Abstract
Toll-like receptor 4 (TLR4) and cellular Src (c-Src) are closely associated with inflammatory cytokines and oxidative stress in hypertension, so we designed this study to explore the exact role of c-Src in the mechanism of action of the TLR4 signaling pathway in salt-induced hypertension. Salt-sensitive rats were given a high salt diet for 10 weeks to induce hypertension. This resulted in higher levels of TLR4, activated c-Src, pro-inflammatory cytokines, oxidative stress, and arterial pressure. Infusion of a TLR4 blocker into the hypothalamic paraventricular nucleus (PVN) decreased the activated c-Src, while microinjection of a c-Src inhibitor attenuated the PVN levels of nuclear factor-kappa B, pro-inflammatory cytokines, and oxidative stress. Our findings suggest that a long-term high-salt diet increases TLR4 expression in the PVN and this promotes the activation of c-Src, which upregulates the expression of pro-inflammatory cytokines and results in the overproduction of reactive oxygen species. Therefore, inhibiting central c-Src activity may be a new target for treating hypertension.
Keywords: Toll-like receptor 4, Cellular Src, Inflammatory cytokines, Oxidative stress, Hypothalamic paraventricular nucleus, Salt-induced hypertension
Introduction
Hypertension is a common cardiovascular disease which is controlled by hereditary and environmental factors. It is also a disease with high rates of morbidity, disability, and mortality. According to epidemiological studies, long-term high-salt intake as an important environmental factor can raise blood pressure and lead to hypertension. In high salt-induced hypertensive rats, there are not only organizational and functional changes in the heart, but the central and peripheral rennin-angiotensin system is also activated. Meanwhile, a long-term high-salt diet is closely associated with inflammatory cytokines and oxidative stress. Hypertension is accompanied by chronic low-grade inflammation, and inflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-10 are considered to be important factors in the occurrence and development of hypertension. The hypothalamic paraventricular nucleus (PVN) plays important roles in sympathetic nerve activity, blood pressure stability, and neurohumoral regulation [1, 2]. Previous studies in our laboratory have shown that a high-salt intake upsets the balance of inflammatory cytokines peripherally and centrally. Neurons positive for IL-1β and IL-6 show higher expression, but the IL-10 is lower in the PVN of hypertensive rats [3, 4]. This indicates that an imbalance of central pro-inflammatory cytokines (PICs) and anti-inflammatory cytokines contributes to the pathogenesis of hypertension.
In addition, a high salt intake can also induce central oxidative stress. In hypertensive rats, neurons positive for NAD(P)H oxidase isoforms, such as NOX2 and NOX4, in the PVN show higher expression [5, 6]. The fluorescence intensity of dihydroethidium (DHE) in the PVN showed an increasing trend in the high-salt diet group, which means that oxidative stress is activated in the PVN. In addition, other indicators of oxidative stress such as NAD(P)H oxidase and malondialdehyde (MDA) are increased, but glutathione (GSH) and superoxide dismutase (SOD) are decreased after high-salt stimulation [7]. Combined with previous research, the inflammatory cytokines and oxidative stress in the PVN in hypertension play an important role in the pathogenesis of salt-induced hypertension.
Toll-like receptor 4 (TLR4), a member of the TLR family, is a pattern-recognition receptor mainly expressed by immune cells. TLR4 is involved in sterile inflammatory responses and plays an important role in the innate immune system and the subsequent development of adaptive immunity. In a number of recent studies, TLR4 has been shown to have a close relationship with inflammatory cytokines and oxidative stress and is considered to be a modulator of the inflammatory responses in various cardiovascular diseases such as coronary arterial disease, heart failure, and hypertension [8, 9]. Activation of the TLR4 signaling pathway increases the expression of PICs in order to induce hypertension [10]. In previous studies, chronic bilateral PVN infusion of the TLR4 inhibitor telmisartan reduces the inflammatory reaction via inhibiting the TLR4 pathway, which decreases blood pressure [11]. In addition, previous studies found that after stimulating several NAD(P)H oxidase isoforms, oxidative stress is involved in the TLR-dependent signaling pathway in cells from immune and non-immune origins [12]. Meanwhile, the result from our laboratory showed that the central inflammatory cytokines and oxidative stress are decreased by blocking the TLR4 pathway in the PVN, and finally decrease the peripheral renal sympathetic activation [9]. Therefore, TLR4 in the PVN plays an important role in the development of hypertension by promoting the expression of inflammatory cytokines and the production of oxidative stress. However, whether TLR4 regulates inflammatory cytokines and oxidative stress by direct action or by some intermediate is unknown.
Cellular Src (c-Src) is a typical non-receptor membrane-combining tyrosine kinase. Studies have shown that c-Src is involved in cell proliferation, motility, and even apoptosis. And c-Src is also considered to play an important role in a variety of cellular signal transduction pathways [13–15]. Studies have shown that c-Src activated in vascular smooth muscle cells (VSMCs) is involved in the angiotensin II-induced oxidative stress responses, which lead to the overproduction of reactive oxygen species (ROS) [16–18]. And the NAD(P)H oxidase subunit may also be a downstream target of c-Src in human VSMCs [14]. In addition, c-Src is also closely associated with the inflammatory response. Activated c-Src is involved in mediating inflammatory responses in synovial fibroblasts in osteoarthritis and the airway smooth muscle cells of asthmatic patients, leading to up-regulation of IL-6 expression [19, 20]. Recently, c-Src has been found in the brain, and may participate in the central regulation of oxidative stress [15]. However, in high salt-induced hypertensive responses, whether c-Src in the PVN is involved in the pathogenesis of hypertension by regulating inflammatory cytokines and oxidative stress, and whether TLR4 in the PVN regulates peripheral sympathetic activity via c-Src is not clear. And no studies have investigated the relationship between c-Src and TLR4 in salt-induced hypertension. Therefore, we proposed the following hypothesis: in high salt-induced hypertension, TLR4 in the PVN is activated, and this activates c-Src to regulate ROS and PICs, then leading to regulation of peripheral sympathetic excitation, elevation of blood pressure, and participation in the occurrence and development of hypertension.
Materials and Methods
Animals
Male Dahl salt-sensitive (S) rats (150 g–200 g body weight) were fed and operated with approval by Xi’an Jiaotong University Animal Care and Use Committee in accordance with the Guidelines for the Care and Use of Experimental Animals of the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
General Experimental Protocol
The rats were randomly divided into two diet intervention groups (0.3% NaCl, NS and 8% NaCl, HS) for 10 weeks and their blood pressure was monitored. After 10 weeks on a diet, the bilateral PVN was infused via minipump with the selective TLR4 blocker TAK-242 (10 µg/h) or artificial cerebrospinal fluid (aCSF) for two weeks or microinjected with the selective c-Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine (PP2, 0.1 nmol) or vehicle. The rats were randomly assigned to eight groups (n = 7 per group): HS + TAK-242; HS + aCSF; NS + TAK-242; NS + aCSF; HS + PP2; HS + vehicle; NS + PP2; and NS + vehicle. All the procedures were carried out under anesthesia with a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture via intraperitoneal injection, using a stereotaxic apparatus.
Bilateral PVN Osmotic Minipump for Chronic Drug Intervention
After anesthesia, the rat’s head was fixed in the stereotaxic apparatus for sterile surgery. The skull was then exposed through a midline incision, and a stainless steel double cannula was implanted into the PVN according to the Paxinos and Watson [21] rat atlas (1.8 mm posterior to bregma, 0.4 mm from midline, and 7.9 mm below the skull surface). The cannula was fixed to the cranium with dental acrylic and two stainless-steel screws [2]. A minipump (Alzet Osmotic Pumps, Model 2004, 0.25 µL/h) was implanted subcutaneously at the back of the neck and connected to the cannula for two weeks of continuous infusion of TAK-242 or aCSF into the PVN as described previously [9, 22]. Rats received subcutaneous buprenorphine (0.03 mg/kg) immediately after surgery and 12 h later.
Microinjection Through Bilateral PVN Cannulae for Chronic Drug Intervention
Due to the storage conditions (− 20°C), a minipump could not be used to infuse PP2 into the PVN. So we microinjected through bilateral PVN cannulae for chronic PP2 intervention. After anesthesia, the stereotaxic procedure was carried out as described previously [2]. A stainless-steel double cannula (Plastics One, Inc.) with a center-to-center distance of 0.5 mm was implanted into the PVN using an introducer, at the stereotaxic coordinates 1.8 mm caudal to the bregma, 0.4 mm lateral to midline, and 7.9 mm below the skull surface [7], and the cannulae were fixed to the cranium with dental acrylic and two stainless steel screws. PP2 (0.1 nmol) or vehicle was microinjected into each side in a volume of 50 nL completed in 1 min [23]. This microinjection was carried out once per day for 14 days. Rats received buprenorphine (0.03 mg/kg, subcutaneous) immediately after surgery and 12 h later.
Mean Arterial Pressure Measurement
Arterial pressure was measured non-invasively via a tail-cuff instrument and its recording system (BP100A, 113 Chengdu Techman Software Co., Ltd, China) throughout the study. The tail artery blood pressure was measured in conscious rats. The method for mean arterial pressure (MAP) has been previously described [11, 24]. The rats were trained in the daily blood pressure measure for at least 7 days to minimize stress-induced blood pressure fluctuations, and were warmed to an ambient temperature of 32°C by placing them in a holding device mounted on a thermostatically-controlled warming plate to achieve the steady pulse. Animals were allowed to acclimate to the tail cuffs for 10 min prior to each pressure recording session. The data collected by the tail-cuff instrument system were the mean value from each group rats each day.
Electrophysiological Recordings
Under anesthesia and after a retroperitoneal laparotomy, the left renal sympathetic nerve was isolated under a stereomicroscope, placed on a platinum electrode, and covered by paraffin oil tampons to record the renal sympathetic nerve activity (RSNA) as described previously [25]. The maximum RSNA was induced by sodium nitroprusside (SNP, 10 µg, intravenous). At the end of the experiment, the background noise, defined as the signal recorded postmortem, was subtracted from the actual RSNA and subsequently expressed as a percentage of maximum (in response to SNP).
Collection of Blood and Tissue Samples
At the end of two weeks of drug intervention and blood pressure measurements, one group of rats was perfused through the left ventricle under anesthesia to collect the brain. The brains were embedded in OCT and cut into 18-μm transverse sections at ~ 1.8 mm from bregma (the PVN) for immunofluorescence staining. Each of the remaining rats was decapitated under anesthesia to collect PVN tissue and blood samples. The PVN tissue was isolated according to microdissection procedures and immediately stored in liquid nitrogen for western blotting and real-time PCR (RT-PCR). The blood samples were centrifuged at 3000 rpm for 15 min and stored at −80°C.
Immunofluorescence Staining
Under anesthesia and after thoracotomy, rats were transcardially perfused through the left ventricle with 200 mL of 1% phosphate-buffered saline (PBS), followed by 400 mL of 4% buffered paraformaldehyde (pH 7.4). The brain was removed and immersed in 4% buffered paraformaldehyde for 48 h, and then immersed in 30% sucrose for at least 2 days. The brain was embedded in OCT and cut into 18-μm transverse sections ~ 1.8 mm from bregma (the PVN). The free-floating sections were incubated with 0.3% Triton-X for 20 min and with 5% goat serum for 30 min. The sections were then incubated with primary antibody diluted in 0.01 mol/L PBS at 4°C overnight followed by incubation with secondary antibody (1:200, green or red fluorescence; Invitrogen, Carlsbad, CA) for 60 min at 37°C. The primary antibodies TLR4 (sc-293072, 1:200), nuclear factor-kappa B (NF-κB) p65 (ab16502, 1:500), NOX2 (sc-130549, 1:200), and NOX4 (ab133303, 1:500) were from Santa Cruz Biotechnology and Abcam. Positive immunofluorescence-stained cells in the PVN were observed under a fluorescence microscope (Nikon, Tokyo, Japan). Superoxide anion levels in the PVN were determined by fluorescence-labeled dihydroethidium staining (DHE, Molecular Probes). All the procedures were performed as previously described [26].
Western Blotting
After measurement of RSNA, the rats were euthanized to collect PVN tissue. The tissue homogenates were subjected to Western blotting for measurement of IL-1β, IL-10, NOX2, NOX4, SOD1, c-Src, and phosphorylated Src (Tyr416) (p-Src) expression in the PVN. The tissue samples were lysed in a RIPA buffer with protease inhibitor and phosphatase inhibitor cocktail. After sonication, the protein content of the resulting samples was determined by a modified BCA protein assay. The samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using Electrophoresis and Blotting Apparatus (Bio-Rad). Protein products were separated by SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. Then the membranes were blocked with 3.0% BSA in TBST buffer (TBS plus 0.1% Tween-20) for 1.5 h at room temperature and incubated with primary antibodies overnight at 4°C [7]. The primary antibodies against IL-1β (sc12742, 17 kDa, 1:500), IL-10 (sc-365858, 20 kDa, 1:500), NOX2 (sc-130549, 91 kDa, 1:500), NOX4 (ab133303, 67 kDa, 1:2000), and SOD1 (sc-8637, 23 kDa, 1:500) were from Santa Cruz Biotechnology and Abcam. Protein loading was controlled by probing all western blots with β-actin antibody and normalizing the IL-1β, IL-10, NOX2, NOX4, and SOD1 protein intensities to that of β-actin. The p-Src (Tyr416) has activity and the ratio of p-Src (Tyr416) to c-Src represents the activation of c-Src. The c-Src antibody (2109s, 60 kDa, 1:1000) and p-Src (Tyr416) antibody (6943s, 60 kDa, 1:1000) were from Gene Company Ltd. The protein intensity of p-Src (Tyr416) was normalized to that of c-Src. Band densities were analyzed using NIH ImageJ software [27].
Real-Time PCR
The rat brains were isolated and cut into a coronal segment from − 0.92 mm to − 2.13 mm posterior to bregma, and a block of the hypothalamus containing the PVN was excised from the coronal segment. The details of PVN microdissection were as described previously [2]. Total RNA isolation, cDNA synthesis, and RT-PCR were performed as previously described [8]. Total RNA was isolated using RNeasy kits (Qiagen) according to the manufacturer’s instructions, and 1 μg of purified RNA was reverse-transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad). Specific primers for NOX2, NOX4, and SOD1 are shown in Table 1. The fold-changes in mRNA expression were determined relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels in each corresponding group [28].
Table 1.
Primers used for real-time PCR.
| Rat genes | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| NOX2 | CTGCCAGTGTGTCGGAATCT | TGTGAATGGCCGTGTGAAGT |
| NOX4 | GGATCACAGAAGGTCCCTAGC | AGAAGTTCAGGGCGTTCACC |
| SOD1 | GGTGGGCCAAAGGATGAAGAG | CCACAAGCCAAACGACTTCC |
| GAPDH | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT |
NOX2, NADPH oxidase subunit 2; NOX4, NADPH oxidase subunit 4; SOD1, superoxide dismutase 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
ELISA Studies
The MDA, SOD, GSH, and NAD(P)H oxidase activity in the PVN was quantified using rat ELISA kits (Invitrogen Corp., Carlsbad, CA). The norepinephrine (NE) in plasma was quantified using rat ELISA kits (Invitrogen) [29]. All the protocols were according to the manufacturer’s instructions. The standards or sample diluents were added and incubated in the appropriate well of a microtiter plate pre-coated with a specific antibody. Conjugate was added and incubated for 1 h at 37°C and then washed. The reactions were stopped with stop solution and read using a microtiter plate reader (MK3, Thermo Fisher Scientific, Waltham, MA).
Statistical Analysis
Data are expressed as the mean ± SEM and P < 0.05 was considered statistically significant. Statistical analyses were performed using Prism (GraphPad Software, Inc.; version 5.0). For the tail blood pressures, the MAP was analyzed by repeated measures ANOVA. One-way ANOVA with Tukey’s post hoc test was applied to the statistical analyses for RSNA, protein levels in the PVN, plasma NE, numbers of positive neurons, fluorescence intensity, and western blotting data.
Results
Effect of Infusion of TAK-242 into the PVN on Mean Arterial Pressure
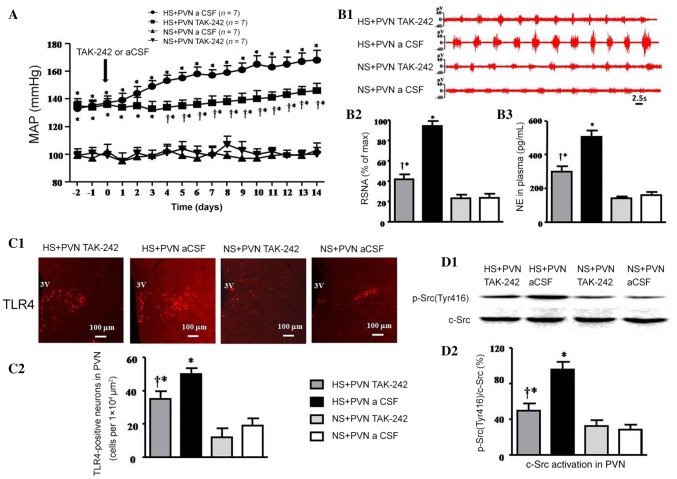
The high-salt diet induced a significant increase in MAP compared with control rats (at day 14, 168 ± 7 mmHg vs 103 ± 5 mmHg, P < 0.05). Bilateral PVN infusion of TAK-242 significantly decreased the MAP compared with the salt-induced hypertensive rats (146 ± 5 mmHg vs 168 ± 7 mmHg, P < 0.05; Fig. 1A).
Fig. 1.
Bilateral PVN infusion of TAK-242 or aCSF on mean arterial pressure, sympathetic nerve activity, TLR4 expression, and c-Src activation in salt-induced hypertensive rats. A Mean arterial pressure (MAP) increased in the high-salt (HS) group. Bilateral PVN infusion of TAK-242 attenuated the HS-induced increase in blood pressure compared with the HS + PVN aCSF group. B1 Representative renal sympathetic nerve activity (RSNA) in different groups. B2 Bar graph comparing the RSNA in different groups. RSNA was increased in the HS groups, and bilateral PVN infusion of TAK-242 made the RSNA lower than in the control group. B3 Bar graph comparing norepinephrine (NE) in the plasma of different groups. The plasma NE in the HS + PVN TAK-242 group was lower than in the control group. C1 Immunofluorescence for TLR4 (red) in the PVN in different groups. C2 Numbers of TLR4-positive neurons in the PVN in different groups. D1 Representative immunoblots of p-Src(Tyr416) and c-Src. D2 Densitometric analysis of protein expression of p-Src(Tyr416)/c-Src in the PVN in different groups. Values are expressed as the mean ± SEM. *P < 0.05 vs NS groups (NS + PVN TAK-242 or NS + PVN aCSF); †P < 0.05 HS + PVN TAK-242 vs HS + PVN aCSF.
Effect of Infusion of TAK-242 into the PVN on Sympathetic Nerve Activity
The RSNA (% of max) and plasma NE induced by HS diet were 395% and 315% of control rats (P < 0.05). Bilateral PVN infusion of TAK-242 resulted in a 55.3% decrease in RSNA and a 40.5% decrease in plasma NE in salt-induced hypertensive rats (P < 0.05). NS + PVN TAK-242 did not attenuate RSNA when compared with NS + PVN aCSF (Fig. 1B1–B3).
Effect of Infusion of TAK-242 into the PVN on TLR4 and c-Src Activation
The HS groups had a high level of expression of TLR4 and p-Src(Tyr416)/c-Src, and they were lower in the HS + PVN TAK-242 group. The immunofluorescence results revealed that the number of TLR4-positive neurons induced by HS diet was 263% of control rats (P < 0.05; Fig. 1C1, C2). Bilateral PVN infusion of TAK-242 caused a 30% decrease in the number of TLR4-positive neurons in hypertensive rats (P < 0.05). Western blotting indicated that the protein expression level of activated c-Src (p-Src(Tyr416)/c-Src) in the PVN of the hypertensive rats was 343% of that in the control group (P < 0.05; Fig. 1D1, D2). Bilateral PVN infusion of TAK-242 resulted in a 47.9% decrease in the protein expression of activated c-Src compared with salt-induced rats (P < 0.05).
Effect of Microinjection of PP2 into the PVN on Mean Arterial Pressure
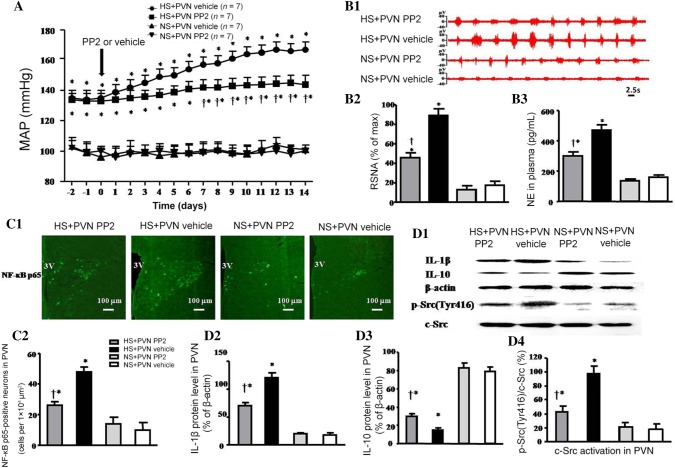
The MAP in the HS + PVN Vehicle group was higher than that in control animals (at day 14, 167 ± 5 mmHg vs 101 ± 3 mmHg, P < 0.05). Bilateral PVN microinjection of PP2 decreased the MAP compared with the HS + PVN Vehicle group (at day 14, 144 ± 6 mmHg vs 167 ± 5 mmHg, P < 0.05) (Fig. 2A).
Fig. 2.
Effects of bilateral PVN microinjection of PP2 or vehicle on mean arterial pressure, sympathetic nerve activity, NF-κB p65 activation, inflammatory cytokines, and c-Src activation in salt-induced hypertensive rats. A The MAP in the HS groups was higher than that in the NS groups. Bilateral PVN microinjections of PP2 for 14 days attenuated the HS-induced increase in blood pressure compared with HS + PVN vehicle. B1 Representative renal sympathetic nerve activity (RSNA) in the different groups. B2 Bar graph comparing RSNA in the different groups. The RSNA was higher in the HS groups, and bilateral PVN microinjection of PP2 reduced it to lower than the control group. B3 Bar graph comparing plasma NE in the different groups. The plasma NE in the HS + PVN PP2 group was lower than in the control group. C1 Immunofluorescence for NF-κB p65 (green) in the PVN in the different groups. C2 Numbers of NF-κB p65-positive neurons in the PVN in the different groups. D1 Representative immunoblots of IL-1β, IL-10, p-Src(Tyr416), and c-Src. D2–D4 Densitometric analysis of protein expression of IL-1β, IL-10, and p-Src(Tyr416)/c-Src in the PVN in the different groups. Values are expressed as the mean ± SEM. *P < 0.05 vs NS groups (NS + PVN PP2 or NS + PVN Vehicle); †P < 0.05 HS + PVN PP2 vs HS + PVN Vehicle.
Effect of Microinjection of PP2 into the PVN on Sympathetic Nerve Activity
The RSNA and plasma NE induced by HS diet were 518% and 294% of the control (P < 0.05). Bilateral PVN microinjection of PP2 resulted in a 48.7% decrease in RSNA and a 36.2% decrease in plasma NE in salt-induced hypertensive rats (P < 0.05). NS + PVN PP2 did not show any significant change in RSNA and plasma NE compared with NS + PVN Vehicle at the end of the experiment (Fig. 2B1–B3).
Effect of Microinjection of PP2 into the PVN on NF-κB p65, IL-1β, IL-10, and c-Src Activation
The HS groups had high levels of NF-κB p65, IL-1β, and p-Src(Tyr416)/c-Src expression, but lower level of IL-10 expression. And the p-Src(Tyr416)/c-Src of HS + PVN PP2 was lower than that of the HS + PVN Vehicle. The immunofluorescence results (Fig. 2C1, C2) revealed that the number of NF-κB p65-positive neurons induced by the HS diet was 480% of the control (P < 0.05). Bilateral PVN microinjection of PP2 caused a 45.8% decrease in the number of NF-κB p65-positive neurons in the PVN in hypertensive rats at the end of the experiment. The Western blotting results (Fig. 2D1–D4) indicated that the protein level of IL-1β and the activated c-Src (p-Src (Tyr416)/c-Src) of salt-induced hypertensive rats were 667% and 544% in the PVN compared with that in the control rats (P < 0.05), but the IL-10 in the PVN was lower than that in control rats. Bilateral microinjection of PP2 into the PVN caused a 41.7% decrease in IL-1β and a 56.1% decrease in activated c-Src, but increased the IL-10 protein level compared with salt-induced hypertensive rats (P < 0.05).
Effect of Microinjection of PP2 into the PVN on NOX2 and SOD1
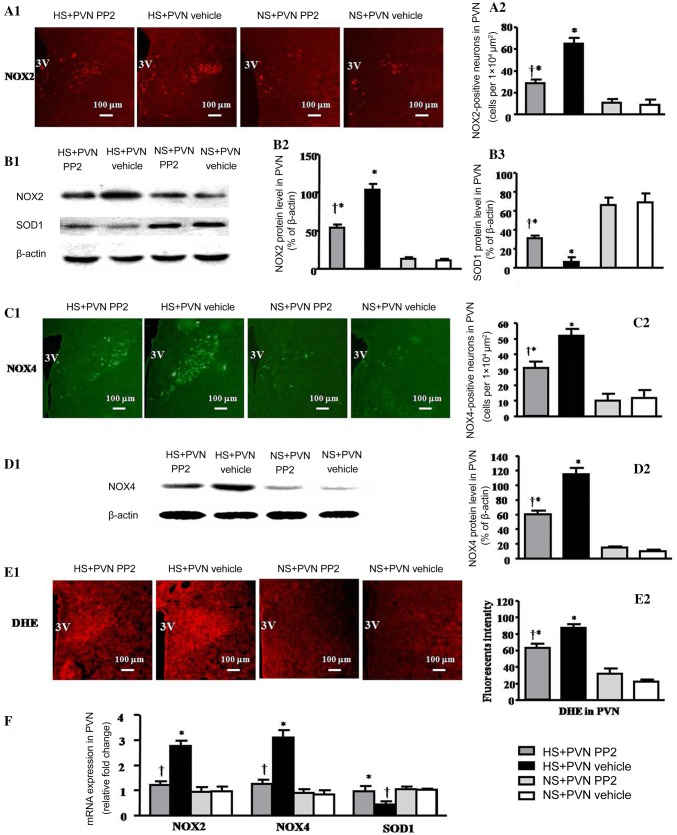
The immunofluorescence results revealed that the number of NOX2-positive neurons induced by the HS diet was 525% of the control (P < 0.05). However, bilateral PVN microinjection of PP2 caused a 49.2% decrease in NOX2-positive neurons in the PVN in hypertensive rats at the end of the experiment (Fig. 3A1, A2). The western blotting results indicated that the protein level of NOX2 in salt-induced hypertensive rats was 945% of the control, but SOD1 was decreased in the PVN compared with control rats (P < 0.05). Bilateral PVN microinjection of PP2 into the PVN caused a 48.1% decrease in NOX2 but increased the SOD1 protein level compared with salt-induced hypertensive rats (P < 0.05) (Fig. 3B1–B3).
Fig. 3.
Effects of bilateral PVN microinjection of PP2 or vehicle on oxidative stress in salt-induced hypertensive rats. A1 Immunofluorescence for NOX2 (red) in the PVN in the different groups. A2 Numbers of NOX2-positive neurons in the PVN in the different groups. B1 Representative immunoblots of NOX2 and SOD1. B2, B3 Densitometric analysis of protein expression of NOX2 and SOD1 in the PVN in the different groups. C1 Immunofluorescence for NOX4 (green) in the PVN in the different groups. C2 Numbers of NOX4-positive neurons in the PVN in the different groups. D1 Representative immunoblots of NOX4. D2 Densitometric analysis of protein expression of NOX4 in the PVN in the different groups. E1 Immunofluorescence for superoxide in the PVN in the different groups. E2 Immunofluorescence intensity of DHE in the PVN in the different groups. F mRNA expression of NOX2, NOX4, and SOD1 in the PVN in the different groups. Values are expressed as the mean ± SEM. *P < 0.05 vs NS groups (NS + PVN PP2 or NS + PVN Vehicle); †P < 0.05 HS + PVN PP2 vs HS + PVN Vehicle.
Effect of Microinjection of PP2 into the PVN on NOX4
The immunofluorescence results (Fig. 3C1, C2) revealed that the number of NOX4-positive neurons induced by the HS diet was 722% compared with control rats (P < 0.05). However, bilateral PVN microinjection of PP2 resulted in a 55.4% decrease in NOX4 in the PVN in hypertensive rats at the end of the experiment. The western blotting results (Fig. 3D1, D2) indicated that the protein level of NOX4 in salt-induced hypertensive rats was 115% in the PVN compared with control rats (P < 0.05). Bilateral microinjection of PP2 into the PVN resulted in a 47.8% decrease in the NOX4 protein level compared with salt-induced hypertensive rats (P < 0.05).
Effect of Microinjection of PP2 into the PVN on the Superoxide Level and the mRNA Levels of NOX2, NOX4, and SOD1
The HS groups had higher ROS activity as measured by DHE (Fig. 3E1, E2). Bilateral PVN microinjection of PP2 reduced the levels of superoxide in the PVN of HS rats. The superoxide level in the HS diet group was 410% of the control (P < 0.05). Bilateral PVN microinjection of PP2 caused a 25.6% decrease in superoxide in the PVN of hypertensive rats at the end of the experiment (P < 0.05). The RT-PCR results (Fig. 3F) indicated that the protein levels of NOX2 and NOX4 induced by the HS diet were 288% and 365% in the PVN compared with control rats, but SOD1 was decrased (P < 0.05). Bilateral PVN microinjection of PP2 resulted in a 56.7% decrease in the mRNA level of NOX2 and a 59.7% decrease in NOX4, but SOD1 was increased compared with the salt-induced hypertensive rats (P < 0.05).
Effect of TAK-242 and PP2 on Oxidative Stress in the PVN
Compared with the NS rats, the hypertensive rats had higher levels of MDA and NAD(P)H oxidase activity, but lower levels of SOD and GSH in the PVN. Infusion of TAK-242 or microinjection of PP2 into the PVN attenuated the levels of the MDA and NAD(P)H oxidase activity, and also augmented the levels of the SOD activity and GSH in the PVN of the HS groups (Table 2).
Table 2.
Effects of TAK-242 and PP2 on MDA, SOD, and GSH levels, and NAD(P)H oxidase activity.
| Parameters | MDA (mmol/mgp) | SOD (U/mgp) | GSH (μmol/mgp) | NAD(P)H oxidase (RLU/mg/s) |
|---|---|---|---|---|
| HS + PVN TAK-242 | 4.4 ± 0.5*† | 7.03 ± 0.34*† | 3.27 ± 0.23*† | 2.21 ± 1.24*† |
| HS + PVN aCSF | 6.03 ± 0.37* | 3.79 ± 0.11* | 0.853 ± 0.20* | 4.14 ± 1.17* |
| NS + PVN TAK-242 | 2.11 ± 0.76 | 9.18 ± 0.39 | 4.27 ± 0.25 | 1.13 ± 0.43 |
| NS + PVN aCSF | 1.94 ± 0.4 | 9.16 ± 1.27 | 4.736 ± 0.27 | 1.15 ± 0.15 |
| HS + PVN PP2 | 3.97 ± 0.47*† | 4.91 ± 0.28*† | 1.84 ± 0.26*† | 3.10 ± 1.1*† |
| HS + PVN vehicle | 5.7 ± 0.38* | 3.5 ± 0.22* | 1.00 ± 0.15* | 4.93 ± 1.73* |
| NS + PVN PP2 | 2.65 ± 0.7 | 7.83 ± 0.47 | 3.4 ± 0.18 | 1.435 ± 0.52 |
| NS + PVN vehicle | 2.6 ± 0.36 | 7.79 ± 0.59 | 3.00 ± 0.21 | 1.56 ± 0.22 |
n = 7/group; NS, normal salt diet; HS, high salt diet; mgp, mg protein; *P < 0.05 vs NS groups, †P < 0.05 HS + PVN TAK-242 or HS + PVN PP2 vs HS control groups.
Discussion
In this study, we mainly investigated how activated c-Src acts in the PVN during the hypertensive responses by an induced high-salt diet. The novel findings of this study are: (1) the HS diet increased the expression of TLR4 and PICs, increased ROS production, and enhanced the activation of c-Src in the PVN; (2) TLR4 regulated blood pressure by increasing the activation of c-Src in the PVN; (3) activation of c-Src in the PVN increased the expression of PICs by regulating the activation of NF-κB; and (4) in the PVN, the activated c-Src increased the production of the ROS in salt-induced hypertension. Thereby, c-Src participates in the development of salt-induced hypertension through regulating the PICs and ROS. We conclude that in salt-induced hypertension, TLR4 in the PVN increases the c-Src activity, and then the activated c-Src increases the PICs and ROS, which lead to increased sympathetic nerve activity and blood pressure. Therefore, inhibition of c-Src activity in the PVN may be a new target for the treatment of salt-induced hypertension.
It is well known that a long-term salt diet increases BP. Previous research focused on peripheral regulation of hypertension, but in recent studies, the central regulation of blood pressure has received more attention, and the PVN as a cardiovascular regulatory center plays a very important role in sympathetic nervous activity, BP control, and neurohumoral regulation. Our previous studies showed that oxidative stress and inflammatory cytokines in the PVN are involved in the regulation of sympathetic activity and blood pressure [2–4]. Chronic inflammatory responses have been noted as a factor in hypertension. The inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-10, are also considered to be important factors in the development of hypertension [30, 31]. Blocking TNF-α in the PVN reduces MAP and improves cardiac hypertrophy [32]. Chronic bilateral PVN intervention with an IL-1β inhibitor reduces the expression of NLRP3 (NOD-like receptor family pyrin domain-containing 3) protein on sympathetic neurons and thus affects the occurrence and development of hypertension [3]. In this study, we found that, in the high salt-induced hypertension, the expression of IL-1β and activated NF-κB in the PVN were increased and the expression of the anti-inflammatory cytokine IL-10 was decreased. So inflammatory cytokines in the PVN are involved in the process of hypertension. In addition, oxidative stress is an important link in the pathogenesis and development of hypertension, and an HS diet induces ROS production in central and peripheral tissues. Our results also indicated that the levels of NAD(P)H oxidase isoforms in the PVN were increased, and the excessive ROS production stimulated hypertensive responses and increased sympathetic outflow in high salt-induced hypertensive rats. The above results are consistent with the studies from our laboratory and other laboratories [4, 33].
TLR4, an important innate immune system receptor, is closely associated with inflammation [34, 35]. Studies have shown that TLR4 regulates the expression of PICs by increasing the activity of NF-κB [10], and TLR4 plays an important role in the progression of hypertension [32]. Other studies have shown that TLR-dependent signaling pathways in some immune or non-immune cells induce oxidative stress by stimulating several NAD(P)H oxidase isoforms [12, 36]. In this study, the HS diet increased the levels of TLR4 in the PVN, and blocking the expression of TLR4 suppressed the effect of HS on the hypertensive responses. Previous experiments in our laboratory also demonstrated that inhibiting TLR4 expression down-regulates PICs and ROS, and regenerates neurotransmitter balance in the PVN [9]. Thus, combined with this study and previous studies from our laboratory, it has been demonstrated that TLR4 regulates PICs and ROS in salt-induced hypertension. However, we do not know the specific mechanism by which TLR4 acts on PICs and ROS, so we suggest the following experiments and speculations.
c-Src is a non-receptor membrane-bound tyrosine kinase that plays an important role in various cell signaling pathways [13–15]. c-Src is closely associated with both inflammatory cytokines [19, 20] and oxidative stress [16–18, 37]. In this study, we found that the expression of activated c-Src in the PVN was higher in the high salt-induced hypertensive rats than in the control group, and the RSNA and BP were higher in the salt-induced hypertension group. After chronic bilateral PVN intervention with a TLR4 blocker, the activation of c-Src in the PVN was decreased, along with the sympathetic excitability and BP. These results indicated that TLR4 regulates the activity of c-Src in the PVN in salt-induced hypertension. After bilateral PVN administration of a c-Src blocker, the activation of NF-κB, the expression of PICs, and the level of ROS were decreased in the PVN. At the same time, the RSNA, NE, and BP were decreased. The results of these experiments indicated that the activated c-Src regulates NF-κB, PICs, and ROS in the PVN in salt-induced hypertension. Therefore, combining the results of the two sets of experiments, we can infer that TLR4 augments the activation of c-Src, and the activated c-Src increases the NF-κB, PICs, and ROS to regulate the RSNA and BP in salt-induced hypertension. Previous research in our laboratory found that PICs and ROS in the PVN are down-regulated after blocking TLR4 [9] and that TLR4 regulates PICs and ROS via regulating the activation of NF-κB, and then regulates hypertensive responses [9, 11]. Meanwhile, some studies have suggested that c-Src has an effect on the ROS and PICs in cells by regulating the activation of NF-κB [19] and the levels of NAD(P)H oxidase [38]. Therefore, we conclude that in salt-induced hypertension, the activated c-Src in the PVN is augmented by TLR4, and then the activated c-Src regulates PICs and ROS via enhancing the activation of NF-κB and NAD(P)H oxidase, so as to regulate sympathetic activity and BP (Fig. 4).
Fig. 4.
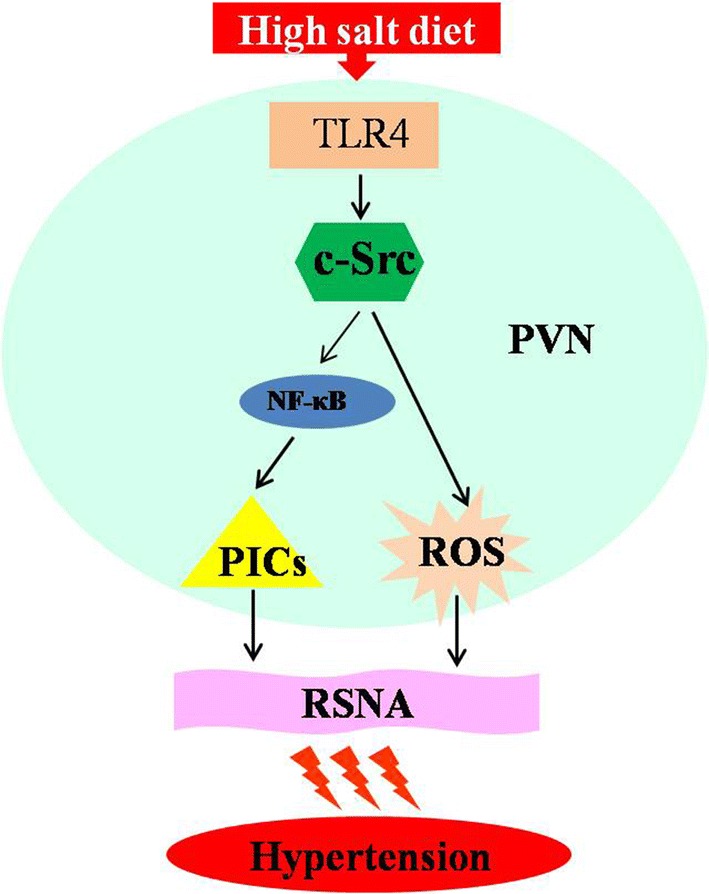
Schematic representation of the hypothesis. c-Src acts as a modulator in the TLR4 signal pathway which regulates inflammatory cytokines and oxidative stress responses, and then increases peripheral sympathetic activation and raises blood pressure in salt-induced hypertension.
Conclusion
We found that a long-term high-salt diet increases TLR4 expression in the PVN and activates the TLR4 signal pathway. And the high expression of TLR4 promotes the activation of c-Src. Blocking the activation of c-Src attenuates the effectiveness of the TLR4 pathway. This result suggests that c-Src may be a modulator in the TLR4 signaling pathway which regulates inflammatory cytokines and oxidative stress to increase the sympathetic activity and blood pressure in salt-induced hypertension. Therefore, inhibiting central c-Src activity may become a new target for treating salt-induced hypertension.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81770426, 81600333, 81600330, and 81800373), China Postdoctoral Science Foundation (2016M602835), and Shaanxi Postdoctoral Science Foundation (2016BSHEDZZ91).
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Qing Yang and Xiao-Jing Yu have contributed equally to this work.
Contributor Information
Jie Qi, Email: jie-qi@xjtu.edu.cn.
Yu-Ming Kang, Email: ykang@mail.xjtu.edu.cn.
References
- 1.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li HB, Qin DN, Cheng K, Su Q, Miao YW, Guo J, et al. Central blockade of salusin beta attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep. 2015;5:11162. doi: 10.1038/srep11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi J, Zhao XF, Yu XJ, Yi QY, Shi XL, Tan H, et al. Targeting interleukin-1 beta to suppress sympathoexcitation in hypothalamic paraventricular nucleus in Dahl salt-sensitive hypertensive rats. Cardiovasc Toxicol. 2016;16:298–306. doi: 10.1007/s12012-015-9338-7. [DOI] [PubMed] [Google Scholar]
- 4.Su Q, Liu JJ, Cui W, Shi XL, Guo J, Li HB, et al. Alpha lipoic acid supplementation attenuates reactive oxygen species in hypothalamic paraventricular nucleus and sympathoexcitation in high salt-induced hypertension. Toxicol Lett. 2016;241:152–158. doi: 10.1016/j.toxlet.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Gao HL, Yu XJ, Qi J, Yi QY, Jing WH, Sun WY, et al. Oral CoQ10 attenuates high salt-induced hypertension by restoring neurotransmitters and cytokines in the hypothalamic paraventricular nucleus. Sci Rep. 2016;6:30301. doi: 10.1038/srep30301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Qin DN, Suo YP, Su Q, Li HB, Miao YW, et al. Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicol Lett. 2015;235:206–215. doi: 10.1016/j.toxlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Su Q, Huo CJ, Li HB, Liu KL, Li X, Yang Q, et al. Renin-angiotensin system acting on reactive oxygen species in paraventricular nucleus induces sympathetic activation via AT1R/PKCgamma/Rac1 pathway in salt-induced hypertension. Sci Rep. 2017;7:43107. doi: 10.1038/srep43107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation. 2015;12:31. doi: 10.1186/s12974-015-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ML, Yu XJ, Li XG, Pang DZ, Su Q, Saahene RO, et al. Blockade of TLR4 within the paraventricular nucleus attenuates blood pressure by regulating ROS and inflammatory cytokines in prehypertensive rats. Am J Hypertens. 2018;31:1013–1023. doi: 10.1093/ajh/hpy074. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 11.Li HB, Li X, Huo CJ, Su Q, Guo J, Yuan ZY, et al. TLR4/MyD88/NF-kappaB signaling and PPAR-gamma within the paraventricular nucleus are involved in the effects of telmisartan in hypertension. Toxicol Appl Pharmacol. 2016;305:93–102. doi: 10.1016/j.taap.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 13.Touyz RM, He G, Wu XH, Park JB, Mabrouk ME, Schiffrin EL. Src is an important mediator of extracellular signal-regulated kinase 1/2-dependent growth signaling by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Hypertension. 2001;38:56–64. doi: 10.1161/01.HYP.38.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 15.Khanna S, Roy S, Park HA, Sen CK. Regulation of c-Src activity in glutamate-induced neurodegeneration. J Biol Chem. 2007;282:23482–23490. doi: 10.1074/jbc.M611269200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng SE, Lee IT, Lin CC, Kou YR, Yang CM. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radic Biol Med. 2010;48:1410–1422. doi: 10.1016/j.freeradbiomed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 18.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.e08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou CH, Fong YC, Tang CH. HMGB-1 induces IL-6 production in human synovial fibroblasts through c-Src, Akt and NF-kappaB pathways. J Cell Physiol. 2011;226:2006–2015. doi: 10.1002/jcp.22541. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CK, Lee IT, Lin CC, Hsiao LD, Yang CM. Sphingosine-1-phosphate mediates COX-2 expression and PGE2 /IL-6 secretion via c-Src-dependent AP-1 activation. J Cell Physiol. 2015;230:702–715. doi: 10.1002/jcp.24795. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson G. The Rat Brain in Stereotaxic Coordinates. 6th ed. San Diego: Academic Press, 2007.
- 22.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Y, Yuan N, Zhang SJ, Gao J, Shi Z, Zhou YB, et al. c-Src in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. Pflugers Arch. 2011;461:437–446. doi: 10.1007/s00424-011-0932-7. [DOI] [PubMed] [Google Scholar]
- 24.Gu JW, Bailey AP, Tan W, Shparago M, Young E. Long-term high salt diet causes hypertension and decreases renal expression of vascular endothelial growth factor in Sprague-Dawley rats. J Am Soc Hypertens. 2008;2:275–285. doi: 10.1016/j.jash.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol. 2014;276:115–120. doi: 10.1016/j.taap.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, You Z, Li M, Pang L, Cheng J, Wang L. Protective effect of resveratrol on the brain in a rat model of epilepsy. Neurosci Bull. 2017;33:273–280. doi: 10.1007/s12264-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong X, Wei J, Wang D, Zhu X, Zhou Y, Wang S, et al. Upregulation of spinal voltage-dependent anion channel 1 contributes to bone cancer pain hypersensitivity in rats. Neurosci Bull. 2017;33:711–721. doi: 10.1007/s12264-017-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, et al. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol. 2014;279:141–149. doi: 10.1016/j.taap.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3beta in neuronal culture. Br J Pharmacol. 2013;169:860–874. doi: 10.1111/bph.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su Q, et al. Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol. 2014;274:436–444. doi: 10.1016/j.taap.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaB in the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iranloye BO, Oludare GO, Morakinyo AO, Esume NA, Ekeh LC. Reproductive parameters and oxidative stress status of male rats fed with low and high salt diet. J Hum Reprod Sci. 2013;6:267–272. doi: 10.4103/0974-1208.126308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Wang Y, Liu C, Hu Y, Wu M, Li J, et al. MyD88-dependent nuclear factor-kappaB activation is involved in fibrinogen-induced hypertrophic response of cardiomyocytes. J Hypertens. 2009;27:1084–1093. doi: 10.1097/HJH.0b013e3283293c93. [DOI] [PubMed] [Google Scholar]
- 35.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 36.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, Sanchez-Armass S, Olivares-Reyes JA, Diebold B, et al. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta. 2013;1830:4650–4659. doi: 10.1016/j.bbagen.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Igwe OJ. Prooxidant-induced c-Src/nuclear factor kappa B-coupled signalling in sensory ganglia mediates cutaneous hyperalgesia. Eur J Pain. 2013;17:1027–1038. doi: 10.1002/j.1532-2149.2012.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]