Abstract
Plantago major L. leaves have been used as a wound healing remedy for centuries in almost all parts of the world and in the treatment of a number of diseases apart from wound healing. These include diseases related to the skin, respiratory organs, digestive organs, reproduction, the circulation, against cancer, for pain relief and against infections. P. major contains biologically active compounds such as polysaccharides, lipids, caffeic acid derivatives, flavonoids, iridoid glycosides and terpenoids. Alkaloids and some organic acids have also been detected. A range of biological activities has been found from plant extracts including wound healing activity, anti-inflammatory, analgesic, antioxidant, weak antibiotic, immuno modulating and antiulcerogenic activity. Some of these effects may attribute to the use of this plant in folk medicine.
Keywords: Traditional uses, Chemical constituents, Biological activities, Plantago major L
1. Botany
Plantago major L. (Plantago major ssp. major L.) is a perennial plant that belongs to the Plantagináceae family. It can become about 15 cm high, but the size varies a lot depending on the growth habitats. The leaves grow in rosettes, and they are ovate to elliptical with parallel venation (5–9). The leaves are glabrous and have an entire or irregularly dentate margin. The flowers are small, brownish-green on long non-ramified spikes.
P. major is pollinated by wind, and large amounts of seeds are produced, up to 20 000 per plant (Fægri, 1970, Tutin et al., 1976). The seeds are quite small with an ovate shape (0.4–0.8×0.8–1.5 mm) and a slightly bitter taste. The seed endosperm has highly thickened cellulosic walls with the cell lumen filled with oil and protein. It forms the major part of the seeds and surrounds the embryo completely. The seeds are located in capsules (8–16 per capsule) and become sticky in humid weather due to the swelling of the polysaccharides present in the seed coat (Qadry, 1963). In this manner the seeds can become attached to animals and humans and thereby be spread.
2. History
Research on pollen has shown that P. major was introduced to the Nordic countries parallel to the introduction to the first primitive cultivated fields in the stone age nearly 4000 years ago (Jonsson, 1983). P. major was spread by man from Europe throughout the world. The Indians named it ‘White man’s footprint’ because it was found everywhere the Europeans had been. This has been adapted into the genus name Plantago that is from Latin planta, meaning sole of the foot.
P. major is a plant that many people know only as a weed, but P. major is also an old medicinal plant that has been known for centuries. In Scandinavia this plant is mostly known for its wound healing properties. The common Norwegian and Swedish name for P. major is groblad meaning ‘healing leaves’.
The traditional use of P. major in wound healing is quite old. It was described by the Greek physician Dioscorides in ‘De materia medica’ in the first century. The leaves were prescribed for treatment of dog bites (Roca-Garcia, 1972). From the ‘Vølsuga saga’ it is known that the Vikings used P. major leaves for wound healing (Nielsen, 1969). P. major was also described in the 12–13th century by the Islamic author Ibn El Beithar having adopted the knowledge from Greek medicine (Fleurentin et al., 1983). Henrik Harpestreng († 1244) from Denmark wrote in ‘Liber Harbarum’ that P. major could heal everything that was torn apart. Mixed with honey it was recommended on wounds. Boiled with butter and eaten, it could heal any organ in the human body (Nielsen, 1969).
It was also commonly used in the time of Shakespeare and is mentioned in his play ‘Romeo and Juliet’, Act I, Scene II from the period 1592–1609: Romeo: Your plantain leaf is excellent for that. Benvoleo: For what, I pray thee? Romeo: For your broken shin.
P. major was described in ‘Flora Danica’ by Simon Paulli in 1648 as a very efficient wound healing remedy. At that time it was so common in use that even small children knew about it. The nerves were pulled out of the leaves, and then the leaves were applied on the wounds morning and evening. For superficial wounds to heal, it was sufficient to apply the juice from the plant (Brøndegaard, 1987). The English apothecary Nicholas Culpeper published ‘The Complete Herbal’ in 1649. The use of plants in the treatment of diseases was based on astrology. At that time people lacked other explanations as to why some plants had certain effects and others not. According to this theory P. major is under Venus: ‘It cures the head by its antipathy to Mars and the privities by its sympathy to Venus. There is not a martial disease that it does not cure’. About the medicinal effects he wrote: ‘It is good to stay spitting of blood and bleedings at the mouth, or the making of foul and bloody water, by reason of any ulcer in the reins or bladder’ (Potterton, 1983).
3. Use in traditional medicine
More recent ethnopharmacological studies show that P. major is used in many parts of the world and in the treatment of a number of diseases ( Table 1): skin diseases, infectious diseases, problems concerning the digestive organs, respiratory organs, reproduction, the circulation, against tumours, for pain relief and for reducing fever.
Table 1.
Some uses of Plantago major L. in traditional medicine
| Traditional use | Part of planta | Country | References |
|---|---|---|---|
| Skin | |||
| Abscesses | l | Hawaii, Norway, Turkey | Nagata (1971), Høeg (1974), Yesilada et al. (1995) |
| l, w | Guatemala, Turkey | Cáceres et al. (1987b), Tabata et al. (1994) | |
| Acne | l, w | Guatemala | Cáceres et al. (1987b) |
| Anti-inflammatory | l, j | Madeira | Rivera and Obón (1995) |
| ng | Cuba | Ruiz et al. (1996) | |
| l | Norway | Høeg (1974) | |
| p, w | Chile, Panama, Rodrigues | Rodriguez et al. (1994), Gupta et al. (1979), Gurib-Fakim et al. (1993) | |
| l, c+p, c | India | Jain (1991), Tiwari et al. (1979) | |
| Bee, wasp and nettle stings | l | India, Iran | Joshi et al. (1982), Zagari (1992) |
| l, j | Denmark, Norway | Brøndegaard (1987), Høeg (1974) | |
| p | USA | Hussey (1974) | |
| Bruises | p | USA | Hussey (1974) |
| l, c | Iran | Zagari (1992) | |
| l | Guatemala | Cáceres et al. (1987b) | |
| Burns | p, c | India | Saklani and Jain (1989), Rao (1981), Jain (1991) |
| l | Guatemala, Iran, Norway | Cáceres et al. (1987b), Zagari (1992), Høeg (1974) | |
| l, j | Cook Isl., Denmark, Rarotonga | Holdsworth (1991), Brøndegaard (1987) | |
| Cutaneous leishmaniasis | l, w + l, c | Brazil | Franca et al. (1996) |
| Cuts | l | India | Saklani and Jain (1989) |
| l, c | Thailand | Anderson (1986a) | |
| l+l, c | Denmark, Norway | Brøndegaard (1987), Høeg (1974) | |
| Dermatitis | l, w | Guatemala | Cáceres et al. (1987b) |
| l | Norway | Høeg (1974) | |
| Desinfectant for wounds | l+l, c+l, w+l, j | Denmark, Norway | Brøndegaard (1987), Høeg (1974) |
| l, c+l, w | Madeira | Rivera and Obón (1995) | |
| l, mix+a | Italy | Leporatti and Pavesi (1990) | |
| ng | Cuba | Ruiz et al. (1996) | |
| l, c | Thailand | Anderson (1986b) | |
| l, w | Chile | Houghton and Manby (1985) | |
| Emollient | l, w+s, w | Europe | Roca-Garcia (1972) |
| l, j | Madeira | Rivera and Obón (1995) | |
| l, w+r | Iran | Zagari (1992) | |
| Exanthema | l | Denmark, Guatemala | Brøndegaard (1987), Cáceres et al. (1987b) |
| Haemostatic on wounds | l, c | India | Rao and Jamir (1982), Jain (1991) |
| l, h+l, c | Denmark, Norway | Brøndegaard (1987), Høeg (1974) | |
| On poison ivy dermatitis | l | USA | Duckett (1980) |
| Pruritus | l, c | Iran | Zagari (1992) |
| Pusformation in impetigo | l+vaseline | India | Joshi et al. (1982) |
| Rosen | l | Guatemala | Cáceres et al. (1987b) |
| Soothing effect | l, w | Iran, Phillippines | Zagari (1992), Lim-Sylianco and Shier (1985) |
| r | Iran | Zagari (1992) | |
| l | Europe | Roca-Garcia (1972), Høeg (1974) | |
| Wound healing | l, w | Canary Islands, Chile, Turkey | Darias et al. (1986), Houghton and Manby (1985), Tabata et al. (1994) |
| Phillippines | Lim-Sylianco and Shier (1985) | ||
| p | USA | Hussey (1974) | |
| l, c | Brazil, Iran | Guillén et al. (1997), Zagari (1992) | |
| l | Guatemala, Russia | Cáceres et al. (1987b), Mironov et al. (1983) | |
| l+l, c+l, w+l, j | Denmark, Norway | Brøndegaard (1987), Høeg (1974) | |
| l, j | Cook Islands, Rarotonga | Holdsworth (1991) | |
| Respiratory organs | |||
| Anti tussive | l, w, mix | Iran | Zagari (1992) |
| l, j+honey | Iran | Zagari (1992) | |
| Asthma, bronchitis | l, r, w | Iran, Bulgaria | Zagari (1992), Markov (1992) |
| Colds | p, w | Panama | Gupta et al. (1979) |
| l, w | Norway | Høeg (1974) | |
| Ear ache | l, r, w | Iran | Zagari (1992) |
| Expectoract | l,w | Brazil | Guillén et al. (1997) |
| Pulmonary diseases | l | Hawaii | Nagata (1971) |
| l, w | Norway, Peru | Høeg (1974), Ramirez et al. (1988) | |
| s, w | Europe | Roca-Garcia (1972) | |
| Throat inflammation | l, w | Brazil, Chile | Guillén et al. (1997), Houghton and Manby (1985) |
| f, mix, w | Iran | Zagari (1992) | |
| Digestive organs | |||
| Cholera | l, w | Haiti | Weniger et al. (1986) |
| Constipation | r, w | California, USA | Bocek (1984) |
| s | India | Jain (1991) | |
| Diarrhea | ng | Mexico | Ponce-Macotela et al. (1994) |
| l, w | Canary Islands | Darias et al. (1986) | |
| j+l, w | India | Joshi et al. (1982), Jain and Puri (1984), Jain (1991) | |
| l, r, w | Iran | Zagari (1992) | |
| Dysenteri | j | USA | Eli Lilly (1898) |
| s, w | India | Joshi et al. (1982) | |
| Gastritis and colitis | j, a | Russia | Mironov et al. (1983) |
| Gum inflammation | l, w | Phillippines | Lim-Sylianco and Shier (1985) |
| Oral wounds | l, w | Brazil | Guillén et al. (1997) |
| Stomach ache | p, w | Argentina, USA (Hmong refug.) | Spring (1989), Bustos et al. (1996) |
| Stomach cramps | l, w | Guatemala | Logan (1973) |
| Stomatitis | l, r, w | Iran | Zagari (1992) |
| l | Guatemala | Cáceres et al. (1987b) | |
| Ulcer | l, w | Brazil, Norway, Turkey | Høeg (1974), Yesilada et al. (1993), Guillén et al. (1997) |
| p, w | Argentina, Panama | Gupta et al. (1979), Bustos et al. (1996) | |
| l, w+j | Russia | Mironov et al. (1983) | |
| s, w | India | Joshi et al. (1982) | |
| Urogenital system | |||
| Abortifacient | r | New Mexico, USA | Conway and Slocumb (1979) |
| s | India | Saklani and Jain (1989) | |
| Contraseptive | l, w | Afghanistan | Hunte et al. (1975) |
| p, w | USA (Hmong refugees) | Spring (1989) | |
| Inhibit menstrual period | l, w | Afghanistan | Hunte et al. (1975) |
| Kidney stones | l, w | Greece | Lawrendiadis (1961) |
| l, w, mix | Venezuela | Morton (1975) | |
| Menstrual disorders | j | USA | Eli Lilly (1898) |
| s | India | Fazal (1979) | |
| Pregnancy and childbirth | r | South Africa | Veale et al. (1992) |
| Renal bladder ailments | l, j | Panama | Gupta et al. (1979) |
| p, w | USA (Hmong refugees) | Spring (1989) | |
| Urinary tract infections | l, r, w | Iran | Zagari (1992) |
| l | Guatemala | Cáceres et al. (1987b) | |
| s, w | India | Joshi et al. (1982) | |
| Uterine problems | p, w | Rodrigues | Gurib-Fakim et al. (1993) |
| Vaginitis | l | Guatemala | Cáceres et al. (1987b) |
| Heart and circulation | |||
| Astringent effect | l, w+r, mix | Iran | Zagari (1992) |
| l, r, w | India | Kapur (1983) | |
| Blood rectifier | l, w+r | Iran | Zagari (1992) |
| Diabetes | l, w+p, w | Chile | Houghton and Manby (1985), Rodriguez et al. (1994) |
| Diuretic | s, w | Vietnam | Doan et al. (1992) |
| ng | New Mexico, USA | Conway and Slocumb (1979) | |
| p, w | Chile, Rodrigues, Thailand | Rodriguez et al. (1994), Gurib-Fakim et al. (1993), Wasuwat (1967) | |
| l, w | Guatemala | Cáceres et al. (1987a) | |
| w, mix | China | Pan and Lay (1966) | |
| j | USA | Eli Lilly (1898) | |
| l, w | India | Joshi et al. (1982) | |
| Edema | l | Turkey | Yesilada et al. (1995) |
| Hemorroides | l, w | Brazil, India | Guillén et al. (1997), Joshi et al. (1982) |
| r,w | Denmark | Brøndegaard (1987) | |
| Hypertention | w | Burma | Kyi et al. (1971) |
| l, w | Hawaii | Nagata (1971) | |
| Sense organs | |||
| Eye infections | p, w | Rodrigues | Gurib-Fakim et al. (1993) |
| l | Guatemala | Cáceres et al. (1987b) | |
| w | Panama | Gupta et al. (1979) | |
| Eye problems | l, j | Haiti, Madeira | Weniger et al. (1986), Rivera and Obón (1995) |
| l | Norway | Høeg (1974) | |
| l, w | Peru, Tobago | Ramirez et al. (1988), Seaforth et al. (1998) | |
| Nerve system | |||
| Analgesic | l, w | Brazil, Peru | Guillén et al. (1997), Ramirez et al. (1988) |
| p, w | USA (Hmong refugees) | Spring (1989) | |
| Antipyretic | r, w | California, USA | Bocek (1984) |
| p, w | Brazil | Brandao et al. (1985) | |
| l, w | Brazil, Columbia | Guillén et al. (1997), Schultes and Raffauf (1994) | |
| l, r, w | India | Joshi et al. (1982), Jain (1991) | |
| Hypnotic | l, w, mix | Venezuela | Morton (1975) |
| Nervous shock | l, w | Haiti | Weniger et al. (1986) |
| Physical weakness | l | Hawaii | Nagata (1971) |
| Stimulant | s, w | India | Joshi et al. (1982), Jain (1991) |
| l | Hawaii | Nagata (1971) | |
| p, w | Rodrigues | Gurib-Fakim et al. (1993) | |
| Toothache | p, w | Rodrigues | Gurib-Fakim et al. (1993) |
| l, r, w | Iran | Zagari (1992) | |
| Antineoplastic | |||
| Tumors | l, w | Canary Islands | Darias et al. (1986) |
| p | Chile, Venezuela | Morton (1975), Bhakuni et al. (1976), Rodriguez et al. (1994) | |
| l, j | Panama | Gupta et al. (1979) | |
| Parasitic infections | |||
| Antihelmintic | p, w | Argentina, Rodrigues | Gurib-Fakim et al. (1993), Bustos et al. (1996) |
| l, w | Guatemala | Logan (1973) | |
| Antimalaria | p | Tanzania | Weenen et al. (1990) |
| Parasites | w | Mexico | Ponce-Macotela et al. (1994) |
| Skeleton | |||
| For bone fractures | p | USA (Hmong refugees) | Spring (1989) |
| Antidote | |||
| Snake poison | p | USA | Hussey (1974) |
| l, p, c, j | India | Jain and Puri (1984), Selvanayahgam et al. (1994) | |
f, Flowers; l, leaves; s, seeds, r, root; p, whole plant; c, crushed; j, juice; w, water extract; a, alcohol extract; mix, mixed with other plants; and ng, not given.
4. Chemical constituents and their biological activities
4.1. Carbohydrates
The seeds contain the monosaccharides glucose, fructose, xylose and rhamnose as well as the disaccharide sucrose and the trisaccharide planteose (O-α-d-Galp-(1→6)-O-β-d-Fruf-(2→1)-α-d-Glcp) (Ahmed et al., 1965). Planteose acts as a reserve carbohydrate in the seeds (Rohrer, 1972).
The outer seed coat contains polysaccharides that swell in contact with water and form mucilage with high viscosity. Polysaccharides extracted from the seeds with cold water are composed of 61% xylose, 13.2% arabinose and 24% galacturonic acid, and the hot water extract of the residue contains 78% xylose, 13.2% arabinose, 3% galactose and 6.2% galacturonic acid (Ahmed et al., 1965). Samuelsen et al. (1999a) found that the polysaccharides in the 50°C water extract are composed of 39.7% xylose, 13.1% arabinose, 17.2% galacturonic acid, 15.5% glucuronic acid, 2.1% rhamnose, 2.5% galactose and 9.9% glucose. The acidic fractions are heteroxylans that consist of blocks of β-(1→4)- linked xylose residues and blocks of β-(1→3)- linked xylose residues in the polymer backbone. Small side chains such as single xylose and arabinose residues and the disaccharides α-l-Araf-(1→3)-β-d-Xylp and α-d-GlcpA-(1→3)-β-d-Xylp are linked to position 2 or 3 of (1→4)- linked xylose residues in the backbone (Samuelsen et al., 1999a, Samuelsen et al., 1999b). The acidic fractions that had the highest Mw in size exclusion chromatography had relatively high anti-complementary activity (Samuelsen et al., 1999a).
The trisaccharide raffinose (0.3 mg/g dry weight) and the tetrasaccharide stachyose (4.5 mg/g dry weight) have been isolated from the leaves. Stachyose acts as temporary carbohydrate storage in the plant (Chatterton et al., 1990).
Gorin (Gorin, 1966a, Gorin, 1966b) isolated polysaccharides composed of galacturonic acid, galactose, arabinose and rhamnose in addition to small amounts of glucose and xylose. By separation on a DEAE-cellulose column a pectic acid polysaccharide, a galactoarabinan and a galactan were isolated. These substances are sometimes referred to as ‘plantaglucid’ and have been used to treat ulcers at 1.5–3 g/day (Gorin et al., 1966). Given in a dose of 1 mg/kg, plantaglucid reduced the ulceration index in rats stomachs 20 times. In dogs it intensified the secretion of gastric juice. Plantaglucid lowered the tone and reduced the range of contractions in isolated rabbit intestine and also had spasmolytic effect. It helped to reduce inflammatory oedema provoked by formalin and dextran. No toxic effects were observed after prolonged enteral administration to rats and dogs (Obolentseva and Khadzhai, 1966).
A highly esterified pectin polysaccharide with Mw 46–48 kDa, PMII was isolated from a 50°C water extract (Samuelsen et al., 1995, Samuelsen et al., 1996). PMII contains both smooth polygalacturonan and two different ramified regions; one (PVa) that has relatively high amounts of (1→4)- and (1→3,6)- linked galactose residues with arabinose linked to position 6. The side chains in PVa were linked to position 4 of the rhamnose residues in the backbone. The other ramified region (PVb) contained arabinose side chains attached to position 3 of the galacturonic acid residues in the backbone. PMII had high anti-complementary activity, and PVa was the part of PMII that had the highest activity. PMII also activated human monocytes in vitro for increased production of tumour necrosis factor α (TNFα). The pectin fraction that was isolated from the 100°C water extract had very low anti-complementary activity compared to PMII, and this may be due to less of the side chains that were in PVa (Samuelsen et al., 1995, Samuelsen et al., 1996). Lately it was shown that PMII activates complement mainly via the classical pathway (Michaelsen et al., 1999) and that it has prophylactic activity against Streptococcus pneumoniae infection in mice (Hetland et al., 1999). From the 50°C water extract an anti-complementary acidic arabinogalactan, PMIa, was isolated (Samuelsen et al., 1998). It was composed of arabinose (31%), galactose (32%), rhamnose (6%) and galacturonic acid (7%). This arabinogalactan consists of a (1→3)- linked galactan backbone with (1→6)- linked galactan side chains with arabinose residues attached to position 3 of galactose residues in the side chains. It also contains 1.5% protein with relatively high amounts of hydroxy proline (28.7%), alanine (14.9%) and serine (10.9%) indicating that this is an arabinogalactan type II due to the classification made by Aspinall (1973). The neutral fraction of the water extract had very low anti-complementary activity and consisted of high amounts of glucose and mannose (Samuelsen et al., 1995).
According to a review article on immuno stimulants from higher plants by Wagner (1987) P. major was previously investigated for immunologically active polysaccharides. The isolated polysaccharides increased phagocytosis 15–50% in two in vitro phagocytosis models, and the highest rate of stimulation was achieved with a 0.1 mg/ml aqueous solution. The types of polysaccharides investigated were not stated. The polysaccharides that have been isolated from P. major are summarised in Table 2 .
Table 2.
Polysaccharides in Plantago major L.
| Polysaccharide | References | |
|---|---|---|
| In leaves | ||
| Plantaglucide | Pectic acid, galactoarabinan, galactan | Gorin (1966a), Gorin (1966b) |
| PMII | Pectin with smooth and hairy regions | Samuelsen et al. (1996) |
| PMIa | Arabinogalactan type II | Samuelsen et al. (1998) |
| Glucomannan | Samuelsen et al. (1995) | |
| In seeds | ||
| Starch | Samuelsen et al. (1999a) | |
| Acidic heteroxylans | Samuelsen et al. (1999a) | |
4.2. Lipids
Fatty acids, both free and after hydrolysis of triglycerides, have been isolated from the seeds and are listed in Table 3 . According to Ahmed et al. (1968) 64.8% of the fatty acids are unsaturated.
Table 3.
Fatty acids isolated from the seeds of Plantago major L.
| Fatty acid | Percent of total fatty acids | References | |
|---|---|---|---|
| Myristic acid | 14:0 | Swiatek et al. (1980) | |
| Palmitic acid | 16:0 | Ahmed et al. (1968), Swiatek et al. (1980) | |
| Stearic acid | 18:0 | Ahmed et al. (1968), Swiatek et al. (1980) | |
| Oleic acid | 18:1 | 37.4 | Ahmed et al. (1968), Swiatek et al. (1980) |
| Linoleic acid | 18:2 | 25.3 | Ahmed et al. (1968), Swiatek et al. (1980) |
| Linolenic acid | 18:3 | 0.9 | Ahmed et al. (1968), Swiatek et al. (1980) |
| Arachidic acid | 20:0 | Ahmed et al. (1968) | |
| Behenic acid | 22:0 | Ahmed et al. (1968) | |
| Lignoceric acid | 24:0 | Pailer and Haschke-Hofmeister (1969) | |
| 9-Hydroxy-cis-11-octadecenoic acid | 18:1 | 1.5 | Ahmad et al. (1980) |
Arachidic acid was isolated from P. major seeds only and not from any other Plantago species investigated. Most of the fatty acids present are generally found in plant seeds. One unusual hydroxyolefinic fatty acid, 9-hydroxy-cis-11-octadecenoic acid which is an isomer of ricinoleic acid was isolated by Ahmad et al. (1980). It is a minor constituent (1.5%) of the seed oil.
From the fresh leaves 0.18% lipids were isolated, and the distributions of the different fatty acids are listed in Table 4 . The unsaturated fatty acids, 18:3ω3 and 18:2ω6 and the saturated fatty acid palmitic acid were most abundant in the leaves.
Table 4.
Fatty acids in Plantago major L. leaves (Guil et al., 1996)
| Fatty acid | % | |
|---|---|---|
| Myristic acid | 14:0 | 1.8 |
| Palmitic acid | 16:0 | 15.9 |
| 16:1ω7 | 1.5 | |
| 16:1ω9 | 0.1 | |
| 16:2ω6 | 0.4 | |
| 16:3ω3 | 1.0 | |
| Stearic acid | 18:0 | 2.1 |
| 18:1ω9 | 2.3 | |
| 18:2ω6 | 11.2 | |
| 18:3ω3 | 33.3 | |
| 18:4ω3 | 2.0 | |
| Arachidic acid | 20:0 | 1.3 |
| 20:4ω6 | 1.0 | |
| 20:5ω3 | 1.3 | |
| Behenic acid | 22:0 | 1.3 |
| 22:1ω9 | 3.5 | |
| 22:6ω3 | 1.5 | |
| 24:0 | 1.0 |
The major components of the leaf wax are the free triterpene acids, oleanolic and ursolic acid (see Other terpenoids), and the linear alkanes C27H56-C33H58. The chloroform extract was composed of about 63% triterpenic acids, 17% linear hydrocarbons, 1% linear alcohols and 19% unidentified compounds independently of the plants age (Bakker et al., 1998).
Clinical and histological studies made by Mironov et al. (1983) showed that saturated C26–C30 primary alcohols with even numbers of carbon atoms from the n-hexane extract and the non-hydrolysable fractions of the n-hexane extract had powerful curative effects on superficial injuries in rabbits.
4.3. Alkaloids
P. major has been tested positive for alkaloids (Rojas, 1968, Smolenski et al., 1974). Schneider (1990) identified them as indicain and plantagonin (Fig. 1 ).
Fig. 1.
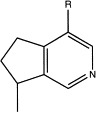
Alkaloids in P. major L. Indicain: R=CHO; plantagonin: R=COOH.
4.4. Caffeic acid derivatives
The ethyl and methyl esters of caffeic acid were isolated from the methanolic extract (Pailer and Haschke-Hofmeister, 1969), and chlorogenic and neochlorogenic acid were isolated from the aqueous extract (Maksyutina, 1971b). According to Noro et al. (1991) plantamajoside is the main caffeic acid derivative in P. major L., and only small amounts of acteoside (synonym to verbascoside) are present. Skari et al. (1999a) on the other hand isolated equal amounts of each compound from the 80% ethanol extract of the plant. According to Mølgaard (1986), plantamajoside and acteoside are not found together in the same plant. In Denmark, there are two subspecies of P. major, P. major ssp. major and ssp. spleiosperma. Plantamajoside is present in both subspecies, while acteoside is found only in ssp. spleiosperma (Mølgaard, 1986). Plantamajoside is glycosylated with glucose to the central glucose while in acteoside it is glycosylated with rhamnose (Fig. 2 ).
Fig. 2.
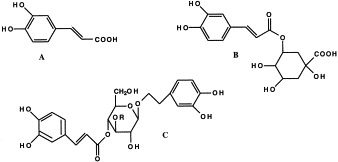
Caffeic acid derivatives in P. major L. (A) Caffeic acid, (B) chlorogenic acid, (C) Plantamajoside R=Glc, acteoside R=Rha.
Plantamajoside has some known biological activities. It has an inhibitory effect on arachidonic acid-induced mouse ear oedema, i.e. anti-inflammatory activity (Murai et al., 1995), inhibitory activity on 5-lipoxygenase (Ravn et al., 1990), 15-lipoxygenase (Skari et al., 1999a) and cAMP phosphodiesterase (Ravn et al., 1990) and antioxidant activity (Miyase et al., 1991). Skari et al. (1999a) found that plantamajoside is a DPPH (diphenylpicrylhydrazyl) radical scavenger. Plantamajoside is also known to have some antibacterial activity (Ravn and Brimer, 1988).
Acteoside has superoxide anion and DPPH radical scavenging activities, has antioxidant activity and inhibits lipid peroxidation (Xiong et al., 1996, Miyase et al., 1991, Zhou and Zheng, 1991, Skari et al., 1999a, Skari et al., 1999b). It inhibits 15-lipoxygenase slightly less efficient than plantamajoside (IC50 117 vs. 96 μM) (Skari et al., 1999a). Acteoside inhibits protein kinase C by interacting directly with the catalytic domain of the enzyme (Herbert et al., 1991). Acteoside inhibits aldose reductase (Ravn et al., 1990) and 5-HETE formation (Kimura et al., 1987). It has antibacterial (Shoyama et al., 1987), immunesuppressant (Sasaki et al., 1989) and analgesic activity (Andary et al., 1982). Acteoside has antihypertensive effect, at a dose of 10 mg/kg on rats a significant decrease in systolic, diastolic and mean arterial blood pressure was observed (Ahmad et al., 1995). The biological activities of these and other caffeic acid derivatives are reviewed in Jiménez and Riguera (1994).
4.5. Flavonoids
Several flavonoids have been isolated from P. major (Table 5 ). According to Kawashty et al. (1994) the amount of each flavonoid isolated from Egyptian P. major can be ranged as follows: luteolin 7-glucoside>hispidulin 7-glucuronide>luteolin 7-diglucoside>apigenin 7-glucoside≈nepetin 7-glucoside>luteolin 6-hydroxy 4′-methoxy 7-galactoside. Skari et al. (1999b) isolated plantaginin and homoplantaginin in addition to several flavonoids having structures that have not been found in P. major earlier. Their structures remain to be published.
Table 5.
Flavonoids in Plantago major L. compound
| Compound |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
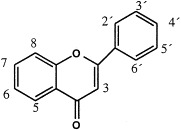 | |||||||||||
| 3 | 5 | 6 | 7 | 8 | 2′ | 3′ | 4′ | 5′ | 6′ | Referencesa | |
| Apigenin 7-glucosid | H | OH | H | OGlc | H | H | H | OH | H | H | 3 |
| Baicalein | H | OH | OH | OH | H | H | H | H | H | H | 1 |
| Hispidulin | H | OH | Ome | OH | H | H | H | OH | H | H | 2 |
| Hispidulin 7-glucuronide | H | OH | Ome | OGlcA | H | H | H | OH | H | H | 3 |
| Homoplantaginin | H | OH | Ome | OGlc | H | H | H | OH | H | H | 4, 5 |
| Luteolin 7-glucosid | H | OH | H | OGlc | H | H | OH | OH | H | H | 3 |
| Luteolin 7-diglucosid | H | OH | H | OGlc-Glc | H | H | OH | OH | H | H | 3 |
| Luteolin 6-hydroxy-4′-methoxy-7-galactoside | H | OH | OH | OGal | H | H | OH | OMe | H | H | 3 |
| Nepetin 7-glucoside | H | OH | Ome | OGlc | H | H | OH | OH | H | H | 3 |
| Plantaginin | H | OH | OH | OGlc | H | H | H | OH | H | H | 4, 5 |
| Scutellarein | H | OH | OH | OH | H | H | H | OH | H | H | 1, 2 |
References: 1, Maksyutina (1971a); 2, Harborne and Williams (1971); 3, Kawashty et al. (1994); 4, Nishibe et al. (1995); and 5, Skari et al. (1999b).
Many flavonoids are antioxidants (Rice-Evans et al., 1996, Bohm et al., 1998). Examples of such compounds in P. major are baicalein, hispidulin and plantaginin (Yuting et al., 1990, Yokozawa et al., 1997, Skari et al., 1999b). A number of flavonoids are also known to have free radical scavenging activity (Kandaswami and Middleton, 1994). Baicalein, hispidulin, scutallarein and plantaginin are free radical scavengers and inhibit lipid peroxidation (Sanz et al., 1994, Yoshino et al., 1997, Gao et al., 1999, Skari et al., 1999b). Both baicalein and hispidulin have anti-inflammatory activities. Baicalein inhibits carrageenan-induced rat paw edema (Lin and Shieh, 1996a), 12-lipoxygenase (You et al., 1999) and LPS induced production of nitric oxide in macrophages (Wakabayashi, 1999) while hispidulin has been shown to be an inhibitor of 5-lipoxygenase (Moongkarndi et al., 1991). Baicalein has hepatoprotective effect against CCl4-induced liver injuries in rats (Lin and Shieh, 1996b). Baicalein can induce cell death of carcinoma cells (Matsuzaki et al., 1996), cause inhibition of cell growth of human hepatoma cells (Motoo and Sawabu, 1994) and has shown strong antiproliferative effect in rat hepatic stellate cells (Inoue and Jackson, 1999). Scutallarein and baicalein have antiallergic activities (Kawasaki et al., 1994, Toyoda et al., 1997). In addition, they are HIV-reverse transcriptase inhibitors in vitro; (IC50 2.5 and 5.6 μM, respectively). The glucosides plantaginin, luteolin 7-glucoside and homoplantaginin are also potent inhibitors (IC50 9.8, 40.2 and 43.3 μM, respectively) while apigenin 7-glucoside had no inhibitory effect on HIV-reverse transcriptase (Nishibe et al., 1997).
4.6. Iridoid glycosides
The iridoid glycosides isolated from P. major are listed in Table 6 , and the structure formulas are given in Fig. 3 . The major iridoid glycoside found is aucubin, but its content varies over the seasons. The highest aucubin level registered (1.3% in dried leaves) was in June. P. major contains less aucubin than P. lanceloata (Long et al., 1995). Three unusual iridoid glycosides with 8,9 double bonds, majoroside (Handjieva et al., 1991), 10-hydroxymajoroside and 10-acetoxymajoroside have been isolated from the aerial parts of the plant (Taskova et al., 1999).
Table 6.
Iridoidglycosides from Plantago major L.
| Iridoidglycoside | Part of plant | % | References |
|---|---|---|---|
| Asperuloside | Flowers | 0.023 | Bianco et al. (1984) |
| Aucubin | Leaves | 0–1.3 | Long et al. (1995) |
| Catapol | Aerial parts | 0.003 | Murai et al. (1996) |
| Gardoside | Aerial parts | 0.001 | Murai et al. (1996) |
| Geniposidic acid | Aerial parts | 0.005 | Murai et al. (1996) |
| Majoroside | Aerial parts | 0.004 | Handjieva et al. (1991) |
| 10-Actoxymajoroside | Aerial parts | 0.03 | Taskova et al. (1999) |
| 10-Hydroxymajoroside | Aerial parts | 0.02 | Taskova et al. (1999) |
| Melittoside | Aerial parts | 0.004 | Murai et al. (1996) |
Fig. 3.
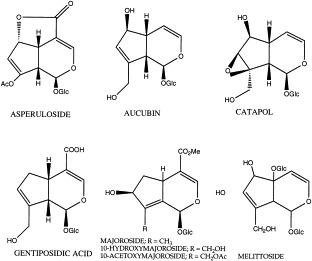
Iridoid glycosides from P. major L.
Aucubin has anti-inflammatory properties: when applied topically aucubin has an inhibitory effect on TPA (12-O-tetradecanoylphorbol acetate) induced mouse ear oedema with a maximum effect at a dose of 1 mg/ear. This effect is close to that of indomethacin at 0.5 mg/ear (Recio et al., 1994).
Aucubin has also spasmolytic properties on acetylcholine induced contraction on rat uterus and rat vas deferens (Oritz de Urbina et al., 1994). Aucubin has antidote activity for poisonous amanita mushrooms in mice by protection against liver damage induced by α-amanitin. The mechanism is thought to be due to a competitive effect of aucubin on α-amanitin inhibition of liver RNA synthesis (Chang et al., 1984). It also has liver protective activity against CCl4-induced hepatic damage in mice (Chang, 1998) in addition to antiviral activity against hepatitis B virus (Chang, 1997).
The aglycon of aucubin, aucubigenin, has antimicrobial activity against bacteria and moulds (Davini et al., 1986).
4.7. Other terpenoids
The terpenoid loliolid has been isolated from the leaves (Pailer and Haschke-Hofmeister, 1969). The triterpenoids oleanolic acid, ursolic acid, 18β-glycyrrhetinic acid and sitosterol were isolated from the leaf wax (Hiltibran et al., 1953, Ringbom et al., 1998). Ursolic acid inhibits cyclooxygenase-2 (IC50 130 μM) and cyclooxygenase-1 (IC50 295 μM) catalysed prostaglandin biosynthesis in vitro while the structural isomer oleanolic acid is less active. 18β-Glycyrrhetinic acid had no significant inhibitory effect (Ringbom et al., 1998). The mechanisms of the anti-inflammatory effects also include inhibition of histamin release from mast cells, inhibition of elastase and inhibition of complement activity. Ursolic acid and oleanolic acid also have hepatoprotective, tumor promotion inhibiting activity and an anti-hyperlipidemic effect (Liu, 1995).
4.8. Glucosinolates
Intact glucosinolates have not been isolated from P. major seeds or leaves (Larsen et al., 1983).
4.9. Vitamins
P. major has been used as a food supply, especially during spring before the harvest of the common vegetables. The vitamin contents have, therefore, been examined. The fresh leaves of old plants that had gone to seed, collected in early spring, were reported to contain 6 mg β-carotene (provitamin A)/100 g and 19 mg ascorbic acid/100 g (Zennie and Ogzewalla, 1977). According to a study of young plants P. major contains 25 mg ascorbic acid, 31 mg dehydroascorbic acid and 8.5 mg carotenoids/100 g young leaves. Thus, P. major can be considered as a good source of vitamin C and carotenoids. In addition, the oxalic acid, nitrate and erucic acid were present in low amounts (67±36 mg/100 g, 101±18 mg/100 g and 3.45%, respectively) indicating a low toxicity of the plant (Guil et al., 1997).
Shoots of P. major collected in June contained 37 mg/g dried leaf material of phylloquinone (vitamin K1). A high vitamin K level might be of importance in the resistance of weeds to the herbicide 2,4 dichlorophenoxyacetic acid. The vitamin K level in P. major was intermediate compared to other plant species, and it was also moderately resistant towards the herbicide (Jansson, 1974).
4.10. Other organic acids
From the methanol extract the following organic acids were isolated: fumaric acid, syringic acid, vanillic acid, p-hydroxy benzoic acid, ferulic acid, p-coumaric acid, gentisic acid, traces of salicylic acid, benzoic acid and cinnamic acid (Pailer and Haschke-Hofmeister, 1969).
5. Biological activity of extracts
P. major is used for different purposes in traditional medicine around the world, therefore, researchers have tested it for different types of biological activities. Most tests have been performed on crude extracts without examining the nature of the active compounds. The results of these studies are listed below and include both positive and negative results.
5.1. Antiulcerogenic activity
P. major has been used in Turkey in the treatment of ulcers. The powdered dried leaves were taken together with honey daily before breakfast. A water immersion-stress ulcer model was used on rats to test the plant extract’s ability to inhibit ulcers. A test sample was given just before immobilisation in a stress cage. After 7 h immersed in a water-bath the rats were killed and the stomachs were taken out for examination. The combined methanol- and water extract (1.2 g/kg) inhibited ulcer formation by 40% relative to the control group which received only the vehicle. The water extract (1 g/kg) inhibited ulcer formation by 37% and the methanol extract inhibited it by 29%. P. major was not among the most active plants tested (Yesilada et al., 1993).
5.2. Anticancer activity
In a screening of anticancer activity of Chilean plants a 50% ethanol extract of leaves, stems and seeds of P. major had no activity in vivo against lymphocytic leukaemia in mice (Bhakuni et al., 1976).
A P. major preparation was reported to be effective in a screening system for prophylactic oncology. The effect included antimetastatic activity in models of tumour metastasis in mice. The details in this study were not described (Yaremenko, 1990). In another study, an aqueous extract was shown to have a prophylactic effect on mammary cancer in mice (Lithander, 1992). The leaves were extracted with phosphate buffer pH 7 containing 0.9% NaCl and injected subcutaneously in mice of the C3H Strong strain. Among mice of this strain more than 90% develop cancer induced by a virus infection. After 60 weeks, 93.3% of the untreated and 18.2% of the treated mice had tumours. The observed effect is thought to be due to stimulation of the immune system rather than a direct effect on the virus. No experimental results support this idea, only some observations made without experimental verification. The P. major extract had good effect on human herpes infections but had no effect on the herpes virus in in vitro tests. The same observations have been made for bacteria; only weak antibacterial activity of P. major extracts in vitro, but they had an effect on infected wounds in vivo. While antibiotics on infected wounds had no effect, topical treatment with P. major extract eradicated the infections and healed the wounds.
5.3. Immunomodulatory activity
The leaves extracted in saline for 2 h at 50°C had chemotactic activity on neutrophils using the Boyden migration chamber method, but it did not enhance neutrophil intracellular killing activity by the nitrozoblue tetrazolium reduction test (Basaran et al., 1997).
5.4. Antiinfective testing in vitro
5.4.1. Antibiotic and antifungal activity
P. major has been included in screening studies of plants used in folk medicine in fighting bacterial and fungal infections in the skin or in the treatment of gastrointestinal disorders.
Discs containing plant extracts were applied to bacteria cultured on agar plates, and the inhibition zones measured after some time. Water extracts, methanol extracts, 50% and 70% ethanol extracts were tested.
The methanol extracts were most active against Salmonella typhimurium (Table 7 ) and had weaker activity against methicillin resistant S. aureus and M. phlei. The methanol extracts were active (8–10 mm inhibition zone) against the fungi F. tricuictum and M. gypseum, and an incomplete inhibition of C. albicans and S. cerevisiae was observed (Table 8 ). The antifungal activity was weaker than the antimycoticum nystatin (15–20 mm inhibition zone).
Table 7.
Antibiotic activity of Plantago major L. water extract, methanol extract (MeOH), 50% and 70% ethanol extract (EtOH) determined by measurement of inhibition zones of discs containing extracts on bacteria cultures on agar platesa
| Bacteria | H2O | MeOH | 50% EtOH | 70% EtOH | Referencesb |
|---|---|---|---|---|---|
| Staphylococcus aureus | − | ++ | + | 1, 2, 3 | |
| S. aureus, methicillin resistant | + | 5 | |||
| S. aureus, methicillin sensitive | − | 5 | |||
| Streptococcus pyogenes | − | 3 | |||
| Bacillus subtilis | − | ++ | − | 2, 3, 5 | |
| Shigella sonnei | + | 2 | |||
| S. flexneri | −/+ | ++ | 2, 3, 4 | ||
| S. dysenteriae | ++ | 4 | |||
| Salmonella typhi | −/+ | 3, 4 | |||
| S. enteritidis | − | 4 | |||
| S. typhimurium | ++ | 5 | |||
| Serratia marcescens | − | 5 | |||
| Enterobacter aerogenes | − | 5 | |||
| Esherichia coli | − | − | ++ | + | 1, 2, 3, 4, 5 |
| Escherichia “crim” | + | 2 | |||
| Klebsiella pneumonia | − | 5 | |||
| Pseudomonas aeruginosa | − | − | 3, 5 | ||
| Proteus vulgaris | − | 3 | |||
| Mycobacteriumphlei | + | 5 | |||
| M. smegmatis | + | 2 |
Effects as defined by the authors: −, inhibition zone <6–8 mm; +, inhibition zone 6–10 mm; ++, inhibition zone 10–15 mm.
References: 1, Gaw and Wang (1949); 2, Moskalenko (1986); 3, Cáceres et al. (1987b); 4, Cáceres et al. (1990); and 5, McCutcheon et al. (1992).
Table 8.
Antifungal activity of Plantago major L. methanol extract (MeOH), 50% ethanol extract (EtOH) determined by measurement of inhibition zones of discs containing extracts on bacteria cultures on agar plates (Cáceres et al., 1987b, McCutcheon et al., 1994)a
| Fungi | MeOH | 50% EtOH |
|---|---|---|
| Aspergillus flavus | − | |
| A. fumigatus | − | |
| Fusarium tricuictum | + | |
| Sacchariomyces cerevisiae | +i | |
| Trichoderma viridae | − | |
| Microsporum cookerii | − | |
| M. gypseum | + | |
| Trichophyton mentagrophytes | − | |
| Candida albicans | +i | − |
Effects as defined by the authors: −, inhibition zone <6–8 mm; +, inhibition zone 6–10 mm; I, incomplete inhibition.
The 50% ethanol extracts were active against S. aureus, B. subtilis, S. dysenteriae and E. coli . These include both gram negative and gram positive bacteria. The 70% ethanol extracts were most effective against S. flexneri and had weaker activity against S. aureus, S. sonnei, E. coli, Esherichia ‘crim’ and M. smegmatis.
The antibiotic activities registered were weaker than the positive controls used. Incubation of gentimicin and a P. major methanol extract gave inhibition zones of >25 mm and 10–15 mm, respectively on S. typhimurium.
In conclusion there seems to be some intermediately polar or nonpolar substances of relatively low molecular weight in P. major that have antibiotic activity against some gram negative and gram positive bacteria in addition to a weak antimycotic activity.
5.4.2. Antigiardiasic activity
P. major is used in Mexico against diarrhoea and/or parasites. A decoction in a saline solution was made of the plant, and this was incubated with trophozoides of Giardia duedenalis. The mortality was 76±1.2 which was at the level of the positive control tinidazol (79±1.9) (Ponce-Macotela et al., 1994).
5.4.3. Antimalarial activity
P. major has been used in the treatment of malaria in Tanzania. In vitro activity against Plasmodium falciparum strain K1 which is multidrug resistant was performed by measurement of the ability of the extracts to inhibit the incorporation of [3H]-hypoxantine into the malaria parasites. The dichloromethane extract of the whole plant had some effect (IC50 10–49 mg/ml), the petroleum ether extract and the methanol extract had little activity (IC50 100–499 mg/ml and >499 mg/ml, respectively). For comparison, the methanol extract of a Cinchona species had an IC50 of 0.5 mg/ml (Weenen et al., 1990).
5.4.4. Antiviral activity
No antiviral activity against herpes and polio virus of ethanol extracts of the entire plant was registered in the in vitro study of Suganda et al. (1983). Neither was the methanol extract of the plant active in vitro against bovine coronavirus, bovine herpesvirus type 1, bovine parainfluenza virus type 3, bovine rotavirus, bovine respiratory syncytial virus, vaccinia virus or vesicular stomatitis virus (McCutcheon et al., 1995).
5.5. Anti-inflammatory and analgesic activity
The aqueous extract (72°C, 30 min) of dried P. major leaves given orally has shown anti-inflammatory and analgesic activities related to inhibition of prostaglandin synthesis in mice and rats. Anti-inflammatory activity in rats was demonstrated by the inhibition of paw oedema induced by carrageenan. The extract did not affect oedema produced by dextran, indicating that the mechanism involved inhibition of cyclooxygenase synthesis rather than an antihistamine activity. The extract also inhibited the formation of exudate and leucocyte mobilisation induced by intrapleural injection of carrageenan, the latter being a known activity of non-steroidal anti-inflammatory compounds. Activity against chronic inflammation was measured as the inhibition of exudate in the air pouch after oral treatment with extract.
Peroral treatment of mice with extract inhibited acetic acid induced writhing (i.e. non-steroid anti-inflammatory activity) but had no effect on the tail flick test (i.e. no opioid-like analgesic activity) (Guillén et al., 1997).
5.6. Antioxidant and free radical scavenger activity
Antioxidant capacity by bleaching of the absorbance of pre-formed 2,2′-azinobis (3-ethylbenzthiazolinesulfonic acid) radical cation in the presence of infusions made from P. major herbal tea bags and P. major leaves were determined. The infusion of P. major tea contained small amounts of free radical scavengers compared to black tea. The antioxidants had low reactivity, measured as a relatively high t 1/2. The antioxidant capacity of the green leaves was higher than that of the P. major tea indicating that processing can lead to significant loss of activity (Campos and Lissi, 1995).
5.7. Diuretic effect
In Guatemala the leaves are used as a diuretic agent. In a screening study of 67 plants a 10% decoction of the dried leaves of P. major was tested on rats. The decoction was administered by a nasogastric catheter at a dose of 1 g/kg. It had an intermediate diuretic activity; urinary output increased by 108±44% after 6 h. Hydrochlorothiazide increased urinary output by 286±38 % (Cáceres et al., 1987a).
In Vietnam, the extracts of the seeds of P. major taken orally are said to have a diuretic effect. A possible diuretic activity was tested on healthy human volunteers in a placebo controlled double-blind crossover model. No significant diuretic effect through increased urinary output or sodium excretion was registered in this study (Doan et al., 1992).
5.8. Hypotensive effect
In Burma, the infusion of P. major is taken orally to produce a fall in blood pressure. Lipophilic compounds were removed from a P. major water extract containing high molecular weight compounds and injected at doses of 15, 20 and 25 mg/kg into anaesthetised dogs. The dose–response effect was not very consistent, and there were large individual variations in the response. The study was of a preliminary nature and without any statistics (Kyi et al., 1971).
In another study normotensive rats were given a P. major extract intravenously. The extract was lyophilised 70% ethanol extracts dissolved in a physiological solution. Maximum effect was obtained 0.2 min after injection and lasted for 0.5 min. The reduction in arterial blood pressure was not significant (Schmeda-Hirschmann et al., 1992).
5.9. Hypoglycaemic activity
Rodriguez et al. (1994) have tested a 70% ethanol extract for its hypoglycaemic activity in normoglycaemic rats without finding any significant effect. The extract was given orally at a dose of 500 mg/kg. The background for the testing was that the Mapuche Indians in Chile have used the infusion of P. major in the treatment of diabetes (Houghton and Manby, 1985).
6. Toxicity
As shown in Table 9 the genotoxicity of P. major extracts on prokaryotes are somewhat contradictory. In the Ames test (S. typhimurium microsomal activation assay), water extracts caused reversions of tester strains TA1537 and TA98. This indicates the presence of direct frameshift mutagens (Lim-Sylianco and Shier, 1985). The P. major saline extract had, however, no response in the Ames test with strains TA98 and TA100 (Basaran et al., 1996).
Table 9.
The toxicity of Plantago major L. leaf extracts
| Extract | Test | Toxic | Comments | Ref.a |
|---|---|---|---|---|
| Decoction | Ames test, strains TA 1537 and TA 98 | + | Direct frameshift mutagens | 1 |
| Saline extract | Ames test strains TA 100 and TA 98 | − | 2 | |
| Alcohol extract | Plate incorporation assay with Aspergillus nidulans D-30 | − | Stimulation of colony growth | 3 |
| Alcohol extract | Aspergillus nidulans somatic segregation assay | − | 3 | |
| 70% Ethanol extract | Brine shrimp (Artemia salina) | + | LC50=7 μg/ml | 4 |
| Not stated | i.p. and oral administration in rats | − | LD50=1000 mg/kg i.p., LD50>4000 mg/kg oral | 5 |
| Saline extract | COMET assay in human lymphocytes | + | DNA strand brakeage | 2 |
References: 1, Lim-Sylianco and Shier (1985); 2, Basaran et al. (1996); 3, Ruiz et al. (1996); 4, Schmeda-Hirschmann et al. (1992); and 5, Angelov et al. (1980).
An alcohol extract showed no toxicity on the diploid strain Aspergillus nidulans D-30, on the contrary, a stimulation of colony growth was observed. The A. nidulans strain used in the somatic segregation assay carry four recessive mutations for conodial colour, and coloured sectors are used as an indicator of genotoxic events leading to somatic segregation. No significant differences in frequency of coloured sectors per colony compared to the negative control were observed. Thus, no genotoxic effect was found of the plant extract (Ruiz et al., 1996).
The 70% ethanol extract was found to be toxic to shrimps (Schmeda-Hirschmann et al., 1992) but P. major possesses a low toxicity in rats at oral and i.p. administration (Angelov et al., 1980).
DNA damage by strand breakage was suggested after examination of human lymphocytes treated with the saline extract. It had an increased activity in the alkaline COMET assay compared to the negative control (Basaran et al., 1996).
7. Concluding remarks
Taking the claimed wound healing activity of P. major into consideration, it is not necessarily only one single compound that is responsible for this effect, the effect may as well be due to several compounds that act in a synergistic manner or to compounds which regulate one another.
There are several of the isolated compounds that may aid the healing of wounds. Plantamajoside and acteoside have antibacterial activities. Some flavonoids and the caffeic acid derivatives plantamajoside and acteoside have antioxidative and free radical scavenging activities. Pectic polysaccharides have been reported to be effective against ulcers in rats and for having immunostimulatory activities. Finally, the long chained saturated primary alcohols that are present in the leaf wax aid the healing of superficial wounds. However, the leaves also contain compounds with anti-inflammatory activity, namely plantamajoside, baicalein, hispidulin, aucubin, ursolic acid and oleanolic acid. Since the inflammatory phase in general is necessary in the wound healing process, anti-inflammatory activity may be undesirable. On the other hand, these substances’ activities when acting together with other compounds present in the leaves are not known at present. Thus, the full picture of P. major as a wound healing remedy may be rather intricate.
Due to the very long tradition in using P. major for wound healing and also because of what is known today about its chemical constituents and biological activities, it seems to be worth the effort of exploring this plant further.
References
- Ahmad M.S., Ahmad M.U., Osman S.M. A new hydroxyolefinic acid from Plantago major seed oil. Phytochemistry. 1980;19:217–2139. [Google Scholar]
- Ahmad M., Rizwani G.H., Aftab K., Ahmad U.V., Gilani A.H., Ahmad S.P. Acteoside: a new antihypertensive drug. Phytotherapy Research. 1995;9:525–527. [Google Scholar]
- Ahmed Z.F., Rizk A.M., Hammouda F.M. Phytochemical studies of egyptian Plantago species (Glucides) Journal of Pharmaceutical Sciences. 1965;54:1060–1062. doi: 10.1002/jps.2600540727. [DOI] [PubMed] [Google Scholar]
- Ahmed Z.F., Hammouda F.M., Rizk A.M., Wassel G.M. Phyochemical studies of egyptian Plantago species. Planta Medica. 1968;4:404–410. doi: 10.1055/s-0028-1099927. [DOI] [PubMed] [Google Scholar]
- Andary C., Wylde R., Laffite C., Privat G., Winternitz F. Structures of verbascoside and oroban choside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry. 1982;21:1123–1127. [Google Scholar]
- Anderson E.F. Ethnobotany of hill tribes of northern Thailand. I. Medicinal plants of Akha. Economic Botany. 1986;40:38–53. [Google Scholar]
- Anderson E.F. Ethnobotany of hill tribes of northern Thailand. II. Lahu medicinal plants. Economic Botany. 1986;40:442–450. [Google Scholar]
- Angelov A., Lambev I., Markov M., Yakimova K., Leseva M., Yakimov A. Study of acute and chronical toxicity of dispergue of Plantago major. Medical Archives. 1980;18:47–52. [Google Scholar]
- Aspinall, G.O., 1973. Carbohydrate polymers of plant cell walls. In: Loewus, F. (Ed.), Biogenesis of Plant Cell Wall Polysaccharides. Academic Press, New York pp. 95–115.
- Bakker M.I., Baas W.J., Sum D.T.H.M., Koloffel C. Leaf wax of Lactuca sativa and Plantago major. Phytochemistry. 1998;47:1489–1493. [Google Scholar]
- Basaran A.A., Yu T.-W., Plewa M.J., Anderson D. An investigation of some Turkish herbal medicines in Salmonella typhimurium and in the COMET assay in human lymphocytes. Teratogenesis. Carcinogenesis and Mutagenesis. 1996;16:125–138. doi: 10.1002/(SICI)1520-6866(1996)16:2<125::AID-TCM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Basaran A.A., Ceritoglu I., Undeger U., Basaran N. Immunomodulatory activities of some Turkish medicinal plants. Phytotherapy Research. 1997;11:609–611. [Google Scholar]
- Bhakuni D.S., Bittner M., Marticorena C. Screening of Chilean plants for anticancer activity. Lloydia. 1976;39:225–243. [PubMed] [Google Scholar]
- Bianco A., Guiso M., Passacantilli P., Francesconi A. Iridoid and phenylpropanoid glycosides from new sources. Journal of Natural Products. 1984;47:901–902. [Google Scholar]
- Bocek B.R. Ethnobotany of Costanoan indians, California, based on collections by John P. Harrington. Economic Botany. 1984;38:241–255. [Google Scholar]
- Bohm H., Boeing H., Hempel J., Raab B., Kroke A. Flavonols, flavones and anthocyanins as native antioxidants of food and their possible role in the prevention of chronic diseases. Zeitschrift fur Ernahrungswissenschaft. 1998;37:147–163. doi: 10.1007/pl00007376. [DOI] [PubMed] [Google Scholar]
- Brandao M., Botelho M., Krettli E. Antimalarial experimental chemotherapy using natural products. Ciência e Cultura. 1985;37:1152–1163. [Google Scholar]
- Brøndegaard, V.J., 1987. Folk og Flora, Vol. 4. Rosenkilde and Bagger, Kobenhavn, pp. 68-77.
- Bustos D.A, Tapia A.A., Feresin G.E., Espinar L.A. Ethnopharmacobotanical survey of Bauchazeta district, San Juan Province, Argentina. Fitoterapia. 1996;5:411–415. [Google Scholar]
- Cáceres A., Giron L.M., Martinez A.M. Diuretic activity of plants used for the treatment of urinary ailments in Guatemala. Journal of Ethnopharmacology. 1987;19:223–245. doi: 10.1016/0378-8741(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Cáceres A., Giron L.M., Alvarado S.R., Torres M.F. Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. Journal of Ethnopharmacology. 1987;20:223–237. doi: 10.1016/0378-8741(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Cáceres A., Cano O., Samayoa B., Aguilar L. Plants used in Guatemala for the treatment of gastrointestinal disorders. A. Screening of 84 plants against enterobacteria. Journal of Ethnopharmacology. 1990;30:55–73. doi: 10.1016/0378-8741(90)90017-n. [DOI] [PubMed] [Google Scholar]
- Campos A.M., Lissi E.A. Evaluation of the antioxidant capacity of herbal teas by a procedure based on the bleaching of ABTS radical cations. Boletin de la Sociedad Chilena de Quimica. 1995;40:375–381. [Google Scholar]
- Chang I.M., Yun H.S., Kim Y.S., Ahn J.W. Aucubin: potential antidote for alpha-amanitin poisoning. Clinical Toxicology. 1984;22:77–85. doi: 10.3109/00099308409035083. [DOI] [PubMed] [Google Scholar]
- Chang I.M. Antiviral activity of aucubin against hepatitis B virus replication. Phytotherapy Research. 1997;11:189–192. [Google Scholar]
- Chang I.M. Liver-protective activities of aucubin derived from traditional oriental medicine. Research Communications in Molecular Pathology and Pharmacology. 1998;102:189–204. [PubMed] [Google Scholar]
- Chatterton N.J., Harrison P.A., Thornley W.R., Bennett J.H. Sucrosyloligosaccharides and cool temperature growth in 14 forb species. Plant Physiology and Biochemistry. 1990;28:167–172. [Google Scholar]
- Conway G.A., Slocumb J.C. Plants used as abortifacients and emmenagogues by spanish New Mexicans. Journal of Ethnopharmacology. 1979;1:241–261. doi: 10.1016/s0378-8741(79)80014-8. [DOI] [PubMed] [Google Scholar]
- Darias V., Bravo L., Barquin E., Herrera D.M., Fraile C. Contribution to the ethnopharmacological study of the Canary Islands. Journal of Ethnopharmacology. 1986;15:169–193. doi: 10.1016/0378-8741(86)90154-6. [DOI] [PubMed] [Google Scholar]
- Davini E., Lavarone C., Trogolo C., Aureli P., Pasolini B. The quantitative isolation and antimicrobial activity of the aglycone of aucubin. Phytochemistry. 1986;25:2420–2422. [Google Scholar]
- Doan D.D., Nguyen N.H., Doan H.K., Nguyen T.L. Studies on the individual and combined diuretic effects of four Vietnamese traditional hermal remedies (Zea mays, Imperata cylindrica, Plantago major and Orthosiphon stamineus) Journal of Ethnopharmacology. 1992;36:225–231. doi: 10.1016/0378-8741(92)90048-v. [DOI] [PubMed] [Google Scholar]
- Duckett S. Plantain leaf for poison ivy. New England Journal of Medicine. 1980;303:583. doi: 10.1056/NEJM198009043031013. [DOI] [PubMed] [Google Scholar]
- Eli Lilly, 1898. Lilly’s Handbook of Pharmacy and Therapeutics. 5th rev. Eli Lilly and Company, Indianapolis, In 46206, USA
- Fazal U. Preliminary clinical study of the treatment of Kasrat-e-Tams (menorrhigie) with tukhm-e-bartang (Plantago major Linn.) Journal of Research in Indian Medicine Yoga and Homeopathy. 1979;14:1–6. [Google Scholar]
- Fleurentin J., Mazars G., Pelt J.-M. Additional information on the cultural background of drugs and medicinal plants of Yemen. Journal of Ethnopharmacology. 1983;8:335–344. doi: 10.1016/0378-8741(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Franca F., Lago E.L., Marsden P.D. Plants used in the treatment of leishmanial ulcers due to Leishmania (Viannia) braziliensis in an endemic area of Bahia, Brazil. Revista da Sociedade Brasileira de Medicina Tropica. 1996;29:229–232. doi: 10.1590/s0037-86821996000300002. [DOI] [PubMed] [Google Scholar]
- Fægri K. Norges Planter: Blomster og Trær i Naturen. Cappelen; Oslo, Norway: 1970. [Google Scholar]
- Gao Z.H., Huang K.X., Yang X.L., Xu H.B. Free radical scavenging and antoxidant activites of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochimica et Biophysica Acta — General Subjects. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Gaw H.Z., Wang H.P. Survey of Chinese drugs for presence of antibacterial substances. Science. 1949;110:11–12. doi: 10.1126/science.110.2844.11-a. [DOI] [PubMed] [Google Scholar]
- Gorin A.G. Polysaccharides from Plantago major leaves. I. Analysis of monosaccharide composition of polysaccharide complex. Chemical Abstracts. 1966;64:8277. [Google Scholar]
- Gorin A.G. Polysaccharides from Plantago major leaves. II. Pectic acid. Chemical Abstracts. 1966;64:11552. [Google Scholar]
- Gorin A.G., Maksyutina N.P., Kolesnikov D.G. New ulcer remedy from the leaves of Plantago major. Chemical Abstracts. 1966;65:17581. [Google Scholar]
- Guil J.L., Torija M.E., Giménez J.J., Rodríguez I. Identification of fatty acids in edible wild plants by gas chromatography. Journal of Chromatography A. 1996;719:229–235. doi: 10.1016/0021-9673(95)00414-9. [DOI] [PubMed] [Google Scholar]
- Guil J.L., Rodriguez-Garcia I., Torija E. Nutritional and toxic factors in selcted wild edible plants. Plant Foods for Human Nutrition. 1997;51:99–107. doi: 10.1023/a:1007988815888. [DOI] [PubMed] [Google Scholar]
- Guillén M.E.N., Emim J.A.S., Souccar C., Lapa A.J. Analgesic and antiinflammatroy activities of the aqueous extract of Plantago major L. International Journal of Pharmacognosy. 1997;35:99–104. [Google Scholar]
- Gupta M.P., Arias T.D., Correa M., Lamba S.S. Ethnopharmacognostic observations on Panamanian medicinal plants. Part I. Quarterly Journal of Crude Drug Research. 1979;17:115–130. [Google Scholar]
- Gurib-Fakim A., Sewrey M., Gueho J., Dulloo E. Medical ethnobotany of some weeds of Mauritius and Rodrigues. Journal of Ethnopharmacology. 1993;39:175–185. doi: 10.1016/0378-8741(93)90034-3. [DOI] [PubMed] [Google Scholar]
- Handjieva N., Spassov S., Bodurova G. Majoroside, an iridoid glucoside from Plantago major. Phytochemistry. 1991;30:1317–1318. [Google Scholar]
- Harborne J.B., Williams C.A. 6-Hydroxyluteolin and scutellarein as phyletic markers in higher plants. Phytochemistry. 1971;10:367–378. [Google Scholar]
- Herbert H.M., Maffrand F.P., Taoubi K., Augereau J.M., Fouraste I., Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of portein kinase C. Journal of Natural Products. 1991;54:1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- Hetland, G., Samuelsen, A.B., Løvik, M. et al., 1999. Protective effect of Plantago major L. pectin polysaccharide against Streptococcus pneumoniae infection in mice (submitted). [DOI] [PubMed]
- Hiltibran R.C., Wadkins C.L., Nicholas H.J. Journal of the American Society. 1953;75:5125. [Google Scholar]
- Holdsworth D.K. Traditional medical plants of Rarotonga, Cook Islands. Part II. International Journal of Pharmacognosy. 1991;29:71–79. [Google Scholar]
- Houghton P.J., Manby J. Medicinal plants of the Mapuche. Journal of Ethnopharmacology. 1985;13:89–103. doi: 10.1016/0378-8741(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Hunte P., Safi M., Macey A., Kerr G.B. Indigenous methods of voluntary fertility regulation in Afghanistan. National Demographic Family Guidance Survey of Settled Population Afghanistan. 1975;4:1. [Google Scholar]
- Hussey J.S. Some useful plants of early New England. Economic Botany. 1974;28:311. [Google Scholar]
- Høeg O.A. Planter og Tradisjon. Universitetsforlaget; Oslo: 1974. pp. 507–511. [Google Scholar]
- Inoue T., Jackson E.K. Strong antiproliferative effects of baicalein in cultured rat hepatic stellate cells. European Journal of Pharmacology. 1999;378:129–135. doi: 10.1016/s0014-2999(99)00418-5. [DOI] [PubMed] [Google Scholar]
- Jain S.P., Puri H.S. Ethnomedicinal plants of Jaunsar-Bawar hills, Uttar Pradesh, India. Journal of Ethnopharmacology. 1984;12:213–222. doi: 10.1016/0378-8741(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Jain S.K. Dictionary of Indian Folk Medicine and Ethnobotany. Deep Publications; New Delhi: 1991. p. 145. [Google Scholar]
- Jansson O. Phylloquinone (vitamin K1) levels in leaves of plant species differing in susceptibility to 2,4-dichlorophenoxyacetic acid. Physiologia Plantarum. 1974;31:323–325. [Google Scholar]
- Jiménez C., Riguera R. Phenylethanoid glycosides in plants: structure and biological activity. Natural Product Reports. 1994;11:591–606. doi: 10.1039/np9941100591. [DOI] [PubMed] [Google Scholar]
- Jonsson S. Blomsterboken. Markens Urter, Lyng og Trær. Teknologisk Forlag; Oslo: 1983. [Google Scholar]
- Joshi D.N., Sah B.C.L., Suri R.K. Some medicinal plants of Rudranath Bugyal (dist. Chamoli), U.P. Bulletin of Medicoethnobotanical Research. 1982;3:27–42. [Google Scholar]
- Kandaswami C., Middleton E. Free radical scavenging and antioxidant activity of plant flavonoids. Advanses in Experimental Medicine and Biology. 1994;366:351–376. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- Kapur S.K. Medico-botanic survey of medicinal and aromatic plants of Mawphlang (Shillong) Indian Drugs. 1983;21:1–5. [Google Scholar]
- Kawasaki M., Toyoda M., Teshima R. In vitro antiallergenic acitivity of flavonoids in histamine-release assay using rat basophilic leukemia (RBL-2H3) cells. Journal of the Food Hygienic Society of Japan. 1994;35:497–503. [Google Scholar]
- Kawashty S.A., Gamal-el-din E., Abdalla M.F., Saleh N.A.M. Flavonoids of Plantago species in Egypt. Biochemical Systematics and Ecology. 1994;22:729–733. [Google Scholar]
- Kimura Y., Okuda S., Nishibe S., Arichi S. Effects of caffeoylglycosides on arachidonate metabolism in leukocytes. Planta Medica. 1987;53:148–153. doi: 10.1055/s-2006-962658. [DOI] [PubMed] [Google Scholar]
- Kyi, K.K., Mya-Bwin, Sein-Gwan, Chit-Maung, Aye-Than, Mya-Tu, M., Tha, S.J., 1971. Hyoptensive property of Plantago major Linn. Union of Burma Journal of Life Sciences 4, 167–171.
- Larsen L.M., Olsen O., Sørensen H. Failure to detect glucosinolates in Plantago species. Phytochemistry. 1983;22:2314–2315. [Google Scholar]
- Lawrendiadis G. Contribution to the knowledge of the medicinal plants of Greece. Planta Medica. 1961;9:164–169. [Google Scholar]
- Leporatti M.L., Pavesi A. New or uncommon uses of several medicinal plants in some areas of central Italy. Journal of Ethnopharmacology. 1990;29:213–223. doi: 10.1016/0378-8741(90)90058-2. [DOI] [PubMed] [Google Scholar]
- Lim-Sylianco C.Y., Shier W.T. Mutagenic and antimutagenic activities in Philippine medicinal and food plants. Journal of Toxicology — Toxin Reviews. 1985;4:71–105. [Google Scholar]
- Lin C.C., Shieh D.E. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. American Journal of Chinese Medicine. 1996;24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- Lin C.C., Shieh D.E. In vivo hepatoprotective effect of baicalein, baicalin and wogonin from Scutellaria rivularis. Phytotherapy Research. 1996;10:651–654. [Google Scholar]
- Lithander A. Intracellular fluid of waybread (Plantago major) as a prophylactic for mammary cancer in mice. Tumor Biology. 1992;13:138–141. doi: 10.1159/000217757. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. Journal of Ethnopharmacology. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Logan M.H. Digestive disorders and plant medicine in highland Guatemala. Anthropos. 1973;68:537–543. [Google Scholar]
- Long C., Moulis C., Stanislas E., Fourasté E. L′aucuboside et le catapol dans les feuilles de Plantago lanceolata L., Plantago major L. et Plantago media L. Journal de Pharmacie de Belgique. 1995;50:484–488. [Google Scholar]
- Maksyntina N.P. Baicalein and scutellarein derivatives in the leaves of Plantago major. Chemistry of Natural Compounds. 1971;7:352. [Google Scholar]
- Maksyutina, N.P., 1971b. Hydroxycinnamic acids of Plantago major and Pl. lanceolata, Chemistry of Natural Compounds, 7, 795.
- Markov, M., 1992. On the pharmacology of Plantago major. Poster 6 at the 2nd Int. Congr. on Ethnopharmacology, Uppsala, Sweden.
- Matsuzaki Y., Kurokawa N., Terai S., Matsumura Y, Kobayashi N., Okita K. Cell death induced by baicalein in human hepatocellular carcinoma cell lines. Japanese Journal of Cancer Research. 1996;87:170–177. doi: 10.1111/j.1349-7006.1996.tb03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon A.R., Ellis S.M., Hancock R.E.W., Towers G.H.N. Antibiotic screening of medicinal plants of the British Colombian native peoples. Journal of Ethnopharmacology. 1992;37:213–223. doi: 10.1016/0378-8741(92)90036-q. [DOI] [PubMed] [Google Scholar]
- McCutcheon A.R., Ellis S.M., Hancock R.E.W., Towers G.H.N. Antifungal screening of medicinal plants of British Columbian native peoples. Journal of Ethnopharmacology. 1994;44:157–169. doi: 10.1016/0378-8741(94)01183-4. [DOI] [PubMed] [Google Scholar]
- McCutcheon A.R., Roberts T.E., Gibbons E. Antiviral screening of British Colombian medicinal plants. Journal of Ethnopharmacology. 1995;49:101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen, T.E., Gilje, A., Samuelsen, A.B., Hoegaasen, K., Paulsen, B.S., 1999. Complement activation of a pectin type polysaccharide fraction, PMII from the leaves of Plantago major L., (submitted). [DOI] [PubMed]
- Mironov V.A., Vasil’ev G.S., Matrosov V.S. Physiologically active alcohols from great plantain. Khimiko-farmatsevticheskii Zhurnal. 1983;17:1321–1325. [Google Scholar]
- Miyase T., Ishino M., Akahori C., Ueno A., Ohkawa Y., Tanizawa H. Phenylethanoid glycosides from Plantago asiatica. Phytochemistry. 1991;30:2015–2018. [Google Scholar]
- Moongkarndi P., Bunyapraphatsara N., Srisukh V., Wagner H. The inhibitory activity in 5-lipoxygenase pathway of hispidulin from Millingtonia hortensis Linn. f. Journal of the Science Society of Thailand. 1991;17:51–56. [Google Scholar]
- Morton J.F. Current folk remedies of northern Venezuela. Quaterly Journal of Crude Drug Research. 1975;13:97–121. [Google Scholar]
- Moskalenko S.A. Preliminary screening of far-eastern ethnomedicinal plants for antibacterial activity. Journal of Ethnopharmacology. 1986;15:231–259. doi: 10.1016/0378-8741(86)90163-7. [DOI] [PubMed] [Google Scholar]
- Motoo Y., Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma-cell lines. Cancer Letters. 1994;86:91–95. doi: 10.1016/0304-3835(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Murai M., Tamayama Y., Nishibe S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Medica. 1995;61:479–480. doi: 10.1055/s-2006-958143. [DOI] [PubMed] [Google Scholar]
- Murai M., Takenaka T., Nishibe S. Iridoids from Plantago major. Natural Medicines. 1996;50:306. [Google Scholar]
- Mølgaard P. Population genetics and geographical distribution of caffeic acid esters in leaves of Plantago major in Denmark. Journal of Ecology. 1986;74:1127–1137. [Google Scholar]
- Nagata K.M. Hawaiian medicinal plants. Economic Botany. 1971;25:245–254. [Google Scholar]
- Nielsen, H., 1969. Lægeplanter og trolddomsurter. In: Kehler, S. (Ed.). Politikens Forlag, København, pp. 321-324.
- Nishibe S., Murai M., Tamayama Y. Studies on constituents of plantaginis herba 7: Flavonoids from Plantago asiatica and P. augustifolia. Natural Medicines. 1995;49:340–342. [Google Scholar]
- Nishibe S., Ono K., Nakane H., Kawamura T., Noro Y., Tanaka T. Studies on constituents of Plantaginis herba 9. Inhibitory effects of flavonoids from Plantago herb on HIV-reverse transcriptase activity. Natural Medicines. 1997;51:547–549. [Google Scholar]
- Noro Y., Hisata Y., Okuda K. Pharmacognostical studies of Plantaginis herba (VII) on the phenylethanoid contents of Plantago spp. Japanese Journal of Pharmacognosy. 1991;1:24–28. [Google Scholar]
- Obolentseva G.V., Khadzhai Y.I. Pharmacological study of Plantaglucide (Plantago major leaf extract) used in the treatment of an acid gastritis and peptic ulcer. Farmakologiya i Toksikologiya. 1966;29:469–472. (English abstract) [PubMed] [Google Scholar]
- Oritz de Urbina A.V., Martin M.L., Fernández B., San Román L., Cubillo L. In vitro antispasmodic activity of peracetylated penstemonoside, aucubin and catapol. Planta Medica. 1994;60:512–515. doi: 10.1055/s-2006-959561. [DOI] [PubMed] [Google Scholar]
- Pailer V.M., Haschke-Hofmeister E. Inhaltstoffe aus Plantago major. Planta Medica. 1969;17:139–145. doi: 10.1055/s-0028-1099839. [DOI] [PubMed] [Google Scholar]
- Pan P.C., Lay Y.C. Application of chinese traditional medicine in the treatment of liver cancer. Zhongyi Zazhi. 1966;5:33–37. [Google Scholar]
- Ponce-Macotela M., Navarro-Alegria I., Matinez-Gordillo M.N., Alvarez-Chacon R. In vitro antigiardiasic activity of plant extracts. La Revista de Investigación Clínica. 1994;46:343–347. [PubMed] [Google Scholar]
- Potterton D. Culpeper’s Colour Herbal. Sterling Publishing; New York: 1983. [Google Scholar]
- Qadry S.M.J.S. A note on Plantago major seeds: a substitute for ispaghula. Journal of Pharmacy and Pharmacology. 1963;15:552–555. doi: 10.1111/j.2042-7158.1963.tb12833.x. [DOI] [PubMed] [Google Scholar]
- Ramirez V.R., Mostacers L.J., Garcia A.E. Vegetales Empleados en Medicina Tradicional Norperuana. Banco Agrariondel Peru & NACL University; Trujillo, Peru: 1988. p. 54. [Google Scholar]
- Rao R.R. Ethnobotany of Maghalaya: medicinal plants used by Khasi and Garo tribes. Economic Botany. 1981;35:4–9. [Google Scholar]
- Rao R.R., Jamir N.S. Ethnobotanical studies in Nagaland. I. Medicinal plants. Economic Botany. 1982;36:176–181. [Google Scholar]
- Ravn H., Brimer L. Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major subsp. major. Phytochemistry. 1988;27:3433–3437. [Google Scholar]
- Ravn H., Nishibe S., Sasahara M., Li X. Phenolic compounds from Plantago asiatica. Phytochemistry. 1990;29:3627. [Google Scholar]
- Recio M.C., Giner R.M., Ríos J.L. Structural considerations on the iridoids as anti-inflammatory agents. Planta Medica. 1994;60:232–234. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Ringbom T., Segura L., Noreen Y., Perera P., Bolin L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. Journal of Natural Products. 1998;61:1212–1215. doi: 10.1021/np980088i. [DOI] [PubMed] [Google Scholar]
- Rivera D., Obón C. The ethnopharmacology of Madeira and Porto Santo Islands, a review. Journal of Ethnopharmacology. 1995;46:73–93. doi: 10.1016/0378-8741(95)01239-a. [DOI] [PubMed] [Google Scholar]
- Roca-Garcia H. Weeds: a link with the past. Arnoldia. 1972;30:23–24. [Google Scholar]
- Rodriguez J., Loyola J.I., Maulén G., Schmeda-Hirschmann G. Hypoglycaemic activity of Geranium core-core, Oxalis rosea and Plantago major extract in rats. Phytotherapy Research. 1994;8:372–374. [Google Scholar]
- Rohrer D.C. The crystal and molecular structure of planteose dihydrate. Acta Crystallographica B. 1972;28:425–433. [Google Scholar]
- Rojas I.R. Contribucion al estudio quimico del llanten (Plantago major L.) Anales de la Facultad de Quimicay Farmacia. 1968;20:146–150. [Google Scholar]
- Ruiz A.R., De la Torre R.A., Alonso N., Villaescusa A., Betancourt J., Vizoso A. Screening of medicinal plants for induction of somatic segragation activity in Aspergillus nidulans. Journal of Ethnopharmacology. 1996;52:123–127. doi: 10.1016/0378-8741(96)01394-3. [DOI] [PubMed] [Google Scholar]
- Saklani A., Jain S.K. Ethnobotanical observations on plants used in northeastern India. International Journal of Crude Drug Research. 1989;27:65–73. [Google Scholar]
- Samuelsen A.B., Paulsen B.S., Wold J.K., Otsuka H., Yamada H., Espevik T. Isolation and partial characterization of biologically active polysaccharides from Plantago major L. Phytotherapy Research. 1995;9:211–218. [Google Scholar]
- Samuelsen A.B., Paulsen B.S., Wold J.K. Characterzation of a biologically active pectin from Plantago major L. Carbohydrate Polymers. 1996;30:37–44. [Google Scholar]
- Samuelsen A.B., Paulsen B.S., Wold J.K., Knutsen S.H., Yamada H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydrate Polymers. 1998;35:145–153. [Google Scholar]
- Samuelsen A.B., Lund I., Djahromi J.M., Paulsen B.S., Wold J.K., Knutsen S.H. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydrate Polymers. 1999;38:133–143. [Google Scholar]
- Samuelsen A.B., Cohen E.H., Paulsen B.S., Brüll L.P., Thomas-Oates J.E. Structural studies of a heteroxylan from Plantago major L. seeds by partial hydrolysis, HPAEC-PAD, methylation and GC-MS, ES-MS and ES-MS/MS. Carbohydrate Research. 1999;315:312–318. doi: 10.1016/s0008-6215(99)00038-5. [DOI] [PubMed] [Google Scholar]
- Sanz M.J., Ferrandiz M.L., Cejudo M. Influence of a series of natural flavonoids on free-radical generating systems and oxidative stress. Xenobiotica. 1994;24:689–699. doi: 10.3109/00498259409043270. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Nishimura H., Morota T. Immunosuppressive principles of Rehmannia glutinosa var. hueichingensis. Planta Medica. 1989;55:458–462. [PubMed] [Google Scholar]
- Schmeda-Hirschmann G., Loyola J.I., Retamal S.R., Rodriguez J. Hypotensive effect and enzyme inhibition activity of Mapuche medicianl plant extracts. Phytotherapy Research. 1992;6:184–188. [Google Scholar]
- Schultes E.V., Raffauf R.F. De plantis toxicariis e mundo novo tropicale commentationes XXXIX. Harvard Papers in Botany. 1994;5:50–68. [Google Scholar]
- Schneider G. Arzneidrogen, Ein Kompendium für Pharmazeuten, Biologien und Chemiker. Wissenschaftsverlag; Mannheim, Germany: 1990. p. 131. [Google Scholar]
- Seaforth C.E., Ballah S., Rollocks S., Craig-James S. Medicinal plants used in Tobago. Fitoterapia. 1998;69:523–527. [Google Scholar]
- Selvanayahgam Z.E., Gnanevendhan S.G., Balakrishna K., Rao R.B. Antisnake venom botanicals from ethnomedicine. Journal of Herbs Spices and Medicinal Plants. 1994;2:45–100. [Google Scholar]
- Shoyama Y., Matsumoto M., Nishioka I. Phenolic glycosides from diseased roots of Rehmannia glutinosa var. purpurea. Phytochemistry. 1987;26:983–986. [Google Scholar]
- Skari K.P., Malterud K.E., Haugli T. Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major, a medicinal plant. In: Kumpulainen J.T., Salone J.T., editors. Proceedings of the 2nd International Conference on Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease. The Royal Society of Chemistry; Cambridge: 1999. pp. 200–202. [Google Scholar]
- Skari, K.P., Malterud, K.E., Haugli, T., 1999b. Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major, a medicinal plant. Poster 495 at 2000 Years of Natural Products Research — Past, Present and Future, Amsterdam, The Netherlands.
- Smolenski S.J., Silinis H., Farnswoth N.R. Alkaloid Screening. IV. Lloydia. 1974;37:30–61. [PubMed] [Google Scholar]
- Spring M.A. Ethnopharmacologic analysis of medicinal plants used by Laotion Hmong refugees in Minnesota. Journal of Ethnopharmacology. 1989;26:65–91. doi: 10.1016/0378-8741(89)90114-1. [DOI] [PubMed] [Google Scholar]
- Suganda A.G., Amoros M., Girre L. Effets inhibiteurs de quelques extraits bruts et semi purifiés de plantes indigènes françaises sur la multiplication de l’herpesvirus humain 1 et du poliovirus humain 2 en culture cellulaire. Journal of Natural Products. 1983;46:626–632. doi: 10.1021/np50029a006. [DOI] [PubMed] [Google Scholar]
- Swiatek K., Kurowska A., Gora J. Chemical composition of some Plantago species seed oil. Herba Polonica. 1980;4:213–217. [Google Scholar]
- Tabata M., Sezik E., Honda G. Traditional medicine in Turkey III. Folk medicine in east Anatolia, Van and Bitlis. International Journal of Pharmacognosy. 1994;32:3–12. [Google Scholar]
- Taskova R., Handjieva N., Evstatieva L., Popov S. Iridoid glucosides from Plantago cornuti, Plantago major and Veronica cymbalaria. Phytochemistry. 1999;52:1443–1445. [Google Scholar]
- Tiwari K.C., Majumder R., Bhattacharjee S. Folklore medicines from Assam and Arunachal Pradesh (District Tirap) International Journal of Crude Drug Research. 1979;17:61–67. doi: 10.3109/13880208209055187. [DOI] [PubMed] [Google Scholar]
- Toyoda M., Tanaka K., Hoshino K., Akiyama H., Tanimura A., Saito Y. Profiles of potentially antiallergic flavonoids in 27 kinds of health tea and green tea infusions. Journal of Agricultural and Food Chemistry. 1997;45:2561–2564. [Google Scholar]
- Tutin T.G., Heywood V.H., Burges N.A. Vol. 4. Cambridge University Press; Cambridge: 1976. p. 39. (Flora Europaea). [Google Scholar]
- Veale D.J.H., Furman K.I., Oliver D.W. South African traditional herbal medicines used during pregnancy and childbirth. Journal of Ethnopharmacology. 1992;36:185–191. doi: 10.1016/0378-8741(92)90043-q. [DOI] [PubMed] [Google Scholar]
- Wagner H., 1987. Immunostimulants from higher plants. In: Hostettmann K., Lea P.J. (Eds.), Biologically Active Natural Products. Clarendan press, Oxford.
- Wakabayashi I. Inhibitory effects of baicalein and wogonin on lipopolysaccharide-induced nitric oxide production in macrophages. Pharmacology and Toxicology. 1999;84:288–291. doi: 10.1111/j.1600-0773.1999.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Wasuwat, S. (1967). A list of Thai medicinal plants. Research Report No. 1 on Research Project 17, A.S.R.C.T., Bankok, pp. 22.
- Weenen H., Nkunya M.H.H., Bray D.H., Mwasumbi L.B., Kinabo L.S., Kilimali V.A.E.B. Antimalarial activity of Tanzanian medicinal plants. Planta Medica. 1990;56:368–370. doi: 10.1055/s-2006-960984. [DOI] [PubMed] [Google Scholar]
- Weniger B., Rouzier M., Daguilh R., Henrys D., Henrys J.H., Anton R. La medecine pupulaire dans le plateau central d’Haiti. 2 inventaire ethnopharmacologique. Journal of Ethnopharmacology. 1986;17:13–30. doi: 10.1016/0378-8741(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Xiong Q.B., Kadota S., Tani T., Namba T. Antioxidative effects of phynyletanoids from Cistanche deserticola. Biological and Pharmaceutical Bulletin. 1996;19:1580–1585. doi: 10.1248/bpb.19.1580. [DOI] [PubMed] [Google Scholar]
- Yaremenko K.V. Adaptogens of the natural origin in prophylactyc oncology. Journal of Cancer Research and Clinical Oncology. 1990;116:82. [Google Scholar]
- Yesilada E., Sezik E., Fujita T., Tanaka S., Tabata M. Screening of some Turkish medicinal plants for their antiulcerogenic activities. Phytotherapy Research. 1993;7:263–265. [Google Scholar]
- Yesilada E., Honda G., Sezik E. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. Journal of Ethnopharmacology. 1995;46:133–152. doi: 10.1016/0378-8741(95)01241-5. [DOI] [PubMed] [Google Scholar]
- Yokozawa T., Dong E., Liu Z.W., Shimizu M. Antioxidative activity of flavones and flavonols in vitro. Phytotherapy Research. 1997;11:446–449. [Google Scholar]
- Yoshino M., Ito M., Okajima H., Haneda M., Murakami K. Role of baicalein compounds as antioxidant in the traditional herbal medicine. Biomedical Research–Tokyo. 1997;18:349–352. [Google Scholar]
- You K.M., Jong H.G., Kim H.P. Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Archives of Pharmacal Research. 1999;22:18–24. doi: 10.1007/BF02976430. [DOI] [PubMed] [Google Scholar]
- Yuting C., Rongliang Z., Zhongjian J., Yong J. Flavonoids as superoxide scavengers and antioxidants. Free Radical Biology and Medicine. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- Zagari A. Medicinal Plants. Iran Book; Tehran: 1992. p. 969. Teheran University Publications No. 1819/4. [Google Scholar]
- Zennie T.M., Ogzewalla C.D. Ascorbic acid and vitamin A content of edible wild plants of Ohio and Kentucky. Economic Botany. 1977;31:76–79. [Google Scholar]
- Zhou Y.-C., Zheng R.-L. Phenolic compounds and analogs as superoxide anion scavengers and antioxidants. Biochemical Pharmacology. 1991;42:1177–1179. doi: 10.1016/0006-2952(91)90251-y. [DOI] [PubMed] [Google Scholar]


