Abstract
Background
Cisplatin (cis-diamminedichloroplatinum, DDP) resistance is identified as the primary obstacle during lung cancer treatment, while DDP resistance is exist extensively. This report was to investigate the roles of propofol in lung cancer cells tolerance to DDP and the potential mechanisms.
Material/Methods
A549 and A549/DDP cells were treated with DDP for 48 hours, and cell proliferation suppression rate was detected by MTT (thiazolyl blue tetrazolium bromide) assay and half maximal inhibitory concentration (IC50) of DDP to lung cancer cells was calculated. Besides, cell proliferation and apoptosis were determined by MTT assay and flow cytometry assay respectively in propofol-treated A549/DDP and A549 cells. Furthermore, we performed MTT assay to determine the influence of propofol on the sensitivity of lung cancer cells to DDP.
Results
The results demonstrated that the IC50 of DDP to A549 cells was lower than that in A549/DDP cells. Propofol dramatically inhibited cell proliferation and promoted cell apoptosis of A549/DDP and A549 cells. In addition, propofol significantly improved the anti-proliferative impact of DDP in A549/DDP and A549 cells, and the value of IC50 for DDP in the A549/DDP and A549 cells were decreased after propofol treatment compare to the control group. Moreover, propofol inhibited the Wnt/β-catenin pathway in a dose-dependent manner in both A549/DDP and A549 cells.
Conclusions
Our report indicated that propofol could control lung cancer cell proliferation and apoptosis, and stimulated the suppression function of DDP on lung cancer cell multiplication via the Wnt/β-catenin signaling pathway, and also provided a new treatment for DDP tolerance to cure lung cancer in clinical.
MeSH Keywords: Cisplatin, Lung Neoplasms, Propofol
Background
Lung cancer is one of the most universal cancers and a primary cause of mortality around the world, with approximately 1 million deaths around the world annually [1,2]. Non-small cell lung cancer (NSCLC) is the main categories in lung cancers, and it is featured by poor prognosis [3]. Recently, tremendous efforts have been made in the treatment of lung cancer, including radiation therapy, immunotherapy, surgery and chemotherapy, however, the long-term survival rate is still very poor because of the ambiguous nosogenesis [4,5]. Chemotherapy has become the most common methods for lung cancer treatment in clinical, and cisplatin (cis-diamminedichloroplatinum, DDP) is the most frequently applied medicine in lung cancer chemotherapy [6]. Unfortunately, chemotherapeutic drug resistance is a serious clinical issue that led to the limited effects on prolonging survival time of patients and improving their life quality. As a result, the 5-year survival rate of lung cancer patient is approximately 17% [7]. Therefore, it is critical to explore the underlying mechanisms of DDP resistance and provide useful approaches to overcome chemotherapeutic drug resistance for successful lung cancer treatment.
Propofol is widely used as intravenous anesthetic for its quick action, and it is frequently used during surgery [8]. Numerous studies have indicated that propofol has an influence on cell proliferation, migration, and invasion of cancer cells, such as pancreatic cancer [9], cervical cancer [10], ovarian cancer [11], and lung cancer [12]. Also, previous reports have demonstrated that propofol exerts many potential non-anesthetic effects. For example, Wu et al. [13] reported that propofol inhibited A549 cell invasion and migration by mediating MMP-2 and p38 MAPK signaling. Chen et al. [14] found propofol could inhibit papillary thyroid cancer cell proliferation and migration via regulating long noncoding RNA (lncRNA) ANRIL. These results indicated that propofol might be a perfect anesthetic agent during cancer surgery. Although previous studies have explored the influence of propofol on lung cancer, the underlying synergy mechanism of DDP and propofol in lung cancer cells are not yet available. Thus, this report was focused on investigating the role of propofol in lung cancer cell biological functions and explored the correlative potential mechanisms. These results will be of great importance for lung cancer clinical treatment.
Many studies have provided evidence that DDP resistance is connected with changed cell signaling pathway, including Akt/mTOR, MAPK2, and Wnt/β-catenin signaling pathways [15–17]. Numerous studies have demonstrated that the Wnt/β-catenin signaling pathway is normally activated by oncogene in NSCLC. Wnts belongs to secreted glycolipo proteins and launches a signaling cascade to mediate organ development and keep homeostasis of well-developed tissues by combining with its cognate receptors [18]. Abnormal Wnt/β-catenin signaling may result in various human diseases, including Alzheimer’s disease, polycystic kidney disease, osteoarthritis, and various cancers [19–21]. Also, the Wnt/β-catenin signaling pathway is involved in many biological processes, such as cell growth, apoptosis, differentiation, and invasion, and Wnt/β-catenin signaling inhibition results in increased levels of apoptosis [22].
In the present study, A549/DDP and A549 cells were chosen to clarify the role and mechanism of propofol in the sensitivity of lung cancer cells to DDP. Our study might help to elucidate underlying mechanism of propofol in withstanding chemotherapeutic efficiency and provide novel insights into DDP tolerance of lung cancer in clinical
Material and Methods
Cell culture and drug treatment
The human lung cancer cell line A549 and the DDP-resistant lung cell line A549/DDP were from Nanjing Keygen Biotech. All the cells were maintained in RPMI-1640 medium (Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 mg/mL streptomycin and 100 IU/mL penicillin in 5% CO2 at 37°C. Then the A549 and A549/DDP cells were cultured in 0, 10, 20, 40, 80, 160, and 320 μM DDP for 48 hours, or stimulated by 0, 1, 5, and 10 μg/mL propofol [11,23] for 48 hours.
MTT (thiazolyl blue tetrazolium bromide) assay
MTT assay was carried out to assess the chemical sensitivity of A549/DDP and A549 cells to DDP or propofol. In brief, medium supplemented with DDP (0, 10, 20, 40, 80, 160, and 320 μM) or propofol (0, 1, 5, and 10 μg/mL) were used to culture the cells in 96-well plates for 48 hours. Afterward, 10 μL MTT solution (Gibco, USA) was added to each well and incubated at 37˚C for an additional 4 hours and then 100 mL dimethyl sulfoxide (DMSO) was used to dissolve the crystal. The optical density (OD) at 490 nm was determined by a micro-plate reader (Thermo Fisher USA), then we obtained the inhibition rate of cells and calculated the IC50 value. The design formula was as follows: cell inhibition rate (%)=1−(experimental group OD value/normal group OD value)×100%.
Flow cytometry analysis
Flow cytometry was conducted to evaluate the cell apoptosis. A549/DDP and A549 cell apoptosis was detected using an Annexin V-FITC/PI detection kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. Briefly, 0, 1, 5, and 10 μg/mL propofol were used to treat the cells for 48 hours. Subsequently, cells were gathered and re-suspended in Annexin V/FITC and propidium iodide (PI) buffer. After that, cell apoptosis was analyzed by a flow cytometer (BD FACSCalibur, USA), and the data were analyzed by WinMDI (version 2.5; Purdue University Cytometry Laboratories, West Lafayette, IN, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from cells with TRIzol reagent (Invitrogen) following the manufacturer’s instructions. Then the total RNA was reversed into cDNA in accordance with the instructions of PrimeScript RT Reagent Kit (Beyotime, China), and quantitative real-time polymerase chain reaction (qRT-PCR) analysis was conducted using the SYBR PrimeScript RT-PCR Kit (TaKaRa) with ABI 7500 Real-Time PCR System (Agilent Technologies, USA). Primer sequences for PCR were listed as following:
-
c-myc forward, 5′CGCTCTAGAGGAAAAGTAAGGAAAACGATTCCTTC3′;
reverse, 5′CGCTCTAGATTGGCTCAATGATATATTTGCCAG3′;
-
β-catenin forward, 5′GTCTGAGGACAAGCCACAGGACTAC-3′;
reverse, 5′AATGTCCAGTCCGAGATCA GCA3′;
-
GAPDH forward, 5′CTTTGGTATCGTGGAAGGACTC3′;
reverse, 5′GTAGAGGCAGGGATGATGTTCT3′.
GAPDH was used as the internal control. The target gene relative levels were analyzed by the 2−ΔΔCq method [24].
Western blot analysis
The protein of lung cancer cells was harvested, centrifuged and degraded by radioimmunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China). Subsequently, the protein samples were subjected to 10% SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) and then transferred onto PVDF membranes refer to the manufacturer’s instructions. Then membranes were incubated in 5% skim milk for 2 hours at room temperature and maintained in the specific primary antibodies against Bax (cat no. ab32503; 1: 1000; Abcam), cleaved caspase-3 (cat no. ab2302; 1: 1000; Abcam), Bcl-2 (cat no. ab32124; 1: 1000; Abcam), pro-caspase3 (cat no. ab32499; 1: 1000; Abcam), β-catenin (cat no. ab32572; 1: 1000; Abcam), c-myc (cat no. Ab32072; 1: 1000; Abcam) and GAPDH (cat no. ab181602; 1: 1000; Abcam) overnight at 4°C. Thereafter, the membranes were incubated with horseradish peroxidase (HRP)-linked secondary antibody (cat no. ab205718; 1: 2,000; Abcam) for 2 hours at room temperature. The proteins were determined by electrochemiluminescent (ECL) system (Pierce, Rockford, USA).
Statistical analysis
These results of multiple experiments were reported as the mean±standard deviation (SD). GraphPad Prism 5 statistical software was carried out for statistical analyses (GraphPad Software, CA, USA). The statistically significant differences were calculated using Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was defined as statistically significant.
Results
The anti-proliferative effect of DDP on A549 and A549/DDP cells
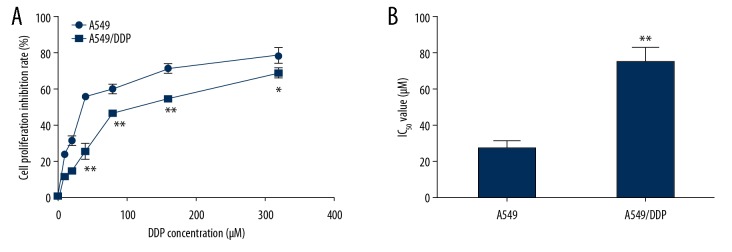
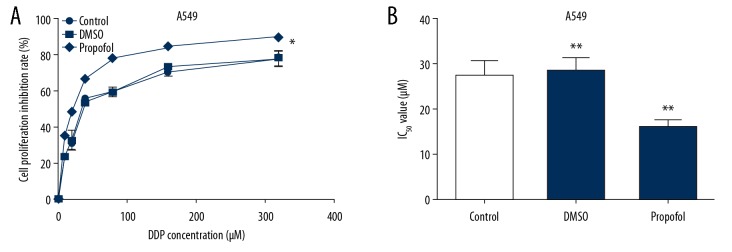
Firstly, A549 and A549/DDP cells were stimulated with indicated DDP (0, 10, 20, 40, 80, 160, and 320 μM) for 48 hours, MTT assay was conducted to measure the cytotoxicity of DDP and the value of IC50 was determined. As presented in Figure 1A, the suppression influence of DDP on A549 cell proliferation was remarkably higher than that on A549/DDP cells. Moreover, the value of IC50 for DDP was obviously lower in A549 cells compared to A549/DDP cells (Figure 1B), indicating that DDP resistance of A549 cells was lower than that in A549/DDP cells.
Figure 1.
DDP suppressed A549/DDP and A549 cells proliferation. The lung cancer cells were stimulated by various concentrations of DDP for 48 hours, and the A549/DDP and A549 cells proliferation suppression rates were determined by MTT assay. (A) The inhibitory effect of DDP on A549/DDP and A549 cell growth. (B) IC50 value of DDP. The results were shown as means±SD. * P<0.05, and ** P<0.01 compared to A549 cells. DDP – cis-diamminedichloroplatinum also known as cisplatin; MTT – thiazolyl blue tetrazolium bromide; SD – standard deviation.
Propofol stimulation decreased cell viability and promoted apoptosis both in A549/DDP and A549 cells
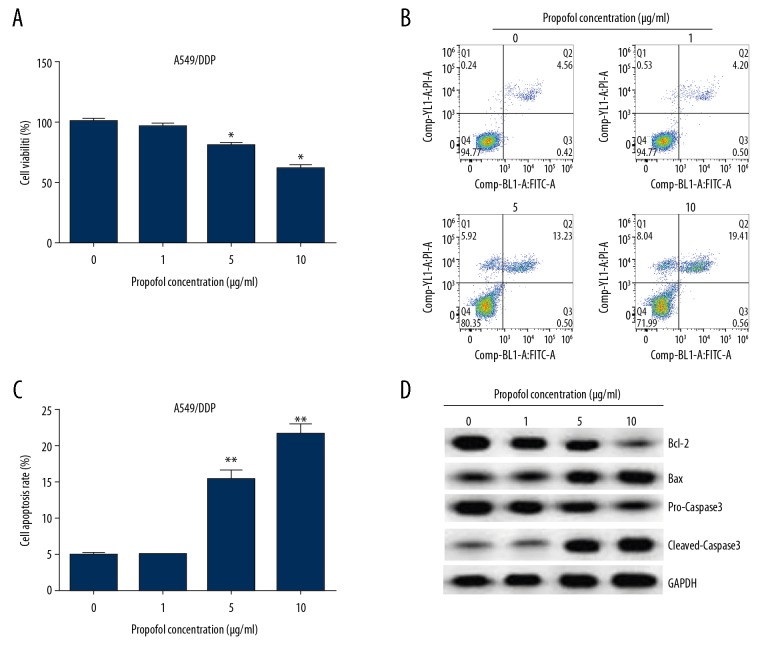
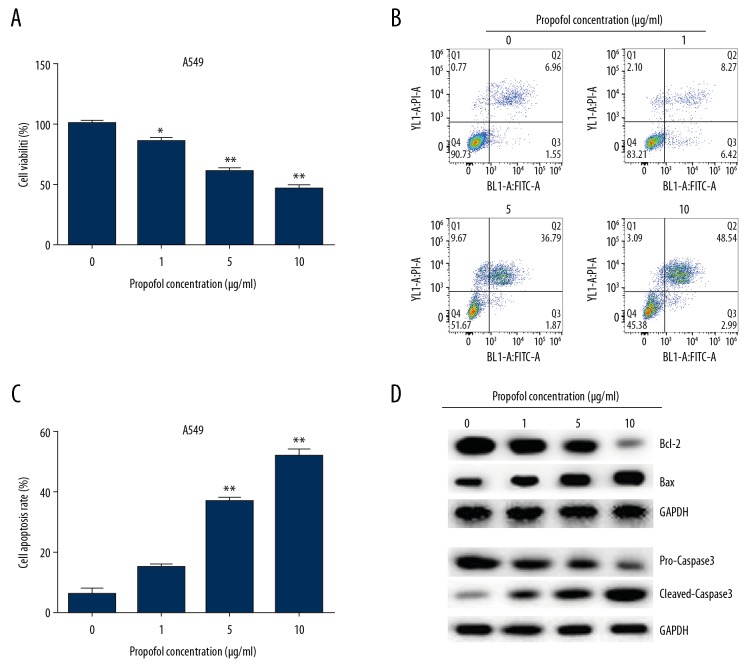
To investigate the roles of propofol in A549/DDP and A549 cells, propofol (0, 1, 5, and 10 μg/mL) was used to induce cells for 48 hours. Then MTT assay and flow cytometry analysis were performed to measure cell viability and apoptosis, respectively. The results from MTT assay showed that propofol notably suppressed cell viability in both A549/DDP and A549 cells compared to the untreated cells. Results presented in Figures 2A and 3A showed that propofol dramatically decreased A549/DDP cell viability at concentrations of 5 and 10 μg/mL (Figure 2A), while 1, 5, and 10 μg/mL propofol significantly suppressed A549 cells viability (Figure 3A). Therefore, 5 μg/mL of propofol was chosen for use in further experiments. Moreover, the results from flow cytometry assay revealed that the ratio of apoptotic cells were induced in propofol-treated A549/DDP (Figure 2B, 2C) and A549 cells (Figure 3B, 3C). Besides, the apoptosis-related protein expressions were determined by western blot, as presented in Figures 2D and 3D, propofol obviously increased the cleaved-caspase-3 and Bax expression in A549/DDP and A549 cells compared to the untreated cells, while, Bcl-2 and pro-caspase-3 expression levels were suppressed after propofol treatment.
Figure 2.
Propofol depressed A549/DDP cells growth and promoted apoptosis. We used 0, 1, 5, and 10 μg/mL propofol to induce A549/DDP cells for 48 hours. (A) Cell viability was evaluated by MTT assay. (B) Flow cytometry was carried out to determine cell apoptosis. (C) Apoptotic cell rate was calculated and presented. (D) The apoptosis-related proteins were assessed by western blot. These data were shown as means±SD. * P<0.05, and ** P<0.01 compared to 0 μg/mL propofol treated cells. DDP – cis-diamminedichloroplatinum also known as cisplatin; MTT – thiazolyl blue tetrazolium bromide; SD – standard deviation.
Figure 3.
Propofol suppressed A549 cells viability and promoted apoptosis. We used 0, 1, 5, and 10 μg/mL propofol to treat A549 cells for 48 hours. (A) Cell viability, (B, C) cell apoptosis, and (D) apoptosis-associated proteins levels were detected by MTT, flow cytometry and western blot assay in A549 cells. The results were shown as means±SD. * P<0.05, and ** P<0.01 compared to 0 μg/ml propofol treated cells. DDP – cis-diamminedichloroplatinum also known as cisplatin; MTT – thiazolyl blue tetrazolium bromide; SD – standard deviation.
Propofol enhanced the sensitivity of lung cancer cells to DDP
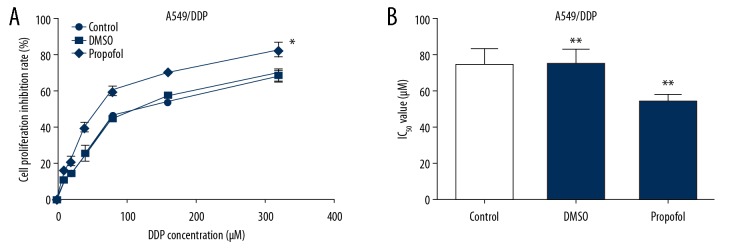
To further explore the role of propofol in the sensitivity of lung cancer cells to DDP, A549/DDP and A549 cells were induced by indicated DDP (0, 10, 20, 40, 80, 160, and 320 μM) and 5 μg/mL propofol or DMSO for 48 hours. MTT was carried out to detect the DDP cytotoxicity and IC50 of DDP. We found that propofol significantly elevated the suppression function of DDP on A549/DDP (Figure 4A) and A549 (Figure 5A) cell proliferation compared to the control. In addition, the value of IC50 for DDP in A549/DDP (Figure 4B) and A549 (Figure 5B) cells were reduced after propofol treatment.
Figure 4.
Propofol promoted the suppression effect of DDP on A549/DDP cell proliferation. We used 0, 10, 20, 40, 80, 160, and 320 μM DDP to stimulate A549/DDP cells and then cultured in 5 μg/mL propofol or DMSO for 48 hours. The influence of propofol on cell growth in A549/DDP cells (A) were determined. MTT assay was conducted to evaluate the IC50 value of DDP in A549/DDP (B) cells after propofol treatment. Results were exhibited as means±SD. * P<0.05 and ** P<0.01 compared to control. DDP – cis-diamminedichloroplatinum also known as cisplatin; MTT – thiazolyl blue tetrazolium bromide; SD – standard deviation.
Figure 5.
Propofol promoted the inhibitory effect of DDP to A549 cell proliferation. We used 0, 10, 20, 40, 80, 160, and 320 μM DDP to treat A549 cells and then cultured in 5 μg/mL propofol or DMSO for 48 hours. MTT assay was conducted to determine cell proliferation (A) and measure the IC50 value (B) in propofol-treated A549 cells. Results were exhibited as means±SD. * P<0.05 and ** P<0.01 compared to control. DDP – cis-diamminedichloroplatinum also known as cisplatin; DMSO – dimethyl sulfoxide; MTT – thiazolyl blue tetrazolium bromide; SD – standard deviation.
Propofol decreased DDP resistance in A549/DDP and A549 cells via inhibiting the Wnt/β-catenin signaling pathway
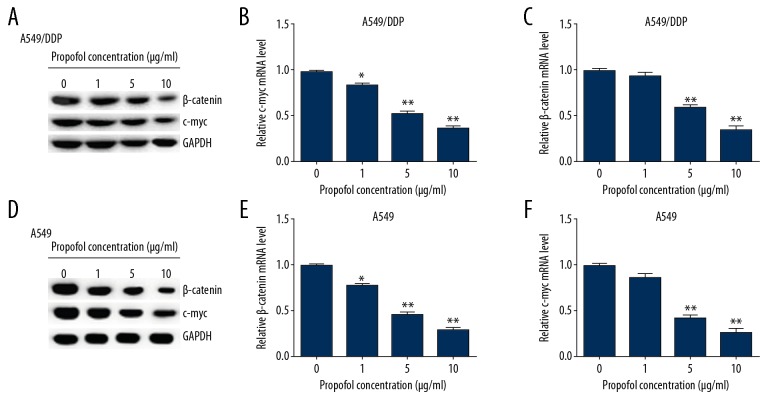
To further explore whether propofol played a significant role in DDP resistance of lung cancer cells, 0, 1, 5, and 10 μg/mL propofol were added into A549/DDP and A549 cells for 48-hour treatment. Then the relative protein and gene levels of the Wnt/β-catenin pathway in A549/DDP and A549 cells were detected by western blot assay and qRT-PCR. As presented in Figure 6, propofol markedly depressed the β-catenin and cmyc protein levels in A549/DDP (Figure 6A) and A549 (Figure 6D) cells in a dose-dependent manner, as compared to the control. Similarly, the mRNA levels of β-catenin and cmyc were significantly decreased in A549/DDP (Figure 6B, 6C) and A549 (Figure 6E, 6F) cells after propofol treatment. These data demonstrated that propofol could enhanced the lung cancer cell sensitivity to DDP by inhibiting the Wnt/β-catenin signaling pathway.
Figure 6.
Effects of propofol on the Wnt/β-catenin signaling pathway in A549/DDP and A549 cells. A549/DDP and A549 cells were stimulated by 0, 1, 5, and 10 μg/mL propofol for 48 hours, then western blot assay and qRT-PCR were performed to assess protein and mRNA levels. (A) β-catenin and c-myc protein expressions were detected in A549/DDP cells. The mRNA levels of β-catenin (B) and c-myc (C) were evaluated by qRT-PCR in A549/DDP cells. (D) β-catenin and c-myc expressions were determined in A549 cells. qRT-PCR assay was used to test β-catenin (E) and c-myc (F) mRNA levels in A549 cells. Results were shown as the mean±SD. * P<0.05 and ** P<0.01 compared to control. DDP – cis-diamminedichloroplatinum also known as cisplatin; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation.
Discussion
DDP, one of the most frequently used drugs in chemotherapy, exerts anti-tumor effects on various cancers, including ovarian cancer, head and neck squamous cell cancer, as well as lung cancer [25–27]. In clinically, DDP plays anti-cancer roles by combing with carboplatin, gemcitabine, paclitaxel, and irinotecan [28]. Lung cancer is identified as a common cancer all over the world with its highest morbidity and mortality yearly. Many efforts have been made in lung cancer treatment over the past few decades, including surgical resection, chemotherapy, and radiation [29]. However, the drug resistance of DDP limits the therapeutic effect in lung cancer patients, and it is identified as the main obstacle in cancer treatment. Therefore, investigation of the underlying mechanism of DDP resistance and exploit efficient therapies are important for solving chemo-resistance.
In this report, A549/DDP and A549 cell lines were chosen to investigate nosogenesis of DDP resistance in lung cancer. Our data suggested that DDP obviously restrained the cell growth of A549/DDP and A549 cells, and the cell proliferation inhibition rate of DDP on A549 cells was significantly higher than that in A549/DDP cells. Meanwhile, compared to A549 cells, DDP IC50 value was greater in A549/DDP cells, suggesting that the resistance of A549/DDP cells to DDP was stronger than A549 cells.
Propofol is a frequently used anesthetic in clinical surgery and cancer treatment. Many reports have demonstrated the anti-tumor character of propofol. Peng et al. [30] reported that propofol suppressed human gastric cancer cell proliferation and promoted apoptosis through regulating microRNA-451 and MMP-2 expression. Moreover, the study of Su et al. [31] showed that propofol stimulated epithelial ovarian cancer cell apoptosis via upregulating microRNA let-7i level. Therefore, to further study the role of propofol in A549/DDP and A549 cells, in our study, cells were stimulated by propofol (0, 1, 5, and 10 μg/mL) for 48 hours. Our study data indicated that propofol suppressed cell viability and accelerated apoptosis both in A549/DDP and A549 cells. Bcl-2 and Bax are known to be vital regulators in cell death signaling and ultimately result in apoptotic cell death [32], which was in accordance with our observation that cleaved-caspase-3 and Bax expression levels were increased, and Bcl-2 and pro-caspase-3 expression levels were suppressed after propofol treatment in lung cancer cells. These data indicated that propofol restrained cell viability and expedited apoptosis by inhibiting expression of Bcl-2 and pro-caspase-3, and it enhanced cleaved-caspase-3 and Bax expression levels, suggesting that propofol might be a new appropriate anesthetic for lung cancer treatment.
Several reports have illustrated that propofol could be used to combine with other chemotherapeutic drug in clinical settings, drugs such as gemcitabine or paclitaxel [33]. Thus, we further evaluated the combined effect of various concentrations of DDP and propofol in lung cancer cells. Different concentration of DDP (0, 10, 20, 40, 80, 160, and 320 μM) combined with 5 μg/mL propofol or DMSO were used to treat A549/DDP or A549 cells for 48 hours. Our findings demonstrated that propofol significantly improved the inhibitory function of DDP in lung cancer cell proliferation compared with the untreated cells. Moreover, the IC50 value for DDP was also reduced after propofol treatment both in 549/DDP and A549 cells. Above all, we provided evidence that propofol enhanced the A549/DDP and A549 cells sensitivity to DDP and might provide novel therapies for anti-DDP-resistant therapy for lung cancer.
Growing evidence has demonstrated the roles of propofol in lung cancer, nevertheless, further explorations are also needed to investigate the potential mechanisms. More and more evidences are being reported that indicates that the excitation of Wnt/β-catenin signaling could block the procession of multiple proteins and affect drug resistance of many cancers including lung cancer [34]. Besides, the Wnt/β-catenin signaling pathway is involved in the cell progression, including cell growth, migration and apoptosis [35]. Nevertheless, the correlation between medicine resistance and the Wnt/β-catenin pathway need to be elucidated. Based on the research of previous reports, in this study we tested the effect of propofol on the drug resistance-associated pathway Wnt/β-catenin in A549/DDP and A549 cells. Recent reports have shown that DDP resistance in lung cancer cells could be reversed via suppressing the PI3K/AKT signaling pathway [36]. Furthermore, Liu et al. [37] indicated that the JNK signaling pathway inactivation could increase sensitivity of hepatocellular carcinoma cells to DDP. Partly in accordance with these reports, our results suggested that propofol remarkably reduced the β-catenin and cmyc mRNA and protein levels in A549/DDP and A549 cells in a dose-dependent manner compared to the control. These data indicated that Wnt/β-catenin might be a vital regulator in the role of propofol in the regulation of DDP resistance of A549/DDP and A549 cells. However, in vivo studies were not performed in our study and this was a limitation that needs to be addressed with further studies.
In summary, these results demonstrated that propofol inhibited lung cancer cell DDP-resistance by regulating the Wnt/β-catenin signaling pathway, suggesting that propofol might be a perfect anesthetic for lung cancer therapy, and provided a new therapeutic strategy for lung cancer DDP-tolerance treatment in clinical settings. However, this study is only a preliminary study of the role of propofol in DDP resistance in lung cancer. In order to make the role of propofol in DDP resistance more convincing, more in-depth research is needed. For example, to determine whether propofol directly or indirectly regulates the Wnt/β-catenin signaling pathway, Wnt/β-catenin signaling pathway inhibitors should be used. Besides, the effect of propofol on lung cancer DDP resistance should be explored in vivo. We will study these issues in the future.
Conclusions
Propofol can enhance the sensitivity of lung cancer cells to DDP by inhibiting the Wnt/β-catenin signaling pathway, and it is a potential compound for the treatment of lung cancer.
Footnotes
Source of support: Departmental sources
References
- 1.Cao X, MacNaughton P, Laurent JC, Allen JG. Radon-induced lung cancer deaths may be overestimated due to failure to account for confounding by exposure to diesel engine exhaust in BEIR VI miner studies. PLoS One. 2017;12:e184298. doi: 10.1371/journal.pone.0184298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plescia M, Henley SJ, Pate A, et al. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. Am J Public Health. 2014;104:S388–95. doi: 10.2105/AJPH.2013.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazandjian D, Gong Y, Keegan P, et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1747. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niyongere S, Saltos A, Gray JE. Immunotherapy combination strategies (non-chemotherapy) in non-small cell lung cancer. J Thorac Dis. 2018;10:S433–50. doi: 10.21037/jtd.2017.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Yin C, Su S, et al. Long-term effects of neoadjuvant radiotherapy, adjuvant radiotherapy, and chemotherapy-only on survival of locally advanced non-small cell lung Cancer undergoing surgery: A propensity-matched analysis. BMC Cancer. 2018;18:1067. doi: 10.1186/s12885-018-4900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jett JR, Hatfield AK, Hillman S, et al. Alternating chemotherapy with etoposide plus cisplatin and topotecan plus paclitaxel in patients with untreated, extensive-stage small cell lung carcinoma: A phase II trial of the North Central Cancer Treatment Group. Cancer. 2003;97:2498–503. doi: 10.1002/cncr.11377. [DOI] [PubMed] [Google Scholar]
- 7.Yan HQ, Huang XB, Ke SZ, et al. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Sci. 2014;105:1220–27. doi: 10.1111/cas.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peskin RM. Contemporary intravenous anesthetic agents and delivery systems: Propofol. Anesth Prog. 1992;39:178–84. [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Zhang J, Hong G, et al. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Wang C, Cui Y, et al. Effects of propofol on several membrane characteristics of cervical cancer cell lines. Cell Physiol Biochem. 2016;40:172–82. doi: 10.1159/000452535. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Teng Y, Yang H, Ma J. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-kappaB signal. Braz J Med Biol Res. 2016;49:e5717. doi: 10.1590/1414-431X20165717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WZ, Liu N. Propofol inhibits lung cancer A549 cell growth and epithelial-mesenchymal transition process by upregulation of microRNA-1284. Oncol Res. 2018;27:1–8. doi: 10.3727/096504018X15172738893959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Wu KC, Yang ST, Hsia TC, et al. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res. 2012;32(11):4833–42. [PubMed] [Google Scholar]
- 14.Chen F, Li M, Zhu X. Propofol suppresses proliferation and migration of papillary thyroid cancer cells by down-regulation of lncRNA ANRIL. Exp Mol Pathol. 2019;107:68–76. doi: 10.1016/j.yexmp.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Teng X, Fan XF, Li Q, et al. XPC inhibition rescues cisplatin resistance via the Akt/mTOR signaling pathway in A549/DDP lung adenocarcinoma cells. Oncol Rep. 2019;41:1875–82. doi: 10.3892/or.2019.6959. [DOI] [PubMed] [Google Scholar]
- 16.Pfankuchen DB, Baltes F, Batool T, et al. Heparin antagonizes cisplatin resistance of A2780 ovarian cancer cells by affecting the Wnt signaling pathway. Oncotarget. 2017;8:67553–66. doi: 10.18632/oncotarget.18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Gao YJ, Hou RZ, et al. MicroRNA-206 facilitates gastric cancer cell apoptosis and suppresses cisplatin resistance by targeting MAPK2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:171–80. doi: 10.26355/eurrev_201901_16761. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Liu Z, Zhang X, et al. Inhibition of cytoplasmic GSK-3beta increases cisplatin resistance through activation of Wnt/β-catenin signaling in A549/DDP cells. Cancer Lett. 2013;336:231–39. doi: 10.1016/j.canlet.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin. 2017;49:853–66. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Rong S, Yuan W, et al. High mobility group Box 1 promotes aortic calcification in chronic kidney disease via the Wnt/β-Catenin pathway. Front Physiol. 2018;9:665. doi: 10.3389/fphys.2018.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Yang H, Hu B, Zhang M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp Ther Med. 2017;14:5057–62. doi: 10.3892/etm.2017.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Lu Q, Xie W, et al. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/β-Catenin signaling. Biochem Biophys Res Commun. 2018;496:443–49. doi: 10.1016/j.bbrc.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 23.Meng C, Song L, Wang J, et al. Propofol induces proliferation partially via downregulation of p53 protein and promotes migration via activation of the Nrf2 pathway in human breast cancer cell line MDA-MB-231. Oncol Rep. 2017;37:841–48. doi: 10.3892/or.2016.5332. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expres- sion data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Wei J, Xu S, et al. MiR2103p regulates cell growth and affects cisplatin sensitivity in human ovarian cancer cells via targeting E2F3. Mol Med Rep. 2019;19:4946–54. doi: 10.3892/mmr.2019.10129. [DOI] [PubMed] [Google Scholar]
- 26.Pushpa NC, Janaki MG, Arul PT, et al. Accelerated radiation therapy using weekend boost with concurrent cisplatin in head and neck squamous cell cancers: An Indian Institutional Experience. J Med Imaging Radiat Sci. 2017;48:307–15. doi: 10.1016/j.jmir.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Wang HJ, Yang ZX, Dai XT, et al. Bisdemethoxycurcumin sensitizes cisplatin-resistant lung cancer cells to chemotherapy by inhibition of CA916798 and PI3K/AKT signaling. Apoptosis. 2017;22:1157–68. doi: 10.1007/s10495-017-1395-x. [DOI] [PubMed] [Google Scholar]
- 28.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–23. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch M, Crawford J, Leopold K, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy with thoracotomy in the treatment of clinically staged IIIA non-small cell lung cancer. Cancer. 1994;74:1243–52. doi: 10.1002/1097-0142(19940815)74:4<1243::aid-cncr2820740411>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Peng Z, Zhang Y. Propofol inhibits proliferation and accelerates apoptosis of human gastric cancer cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol Res. 2016;15(2) doi: 10.4238/gmr.15027078. [DOI] [PubMed] [Google Scholar]
- 31.Su Z, Hou XK, Wen QP. Propofol induces apoptosis of epithelial ovarian cancer cells by upregulation of microRNA let-7i expression. Eur J Gynaecol Oncol. 2014;35:688–91. [PubMed] [Google Scholar]
- 32.Smith CC, Guevremont D, Williams JM, Napper RM. Apoptotic cell death and temporal expression of apoptotic proteins Bcl-2 and Bax in the hippocampus, following binge ethanol in the neonatal rat model. Alcohol Clin Exp Res. 2015;39:36–44. doi: 10.1111/acer.12606. [DOI] [PubMed] [Google Scholar]
- 33.Wang YC, Li N, Zhao Y, Zhang LJ. Effects of female sex hormones on chemotherapeutic paclitaxel-induced neuropathic pain and involvement of inflammatory signal. J Biol Regul Homeost Agents. 2018;32:1157–63. [PubMed] [Google Scholar]
- 34.Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104:1303–8. doi: 10.1111/cas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su N, Wang P, Li Y. Role of Wnt/beta-catenin pathway in inducing autophagy and apoptosis in multiple myeloma cells. Oncol Lett. 2016;12:4623–29. doi: 10.3892/ol.2016.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Bao C, Mu Q, et al. Reversal of cisplatin resistance by inhibiting PI3K/Akt signal pathway in human lung cancer cells. Neoplasma. 2016;63:362–70. doi: 10.4149/304_150806N433. [DOI] [PubMed] [Google Scholar]
- 37.Liu XY, Liu SP, Jiang J, et al. Inhibition of the JNK signaling pathway increases sensitivity of hepatocellular carcinoma cells to cisplatin by down-regulating expression of P-glycoprotein. Eur Rev Med Pharmacol Sci. 2016;20:1098–108. [PubMed] [Google Scholar]