Abstract
The present study investigated the effects of loose farrowing type during gestation and after farrowing on reproductive performance and of lactating sows. A total of 22 primiparous sows (Landrace; average initial body weights [BW], 228.54 ± 12.79 kg) were allotted to one of two treatments on the basis of body weight. Sows were divided into two experimental groups, conventional farrowing crates (CON), and loosed-farrowing pens (LFP). The experiment duration was around 38 days ranging from 10 days before parturition to 28 days after parturition. Gestating sows at the age of 105 d were placed in gestational stalls (group housing). All the sows were fed a common diet according to the National Research Council requirements for lactation. Cross-fostering was performed within 1 day of parturition. From 1 day after weaning, estrus detection was performed twice-daily (0900 and 1730 h) for 10 min by boar exposure. There were significant effects of LFP housing type on the farrowing duration, and farrowing interval. At the farrowing time, none of the litter parameters including total born, stillborn, mummy, born alive piglets and total litter weight and piglet weight were affected. There were no effects of housing type on the mortality of piglets at d 1, 3, 7, 21, and 28. In conclusion, the result of this study showed that there is no performance difference between the crated or LFP sows, which indicate that the LFP housing has the potential to be used as an alternative to the crated house without any detrimental effects in reproduction performance of lactating sows.
Keywords: Farrowing, Litter performance, Mortality, Welfare
INTRODUCTION
Living space for confined farm animals has been known as one of the most important welfare issues worldwide, particularly in highly productive animals including the gestating and lactating sows. During the last decades, there has been a focus on animal welfare within intensive farming based on using loose-house during gestation or lactation periods [1]. Currently, the large number of housing types for lactating sows are shaped as farrowing crates due to the advantages such as saving the space, cost, labor work, higher control on feces handling, and suckling piglet sanitation [2–4]. There is a wide range of confinement periods based on the countries or production potential from one week before farrowing to four weeks after farrowing. Besides the advantages, animal welfare is highly compromised in farrowing crates, particularly that gestating sows intensively display nest-building behavior [1]. The limited area in the crated gestation system compromises the welfare and increases the farrowing period in sows due to lower secretion of oxytocin into the bloodstream [5,6]. Several studies have shown that a prolonged farrowing period has an influence on the number of still-birth piglets [6,7]. Oliviero et al. [8] reported a higher number of stillborn piglets in sows farrowed in farrowing crates than sows placed in loose-housed. However, several studies showed no difference between the farrowing systems [1,9]. Moreover, the provision of high welfare and providing a higher area for gestating sows has been shown to have a positive influence on sow behavior likely to increase the litter performance.
The majority of crushed piglets by lactating sows occur within the first two days postpartum [10]. The style of loose-houses may affect the performance of lactating sows, as several studies reported that the number of crushed piglets increases in the loose sows than restricted sows in crates [8,9]. The higher number of crushed piglets in loose farrowing style can be explained by the more available space for lactating sows to lay in different positions and a higher probability of a sudden overlaying on suckling piglets. Therefore, on the other hand, the movement restriction of sows after farrowing is recommended by crating to decrease the crushed piglet number. However, the literature recommends that the start of the crating of gestating sows have to be 2 or 3 days before farrowing to avoid a prolonged farrowing duration [11]. The contradictory reports in the literature show that satisfactory results may be obtained by further studies considering both welfare issues and economical items. The loose-house used in this experiment provides more space for sows until three days before farrowing and also from the fifth days of lactation afterward to maintain the welfare factors, however, the sows are restricted after farrowing until the fifth day of lactation to minimize the ratio of crushed piglets. The objective of this study was to investigate the effects of the loose housing type with a new design to evaluate the performance of lactating sows and piglets survival rate.
MATERIALS AND METHODS
Animals and management
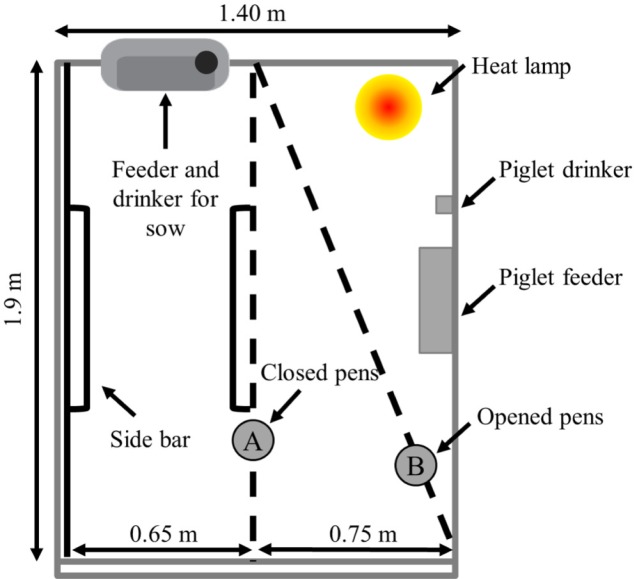
This experiment was conducted at the NIAS institute farm located in Cheonan-si from October to November of 2019. A total of 22 primiparous sows (Landrace; average initial body weight [BW], 228.54 ± 12.79 kg) were allotted to one of two treatments on the basis of body weight. Sows were divided into two experimental groups, conventional farrowing crates, and loosed farrowing pens using a completely randomized design. The experiment duration was around 38 days ranging from 10 days before parturition to 28 days after parturition. Therefore, the experimental sows were moved into the farrowing barn at day 105 of gestation and were raised until day 28 of weaning. The artificial insemination was performed on all sows 2 times after the onset of estrus, and a Pharvision B-mode ultrasound machine (AV 2,100 V; Ambisea Tech. Shenzhen, China) was used to detect pregnancy at day 30 post-breeding [12]. The control treatment had a general farrowing stall (0.65 m × 1.9 m) and creep area (0.75 m × 1.9 m), in contrast, the loosed-farrowing pens (LFP) contained a farrowing stall with openable pens (Fig. 1) that could provide more area for farrowing and lactating sow, when was opened. In the LFP treatment, closed farrowing crates had 0.65 m × 1.9 m of sows’ area (1.24 m2) and 0.75 m × 1.9 m of creep area (1.43 m2), since opened pens sows and piglets had 1.95 m2 and 0.72m2, respectively. To make the loose farrowing facilities, openable pens were opened from the beginning of the experiment before farrowing and for day 5 of lactation to weaning. Both farrowing equivalents had a single feeder. Water was always available through a nipple drinker and a separated feeder and drinker were considered for piglets. Both the conventional farrowing facilities (CON) and LFP farrowing stalls were surrounded by solid metal dividers and the movement of the sows was restricted by lateral bars to control the sudden roll of sows to the side. The routine piglet management and health procedures such as subcutaneous iron dextran injection (50 mg/piglet) and ear notching were performed within the first 24 h. The daily sow feeder check was performed 3 times to be refilled when required. Backfat thickness (BFT) was evaluated at day 105 of gestation, post-partum, and day 28 of lactation (weaning) at the 10th rib, 6.5 cm from one side of the backbone using a medical imaging ultrasound (Loveland, CO, USA). Changes in BFT of sows during gestation and lactation were measured by calculating the difference between BFT at d 1 after placed in a farrowing room and day 1 of lactation and BFT at weaning (day 28 of lactation). The farrowing time was recorded with a camera (HDR-AS50, Sony, Tokyo, Japan) from the expulsion of first birth to the last birth and viewed afterward to calculate the farrowing duration and farrowing interval. The piglets with no sign of breathing were considered as stillborn. Cross‐fostering was conducted within 1 d of parturition. From 1 d after weaning, the detection of estrus was performed twice-daily (0900 and 1730 h) for 10 min by boar exposure. Standard litter traits such as the total number of born, stillbirth, mummy, and born alive, piglet BW (kg) at birth and weaning, and piglet weight gain (kg) and average daily gain (g/d) were recorded individually. Daily feed intake (kg/d) of each sow and weaning to estrus interval (d) were also recorded. All the sows were fed a common diet according to the National Research Council requirements for lactation (Table 1). A commercial feed was provided for suckling piglets from d 21 to d 28 with 4,250 kcal/kg gross energy, 23.1% crude protein, 0.7% calcium, and 0.8% available phosphorus. The dead piglets were collected during morning feeding to calculate piglet mortality. The dead piglets with the signs of broken bone or bruised corpse was marked as crushed piglets. The dead pigs with starvation or hypothermia signs were detected through the recorded video by the evaluation of the location or time of death.
Fig. 1. Illustration of farrowing pens design and dimensions for loose housed sows.
The line A represents housing of confined sow for d 105 of gestation to d 5 after farrowing. Line B represents housing of loosed sows during d 6 after farrowing to weanling.
Table 1. Composition of basal diets for gestation and lactation (as-fed basis).
| Items | Gestation | Lactation |
|---|---|---|
| Ingredients (%) | 100.00 | 100.00 |
| Corn | 40.49 | 40.95 |
| Wheat | 12.00 | 10.00 |
| Wheat bran | 4.00 | - |
| Palm kernel meal | 5.00 | 3.00 |
| Distiller’s dried grains with solubles | 12.00 | 8.00 |
| Canola meal | 3.00 | - |
| Soybean meal | 6.95 | 27.84 |
| Coconut meal | 4.00 | - |
| Corn gluten feed | 2.00 | - |
| Animal fat | 5.21 | 4.00 |
| Molasses | 2.00 | 3.00 |
| Mono dicalcium phosphate | 0.85 | 0.85 |
| Limestone | 1.47 | 1.38 |
| Salt | 0.55 | 0.50 |
| Choline chloride (50%) | 0.06 | 0.06 |
| L-Lysine · HCl (78%) | 0.09 | 0.12 |
| DL-Methionine (99.8%) | - | 0.02 |
| Vitamin premix1) | 0.20 | 0.15 |
| Mineral premix2) | 0.10 | 0.10 |
| Phytase | 0.03 | 0.03 |
| Calculated composition (%) | ||
| Metabolizable energy (MJ/kg) | 13.68 | 14.01 |
| Crude protein | 14.60 | 20.10 |
| Calcium | 0.75 | 0.73 |
| Available phosphorus | 0.32 | 0.32 |
| Lysine | 0.67 | 1.13 |
| Methionine + Cysteine | 0.56 | 0.70 |
Supplied per kilogram diet: vitamin A, 9,600 IU; vitamin D3, 1,800 IU; vitamin E, 24 mg; vitamin K3, 1.5 mg; vitamin B1, 1.5 mg; vitamin B2, 12 mg; vitamin B6, 2.4 mg; vitamin B12, 0.045 mg; pantothenic acid, 24 mg; niacin, 45 mg; biotin, 0.09 mg; folic acid, 0.39 mg.
Supplied per kilogram diet: Fe, 150 mg; Cu, 96 mg; Zn, 72 mg; Mn, 46.5 mg; I, 0.9 mg; Se, 0.3 mg.
Blood samplings
On day 1 (post farrowing), and day 28 (weaning) of lactation, 10 mL blood samples from six randomly selected sows per treatment were collected by ear vein catheter before the morning feeding at 0900 h using a disposable vacutainer tube containing sodium heparin as an anticoagulant to separate the plasma (Becton Dickinson, Franklin, NJ, USA). The plasma automatic biochemical analyzer (Fuji Dri-chem 3500i, Fujifilm, Tokyo, Japan) was applied to evaluate the concentration of blood urea nitrogen, glucose, and triglyceride. Swine cortisol kit (Endocrine Technologies Inc., USA) was used, and concentration was determined in duplicate by ELISA using Biolog MicroStation system. After centrifugation (3,000 ×g for 20 min), all plasma samples were separated and stored at −20°C and later analyzed for blood parameters [13].
Statistical analyses
Data generated in the present experiment were analyzed by the SAS statistical package (SAS 9.1, SAS Institute, Cary, NC, USA). Sow was considered as experimental unit and distributed between the treatments in a completely randomized design using initial body weight as a covariate and removed from the model when insignificant. The main effects of farrowing facilities were determined by the Student’s t-test. A p-value ≤ 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The body weight, backfat thickness, daily feed intake, and weaning-to-estrus interval of sows are presented in Table 2. There were no housing effects on sow body weight and backfat thickness at gestation, lactation, and weaning. The changes in body weight and backfat thickness from gestation to lactation, and lactation to weaning were insignificant between the treatments. Moreover, the housing type had no effect on daily feed intake and weaning to estrus interval. The effect of housing type on the farrowing duration is shown in Table 3. The gestation length was not affected by the treatments, however, the farrowing duration and farrowing interval were increased (p < 0.05) in LFP sows. The effect of housing type on litter size and litter growth are presented in Table 4 and Table 5. At the farrowing time, housing type had no effects total born, stillborn, mummy, born alive piglets and total litter weight and piglet weight. The initial litter size, piglets weaned, survival rate, initial litter weight, piglets weaned weight, total weight gain, and average daily gain were not affected by the treatments. The farrowing duration was 326 min for the CON sows and 151 min for the LFP sows. These durations are much lower than the average duration of 462 min, and 394 min that has been shown in lactating sows by Hales et al. [14]. The farrowing duration in this study is shorter than what has normally been reported in the recent scientific literature. The average farrowing duration in recent studies was raged from 174 to 311 min in crated sows and varied between 146 to 218 min in sows in pens [2,8,15]. The main aim of the current experiment was to evaluate the ratio of piglet mortality in a loose-housing with a few days confinement after farrowing in comparison to crated sows. Nevertheless, our result indicated no significant differences between treatments in the gestation length of sows, the significantly shorter farrowing duration may confirm the positive effects of loose-housing in sows. The provision of the loose house with a wider area in farrowing pens led to the shorter farrowing duration and farrowing interval. Previous reports indicated that confinement of gestating sows before farrowing decreased the circulating oxytocin concentrations in plasma at parturition [8,11]. We did not investigate the concentration of oxytocin, however, the concentration of cortisol was numerically decreased around 28% in LFP sows. The LFP sows would thereby be less under stress compared to sows in the crated-house. Besides the shorter farrowing duration, the number of stillbirth piglets was tended to be decreased in LFP sows. In a previous study, it was shown that the allocation of more space to confined gilts before farrowing only numerically increased the number of born piglets [15]. However, several authors mentioned a positive relationship between the numbers of stillbirth piglets and farrowing duration [16,17]. One of the weak points of our study is the number of used sows in the experiment. This would explain why most of the differences in the parameters just appeared as tendencies.
Table 2. Effects of different farrowing facilities on body weight, backfat thickness, feed intake and weaning to estrus interval in primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Sow body weight (kg) | ||||
| Gestation (d 105) | 229.25 | 227.82 | 3.86 | 0.810 |
| Lactation (d 1) | 208.03 | 206.80 | 3.73 | 0.828 |
| Weaning (d 28) | 200.53 | 200.31 | 4.62 | 0.974 |
| G to L change (-) | 21.23 | 21.02 | 1.31 | 0.912 |
| L to W change (-) | 7.50 | 6.49 | 2.07 | 0.734 |
| Sow backfat thickness (mm) | ||||
| Gestation (d 105) | 26.27 | 28.23 | 0.98 | 0.171 |
| Lactation (d 1) | 25.68 | 27.50 | 1.08 | 0.251 |
| Weaning (d 28) | 21.64 | 23.86 | 0.82 | 0.070 |
| G to L change (-) | 0.59 | 0.73 | 0.21 | 0.657 |
| L to W change (-) | 4.05 | 3.64 | 1.03 | 0.781 |
| Daily feed intake (kg/d) | 4.00 | 4.16 | 0.14 | 0.475 |
| Weaning to estrus interval (d) | 5.59 | 5.45 | 0.20 | 0.622 |
Data represents means based on eleven replicates primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means; G, gestation; L, lactation; W, weaning.
Table 3. Effects of different farrowing facilities on gestation length, farrowing duration and farrowing interval in primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Gestation length (d) | 115.27 | 114.91 | 0.60 | 0.676 |
| Farrowing duration (min) | 326.2 | 151.0 | 40.3 | 0.017 |
| Farrowing interval (min) | 25.9 | 12.6 | 2.89 | 0.011 |
Data represents means based on eleven replicates primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means.
Table 4. Effects of different farrowing facilities on litter size and litter weight of primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Litter size (n) | ||||
| Total born | 12.00 | 12.09 | 0.85 | 0.940 |
| Stillbirth | 2.55 | 0.64 | 0.69 | 0.092 |
| Mummy | 0.55 | 0.18 | 0.22 | 0.250 |
| Born alive | 9.00 | 9.91 | 0.62 | 0.319 |
| Litter weight (kg) | ||||
| Total born | 13.82 | 13.56 | 1.08 | 0.869 |
| Born alive | 12.59 | 12.25 | 1.32 | 0.867 |
| Piglets weight (kg) | ||||
| Total born | 1.17 | 1.11 | 0.06 | 0.519 |
| Born alive | 1.37 | 1.26 | 0.11 | 0.478 |
Data represents means based on eleven primiparous sows per treatment.
CON, conventional farrowing facilities, LFP, loosed farrowing pens; SEM, standard error of means.
Table 5. Effects of different farrowing facilities on piglet performance in primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Initial litter size (n) | 9.35 | 9.55 | 0.53 | 0.810 |
| Piglets weaned (n) | 8.09 | 7.82 | 0.38 | 0.622 |
| Survival rate (%) | 87.72 | 83.05 | 3.62 | 0.374 |
| Weight (kg) | ||||
| Initial litter | 11.46 | 11.32 | 0.66 | 0.877 |
| Initial piglet | 1.24 | 1.18 | 0.06 | 0.505 |
| Litter weaned | 67.03 | 67.80 | 3.66 | 0.883 |
| Piglets weaned | 8.28 | 8.71 | 0.30 | 0.317 |
| Litter weight gain | 55.55 | 56.47 | 3.36 | 0.850 |
| Piglet average daily gain (g/d) | 240.79 | 254.10 | 10.04 | 0.362 |
Data represents means based on eleven primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means.
The effect of housing type on piglet mortality is shown in Table 6. There were no effects of housing type on the mortality of piglets at d 1, 3, 7, 21, and 28. Moreover, no difference was detected in the number of crushed and starved piglets. In this study, after the farrowing, all the LFP sows remained in the crated house without any extra space as same as CON sows for five days. The insignificant difference in piglet mortality is in contrast to the study of Hales et al. [14] who reported that the confinement of sows from d 114 of gestation to d 4 after parturition decreased piglet mortality due to less died piglets before the equalization of litter, however, the confinement did not improve the performance of lactating sows after farrowing. Our result also showed numerically lower mortality in CON sows than the LFP sows during the first third days after farrowing. However, the average mortality in this experiment (14.61%) was much lower than the records from other literature with 21% or 17.9% mortality in the loose house or confine house [14]. After d 5 of lactation, higher numerical piglet mortality was observed. It is confirmed that loose housing, regardless of the type, significantly increase the mortality of piglets [7,10]. One of the issues with a loose house is about providing a wider space for suckling piglets that encourage them to wander around and become chilled due to staying far from the mother sow and heating source [18].
Table 6. Effects of different farrowing facilities on piglet mortality in primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Mortality (%) | ||||
| Day 1 | 0.00 | 1.01 | 0.51 | 0.341 |
| Day 3 | 1.66 | 2.53 | 1.43 | 0.681 |
| Day 7 | 4.41 | 4.37 | 1.76 | 0.989 |
| Day 21 | 5.29 | 8.21 | 2.87 | 0.481 |
| Day 28 | 0.91 | 0.83 | 0.87 | 0.948 |
| Overall | 12.28 | 16.95 | 3.61 | 0.317 |
| Primary cause of death (n) | ||||
| Crushed | 0.55 | 1.09 | 0.25 | 0.137 |
| Starvation/hypothermia | 0.45 | 0.55 | 0.27 | 0.811 |
| Unknown | 0.27 | 0.09 | 0.12 | 0.293 |
Data represents means based on eleven primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means.
The effect of housing type on blood metabolite and cortisol concentration is shown in Table 7 and Table 8. There were no effects of housing type on the concentration of blood urea nitrogen, glucose, triglyceride, creatinine, and cortisol at post-farrowing and weaning time. The activity and behavior of sows can affect the cortisol levels and normally the concentration of cortisol dramatically increases at parturition [8,19]. They emphasized that the increased cortisol level after parturition in the crated sows is associated with constraints such as the restrictions on treating the suckling piglets.
Table 7. Effects of different farrowing facilities on blood metabolites in primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Post farrowing (mg/dL) | ||||
| Blood urea nitrogen | 6.00 | 5.50 | 0.67 | 0.613 |
| Glucose | 74.67 | 76.50 | 1.85 | 0.503 |
| Triglyceride | 48.17 | 55.00 | 5.56 | 0.405 |
| Creatinine | 2.12 | 2.14 | 0.14 | 0.916 |
| Weanling (mg/dL) | ||||
| Blood urea nitrogen | 12.17 | 14.00 | 1.06 | 0.267 |
| Glucose | 92.00 | 99.50 | 4.15 | 0.248 |
| Triglyceride | 17.00 | 17.83 | 2.26 | 0.817 |
| Creatinine | 2.02 | 2.01 | 0.10 | 0.991 |
Data represents means based on eleven primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means.
Table 8. Effects of different farrowing facilities on cortisol concentration of primiparous sows.
| Items | CON | LFP | SEM | p-value |
|---|---|---|---|---|
| Cortisol (ng/ mL) | ||||
| Post farrowing | 3.19 | 2.50 | 0.35 | 0.196 |
| Weanling | 3.33 | 2.76 | 0.57 | 0.530 |
Data represents means based on eleven primiparous sows per treatment.
CON, conventional farrowing facilities; LFP, loosed farrowing pens; SEM, standard error of means.
In conclusion, the result of this study showed that there is no performance difference between the crated or LFP sows, which indicate that the LFP housing has the potential to be used as an alternative to the crated house without any detrimental effects in reproduction performance of lactating sows.
Acknowledgements
This study was supported by 2020 the RDA Fellowship Program of National Institute of Animal Science, Rural Development Administration, Korea.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01198001)” Rural Development Administration, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Choi Y, Jung HJ.
Data curation: Choi Y, Min YJ.
Formal analysis: Choi Y, Kim, YH.
Methodology: Jeong YD.
Software: Kim DW, Kim JE.
Validation: Jung HJ.
Investigation: Min YJ, Jeong YD, Kim DW.
Writing - original draft: Choi Y.
Writing - review & editing: Choi Y, Jung HJ.
Ethics approval and consent to participate
This study was approved by IACUC of Rural Development Administration (No. NIAS-2019-388).
REFERENCES
- 1.Kim KH, Hosseindoust A, Ingale SL, Lee SH, Noh HS, Choi YH, et al. 2016. Effects of gestational housing on reproductive performance and behavior of sows with different backfat thickness. Asian-Australas J Anim Sci. 2016;29:142–8. doi: 10.5713/ajas.14.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thodberg K, Jensen KH, Herskin MS. Nest building and farrowing in sows: relation to the reaction pattern during stress, farrowing environment and experience. Appl Anim Behav Sci. 2002;77:21–42. doi: 10.1016/S0168-1591(02)00026-6. [DOI] [Google Scholar]
- 3.Lee SH, Hosseindoust AR, Kim JS, Choi YH, Lee JH, Kwon IK, et al. Bacteriophages as a promising anti-pathogenic option in creep-feed for suckling piglets: targeted to control Clostridium spp. and coliforms faecal shedding. Livest Sci. 2016;191:161–4. doi: 10.1016/j.livsci.2016.08.003. [DOI] [Google Scholar]
- 4.Choi Y, Hosseindoust A, Shim Y, Kim M, Kumar A, Oh S, et al. Evaluation of high nutrient diets on litter performance of heat-stressed lactating sows. Asian-Australas J Anim Sci. 2017;30:1598–604. doi: 10.5713/ajas.17.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun J, Swan KM, Oliviero C, Peltoniemi O, Valros A. Effects of prepartum housing environment on abnormal behaviour, the farrowing process, and interactions with circulating oxytocin in sows. Appl Anim Behav Sci. 2015;162:20–5. doi: 10.1016/j.applanim.2014.11.006. [DOI] [Google Scholar]
- 6.Oliviero C, Heinonen M, Valros A, Peltoniemi O. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Hales J, Moustsen VA, Devreese AM, Nielsen MBF, Hansen CF. Comparable farrowing progress in confined and loose housed hyper-prolific sows. Livest Sci. 2015;171:64–72. doi: 10.1016/j.livsci.2014.11.009. [DOI] [Google Scholar]
- 8.Oliviero C, Heinonen M, Valros A, Hälli O, Peltoniemi OAT. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim Reprod Sci. 2008;105:365–77. doi: 10.1016/j.anireprosci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LJ, Berg P, Jørgensen G, Andersen IL. Neonatal piglet traits of importance for survival in crates and indoor pens. J Anim Sci. 2011;89:1207–18. doi: 10.2527/jas.2010-3248. [DOI] [PubMed] [Google Scholar]
- 10.KilBride AL, Mendl M, Statham P, Held S, Harris M, Cooper S, et al. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev Vet Med. 2012;104:281–91. doi: 10.1016/j.prevetmed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Yun J, Swan KM, Farmer C, Oliviero C, Peltoniemi O, Valros A. Prepartum nest-building has an impact on postpartum nursing performance and maternal behaviour in early lactating sows. Appl Anim Behav Sci. 2014;160:31–7. doi: 10.1016/j.applanim.2014.08.011. [DOI] [Google Scholar]
- 12.Choi Y, Moturi J, Hosseindoust A, Kim M, Kim K, Lee J, et al. Night feeding in lactating sows is an essential management approach to decrease the detrimental impacts of heat stress. J Anim Sci Technol. 2019;61:333–9. doi: 10.5187/jast.2019.61.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Choi Y, Hosseindoust A, Kim M, Hwang S, Bu M, et al. Evaluation of high nutrient diets and additional dextrose on reproductive performance and litter performance of heat-stressed lactating sows. Anim Sci J. 2019;90:1212–19. doi: 10.1111/asj.13214. [DOI] [PubMed] [Google Scholar]
- 14.Hales J, Moustsen VA, Nielsen MBF, Hansen CF. Temporary confinement of loose-housed hyperprolific sows reduces piglet mortality. J Anim Sci. 2015;93:4079–88. doi: 10.2527/jas.2015-8973. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis S, Reed BT, Lawrence AB, Calvert SK, Stevenson J. Peri-natal environmental effects on maternal behaviour, pituitary and adrenal activation, and the progress of parturition in the primiparous sow. Anim Welf. 2004;13:171–81. [Google Scholar]
- 16.Fraser D, Phillips PA, Thompson BK. Farrowing behaviour and stillbirth in two environments: an evaluation of the restraint-stillbirth hypothesis. Appl Anim Behav Sci. 1997;55:51–66. doi: 10.1016/S0168-1591(97)00007-5. [DOI] [Google Scholar]
- 17.van Dijk AJ, van Rens BTTM, van der Lende T, Taverne MAM. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology. 2005;64:1573–90. doi: 10.1016/j.theriogenology.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Baxter EM, Adeleye OO, Jack MC, Farish M, Ison SH, Edwards SA. Achieving optimum performance in a loose-housed farrowing system for sows: the effects of space and temperature. Appl Anim Behav Sci. 2015;169:9–16. doi: 10.1016/j.applanim.2015.05.004. [DOI] [Google Scholar]
- 19.Osterlundh I, Holst H, Magnusson U. Hormonal and immunological changes in blood and mammary secretion in the sow at parturition. Theriogenology. 1998;50:465–77. doi: 10.1016/S0093-691X(98)00153-8. [DOI] [PubMed] [Google Scholar]