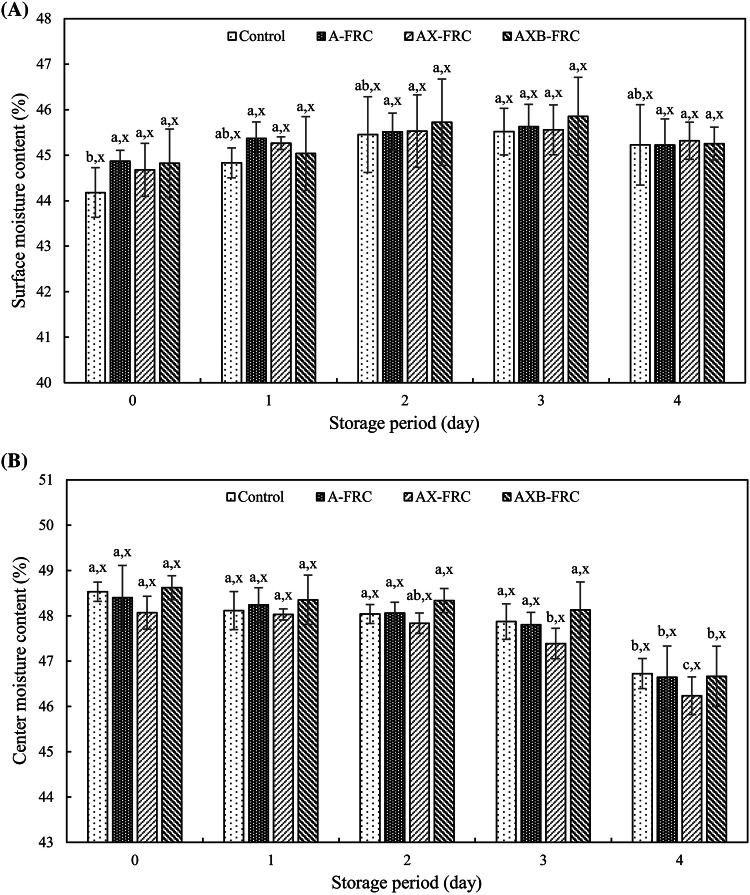
The moisture content (% wet basis) of the FRC surface with ERH was not changed significantly up to 4 days of storage (Fig. 1A). However, the moisture content of the control surface appeared a slight increase during 3 days of storage, and then remained constant. The moisture content of the FRC center with ERH decreased significantly (P < 0.05) after 4 days of storage (Fig. 1B). This was due to water migration from the center to the surface during storage, although moisture was evaporated from the crust of the FRC with different transfer rate of water from center to surface, or from surface to the surroundings. However, in a closed system, water can be equilibrated between the crumb and crust during storage driven by the moisture gradient (Baik and Chinachoti, 2000). On the other hand, the moisture content of the FRC with ERH was not significantly different from that of the control during storage (P > 0.05). Therefore, the carbohydrases did not affect significantly the moisture content of FRC.
Fig. 1.
Moisture content of fermented rice cake during storage at 25 °C. (A) Surface moisture content, (B) Center moisture content. a–bSignificantly different (P < 0.05) between the same samples at different storage period. xNot significantly different (P > 0.05) between different samples at the same storage period
Texture and retrogradation properties
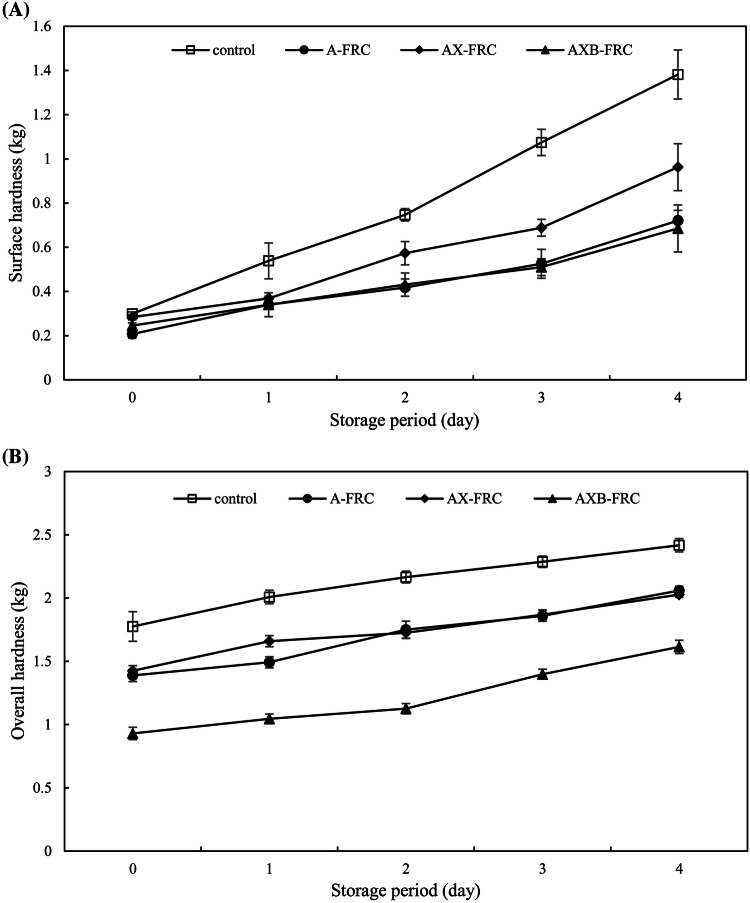
The overall and surface hardness of FRC with different ERH at 25 °C for 4 days are presented in Fig. 2. The overall and surface hardness of all samples increased with increasing storage period. The overall hardness values of fresh FRC with ERH were lower than that of the control. This might be due to higher specific volume of FRC with ERH. On the other hand, the amylase resulted in a change of the amylopectin and amylose structure, which in turn led to changes in the network structure of starch and starch as well as starch and other substances (proteins and lipids, etc.). The effect of amylase on the mechanical properties of bread may be caused by changes in the amylose and amylopectin components (Hug-Iten et al., 2003; Nguyen et al., 2015).
Fig. 2.
The surface and overall hardness of fermented rice cake during storage at 25 °C. (A) Surface hardness, (B) Overall hardness
After 4 days of storage, FRC with ERH had lower overall and surface hardness values than the control. AXB-FRC had the lowest overall hardness values. The Avrami parameters of FRC with ERH was significantly different from those of the control (Table 2). The estimated rate constants (k) of FRC with ERH were significantly (P < 0.05) lower than that of the control. Furthermore, the use of A and AXB reduced effectively the firming rate of FRC. The hardening process of starchy foods is very complicated, the basic mechanism is still not fully understood. However, moisture loss, amylopectin recrystallization and starch network formation during production and storage were often used to explain the increase in hardness of starchy foods (Goesaert et al., 2009). The melting enthalpies (ΔH) of FRC with ERH stored at 25 °C for 4 day were significantly (P < 0.05) lower than that of the control (Table 2). Therefore, the enzyme treatment can effectively inhibit starch retrogradation. However, there were not significantly differences (P > 0.05) between the melting enthalpies of A-, AX- and AXB-FRC. The anti-staling properties of different amylases clearly depend on the properties and action mechanism of the amylases. The addition of a conventional α-amylase affected the molecular weight of both amylose and amylopectin and the amylopectin side-chain distribution to a limited extent and induced degradation of starch and reduced the connectivity between the crystallites in the continuous starch phase (Hug-Iten et al., 2003). Goesaert et al. (2009) also reported that porcine pancreatic α-amylase addition reduced the level of recrystallized amylopectin to a limited extent. In addition, low molecular weight dextrins produced by enzymatic hydrolysis may act as plasticizers and contribute to a lower firmness. Due to an exoacting property of β-amylase, it can shorten the external chain length of amylopectin and retain the overall branch structure of amylopectin, which inhibits the recrystallization of amylopectin (Hickman et al., 2008). Although xylanase has no direct effect on both amylose and amylopectin, the recombination of the crosslinked network of starch molecules may be affected by its hydrolysis of hemicellulose (Ghoshal et al., 2013). In this present study, the combined action of α-amylase, β-amylase and xylanase had the best anti-staling effect on FRC.
Scanning electron microscope
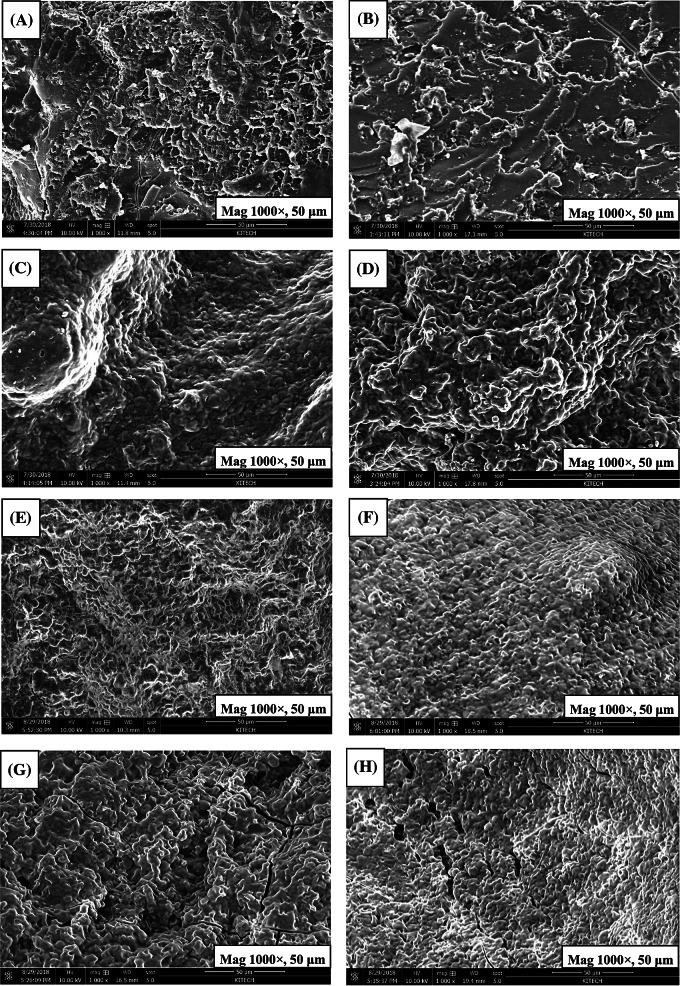
The microstructures of FRC stored for 0 and 4 days determined using a scanning electron microscope (SEM) analysis with 1000×magnification are shown in Fig. 3. The structure of fresh FRC with ERH was significantly different from that of the control (Fig. 3A, C, E, G). In Fig. 3(A), the larger and heterogeneous pores appeared in the control sample, which resulted in more dense and compact structure. This phenomenon probably decreased the specific volume of the control compared to that of FRC with ERH. The coalesced pores and homogenous distribution of tiny air bubbles appeared in the A-FRC (Fig. 3C), in which the swollen starch granules were completely different from the control. Błaszczak et al. (2004) reported that the changes in geometry (granules swell tangentially) and deformation due to high water absorption were the main reasons for structural changes of starch granules. AX-FRC had more disorderly network structure, more small pores and layered starch granules compared with A-FRC (Fig. 3E). This might be due to that the water-insoluble pentosan in rice batter was hydrolyzed by xylanase to be a water-soluble pentosan, thereby improving the interaction of protein, water and pentosane (Eugenia Steffolani et al., 2012). AXB-FRC had more large pores and fluffier network structure, which was different from AX-FRC (Fig. 3G). This conformation may be used to explain the lower hardness and larger specific volume of AXB-FRC. Therefore, the changes in the starch structure in rice batter hydrolyzed with different enzymes affected the starch-starch interaction. On the basis of SEM analysis of FRC stored for 4 days, there was a significant difference in the structures compared with fresh FRC (Fig. 3B, D, F, H). A dense sheet-like laminate structure was shown in the microstructure of the control (Fig. 3B). Błaszczak et al. (2004) reported that the majority of starch granules in the microstructure of the breads stored for 5 days was strongly folded, indicating structural changes related to starch retrogradation. Although the structures of the enzyme-treated FRC stored for 4 days were more compact, the pores were still clearly visible, resulting in a decrease in their hardness, which further confirmed the modification of starch structure by amylase and xylanase.
Fig. 3.
Scanning electron micrographs of the fresh fermented rice cakes (A, C, E, and G) and FRC stored for 4 days (B, D, F, and H). (A, B): control; (C, D): A-FRC; (E, F): AX-FRC; (G, H): AXB-FRC
Sensory evaluation
The average sensory scores of appearance, flavor, taste and texture of the FRC significantly (P < 0.05) decreased during storage (Table 3). The flavor and taste scores of FRC with ERH were not significantly different (P > 0.05) from those of the control. The average softness scores of FRC with ERH were higher than that of the control. AXB-FRC had the highest softness score, indicating that it had the lowest hardness, followed by A- and AX-FRC. After 3 days, sensory evaluation of various properties of FRC with ERH exhibited significant improvement even if these indicators decreased significantly. AX- and AXB-FRC had slightly lower chewiness and color scores, and AXB-FRC had obviously higher softness score than other samples. In particular, the pore uniformity was not significantly different from that of fresh FRC. Therefore, the addition of α-amylase, β-amylase and xylanase decreased the hardness of FRC and increased the uniformity of crumb cell, but had no significant effect on its flavor and mouthfeel.
Table 3.
Sensory scores of the fermented rice cake during storage at 25 °C
| Sample | Color | Uniformity of crumb cell | Flavor | Taste | Softness | Chewiness |
|---|---|---|---|---|---|---|
| 0 day | ||||||
| Control | 8.10 ± 0.55a | 7.60 ± 0.50a | 7.87 ± 0.63a | 7.80 ± 0.36a | 7.53 ± 0.29b | 7.67 ± 0.61a |
| A | 8.23 ± 0.43a | 7.87 ± 0.58a | 7.83 ± 0.59a | 7.77 ± 0.28a | 8.00 ± 0.19ab | 8.21 ± 0.50a |
| AX | 7.73 ± 0.52a | 7.60 ± 0.50a | 7.73 ± 0.58a | 7.67 ± 0.48a | 8.03 ± 0.31ab | 8.00 ± 0.53a |
| AXB | 7.74 ± 0.48a | 7.77 ± 0.51a | 7.80 ± 0.50a | 7.67 ± 0.69a | 8.33 ± 0.21a | 8.40 ± 0.66a |
| 3 days | ||||||
| Control | 7.07 ± 0.47a | 6.53 ± 0.33b | 6.20 ± 0.55a | 6.23 ± 0.52a | 6.07 ± 0.24b | 6.00 ± 0.45a |
| A | 7.00 ± 0.54a | 7.20 ± 0.24a | 6.40 ± 0.47a | 6.53 ± 0.51a | 6.60 ± 0.22a | 6.40 ± 0.59a |
| AX | 6.23 ± 0.38a | 7.40 ± 0.23a | 6.23 ± 0.37a | 6.50 ± 0.31a | 6.57 ± 0.26a | 5.80 ± 0.69a |
| AXB | 6.27 ± 0.44a | 7.33 ± 0.25a | 6.33 ± 0.38a | 6.55 ± 0.40a | 6.90 ± 0.19a | 5.70 ± 0.51a |
a–cMeans in the same column with different superscripts are significantly different (P < 0.05)
In conclusion, ERH had different functional and pasting properties compared with the control rice flour. The fermented rice cake with ERH had larger specific volume, lower hardness and firming rate than the control. The use of ERH changed the internal microstructure of FRC, but had not effect on its moisture content. Therefore, the application of α-amylase, xylanase and β-amylase improved the quality of rice flour, and ERH with AXB had a better anti-staling effect on fermented rice cake than that with A and AX.
Acknowledgements
This work was supported by High Value-added Food Technology Development Program funded by Korean Ministry of Agriculture, Food and Rural Affairs (MAFRA) (117067-03).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ling-Wei Meng, Email: 791202@gwnu.ac.kr.
Sang Moo Kim, Email: smkim@gwnu.ac.kr.
References
- Adedeji AA, Ngadi M. Porosity determination of deep-fat-fried coatings using pycnometer (Fried batter porosity determination by pycnometer) Int. J. Food Sci. Tech. 2011;46:1266–1275. doi: 10.1111/j.1365-2621.2011.02631.x. [DOI] [Google Scholar]
- Ananingsih VK, Gao J, Zhou W. Impact of green tea extract and fungal alpha-amylase on dough proofing and steaming. Food Bioproc. Tech. 2013;6:3400–3411. doi: 10.1007/s11947-012-0986-3. [DOI] [Google Scholar]
- Ariffin F, Baharom MA, Kaur B, Murad M. The physicochemical properties and sensory evaluation of bread made with a composite flour from wheat and tempoyak (fermented durian) Am. J. Appl. Sci. 2015;12:775–784. doi: 10.3844/ajassp.2015.775.784. [DOI] [Google Scholar]
- Baik MY, Chinachoti P. Moisture redistribution and phase transitions during bread staling. Cereal Chem. 2000;77:484–488. doi: 10.1094/CCHEM.2000.77.4.484. [DOI] [Google Scholar]
- Barrera GN, Tadini CC, León AE, Ribotta PD. Use of alpha-amylase and amyloglucosidase combinations to minimize the bread quality problems caused by high levels of damaged starch. J. Food Sci. Technol. 2016;53:3675–3684. doi: 10.1007/s13197-016-2337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavent-Gil Y, Rosell CM. Comparison of porous starches obtained from different enzyme types and levels. Carbohydr. Polym. 2017;157:533–540. doi: 10.1016/j.carbpol.2016.10.047. [DOI] [PubMed] [Google Scholar]
- Błaszczak W, Sadowska J, Rosell CM, Fornal J. Structural changes in the wheat dough and bread with the addition of alpha-amylases. Eur. Food Res. Technol. 2004;219:348–354. doi: 10.1007/s00217-004-0972-8. [DOI] [Google Scholar]
- Courtin C, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J. Cereal Sci. 2002;35:225–243. doi: 10.1006/jcrs.2001.0433. [DOI] [Google Scholar]
- Dura A, Błaszczak W, Rosell CM. Functionality of porous starch obtained by amylase or amyloglucosidase treatments. Carbohydr. Polym. 2014;101:837–845. doi: 10.1016/j.carbpol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Eugenia Steffolani M, Ribotta PD, Pérez GT, León AE. Combinations of glucose oxidase, α-amylase and xylanase affect dough properties and bread quality. Int. J. Food Sci. Tech. 2012;47:525–534. doi: 10.1111/j.1365-2621.2011.02873.x. [DOI] [Google Scholar]
- Ghoshal G, Shivhare U, Banerjee U. Effect of xylanase on quality attributes of whole-wheat bread. J. Food Qual. 2013;36:172–180. doi: 10.1111/jfq.12034. [DOI] [Google Scholar]
- Goesaert H, Leman P, Bijttebier A, Delcour JA. Antifirming effects of starch degrading enzymes in bread crumb. J. Agric. Food Chem. 2009;57:2346–2355. doi: 10.1021/jf803058v. [DOI] [PubMed] [Google Scholar]
- Gujral HS, Haros M, Rosell CM. Starch hydrolyzing enzymes for retarding the staling of rice bread. Cereal Chem. 2003;80:750–754. doi: 10.1094/CCHEM.2003.80.6.750. [DOI] [Google Scholar]
- Heyman B, De Vos WH, Van der Meeren P, Dewettinck K. Gums tuning the rheological properties of modified maize starch pastes: differences between guar and xanthan. Food Hydrocoll. 2014;39:85–94. doi: 10.1016/j.foodhyd.2013.12.024. [DOI] [Google Scholar]
- Hickman BE, Janaswamy S, Yao Y. Properties of starch subjected to partial gelatinization and β-amylolysis. J. Agric. Food Chem. 2008;57:666–674. doi: 10.1021/jf8030698. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brandolini A. Evaluation of heat damage, sugars, amylases and colour in breads from einkorn, durum and bread wheat flours. J. Cereal Sci. 2011;54:90–97. doi: 10.1016/j.jcs.2011.05.002. [DOI] [Google Scholar]
- Hug-Iten S, Escher F, Conde-Petit B. Staling of bread: role of amylose and amylopectin and influence of starch-degrading enzymes. Cereal Chem. 2003;80:654–661. doi: 10.1094/CCHEM.2003.80.6.654. [DOI] [Google Scholar]
- Jang S, Shin WK, Kim Y. Texture of steamed rice cake prepared via soy residue and hydroxypropyl methylcellulose supplementation. Cereal Chem. 2019;96:57–65. doi: 10.1002/cche.10083. [DOI] [Google Scholar]
- Jiang Z, Li X, Yang S, Li L, Tan S. Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritima. Food Res. Int. 2005;38:37–43. doi: 10.1016/j.foodres.2004.07.007. [DOI] [Google Scholar]
- Kaltsa O, Georgopoulos T, Yanniotis S, Mandala L. Effect of enzyme blends and dough strengthening emulsifier on extending the shelf life of sandwich bread applying response surface methodology. Int. J. Innov. Res. Sci. Eng. Technol. 2013;3:149–160. [Google Scholar]
- Kim HY, Park MJ, Woo SI. Development of functional Jeungpyun with dietary fiber and shelf-life studies. Food Sci. Biotechnol. 1999;8:58–64. [Google Scholar]
- Kunamneni A, Singh S. Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem. Eng. J. 2005;27:179–190. doi: 10.1016/j.bej.2005.08.027. [DOI] [Google Scholar]
- Lim SB, Tingirikari J, Kwon YW, Li L, Kim GE, Han NS. Polyphasic microbial analysis of traditional Korean Jeung-pyun sourdough fermented with makgeolli. J. Microbiol. Biotechnol. 2017;27:226–233. doi: 10.4014/jmb.1607.07033. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Santiago DM, Noda T, Tsuboi K, Kawakami S, Yamauchi H. The bread making qualities of bread dough supplemented with whole wheat flour and treated with enzymes. Food Sci. Technol. Res. 2017;23:403–410. doi: 10.3136/fstr.23.403. [DOI] [Google Scholar]
- Mokrzycki W, Tatol M. Colour difference ∆E—a survey. MG. & V. 2011;20:383–411. [Google Scholar]
- Nguyen DHD, Tran PL, Ha HS, Lee JS, Hong WS, Le QT, Oh BC, Park SH. Presence of β-amylase in ramie leaf and its anti-staling effect on rice cake. Food Sci. Biotechnol. 2015;24:37–40. doi: 10.1007/s10068-015-0006-2. [DOI] [Google Scholar]
- Oladele A, Aina J. Chemical composition and functional properties of flour produced from two varieties of tigernut (Cyperus esculentus) Afr. J. Biotechnol. 2007;6:2473–2476. doi: 10.5897/AJB2007.000-2391. [DOI] [Google Scholar]
- Ragaee S, Abdel-Aal E-SM. Pasting properties of starch and protein in selected cereals and quality of their food products. Food Chem. 2006;95:9–18. doi: 10.1016/j.foodchem.2004.12.012. [DOI] [Google Scholar]
- Renzetti S, Arendt E. Effect of protease treatment on the baking quality of brown rice bread: from textural and rheological properties to biochemistry and microstructure. J. Cereal Sci. 2009;50:22–28. doi: 10.1016/j.jcs.2009.02.002. [DOI] [Google Scholar]
- Rocha TdS, Carneiro APdA, Franco CML. Effect of enzymatic hydrolysis on some physicochemical properties of root and tuber granular starches. Food Sci. Technol. 2010;30:544–551. doi: 10.1590/S0101-20612010000200039. [DOI] [Google Scholar]
- Shibuya N, Iwasaki T. Effect of cell wall degrading enzymes on the cooking properties of milled rice and the texture of cooked rice. Nippon Shokuhin Kogyo Gakkaishi. 1984;31:656–660. doi: 10.3136/nskkk1962.31.10_656. [DOI] [Google Scholar]
- Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Song J, Park H. Effect of starch degradation enzymes on the retrogradation of a Korean rice cakes. Prev. Nutr. Food Sci. 2003;32:1262–1269. [Google Scholar]
- Stojceska V, Ainsworth P, Plunkett A, İbanoğlu E, İbanoğlu Ş. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J. Food Eng. 2008;87:554–563. doi: 10.1016/j.jfoodeng.2008.01.009. [DOI] [Google Scholar]
- Wang Y-Y, Norajit K, Ryu G-H. Influence of extruded hemp-rice flour addition on the physical properties of wheat bread. Prev. Nutr. Food Sci. 2011;16:62–66. doi: 10.3746/jfn.2011.16.1.062. [DOI] [Google Scholar]
- Yeboah-Awudzi M, Lutterodt HE, Kyereh E, Reyes V, Sathivel S, Manful J, King JM. Effect of bambara groundnut supplementation on the physicochemical properties of rice flour and crackers. J. Food Sci. Technol. 2018;55:3556–3563. doi: 10.1007/s13197-018-3281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]